Abstract
Background
Vascular endothelial growth factor (VEGF) can induce matrix metalloproteinase (MMP)-9 activities and focal angiogenesis. We hypothesized that VEGF activation of cerebral MMP-9 would require nitric oxide (NO) participation.
Methods
We compared the in vivo effects of: (1) NG-monomethyl-L-arginine (L-NMMA), a non-specific NO synthase (NOS) inhibitor; (2) L-N6-(1-iminoethyl)lysine (L-NIL), an inducible NOS (iNOS) selective inhibitor; and (3) doxycycline, a known non-specific inhibitor of MMP in the mouse brain, using in situ zymography and endothelial marker CD31. 3-nitrotyrosine (3-NT) was used as a surrogate for NO activity. Inflammatory cell markers CD68 and MPO were used to confirm leukocyte infiltration.
Results
VEGF-stimulated MMP-9 activity expressed primarily around cerebral microvessels. L-NMMA suppressed cerebral angiogenesis (p<0.05), especially those microvessels associated with MMP-9 activation (p<0.02) induced by VEGF, comparable to the effect of doxycycline. L-NIL showed similar inhibitory effects. 3-NT confirmed NO levels in the brain. Compared to the lacZ control, VEGF increased inflammatory cell infiltration, especially macrophages, in the induced brain angiogenic focuses.
Conclusions
Inhibition of NO production decreased MMP-9 activity and focal angiogenesis in the VEGF-stimulated brain. Both specific and non-specific inhibition of NOS resulted in similar reductions, suggesting that VEGF-stimulated cerebral MMP activity and angiogenesis are predominantly mediated through iNOS, a specific NOS isoform mediating inflammatory responses.
Keywords: nitric oxide, vascular endothelial growth factor, matrix metalloproteinase
Vascular endothelial growth factor (VEGF) is highly expressed in cerebral vascular malformations. Exaggerated VEGF expression can induce matrix metalloproteinase (MMP)-9 related cerebral angiogenesis contributing to excessive vascular remodeling1 and hemorrhage.2 The exact role of nitric oxide (NO) in MMP activation has been controversial.3, 4 We hypothesized that NO is a critical mediator in VEGF-stimulated MMP-9 activities and angiogenesis in the brain.
Materials and Methods
Male adult CD-1 mice (Charles River,MA) were divided into five groups (approved by University of California, San Francisco Committee on Animal Research): control and VEGF, and three VEGF groups receiving: (1) NG-monomethyl-L-arginine (L-NMMA), a non-specific NOS inhibitor, at 170 mg/kg/day; (2) L-N6-(1-iminoethyl)lysine (L-NIL) (Alexis, CA), an iNOS selective inhibitor, at 17 mg/kg/day, a dosage selected at the plateau of a dose-response curve;5 and (3) doxycycline (Sigma, MO), a non-specific MMP inhibitor, at 30 mg/kg/day, a dose shown to inhibit VEGF-induced MMP-9 activity and angiogenesis.1 Adenoviral-mediated VEGF (AdVEGF) gene transfer in the brain was performed.6 Brain specimens were harvested after two weeks, when the newly formed microvessels increased following AdVEGF transduction6 and while cerebral MMP expression was still elevated.1
For in situ zymography, frozen brain sections were incubated with DQ gelatin conjugate (Molecular Probes). Localization of CD31, 3-nitrotyrosine (3-NT), CD68, MPO expression was assessed following chromogenic staining, or the double-labeled fluorescent staining protocols. Capillary density was used as an index for cerebral angiogenesis.6 Data were collected as the total number of microvessels marked by CD31 and vessels with positive gelatinolytic activities.
Vessel counts were expressed as mean ± standard deviation and analyzed using ANOVA with PLSD. A p value <0.05 was considered statistically significant.
Results
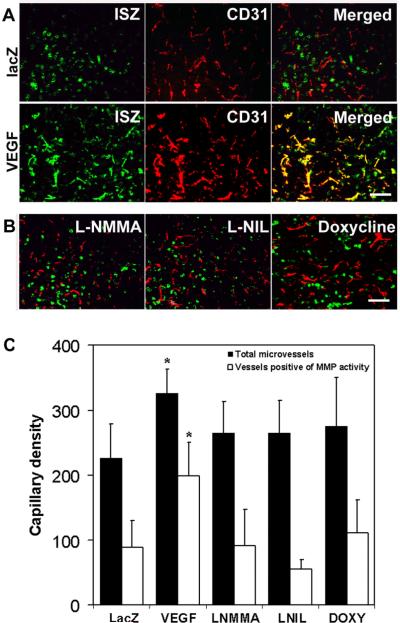
Under VEGF hyperstimulation, gelatinolytic (MMP) activities co-localized with brain microvessels (Figure 1A). Similar to the doxycycline group, gelatinolytic activity around the blood vessels substantially decreased after L-NMMA or L-NIL treatment (Figure 1B). VEGF increased capillary density in the brain in comparison with the lacZ group (326 ± 37 vs. 226 ± 53, microvessel counts, p<0.05), while the number of vessels with positive MMP gelantinolytic activity also increased (199 ± 52 vs. 89 ± 40, p<0.05). Comparable to doxycycline,1 L-NMMA suppressed total capillary density to 265 ± 48 (p<0.05) and vessels positive for MMP activity to 91 ± 56 (p<0.02, Figure 1C). iNOS selective inhibitor, L-NIL, showed similar reduction.
