Abstract
Dysferlin (DYSF) and myoferlin (MYOF), members of the ferlin family of membrane proteins, are co-expressed in human placental syncytiotrophoblast (STB). Although the role of these ferlin proteins in the placenta has yet to be established, it has been suggested that DYSF and MYOF may contribute to the stability of the apical STB plasma membrane. The release of STB-derived cellular debris increases in the setting of preeclampsia (PE), suggesting relative destabilization of the hemochorial interface. To test whether PE was associated with alterations in placental expression of DYSF and/or MYOF, a cross-sectional study was performed using specimens of villous placenta collected form women with severe PE (n = 10) and normotensive controls (n = 10). DYSF and MYOF expression were examined using quantitative real-time RT-PCR, immunoblotting, and immunofluorescence labeling of tissue specimens. Placental DYSF expression was 57% lower at the mRNA level (p = 0.03) and 38% lower at the protein level (p = 0.026) in severe PE as compared to normotensive subjects. There were no differences in placental MYOF protein or mRNA expression between these groups. No appreciable changes in the distribution of DYSF or MYOF within placental villli were observed in PE relative to control specimens. We conclude that DYSF expression is reduced in severe PE relative to gestational age-matched controls. As DYSF has a role in membrane repair, these data suggest a role for DYSF in the stability of the apical STB plasma membrane and may account, at least in part, for the increased shedding of microparticles from this membrane in PE.
Keywords: dysferlin, myoferlin, preeclampsia, placenta
Introduction
Throughout gestation, the human placenta undergoes an impressive amount of membrane damage and repair. As part of routine turnover, sprout-like structures containing clusters of densely heterochromatic nuclei and cellular debris (commonly referred to as syncytial knots or sprouts, although controversy exists with regard to the specificity of these terms [1]) arise from the apical syncytiotrophoblast (STB) and are shed into the maternal circulation [2–4]. It has been estimated that the average term placenta sheds between 1.5 × 105 [5] and 8.5 × 105 [6] STB-derived particles daily. How the apical plasma membrane of the STB repairs itself after syncytial deportation remains unknown, although one assumes the placenta has developed a highly efficient and rapid mechanism for local membrane repair. Indeed, such repair must occur or else damage to the STB would be extensive.
While the mechanisms that contribute to the repair of damaged plasma membranes have not been completely explicated, current consensus suggests that spontaneous resealing (excepting special circumstances) is unlikely [7]. As such, all cells and tissues are believed to require system(s) whereby foci of damaged membranes can be rapidly contained. One widely endorsed hypothesis holds that plasma membrane disruptions are repaired by “patches” arising from intracellular membrane vesicles [7]. In most instances, this is believed to occur through Ca2+-regulated exocytotic fusion of endomembranous material with the damaged membrane bilayer [7;8].
Dysferlin (DYSF) and myoferlin (MYOF) are two proteins of the ferlin family that contribute to the repair of damaged plasma membranes in striated muscle [7;9–11]. Six ferlin paralogs with sequence homology with the Caenorhabditis elegans FER-1 protein have been described in mammalian systems. In addition to DYSF (also known as FER1L1) and MYOF (also known as FER1L3), members include otoferlin (OTOF, also known as FER1L2), FER1L4, FER1L5, and FER1L6. Ferlin family proteins appear to share conserved, Ca2+-responsive mechanisms whereby membrane fusion events are regulated [12]. The importance of ferlin-dependent membrane repair is highlighted by the pathobiological phenotypes that arise when one of these proteins fails in its normal function. In C. elegans and Drosophila melanogaster, ferlin mutations lead to impaired spermatogenesis and infertility [13–15]. In mice and humans, mutations in DYSF result in certain muscular dystrophies and cardiomyopathy [11;16–19], while OTOF deficiency produces sensorineural deafness [20;21]. Given that DYSF and MYOF are co-expressed in placental trophoblast [22;23], it has been speculated that these proteins might also contribute to STB plasma membrane repair [24].
Preeclampsia is associated with an increase in the amount of trophoblast-derived cellular debris within the maternal circulation [25–27]. Microparticles derived from STB shedding, together with other vasculopathic factors, are thought to contribute to the systemic manifestations of preeclampsia [28]. Preeclamptic placentas are characterized histologically by advanced villous maturation and an increase in the density of syncytial knots [29;30]. Such placentas also contain abnormalities at the ultrastructural level, including focal syncytial necrosis and loss or distortion of STB microvilli [3;31;32]. These pathological features are likely to represent events both antecedent and subsequent to trophoblast deportation [6;33], and suggest that preeclampsia is associated with destabilization of the STB plasma membrane. We have speculated that disturbances in the concentration or function of DYSF and/or MYOF might contribute to STB plasma membrane instability in the setting of preeclampsia [24]. The current study was designed to address whether severe preeclampsia is associated with changes in the expression and/or distribution of placental DYSF and MYOF.
Methods
Reagents and Supplies
A mouse monoclonal antibody against DYSF (clone Ham1/7B6) was purchased from Vector Laboratories (Burlingame, CA). A mouse polyclonal antibody against MYOF was obtained from Novus Biologicals (Littleton, CO). A rabbit polyclonal antibody against MYOF was obtained from Sigma-Aldrich (St. Louis, MO). Mouse anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibody was purchased from Chemicon International (Temecula, CA). TaqMan Universal Master Mix and Assays-on-Demand gene expression target assay mixtures for the detection of dysferlin (Hs00243339_m1), myoferlin (Hs01053408_m1), and the RPLPO large ribosomal protein (4310879E) were obtained from Applied Biosystems (Foster City, CA).
Precast Precise 10% polyacrylamide gels and SuperSignal chemiluminescent detection reagents were obtained from Thermo Scientific (Rockford, IL). ProLong antifade mounting reagent and Alexa Fluor-conjugated secondary antibodies were purchased from Molecular Probes-Invitrogen (Eugene, OR). TRIzol reagent and the SuperScript III first-strand synthesis system for RT-PCR were from Invitrogen (Carlsbad, CA). RNeasy mini columns and the RNase-Free DNase Kits were purchased from QIAGEN (Valencia, CA). All other reagents unless otherwise specified were obtained from Sigma-Aldrich (St. Louis, MO).
Tissue Collection
A cross-sectional study was performed using human placental specimens obtained with informed consent according to a protocol approved by the Biomedical Sciences Institutional Review Board at Ohio State University, Columbus, OH. Eligible subjects included English-speaking women 18–45 years of age delivered of a live singleton infant between 20 and 42 weeks of gestation at The Ohio State University Medical Center. Subjects with major structural fetal abnormalities or prenatally diagnosed aneuploidy were excluded. Given that the current study was exploratory in nature, we sought to examine the expression of placental ferlins among the more extreme cases of preeclampsia (PE) relative to controls, with the expectation that any significant differences could be explored in greater detail in subsequent studies. To this end, cases were restricted to women diagnosed with severe preeclampsia (PE), as established by the National High Blood Pressure Education Program Working Group Report on High Blood Pressure in Pregnancy [34]. For each case, a normotensive control subject matched for gestational age at delivery (± 7 days) was recruited. The criterion of matching for gestational age was instated based on prior data, in which DYSF protein expression was found to be approximately three times higher in first-trimester relative to term placental specimens [22].
