Abstract
Background & Aims
Enteroendocrine cells, the largest and most diverse population of mammalian endocrine cells, comprise a number of different cell types in the gut mucosa that produce, store, and secrete small molecules, peptides and/or larger proteins that regulate many aspects of gut physiology. Little is known about less-typical endocrine cells in the intestinal mucosa that do not contain secretory granules, such as brush or caveolated cells. We studied a subset of these enteroendocrine cells in duodenum that produce several peptides, including endogenous opioids, and that also express the Trpm5 cation channel.
Methods
We studied expression patterns of Trpm5 and other molecules by immunohistochemical and ELISA analyses of intestinal tissues from transgenic mice that express green fluorescent protein from theTrpm5 promoter, as well as wild-type and Trpm5-null mice.
Results
We describe a type of enteroendocrine cell in mouse duodenum that is defined by the presence of the Trpm5, that does not contain typical secretory granules, yet expresses endogenous opioids (β-endorphin and Met-enkephalin) and uroguanylin in apical compartments close to the lumen of the gut.
Conclusion
Solitary chemosensory cells that co-express β-endorphin, Met-enkephalin, uroguanylin and Trpm5 exist in mouse duodenum. These cells are likely to secrete the bioactive peptides into the intestinal lumen in response to dietary factors; release of the opioid peptides requires the Trpm5 ion channel.
Introduction
Numerous endocrine cells are scattered throughout the mucosa of the intestine. These cells are thought to provide the first line of luminal sensing and to participate in the regulation of gut physiology, as well as in overall regulation of nutrition and metabolism.1–3 There are twenty or more different subtypes of enteroendocrine cells based on the major products they secrete. Most enteroendocrine cells release their hormones or regulatory peptides into the local environment from which they can reach nerve endings and/or enter the bloodstream; some other enteroendocrine cells directly release their products into the gut lumen. Regulation of enteroendocrine cells is complex and can involve direct stimulation by the luminal contents, stimulation by hormones from other endocrine cells and/or neuronal stimulation.1
While several types of enteroendocrine cells have been well characterized, a few cell types have unknown functions even though their product(s) are known to be present in the gut. In this paper we focus on a particular subset of enteroendocrine cells in duodenum that produce several peptides, including endogenous opioids, and also express the Trpm5 ion channel. Trpm5 is a cation channel that is highly expressed in taste cells and is required for signal transduction of bitter, sweet and umami [amino acid] tasting substances.6,7 The Trpm5 channel in taste cells appears to be involved in the final step of a signaling cascade that leads to membrane depolarization and propagation of the signal (either through synapses or via secreted peptides) to nerve endings. Trpm5 also has been implicated in chemosensory transduction in solitary chemosensory cells found in gut, respiratory epithelium, olfactory epithelium and elsewhere.6–8
Trpm5 RNA has been found in multiple tissues,6,8 however, apart from taste cells, only solitary chemosensory cells, such as those in gut mucosa, express levels of Trpm5 RNA comparable to those observed in taste cells.6,7 Although these Trpm5-expressing cells are present in all parts of the intestine, here we focused on duodenum, where sensory cells play a predominant role in determining the composition and quantity of the contents entering from stomach and send signals to various other organ systems. These signals then help to effectively process and utilize the food or defend the organism against possible harmful chemicals or toxins.
Proopiomelanocortin (POMC) is expressed in several tissues, particularly the nervous system, and its opioid products (β-endorphin and Met-enkephalin) are well known to mediate analgesia.9 Recent findings indicate that endogenous opioids have additional physiological effects, including roles in glucose homeostasis and central glucose sensing10 and in energy balance and homeostasis.11,12 The endogenous opioids and opioid receptors that participate in these physiological processes are highly expressed by neurons in hypothalamus.13 However, another intimate connection between energy supply and opioids exists in the gut, where the opioids16–18 and their receptors13–15 are also plentiful, and mediate functions such as gastric emptying, gut motility and intestinal secretion.14 For example, the antidiarrheal properties of opiates have been well known for thousands of years.15 It has been shown that β-endorphin and Met-enkephalin are produced in the gut and secreted into the gastric, duodenal and jejunal lumina as well as into the gallbladder.19–22 Very little is known, however, about the type(s) of cells that are the source of endogenous opioids delivered to the lumina of the gut, or what physiological roles these cells may play.