Figure 1.
A. VEGF-stimulated MMP gelatinolytic activities co-localized with microvessels in the angiogenic focus in brain caudate putamen. Merged images (yellow): MMP activity (in situ zymography, green) and microvessels (CD31,red). B. Inhibitors decreased mouse cerebral MMP gelatinolytic activity. Bar =20 μm. C. Quantitation of capillary density (mean±SD, n= 5-6. * p<0.05).
3-NT positive cells intensively increased in the VEGF-stimulated brain, which was blocked by L-NMMA and similarly by L-NIL, as well as after doxycycline treatment (Figure 2A). 3-NT positive signals co-localized with cells near the cerebral microvessels and vascular walls (Figure 2B).
Figure 2.
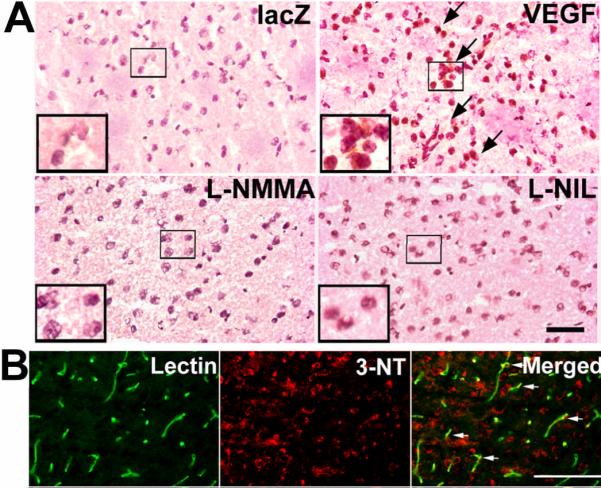
A. 3-NT expression in the brain angiogenic focus (n=3). Arrows point to positive stainings. Inset shows larger magnification. Bar=50 μm. B. 3-NT in cells in close vicinity to and co-localizing with cerebral microvessels (lectin). Bar=100 μm.
Neutrophils (MPO) and especially macrophages (CD68) were increased under VEGF stimulation (Figure 3A). CD68 largely co-localized with 3-NT, further illustrating macrophages as one of the major sources of VEGF-induced NO activity (Figure 3B).
Figure 3.
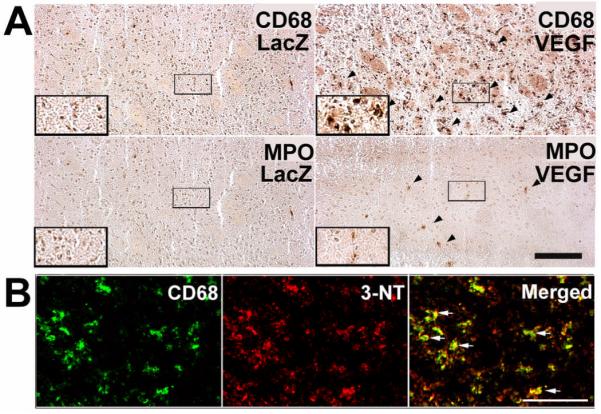
A. Increased inflammatory cells in the VEGF-stimulated brain angiogenic focus (n=3). Arrows point to positive stainings. Insets show larger magnification. Bar=100 μm. B. Macrophage marker CD68 co-localized with 3-NT expressing cells. Bar=100 μm.
Discussion
We demonstrated that inhibition of NOS suppressed VEGF-stimulated MMP activity and angiogenesis in the mouse brain. While in vitro studies have shown that NO can either inhibit or promote MMP activation depending on cell types and stimulants,3, 4 our data from this in vivo mouse model provide direct evidence linking VEGF-induced NO production to increased MMP activity and cerebral angiogenesis.
Although in situ zymography may detect both MMP-2 and -9 activity, increased MMP-9 activation (but not MMP-2 in either pro- or activated form) has been detected by gelatin zymography under VEGF stimulation in vivo1 or in vitro,7 despite MMP-2 involvement in other neurovascular degradation pathways.8 The failure of VEGF to stimulate angiogenesis in MMP-9 knockout mice9 further supports its importance in the VEGF pathway.
iNOS inhibition showed similar effects as non-specific NOS inhibition, suggesting a predominant role of iNOS in VEGF-induced cerebral angiogenesis, although eNOS has been found in vascular endothelial cells in response to VEGF. While NO and MMP can increase VEGF release from tissues, recent evidence indicates that NOS inhibition mainly alters VEGF downstream effector capacity,10 in our case, cerebral MMP activation and angiogenic response. Given that iNOS is a potent source of NO in leukocytes and vascular cells, increased leukocyte infiltration is consistent with VEGF-induced inflammation in brain angiogenesis.