Three representative biopsies of villous placental tissue, free of evident pathology, were collected from the maternal surface of each placenta by dissecting ~2-cm sections between the basal and chorionic surfaces. In all cases, tissue was processed as soon as possible following delivery of the placenta (within 10 min). Placental tissue was either fixed in 4% paraformaldehyde (PFA) or thoroughly washed in PBS and flash frozen in liquid nitrogen and stored at − 80°C until used.
Total RNA Extraction and Real-Time RT-PCR
For total RNA extraction from frozen tissues, samples (~60–120 mg) were ground with a mortar and pestle under liquid N2 and immediately placed in TRIzol reagent (Invitrogen). Total RNA was extracted according to the manufacturer’s instructions, with modification. Briefly, following chloroform extraction and centrifugation, the resulting aqueous phase was applied to an RNeasy mini column (QIAGEN) and processed according to the manufacturer’s protocol. This included on-column DNase digestion using the RNase-Free DNase kit (QIAGEN) to remove contaminating genomic DNA. RNA was quantified by spectrophotometric absorbance at 260 nm, and 2 μg from each sample was then reverse-transcribed to cDNA using oligo dT primers (Invitrogen) and SuperScript III reverse transcriptase (Invitrogen).
For quantitative real-time RT-PCR, cDNA was amplified with Assays-on-Demand gene expression target assay primer/probe mixtures for DYSF and MYOF, and detected with a Prism 7300 sequence detector (Applied Biosystems). Amplification mixtures contained 2.5 μl of the appropriate target assay mixture, 1 μl of first-strand cDNA synthesis mixture (corresponding to 50 ng of reverse-transcribed RNA), 25 μl of TaqMan Universal Master Mix (Applied Biosystems), and nuclease-free distilled, deionized water to a total volume of 50 μl. Amplification was performed over 40 cycles of denaturation at 95°C for 15 sec and annealing/extension at 60°C for 1 min.
Amplification curves were analyzed using software provided by the manufacturer (Applied Biosystems). For each target gene, duplicate amplifications were performed. The technician performing the assay was blinded to the status (severe PE vs. control) of each sample. Using cycle threshold (CT) values, target gene expression was normalized to the expression of RPLPO mRNA (endogenous control), as determined in parallel reactions. The choice of RPLPO as an appropriate endogenous control in villous placental tissue was based on both our prior experience (unpublished observations) as well as the work of others [35]. The fold change in normalized DYSF and MYOF values between case and control groups (the referent group being the control group in each instance) was calculated using the comparative CT method and expressed as 2−ΔΔCt [36]. In control reactions, it was confirmed that there was no amplification when the reverse transcriptase step was omitted.
Protein Extraction and Immunoblotting
Placental tissue (~60–120 mg) was pulverized under liquid N2 using a mortar and pestle and subsequently incubated for 20 min in ice-cold octylglucoside lysis buffer (150 mM Na2PO4, 60 mM n-octyl β-D-glucopyranoside, 10 mM D-gluconic acid lactone, 1 mM EDTA) containing protease inhibitor cocktail. The lysates were clarified by centrifugation for 10 min at 14,000 × g and 4°C, and the supernatants were retained. Aliquots of each sample were assayed for protein concentration using the Bio-Rad protein dye reagent (Bio-Rad) using a standard of bovine serum albumin.
Equal amounts of total protein (40 μg/lane for DYSF and 80 μg/lane for MYOF) from each placental extract were resolved by SDS-PAGE using continuous 10% acrylamide gels (Thermo Scientific). Proteins resolved by gel electrophoresis were transferred electrophoretically to nitrocellulose membranes. Membranes were washed in Tris-buffered saline containing 0.2% Tween 20 (TBST), incubated for 1 h at 22°C with 5% nonfat milk in TBST, and then incubated overnight at 4C with primary antibodies in TBST/milk. The antibody dilutions were anti-DYSF, 1:5000, and mouse polycloncal anti-MYOF, 1:1000. After washing in TBST, membranes were incubated with species-appropriate HRP-labeled secondary antibodies (diluted 1:2000) in TBST/milk for 1 h at 22°C. Antibody binding was detected using SuperSignal chemiluminescent substrate (Thermo Scientific) and detected using a VersaDoc Imaging System (Bio-Rad, Hercules, CA). Following detection of DYSF and MYOF, blots were subsequently reprobed using anti-GAPDH, 1:1000 as a control for protein loading. All chemiluminescent signals were collected within the linear response range of the CCD camera and analyzed using Quantity One software (Bio-Rad). The potential for cross-reactivity between the anti-DYSF and anti-MYOF antibodies was assessed in immunoblot assays [23, and unpublished observations], and was found to be negligible.
Immunofluorescence Microscopy (IFM)
Placental specimens were fixed for 1–2 h at room temperature in 4% paraformaldehyde (PFA)/PBS. To facilitate cryopreservation, the specimens were infiltrated with 20% sucrose/PBS overnight at 4°C. Specimens were cut into small pieces and embedded in Tissue-Tek Optimal Cutting Temperature compound (Electron Microscopy Sciences). Embedded tissues were frozen on dry ice and stored at − 80°C until the time of sectioning (performed by the Pathology Core Facility, The Ohio State University, Columbus, OH). Cryostat sections (~6 μm) were stained with hematoxylin/eosin (to evaluate overall morphological integrity). For immunolabeling, unstained cryosections first were equilibrated in PBS at room temperature for 30 min and then incubated in 0.5% SDS in PBS for 10 min at room temperature for antigen retrieval. Sections washed free of SDS were next incubated in blocking solution (5% non-fat dry milk in PBS) for 1 h at room temperature before the addition of antibodies. The sections were then incubated with primary antibodies overnight at 4°C. The primary antibodies used included monoclonal anti-DYSF (1:100 dilution), mouse polycloncal anti-MYOF (1:50), and rabbit polyclonal anti-MYOF (1:50). The sections were subsequently washed and then incubated with fluorochrome-labeled secondary antibodies diluted 1:200 for 60 min at room temperature. After washing in PBS, the nuclei were stained with 5 μg/ml 4′, 6-diamidino-2-phenylindole (DAPI) for 15 min before mounting in the ProLong anti-photobleaching agent. In control preparations, the primary antibodies were omitted, whereas the secondary antibody incubation was retained. As has been previously reported [22;23], these control preparations lacked appreciable immunolabeling, confirming that each of the primary antibodies provided specific immunolocalization patterns (data not shown). Cross-reactivity between the anti-DYSF and mouse polycloncal anti-MYOF antibodies by IFM was found to be insignificant in pilot studies using BeWo, JEG-3, and JAR cells [23]. In addition, double-labeling experiments using anti-DYSF and mouse polyclonal anti-MYOF antibodies revealed distinct labeling patterns in villous placenta sections (not shown), whereas parallel experiments using a combination of mouse and rabbit polyclonal anti-MYOF antibodies demonstrated identical labeling patterns.