In this paper we focused on Trpm5-expressing cells in mouse duodenum and identified them as a source of β-endorphin and Met-enkephalin released into the gut lumen. We have determined which substances stimulate this release. We have also identified uroguanylin, a peptide from the natriuretic family,23–25 as being produced by the Trpm5-expressing gut cells.
Materials and Methods
Mice
Transgenic mice expressing eGFP from 10kb long Trpm5 promoter, and Trpm5KO mice were described previously.26,5 Their genetic background is C57Bl/6. Control WT mice were C57Bl/6 obtained from Charles Rivers Laboratories or bread at Mount Sinai Animal Facility. All animals were kept on a regular mouse chow and maintained according to the Mount Sinai IACUC policies.
Antibodies
Trpm5, rabbit polyclonal antibody was generated in our laboratory against the epitope CRKEAQHKRQHLERDLPDPLDQK. Chromogranin A, Met-enkephalin, and β-endorphin rabbit polyclonal antibodies were obtained from Immunostar, cat # 20086, #20065, and #20063 respectively. β-endorphin rabbit polyclonal antibodies were also purchased from SIGMA, E1520, and Research Diagnostics, Endorph Abr. Chromogranin B rabbit polyclonal antibody was from Abcam, cat.# ab12242, uroguanylin (Guca2b) rabbit polyclonal antibody was obtained from Orbigen (PAB 11359) (epitope PALPLDLQPVCASQE), and uroguanylin goat polyclonal antibody from Santa Cruz cs-32578 (epitope DDCELCVNVACTGCL).
Immunohistochemistry
Animals were sacrificed by CO2 asphyxia and the intestinal tissues fixed with Zamboni’s or PLP fixative containing 3% paraformaldehyde, 0.019M L-lysine monohydrochloride, and 0.23% sodium m-periodate. The tissues were incubated in 20% sucrose overnight, embedded in OCT and 15 μM sections were prepared using a cryostat. Indirect immunostaining of the sections was Trpm5 cells secrete opioids performed according to protocols of manufacturers of the antibodies. Briefly, the sections were washed in blocking buffer and then incubated with primary antibodies for 2 to 24 hr at RT or at 4 C, respectively. After incubation the sections were washed and incubated with a secondary antibody labeled either with Cy3 or Alexa Fluor 586 for 30 minutes followed by extensive washing. Sections were then viewed and photographed using a Provis AX70 fluorescent microscope (Olympus Optical Co., Ltd., Japan) with a U-CMAD-2 digital camera (Olympus) and Image-pro plus Software v. 3.0 (Media Cybernetics, Maryland USA) and objective lenses: Olympus UPIanFI 10× (Numerical Aperture 0.30), 20× (NA 0.50) and 40× (NA 0.75), or confocal microscope IX-70 Olympus (Olympus Optical Co., Ltd., Japan). In the negative controls, the primary antiserum was omitted. For double labeling with two antibodies, the Zenon Antibody Labeling technique (Molecular Probes) was used and staining performed according to manufacturer’s protocol.
ELISA
A β-endorphin ELISA kit was purchased from MD Biosciences and used according to the manufacturer’s protocol. For most experiments, the intestinal perfusate was diluted 10 to 100 ×in HBSS before the assay. The tested substances were dissolved in HBSS prior to the experiment, with the exception of 20% Intralipid (Sigma I141), which was used as received. Intralipid is an i.v. fluid used in clinical practice for intravenous alimentation. It is an emulsion containing soybean oil, lecithin, and glycerol.