As an important mechanism underlying VEGF-stimulated MMP activity, further studies are needed to explore anti-inflammatory manipulation by local NO inhibition to decrease pathological angiogenesis and stabilize abnormal vasculature to decrease spontaneous hemorrhage risk. On the other hand, accumulating evidence suggests that VEGF-MMP cascade augmentation may be beneficial during stroke recovery.11 The demonstrated participation of NO in the VEGF-stimulation pathway may also open a new window for therapeutic interventions to promote functional revitalization after CNS insults.
Acknowledgments
The authors wish to thank Weizhong Liu, Voltaire Gungab, and members of the UCSF BAVM Study Project (http://avm.ucsf.edu) for their assistance in data collection and manuscript preparation.
Sources of Funding This work was supported in part by a Foundation of Anesthesia Education and Research (FAER) research training grant (C.Z.L.), and by NIH grants R01 NS27713 (W.L.Y.), and P01 NS44155 (W.L.Y.).
References
- 1.Lee CZ, Xu B, Hashimoto T, McCulloch CE, Yang GY, Young WL. Doxycycline suppresses cerebral matrix metalloproteinase-9 and angiogenesis induced by focal hyperstimulation of vascular endothelial growth factor in a mouse model. Stroke. 2004;35:1715–1719. doi: 10.1161/01.STR.0000129334.05181.b6. [DOI] [PubMed] [Google Scholar]
- 2.Lee CZ, Xue Z, Zhu Y, Yang GY, Young WL. Matrix metalloproteinase-9 inhibition attenuates vascular endothelial growth factor-induced intracranial hemorrhage. Stroke. 2007;38:2563–2568. doi: 10.1161/STROKEAHA.106.481515. [DOI] [PubMed] [Google Scholar]
- 3.Gu Z, Kaul M, Yan B, Kridel SJ, Cui J, Strongin A, Smith JW, Liddington RC, Lipton SA. S-nitrosylation of matrix metalloproteinases: Signaling pathway to neuronal cell death. Science. 2002;297:1186–1190. doi: 10.1126/science.1073634. [DOI] [PubMed] [Google Scholar]
- 4.Liu W, Rosenberg GA, Liu KJ. Auf-1 mediates inhibition by nitric oxide of lipopolysaccharide-induced matrix metalloproteinase-9 expression in cultured astrocytes. J Neurosci Res. 2006;84:360–369. doi: 10.1002/jnr.20895. [DOI] [PubMed] [Google Scholar]
- 5.Connor JR, Manning PT, Settle SL, Moore WM, Jerome GM, Webber RK, Tjoeng FS, Currie MG. Suppression of adjuvant-induced arthritis by selective inhibition of inducible nitric oxide synthase. Eur J Pharmacol. 1995;273:15–24. doi: 10.1016/0014-2999(94)00672-t. [DOI] [PubMed] [Google Scholar]
- 6.Yang GY, Xu B, Hashimoto T, Huey M, Chaly T, Jr., Wen R, Young WL. Induction of focal angiogenesis through adenoviral vector mediated vascular endothelial cell growth factor gene transfer in the mature mouse brain. Angiogenesis. 2003;6:151–158. doi: 10.1023/B:AGEN.0000011803.56605.78. [DOI] [PubMed] [Google Scholar]
- 7.Rivilis I, Milkiewicz M, Boyd P, Goldstein J, Brown MD, Egginton S, Hansen FM, Hudlicka O, Haas TL. Differential involvement of mmp-2 and vegf during muscle stretch- versus shear stress-induced angiogenesis. Am J Physiol Heart Circ Physiol. 2002;283:H1430–1438. doi: 10.1152/ajpheart.00082.2002. [DOI] [PubMed] [Google Scholar]
- 8.Wang X, Lee SR, Arai K, Tsuji K, Rebeck GW, Lo EH. Lipoprotein receptor-mediated induction of matrix metalloproteinase by tissue plasminogen activator. Nat Med. 2003;9:1313–1317. doi: 10.1038/nm926. [DOI] [PubMed] [Google Scholar]
- 9.Hao Q, Liu J, Pappu R, Su H, Rola R, Gabriel RA, Lee CZ, Young WL, Yang GY. Contribution of bone marrow-derived cells associated with brain angiogenesis is primarily through leucocytes and macrophages. Arterioscler Thromb Vasc Biol. 2008;28:2151–2157. doi: 10.1161/ATVBAHA.108.176297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhandari V, Choo-Wing R, Chapoval SP, Lee CG, Tang C, Kim YK, Ma B, Baluk P, Lin MI, McDonald DM, Homer RJ, Sessa WC, Elias JA. Essential role of nitric oxide in vegf-induced, asthma-like angiogenic, inflammatory, mucus, and physiologic responses in the lung. Proc Natl Acad Sci U S A. 2006;103:11021–11026. doi: 10.1073/pnas.0601057103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao BQ, Wang S, Kim HY, Storrie H, Rosen BR, Mooney DJ, Wang X, Lo EH. Role of matrix metalloproteinases in delayed cortical responses after stroke. Nat Med. 2006;12:441–445. doi: 10.1038/nm1387. [DOI] [PubMed] [Google Scholar]