Conventional fluorescence images were collected with a Zeiss Axiovert microscope equipped with a Photometrics Cool Snap HQ2 CCD camera (Roper Industries, Pleasanton, CA). Images were captured using Slidebook image analysis software (Version 4.2, Intelligent Imaging Innovations, Denver, CO). All images were collected within the linear response range of the CCD camera. Exported micrographs were compiled with Photoshop CS2 software (Version 9, Adobe Systems).
Statistical Analyses
Statistical analyses were performed using Stata/IC version 10.0 (StataCorp, College Station, TX) and GraphPad Prism version 5.01 (GraphPad Software, La Jolla, CA) software packages. A Shapiro-Wilk test for normality was initially performed on DYSF and MYOF protein and mRNA expression data to confirm the validity of parametric statistical testing. The paired t-test was used to compare expression levels in sets of control and PE placentas matched for gestational age at delivery. To compare demographic and clinical characteristics between the groups, Student’s t-test was used for parametric continuous variables, the Wilcoxon rank-sum test was used for non-parametric continuous variables, and Fisher’s exact test was used for categorical variables (Table). Linear regression analysis was performed to test the association between DYSF or MYOF immunoreactivity and gestational age at delivery. In all instances, a p-value <0.05 was considered significant.
Table 1.
Selected clinical characteristics of severe preeclamptic subjects and controls
| Control n = 10 | Severe PE n = 10 | |
|---|---|---|
| Maternal age (y)* | 26.2 ± 6.1 | 25.1 ± 5.6 |
| Gravidity* | 3.2 ± 2.3** | 1.4 ± 0.7** |
| Parity* | 1.3 ± 1.7** | 0.0 ± 0.0** |
| Gestational age at delivery (weeks)* | 34.0 ± 2.4 | 33.7 ± 2.4 |
| Birthweight (g)* | 2306.8 ± 504.9 | 2228.0 ± 669.7 |
| Max. SBP (mm Hg)* | 135.2 ± 18.9† | 177.1 ± 17.9† |
| Max. DBP (mm Hg)* | 83.3 ± 9.7† | 107.3 ± 9.5† |
| No labor | 20% | 30% |
| Cesarean section | 40% | 70% |
| Maternal race | ||
| White | 70% | 80% |
| Black | 30% | 20% |
Mean ± standard deviation
denotes p ≤ 0.05
denotes p ≤ 0.01
Abbreviations: SBP (systolic blood pressure), DBP (diastolic blood pressure)
Results
Clinical Characteristics of Tissue Donors
Villous placental specimens were collected from 10 cases with severe preeclampsia (PE) and 10 controls matched for gestational age at delivery (± 7 days). All cases had proteinuric hypertension (an increase in blood pressure to at least 140 mm Hg systolic or 90 mm Hg after the 20th week of gestation, combined with urinary protein excretion of at least 0.3 g per 24 hours) in the presence of one or more of the diagnostic criteria for severe PE established by the National High Blood Pressure Education Program Working Group [34]. Specific diagnoses of severe PE were based upon: blood pressure criteria alone (2 cases); thrombocytopenia with laboratory evidence of hepatic dysfunction (1 case); central nervous system (CNS) symptomatology alone (1 case); and blood pressure criteria in addition to proteinuria exceeding 5 g per 24 h, CNS symptomatology, and/or laboratory evidence of hepatic dysfunction (6 cases). In one case, fetal growth restriction was suspected based on third-trimester ultrasound biometric parameters; however, at delivery, the infant was found to have a birthweight appropriate for gestational age. As such, no placentas from cases with intrauterine growth restriction (IUGR) were included in the present analysis.
Selected clinical and demographic data for the severe PE and control groups have been summarized (Table). The two groups were similar with respect to maternal age, gestational age at delivery, infant birthweight, and maternal race. Relative to the control group, subjects with severe PE were more likely to be nulliparous and primigravid. A similar percentage of controls and cases with severe PE were delivered in the absence of labor. A greater proportion of subjects with severe PE were delivered by cesarean section relative to the controls, although this difference was not statistically significant. As expected, the subjects with severe PE exhibited higher maximal systolic and diastolic blood pressures relative to the control group.
Expression of Placental DYSF and MYOF Proteins in Severe Preeclampsia
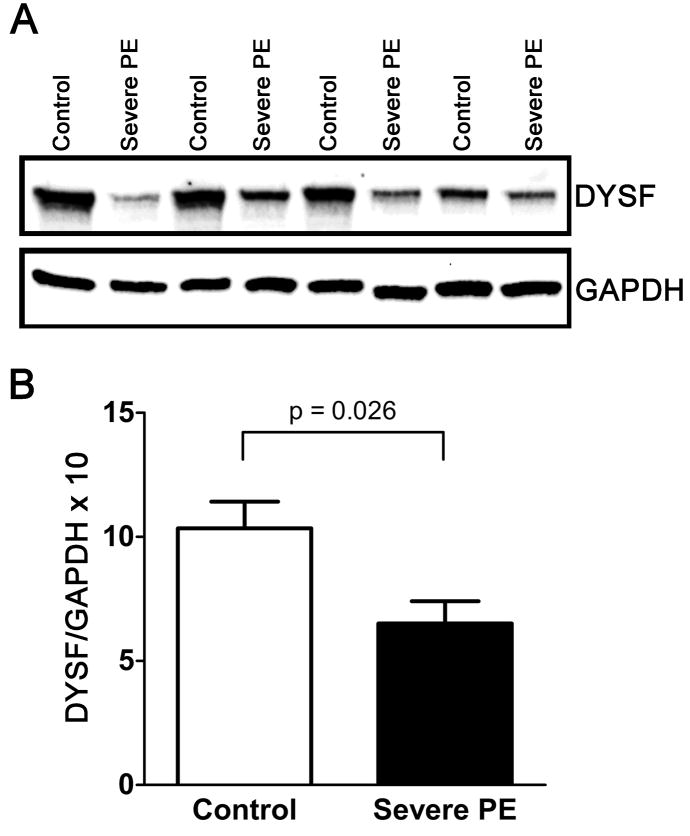
We first examined the relative expression of placental DYSF and MYOF proteins in each group by immunoblot analysis. Using the mouse monoclonal anti-DYSF antibody (Ham1/7B6), a single immunoreactive band with a molecular mass of ~230 kDa was observed (Fig. 1A). Similarly, when detected using a mouse polyclonal antibody, MYOF appeared as a single band also migrating at ~230 kDa (Fig. 2A). In the case of DYSF, pilot experiments demonstrated that delays in sample processing were accompanied by a decrease in the intensity of the major high molecular weight band, coupled with the appearance of multiple lower molecular weight species thought to represent degradation products (Supplemental Fig. 1). Importantly, no prominent lower molecular weight bands were observed in the current series of samples, indicating that sample integrity was sufficiently preserved using the collection methods described to enable reliable quantification.
Figure 1.