Intestinal perfusions
Four WT and KO animals were fasted overnight and then sacrificed by CO2 asphyxia. 10 cm long pieces of small intestine containing duodenum and upper jejunum were excised and perfused through the jejunal end so that the full length of the duodenum was exposed. Perfusion was through a plastic “yellow” tip connected to i.v. tubes. The perfusion rate was controlled by a peristaltic pump set to deliver 0.5ml per minute. Intestines were first perfused with Hanks buffered solution (SIGMA) for 5 minutes after which a control 1-minute sample was collected. The yellow tip was then switched to a tube with test solution and samples were collected in 1-minute intervals for 6 minutes.
Results
Trpm5 expression in mouse intestine
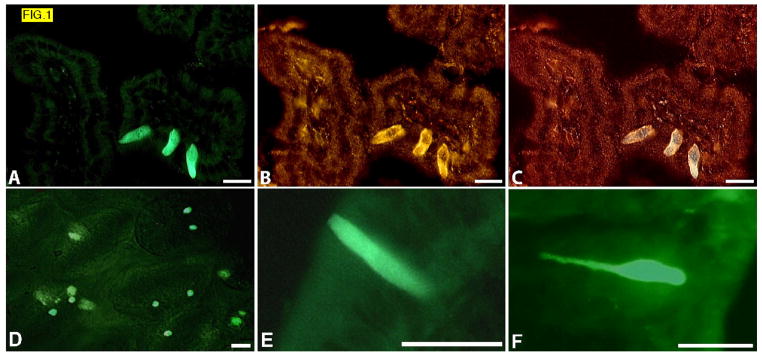
Trpm5 expression in intestinal cells was monitored by immunohistochemistry and expression of a GFP transgene faithfully driven in Trpm5-expressing taste cells by the 12 kb long Trpm5 promoter.26 Both methods revealed a large number of scattered Trpm5-expressing cells throughout the entire gut, from stomach to colon. By comparing GFP transgene fluorescence with Trpm5 immunostaining we determined that all Trpm5-positive GFP-positive gut cells also expressed endogenous Trpm5 protein (FIG. 1A,B,C); the only difference is that fluorescence from the cytoplasmic GFP transgene fills the entire cell body, whereas the immunostaining of the integral membrane protein Trpm5 detects the ion channel in the membrane. Therefore we could reliably use GFP transgene fluorescence as a marker to identify Trpm5-expressing gut cells and to identify other molecules produced by these cells that are co-expressed with Trpm5.
FIG. 1.
(A–C) Sections from duodena of Trpm5-GFP mice. GFP fluorescence (A), indirect immunostaining with Trpm5 antibody (B), overlay (C). GFP fluorescence in freshly dissected “live” epithelium of jejunum (D). Higher magnification view showing typical shape of Trpm5-GFP cells in sections of duodenum (E), and colon (F).
We found that the Trpm5-positive cells are abundant in the gut, predominantly in villi, with highest representation in duodenum, jejunum and ileum where there are several cells per villus. Because of the intensity of the GFP signal, Trpm5-expressing cells could be observed even in live epithelium, providing a good sense of their location within the epithelium (FIG. 1D, Supporting FIG. 1). The Trpm5-positive cells are mostly cylindrical or pear-shaped, with the apical part slightly protruding into the lumen. They often have a long narrow basal extension, particularly in colon, where these extensions are long and resemble small axons (FIG. 1E,F). Interestingly, we also observed numerous Trpm5-positive cells in gallbladder, bile duct and pancreatic ducts, and found particularly heavy clustering of the cells close to and at the papilla of Vater (Supporting FIG. 2).