Expression of immunoreactive DYSF in subjects with severe preeclampsia (n = 10) and normotensive controls (n = 10) matched for gestational age at delivery. A) Representative immunoblots prepared from villous placental samples demonstrating the relative levels of expression of DYSF and GAPDH in control specimens and those delivered of subjects with severe PE. Four of the ten case-control pairs used in the analysis are shown. B) Values obtained from quantitative analysis of immunoreactive DYSF in placentas delivered of control (white bars) and preeclamptic (black bars) subjects. Data were normalized to GAPDH levels for each specimen and multiplied by a factor of ten to facilitate interpretation (mean ± SEM). Placental DYSF protein expression was significantly different between the groups, P < 0.05 (paired t-test).
Figure 2.
Expression of immunoreactive MYOF in subjects with severe preeclampsia (n = 10) and normotensive controls (n = 10) matched for gestational age at delivery. A) Representative immunoblots prepared from villous placental samples demonstrating the relative levels of expression of MYOF and GAPDH in control specimens and those delivered of subjects with severe PE. Four of the ten case-control pairs used in the analysis are shown. It should be noted that the apparent association between severe PE and reduced immunoreactive MYOF suggested in these immunoblots was not observed in all case-control pairs. B) Values obtained from quantitative analysis of immunoreactive MYOF in placentas delivered of control (white bars) and preeclamptic (black bars) subjects. Data were normalized to GAPDH levels for each specimen and multiplied by a factor of ten to facilitate interpretation (mean ± SEM). N.S., not significant (paired t-test).
By immunoblot analysis, placental DYSF expression was 38% lower on average among subjects with severe PE relative to control specimens (Fig. 1B; p = 0.026, paired t-test). Although a trend toward decreased MYOF levels in the PE placentas was observed, this was not statistically significant (Fig. 2B). In a prior report, quantitative IFM revealed that DYSF protein expression was approximately three times higher in first-trimester relative to term placentas [22]. We therefore performed linear regression to determine whether changes in expression of placental DYSF and MYOF were also present within the third trimester and could be correlated to gestational age at delivery. We observed no significant diminution in immunoreactive DYSF or MYOF expression with advancing gestational age in placental specimens delivered between 28 and 37 weeks of gestation (Supplemental Fig. 2). This result suggests that overall expression levels of DYSF and MYOF are relatively stable during the third trimester. Further, the data presented in this regression analysis reflect the overall decrease in DYSF expression levels of the PE samples in comparison to the control specimens.
Expression of Placental DYSF and MYOF mRNA Species in Severe Preeclampsia
We considered that the decrease in placental DYSF protein expression observed in the setting of severe PE might result from reduced gene transcription or increased protein deportation (e.g., during syncytial shedding), among other possibilities. To examine whether changes in the expression of DYSF and MYOF mRNA species accompanied changes at the protein level, we performed quantitative real-time RT-PCR on the placental samples from cases of severe PE and controls (Fig. 3).
Figure 3.
Expression of placental DYSF and MYOF mRNA species in subjects with severe preeclampsia (n = 10) and normotensive controls (n = 10) matched for gestational age at delivery. A,B) Real-time RT-PCR was used to assess DYSF (A) and MYOF (B) mRNA expression in placentas delivered of control (white bars) and preeclamptic (black bars) subjects. For each target gene, the threshold cycle (CT) at which each mRNA species was detected was normalized to that of RPLPO. The fold-change in transcript expression was calculated in relation to the severe PE group (for DYSF) or control group (for MYOF) using the comparative CT method [36], and expressed as mean ± SEM in the graphs. Only placental DYSF mRNA expression was significantly different between the groups, P < 0.05 (paired t-test). N.S., not significant.
Placental DYSF mRNA levels were 57% lower on average among subjects with severe PE as compared to normotensive subjects (Fig. 3A; p = 0.03, paired t-test). There were no statistically significant differences in placental MYOF mRNA levels between subjects with severe PE and normotensive controls (Fig. 3B). These results suggest that DYSF gene expression in the placenta is relatively decreased in the setting of severe PE, and is not accompanied by compensatory MYOF upregulation. The diminution in placental DYSF mRNA is consistent with (and likely accounts for much of) the observed decrease in immunoreactive DYSF in severe PE.
Distribution of Placental DYSF and MYOF in Severe Preeclampsia
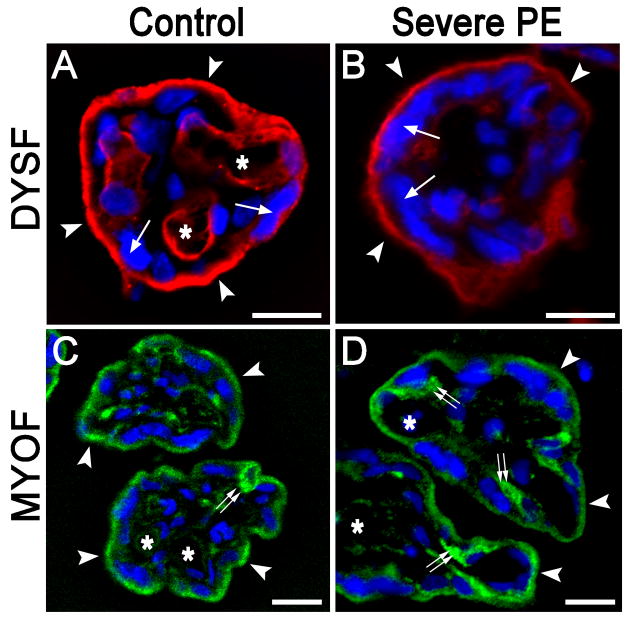
To characterize the expression of DYSF and MYOF in human placental cells in pregnancies complicated by severe PE, IFM was applied to conventional (~6 μm) cryostat sections In pilot experiments, we observed that immunolabeling was highly dependent upon the duration of fixation (Supplemental Fig. 3), such that labeling intensity decreased dramatically with increasing time in fixative. Due to variations in fixation time (between 1 and 2 h) in 50% of the specimens collected, planned quantitative IFM was abandoned.
As we previously documented in term placentas [22], DYSF was expressed in the STB and, to a variable extent, fetal capillary endothelial cells (Fig. 4A). Within the STB, DYSF labeling was largely restricted to the apical plasma membrane, with lesser staining along the basal surface. Interestingly, we observed no significant difference in the general patterns of DYSF localization between PE cases (Fig. 4B) and controls (Fig. 4A), although the overall staining intensity was somewhat lower among the PE samples. Thus, severe PE was not associated with a perturbation in the normal trafficking of DYSF to the apical STB.
Figure 4.
The localization of DYSF and MYOF within villous structures does not change in the setting of severe PE. A and B) Representative IFM localization of DYSF (red) in 6 μm sections of villous placenta from a normotensive subject (A) and a subject with severe PE (B) as observed using conventional epifluorescence microscopy. The most intense signal was found in the STB (the nuclei of which are denoted by single arrows), particularly in the apical plasma membrane regions (arrowheads). Labeling intensity was typically less prominent, but more variable, in fetal capillary endothelial cells (asterisks indicate lumens of vessels). There were no appreciable changes in the distribution of DYSF labeling in either setting. C and D) Representative IFM localization of MYOF (green) in 6 μm placental sections from a normotensive subject (C) and a subject with severe PE (D) as observed using conventional epifluorescence microscopy. MYOF labeling was prominent in STB (arrowheads), with less intense labeling in villous stromal cells and variable labeling in fetal capillary endothelial cells (asterisks). In most instances, cells interior to the STB exhibited pronounced MYOF labeling (double arrows). Nuclei counterstained with DAPI appear blue in all panels. Bars = 20 μm.
MYOF labeling was also evident in the STB (Fig 4C, D) and, to a lesser extent, villous stromal cells and capillary endothelial cells. MYOF intensity was particularly strong among certain cells immediately interior to the STB (double arrows in Figs. 4C and 4D), the identity of which has not been established. Equivalent results were obtained using the either the monoclonal MYOF antibody described previously [23], or a polyclonal MYOF antibody which recently became available from a commercial source (Sigma) (data not shown). As with DYSF, we observed no significant changes in MYOF localization in the setting of severe PE as compared to control specimens by standard epifluorescence.
Discussion
Increased syncytial shedding [37], together with augmented epithelial denudation and ultrastructural abnormalities (e.g., loss or distortion of microvilli at the STB apical surface [3;31;32]), suggest that STB plasma membrane stability may be compromised in the setting of PE. The current study was designed to address whether changes in the amount and/or distribution of ferlin proteins might be associated with this increase in membrane dynamics. We found that placental DYSF expression was reduced in subjects with severe PE relative to gestational age matched controls. By contrast, the expression of MYOF was not reduced significantly, although a slight trend toward decreased protein levels among the PE placentas was observed. No appreciable changes in the distribution of these proteins in severe PE could be discerned. These data suggest a role for DYSF in the stability of the apical plasma membrane of the STB and may account, at least in part, for the increased shedding of microparticles in PE.
While it remains to be established the extent to which DYSF and MYOF contribute to STB apical plasma membrane repair, in light of the function of ferlin family proteins in general, this is not an unreasonable speculation [23;24]. There is compelling evidence that DYSF is required for vesicle fusion during Ca2+-induced skeletal muscle membrane repair [18;19], and MYOF has recently been implicated in the repair of the plasma membrane in cultured endothelial cells [38]. Structural homologs and paralogs of these proteins similarly orchestrate the fusion of membranous vesicles to cell membranes [12–15;21], suggesting a mechanistic theme among ferlin proteins that is consistent with the “patch hypothesis” for membrane repair [7;8]. The assertion that DYSF and MYOF are important for membrane integrity is reinforced by the observation of coincident expression of these proteins at high levels in the STB (this work, and [22;23]); this is to be contrasted with observations in skeletal muscle [39;40], in which DYSF expression alone predominates in multinucleate myofibers, while MYOF is expressed in mononuclear myoblasts. As such, the expression of these Ca2+-dependent membrane repair proteins may be so important in STB that functional redundancy has been selected for during the evolution of primate placentation.
It is interesting to note that, like DYSF, other gene products that are highly expressed in trophoblast are also decreased in the setting of PE; examples include HLA-G [41], placental isoferritin [42], syncytin-1 [43;44], and the GCM1 (glial cells missing) transcription factor [45]. As was suggested by Colbern and colleagues [41], these decreases may be related to an overall reduction in the amount of trophoblastic tissue in placentas from patients with PE. Such findings could be related to increased STB-derived microparticle shedding [27] or impaired CTB fusion [46]. In addition, a functional relationship among GCM1, syncytin-1, and DYSF might contribute to the current findings. In the BeWo choriocarcinoma cell line, mononuclear cells express little DYSF protein until they are induced to form fused, syncytial-like structures through the addition of forskolin, an adenylate cyclase activator [23]. The mechanism of syncytial formation following foskolin treatment in BeWo appears to be mediated through the cAMP/protein kinase A signaling pathway, which enhances GCM1-mediated transcriptional activation [47]. GCM1, in turn, regulates syncytin-1 gene expression [48], which promotes cell-cell fusion. Although the regulatory elements that control DYSF gene expression during syncytialization are unknown, available evidence suggests that DYSF upregulation is subsequent to the activation of GCM1 and syncytin-1 gene expression. Assuming that this model faithfully recapitulates the molecular events of STB formation in vivo, it is reasonable that decreased placental DYSF expression would accompany decreases in syncytin-1 and GCM1 in the setting of PE.
The reduction in mean DYSF expression levels among PE placental specimens was not accompanied by a discernable change in MYOF expression. In part, this finding might reflect a limitation due to the small sample size. Post hoc analysis revealed that 10 pairs was sufficient to detect the observed effect on immunoreactive DYSF expression with 82% power, while the power to detect the more modest reduction in immunoreactive MYOF was only 27%. The lack of a significant decrease in MYOF by immunoblot or real-time RT-PCR analysis might also reflect inherent limitations of these assays, which would tend to underestimate changes in MYOF expression if these were restricted to the STB. Of note, DYSF expression was relatively restricted, being confined largely to the apical STB and, to a lesser extent, fetal capillary endothelial cells; by comparison, MYOF was expressed more broadly among placental cell types. As such, the DYSF expression levels obtained using these methods would be more representative of expression in STB and fetal capillary endothelial cells, whereas MYOF expression would reflect villous expression more broadly. As a consequence, particularly in light of the small sample size, it cannot be discerned from these methods alone whether MYOF expression was actually decreased, or possibly increased, in the STB specifically (as opposed to the placental villi overall). In future studies, a method such as quantitative IFM may provide a more reliable means to assess STB-specific MYOF expression in tissue specimens. However, any such studies must ensure that the specimen collection and fixation procedures are held strictly constant, since stability and immunoreactivity of the ferlin proteins in placental tissue are susceptible to proteolytic degradation and are sensitive to fixation conditions. Further, due to these limitations, careful interpretation of results obtained from retrospective studies of banked fixed tissue must also be considered.
The pathological significance of the observed association between decreased placental DYSF expression in PE requires further investigation. To date, the extent to which pregnancy complications affect mothers carrying fetuses with dysferlin deficiencies (such as those associated with limb girdle limb girdle muscular dystrophy type 2B and Miyoshi Myopathy) has not been addressed. At a minimum, the current data suggest that MYOF may be present in sufficient amounts to compensate for relative DYSF deficiency in the setting of PE. In addition, ferlin-independent compensatory membrane repair pathways might also be invoked in this context. As an example, in clinical cases of dysferlin deficiencies, it has been suggested that skeletal muscle (and possibly other tissues) may utilize a “rescue” membrane repair pathway involving the synaptotagmin like protein Slp2a, which has structural similarities to DYSF [49]. It is entirely plausible that a similar mechanism may be utilized by preeclamptic placentas. Alternatively, villous lesions may also be contained via the deposition of fibrin-rich fibrinoid at sites of STB denudation [50–52]. The utilization of such compensatory repair pathways, and the extent to which these may ameliorate (or exacerbate) pathological sequelae, remains to be determined.
In summary, the current work presents novel evidence suggesting that the expression of placental DYSF, but not placental MYOF, is decreased in the setting of severe PE. While this manuscript was in preparation, we became aware of the preliminary results of a second study in which the expression of DYSF and MYOF in preterm placentas from pregnancies affected by preeclampsia and intrauterine growth restriction was examined [53]. In agreement with our current results, this abstract suggested that DYSF, but not MYOF, mRNA levels were down-regulated in placentas in the setting of PE. To the best of our knowledge, these two reports represent the first to examine this relationship. Although the functional significance of these findings have yet to be corroborated, the current results suggest that relative DYSF deficiency is associated with (and possibly contributes to) STB plasma membrane instability in placentas complicated by PE.
Supplementary Material
Supplemental Figure 1. DYSF integrity is compromised when the time from placental delivery to cryopreservation is increased. Three separate lysates of term human placenta were collected using a protocol previously described [22;54] and probed with an antibody against DYSF. A) With delays of 30 min or longer from the time of delivery to the time of preservation in liquid N2, placental lysates (lanes 1 and 2) exhibited multiple immunoreactive bands migrating below the expected molecular weight of ~230 kDa. B) When samples were snap-frozen within 10 min of delivery of placental delivery (lane 1), most of the prominent molecular weight bands migrating between 33 and 175 kDa were absent. Lanes 2 and 3 represent lysates of BeWo cells cultivated in the absence or presence of forskolin treatment [23], which were used as negative and positive controls for DYSF expression, respectively.
Supplemental Figure 2. The relationship between placental ferlin protein expression and gestational age at delivery. Values obtained from quantitative analysis of immunoreactive DYSF (A) and MYOF (B) in placentas delivered of control (white squares) and preeclamptic (black circles) subjects were plotted against gestational age. Data were normalized to GAPDH levels for each specimen and multiplied by a factor of ten to facilitate interpretation. Linear regression analysis revealed that neither protein was significantly associated with gestational age of delivery between 28 and 37 weeks. The best fit regression lines (solid red line) and the 95% confidence intervals (dashed red lines) for these analyses have been presented.
Supplemental Figure 3. Duration of fixation has a significant influence on fluorescence intensity measurements. To assess the effect of fixation time on antigen-antibody interactions, a single placental specimen from an uncomplicated term cesarean delivery was subjected either to immediate freezing, or varying periods of fixation in 4% PFA, prior to embedding and cryosectioning. Quantitative image analysis was performed on structures within placental villi that were immunofluorescently labeled using anti-DYSF and anti-MYOF antibodies. As shown in the case of DYSF, increases in the duration of fixation were associated with significant decreases in labeling intensity (measurements represent mean ± SEM for three randomly selected images taken from each sample). Simultaneously, increasing fixation time significantly improved the preservation of architectural integrity when sections were stained with hematoxylin/eosin and examined using brightfield microscopy (not shown). Similar results were obtained when MYOF labeling was examined.
Acknowledgments
Funding Sources: The current work was supported by a grant in aid from Perinatal Resources, Inc. (Hilliard, OH) and The Ohio State University Perinatal Research and Development Fund. Additional support was provided by grant HD49628 from the National Institutes of Health.
The authors gratefully acknowledge the help and support of Kelly Senecal, Bruce Nichols, and other members of the Tissue Procurement Lab of The Ohio State University Department of Pathology (Columbus, OH), who assisted with tissue collection. In addition, the authors are indebted to the staff at the Pathology Core Facility at The Ohio State University (Columbus, OH) for technical assistance with cryosectioning and histological staining of placental biopsy specimens. Portions of this work were presented in abstract form at the Annual Meeting of the Society for Gynecologic Investigation, March 17–21, 2009, Glasgow, Scotland, UK.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Castellucci M, Kauffmann P. Basic structure of the villous trees. In: Benirschke K, Kaufmann P, Baergen R, editors. Pathology of the Human Placenta. 5. New York: Springer; 2006. pp. 50–120. [Google Scholar]
- 2.Martin BJ, Spicer SS. Ultrastructural features of cellular maturation and aging in human trophoblast. J Ultrastruct Res. 1973;43:133–149. doi: 10.1016/s0022-5320(73)90074-9. [DOI] [PubMed] [Google Scholar]
- 3.Jones CJ, Fox H. Syncytial knots and intervillous bridges in the human placenta: an ultrastructural study. J Anat. 1977;124:275–286. [PMC free article] [PubMed] [Google Scholar]
- 4.Huppertz B, Tews DS, Kaufmann P. Apoptosis and syncytial fusion in human placental trophoblast and skeletal muscle. Int Rev Cytol. 2001;205:215–253. doi: 10.1016/s0074-7696(01)05005-7. [DOI] [PubMed] [Google Scholar]
- 5.Ikle FA. Dissemination of syncytial trophoblast cells in the maternal blood stream during pregnancy. Bull Schweiz Akad Med Wiss. 1964;20:62–72. [PubMed] [Google Scholar]
- 6.Abumaree MH, Stone PR, Chamley LW. An in vitro model of human placental trophoblast deportation/shedding. Mol Hum Reprod. 2006;12:687–694. doi: 10.1093/molehr/gal073. [DOI] [PubMed] [Google Scholar]
- 7.McNeil PL, Kirchhausen T. An emergency response team for membrane repair. Nat Rev Mol Cell Biol. 2005;6:499–505. doi: 10.1038/nrm1665. [DOI] [PubMed] [Google Scholar]
- 8.Terasaki M, Miyake K, McNeil PL. Large plasma membrane disruptions are rapidly resealed by Ca2+-dependent vesicle-vesicle fusion events. J Cell Biol. 1997;139:63–74. doi: 10.1083/jcb.139.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bansal D, Campbell KP. Dysferlin and the plasma membrane repair in muscular dystrophy. Trends Cell Biol. 2004;14:206–213. doi: 10.1016/j.tcb.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 10.Glover L, Brown RH., Jr Dysferlin in membrane trafficking and patch repair. Traffic. 2007;8:785–794. doi: 10.1111/j.1600-0854.2007.00573.x. [DOI] [PubMed] [Google Scholar]
- 11.Han R, Bansal D, Miyake K, Muniz VP, Weiss RM, McNeil PL, Campbell KP. Dysferlin-mediated membrane repair protects the heart from stress-induced left ventricular injury. J Clin Invest. 2007;117:1805–1813. doi: 10.1172/JCI30848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Washington NL, Ward S. FER-1 regulates Ca2+-mediated membrane fusion during C. elegans spermatogenesis. J Cell Sci. 2006;119:2552–2562. doi: 10.1242/jcs.02980. [DOI] [PubMed] [Google Scholar]
- 13.Achanzar WE, Ward S. A nematode gene required for sperm vesicle fusion. J Cell Sci. 1997;110:1073–1081. doi: 10.1242/jcs.110.9.1073. [DOI] [PubMed] [Google Scholar]
- 14.Ohsako T, Hirai K, Yamamoto MT. The Drosophila misfire gene has an essential role in sperm activation during fertilization. Genes Genet Syst. 2003;78:253–266. doi: 10.1266/ggs.78.253. [DOI] [PubMed] [Google Scholar]
- 15.Smith MK, Wakimoto BT. Complex regulation and multiple developmental functions of misfire, the Drosophila melanogaster ferlin gene. BMC Dev Biol. 2007;7:21. doi: 10.1186/1471-213X-7-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bashir R, Britton S, Strachan T, Keers S, Vafiadaki E, Lako M, Richard I, Marchand S, Bourg N, Argov Z, Sadeh M, Mahjneh I, Marconi G, Passos-Bueno MR, Moreira ES, Zatz M, Beckmann JS, Bushby K. A gene related to Caenorhabditis elegans spermatogenesis factor fer-1 is mutated in limb-girdle muscular dystrophy type 2B. Nat Genet. 1998;20:37–42. doi: 10.1038/1689. [DOI] [PubMed] [Google Scholar]
- 17.Liu J, Aoki M, Illa I, Wu C, Fardeau M, Angelini C, Serrano C, Urtizberea JA, Hentati F, Hamida MB, Bohlega S, Culper EJ, Amato AA, Bossie K, Oeltjen J, Bejaoui K, Kenna-Yasek D, Hosler BA, Schurr E, Arahata K, de Jong PJ, Brown RH., Jr Dysferlin, a novel skeletal muscle gene, is mutated in Miyoshi myopathy and limb girdle muscular dystrophy. Nat Genet. 1998;20:31–36. doi: 10.1038/1682. [DOI] [PubMed] [Google Scholar]
- 18.Lennon NJ, Kho A, Bacskai BJ, Perlmutter SL, Hyman BT, Brown RH., Jr Dysferlin interacts with annexins A1 and A2 and mediates sarcolemmal wound-healing. J Biol Chem. 2003;278:50466–50473. doi: 10.1074/jbc.M307247200. [DOI] [PubMed] [Google Scholar]
- 19.Bansal D, Miyake K, Vogel SS, Groh S, Chen CC, Williamson R, McNeil PL, Campbell KP. Defective membrane repair in dysferlin-deficient muscular dystrophy. Nature. 2003;423:168–172. doi: 10.1038/nature01573. [DOI] [PubMed] [Google Scholar]
- 20.Yasunaga S, Grati M, Chardenoux S, Smith TN, Friedman TB, Lalwani AK, Wilcox ER, Petit C. OTOF encodes multiple long and short isoforms: genetic evidence that the long ones underlie recessive deafness DFNB9. Am J Hum Genet. 2000;67:591–600. doi: 10.1086/303049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roux I, Safieddine S, Nouvian R, Grati M, Simmler MC, Bahloul A, Perfettini I, Le GM, Rostaing P, Hamard G, Triller A, Avan P, Moser T, Petit C. Otoferlin, defective in a human deafness form, is essential for exocytosis at the auditory ribbon synapse. Cell. 2006;127:277–289. doi: 10.1016/j.cell.2006.08.040. [DOI] [PubMed] [Google Scholar]
- 22.Vandre DD, Ackerman WE, Kniss DA, Tewari AK, Mori M, Takizawa T, Robinson JM. Dysferlin is expressed in human placenta but does not associate with caveolin. Biol Reprod. 2007;77:533–542. doi: 10.1095/biolreprod.107.062190. [DOI] [PubMed] [Google Scholar]
- 23.Robinson JM, Ackerman WE, Behrendt NJ, Vandré DD. While dysferlin and myoferlin are co-expressed in the human placenta, only dysferlin expression is responsive to trophoblast fusion in model systems. Biol Reprod. 2009 doi: 10.1095/biolreprod.108.074591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robinson JM, Vandre DD, Ackerman WE. Placental proteomics: a shortcut to biological insight. Placenta. 2009;30 (Suppl A):S83–S89. doi: 10.1016/j.placenta.2008.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Knight M, Redman CW, Linton EA, Sargent IL. Shedding of syncytiotrophoblast microvilli into the maternal circulation in pre-eclamptic pregnancies. Br J Obstet Gynaecol. 1998;105:632–640. doi: 10.1111/j.1471-0528.1998.tb10178.x. [DOI] [PubMed] [Google Scholar]
- 26.Johansen M, Redman CW, Wilkins T, Sargent IL. Trophoblast deportation in human pregnancy--its relevance for pre-eclampsia. Placenta. 1999;20:531–539. doi: 10.1053/plac.1999.0422. [DOI] [PubMed] [Google Scholar]
- 27.Goswami D, Tannetta DS, Magee LA, Fuchisawa A, Redman CW, Sargent IL, von DP. Excess syncytiotrophoblast microparticle shedding is a feature of early-onset pre-eclampsia, but not normotensive intrauterine growth restriction. Placenta. 2006;27:56–61. doi: 10.1016/j.placenta.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 28.Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science. 2005;308:1592–1594. doi: 10.1126/science.1111726. [DOI] [PubMed] [Google Scholar]
- 29.Tenney B, Parker F. The placenta in toxemia of pregnancy. Am J Obstet Gynecol. 1940;39:1000–1005. [Google Scholar]
- 30.Heazell AE, Moll SJ, Jones CJ, Baker PN, Crocker IP. Formation of syncytial knots is increased by hyperoxia, hypoxia and reactive oxygen species. Placenta. 2007;28 (Suppl A):S33–S40. doi: 10.1016/j.placenta.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 31.Anderson WR, McKay DG. Electron microscope study of the trophoblast in normal and toxemic placentas. Am J Obstet Gynecol. 1966;95:1134–1148. doi: 10.1016/s0002-9378(66)80016-9. [DOI] [PubMed] [Google Scholar]
- 32.Jones CJ, Fox H. An ultrastructural and ultrahistochemical study of the human placenta in maternal pre-eclampsia. Placenta. 1980;1:61–76. doi: 10.1016/s0143-4004(80)80016-6. [DOI] [PubMed] [Google Scholar]
- 33.Huppertz B, Frank HG, Kingdom JC, Reister F, Kaufmann P. Villous cytotrophoblast regulation of the syncytial apoptotic cascade in the human placenta. Histochem Cell Biol. 1998;110:495–508. doi: 10.1007/s004180050311. [DOI] [PubMed] [Google Scholar]
- 34.Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Am J Obstet Gynecol. 2000;183:S1–S22. [PubMed] [Google Scholar]
- 35.Founds SA, Conley YP, Lyons-Weiler JF, Jeyabalan A, Hogge WA, Conrad KP. Altered global gene expression in first trimester placentas of women destined to develop preeclampsia. Placenta. 2009;30:15–24. doi: 10.1016/j.placenta.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 37.Redman CW, Sargent IL. Circulating microparticles in normal pregnancy and pre-eclampsia. Placenta. 2008;29 (Suppl A):S73–S77. doi: 10.1016/j.placenta.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 38.Bernatchez PN, Acevedo L, Fernandez-Hernando C, Murata T, Chalouni C, Kim J, Erdjument-Bromage H, Shah V, Gratton JP, McNally EM, Tempst P, Sessa WC. Myoferlin regulates vascular endothelial growth factor receptor-2 stability and function. J Biol Chem. 2007;282:30745–30753. doi: 10.1074/jbc.M704798200. [DOI] [PubMed] [Google Scholar]
- 39.Davis DB, Doherty KR, Delmonte AJ, McNally EM. Calcium-sensitive phospholipid binding properties of normal and mutant ferlin C2 domains. J Biol Chem. 2002;277:22883–22888. doi: 10.1074/jbc.M201858200. [DOI] [PubMed] [Google Scholar]
- 40.de LN, Gallardo E, Soriano M, Dominguez-Perles R, de la TC, Rojas-Garcia R, Garcia-Verdugo JM, Illa I. Absence of dysferlin alters myogenin expression and delays human muscle differentiation “in vitro”. J Biol Chem. 2006;281:17092–17098. doi: 10.1074/jbc.M601885200. [DOI] [PubMed] [Google Scholar]
- 41.Colbern GT, Chiang MH, Main EK. Expression of the nonclassic histocompatibility antigen HLA-G by preeclamptic placenta. Am J Obstet Gynecol. 1994;170:1244–1250. doi: 10.1016/s0002-9378(94)70134-2. [DOI] [PubMed] [Google Scholar]
- 42.Maymon R, Bahari C, Moroz C. Placental isoferritin: a new serum marker in toxemia of pregnancy. Am J Obstet Gynecol. 1989;160:681–684. doi: 10.1016/s0002-9378(89)80058-4. [DOI] [PubMed] [Google Scholar]
- 43.Lee X, Keith JC, Jr, Stumm N, Moutsatsos I, McCoy JM, Crum CP, Genest D, Chin D, Ehrenfels C, Pijnenborg R, van Assche FA, Mi S. Downregulation of placental syncytin expression and abnormal protein localization in pre-eclampsia. Placenta. 2001;22:808–812. doi: 10.1053/plac.2001.0722. [DOI] [PubMed] [Google Scholar]
- 44.Chen CP, Wang KG, Chen CY, Yu C, Chuang HC, Chen H. Altered placental syncytin and its receptor ASCT2 expression in placental development and pre-eclampsia. BJOG. 2006;113:152–158. doi: 10.1111/j.1471-0528.2005.00843.x. [DOI] [PubMed] [Google Scholar]
- 45.Chen CP, Chen CY, Yang YC, Su TH, Chen H. Decreased placental GCM1 (glial cells missing) gene expression in pre-eclampsia. Placenta. 2004;25:413–421. doi: 10.1016/j.placenta.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 46.Langbein M, Strick R, Strissel PL, Vogt N, Parsch H, Beckmann MW, Schild RL. Impaired cytotrophoblast cell-cell fusion is associated with reduced Syncytin and increased apoptosis in patients with placental dysfunction. Mol Reprod Dev. 2008;75:175–183. doi: 10.1002/mrd.20729. [DOI] [PubMed] [Google Scholar]
- 47.Chang CW, Chuang HC, Yu C, Yao TP, Chen H. Stimulation of GCMa transcriptional activity by cyclic AMP/protein kinase A signaling is attributed to CBP-mediated acetylation of GCMa. Mol Cell Biol. 2005;25:8401–8414. doi: 10.1128/MCB.25.19.8401-8414.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yu Z, Zhang W, Kone BC. Signal transducers and activators of transcription 3 (STAT3) inhibits transcription of the inducible nitric oxide synthase gene by interacting with nuclear factor kappaB. Biochem J. 2002;367:97–105. doi: 10.1042/BJ20020588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kesari A, Fukuda M, Knoblach S, Bashir R, Nader GA, Rao D, Nagaraju K, Hoffman EP. Dysferlin deficiency shows compensatory induction of Rab27A/Slp2a that may contribute to inflammatory onset. Am J Pathol. 2008;173:1476–1487. doi: 10.2353/ajpath.2008.080098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mayhew TM, Barker BL. Villous trophoblast: morphometric perspectives on growth, differentiation, turnover and deposition of fibrin-type fibrinoid during gestation. Placenta. 2001;22:628–638. doi: 10.1053/plac.2001.0700. [DOI] [PubMed] [Google Scholar]
- 51.Humphrey RG, Smith SD, Pang L, Sadovsky Y, Nelson DM. Fibrin enhances differentiation, but not apoptosis, and limits hypoxic injury of cultured term human trophoblasts. Placenta. 2005;26:491–497. doi: 10.1016/j.placenta.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 52.Rampersad R, Barton A, Sadovsky Y, Nelson DM. The C5b-9 membrane attack complex of complement activation localizes to villous trophoblast injury in vivo and modulates human trophoblast function in vitro. Placenta. 2008;29:855–861. doi: 10.1016/j.placenta.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kodaman PH, Tang Z, Di Lorenzo A, Guller S, Sessa W. Decreased expression of dysferlin transcript in preterm placentas from pregnancies affected by preeclampsia and intrauterine growth restriction. Reprod Sci. 2009;16 (Supplement):70A. [Google Scholar]
- 54.Robinson JM, Ackerman WE, Tewari AK, Kniss DA, Vandré DD. Isolation of highly enriched apical plasma membranes of the placental syncytiotrophoblast. Anal Biochem. 2009;387:87–94. doi: 10.1016/j.ab.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. DYSF integrity is compromised when the time from placental delivery to cryopreservation is increased. Three separate lysates of term human placenta were collected using a protocol previously described [22;54] and probed with an antibody against DYSF. A) With delays of 30 min or longer from the time of delivery to the time of preservation in liquid N2, placental lysates (lanes 1 and 2) exhibited multiple immunoreactive bands migrating below the expected molecular weight of ~230 kDa. B) When samples were snap-frozen within 10 min of delivery of placental delivery (lane 1), most of the prominent molecular weight bands migrating between 33 and 175 kDa were absent. Lanes 2 and 3 represent lysates of BeWo cells cultivated in the absence or presence of forskolin treatment [23], which were used as negative and positive controls for DYSF expression, respectively.
Supplemental Figure 2. The relationship between placental ferlin protein expression and gestational age at delivery. Values obtained from quantitative analysis of immunoreactive DYSF (A) and MYOF (B) in placentas delivered of control (white squares) and preeclamptic (black circles) subjects were plotted against gestational age. Data were normalized to GAPDH levels for each specimen and multiplied by a factor of ten to facilitate interpretation. Linear regression analysis revealed that neither protein was significantly associated with gestational age of delivery between 28 and 37 weeks. The best fit regression lines (solid red line) and the 95% confidence intervals (dashed red lines) for these analyses have been presented.
Supplemental Figure 3. Duration of fixation has a significant influence on fluorescence intensity measurements. To assess the effect of fixation time on antigen-antibody interactions, a single placental specimen from an uncomplicated term cesarean delivery was subjected either to immediate freezing, or varying periods of fixation in 4% PFA, prior to embedding and cryosectioning. Quantitative image analysis was performed on structures within placental villi that were immunofluorescently labeled using anti-DYSF and anti-MYOF antibodies. As shown in the case of DYSF, increases in the duration of fixation were associated with significant decreases in labeling intensity (measurements represent mean ± SEM for three randomly selected images taken from each sample). Simultaneously, increasing fixation time significantly improved the preservation of architectural integrity when sections were stained with hematoxylin/eosin and examined using brightfield microscopy (not shown). Similar results were obtained when MYOF labeling was examined.