Trpm5-positive cells in duodenum do not express chromogranin A or chromogranin B
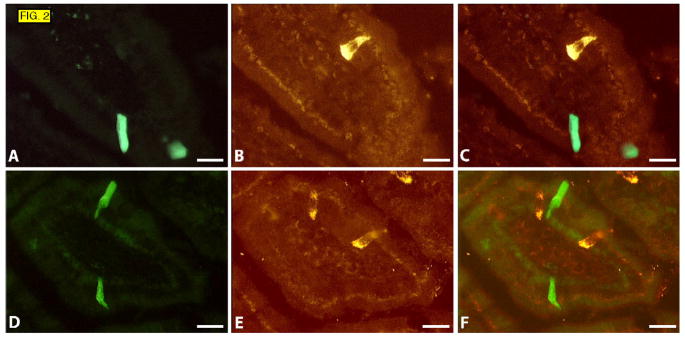
To further characterize the Trpm5-positive cells we tested whether they contain secretory granules characterized by the presence of chromogranin A and/or B. We found that no Trpm5-positive cells expressed these markers (FIG. 2). Moreover, they also did not express some of the peptides and amines known to be present in intestinal enteroendocrine and enterochromaffin cells such as GLP1, secretin, somatostatin, and serotonin (data not shown). Based on their morphological criteria, lack of secretory granules and solitary positioning in the gastrointestinal tract, these cells would appear to be brush cells, also referred to as tufted or caveolated cells.27–29
FIG. 2.
Sections of duodenum from Trpm5-GFP mice. GFP fluorescence (A and D), immunostaining with chromogranin A (B) and chromogranin B antibody (E), overlays (C and F).
Trpm5-positive cells in duodenum express β-endorphin and Met-enkephalin
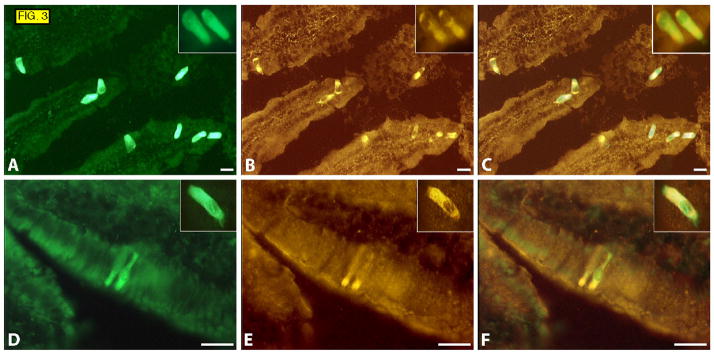
We next carried out more extensive co-expression studies to determine which molecules, known or suggested to play a role in gut physiology, are expressed by the Trpm5-positive cells. We found that almost all of the Trpm5-positive cells in duodenum expressed β-endorphin and Met-enkephalin, though occasional cells seemed to express only Trpm5 or β-endorphin/Met-enkephalin. The latter cells may represent distinct (but very minor) subpopulations, a developmental state, or they may simply be cells in which the immunoreactive target molecule(s) fell below the limit of detection.
Interestingly, β-endorphin and Met-enkephalin immunostaining was strongest in the apical portion of the cells, oriented towards the lumen of the gut (FIG. 3). This is in contrast to the peptides and granules that are located in the stroma-oriented basal part in typical triangular-shaped enteroendocrine cells (see chromogranin-immunoreactive cells in FIG. 2).
FIG. 3.
Sections of duodenum from Trpm5-GFP mice. GFP fluorescence (A and D ), immunostaining with antibodies against Met-enkephalin (B), β-endorphin (E), overlays (C and F). Insets show higher magnification of cells from a different field.
β-endorphin and Met-enkephalin originate from the same precursor protein encoded by the POMC gene. In agreement with previous reports,30,31 we confirmed by RT-PCR that the POMC gene is expressed in the mucosa of mouse duodenum (data not shown). We then looked at expression of other peptides encoded by the POMC gene. By immunohistochemistry we detected a few duodenal mucosal cells that were positive for alpha-MSH, but these cells were not positive for Trpm5-GFP (data not shown). An antibody against beta-MSH detected a weak signal in some Trpm5-GFP expressing cells. However, because the rodent POMC-encoded precursor protein lacks the recognition site to cleave off beta-MSH, this signal likely originated from an incompletely processed precursor protein. We found no immunoreactive cells in intestinal mucosa with antibodies directed against ACTH, dynorphin or Leu-enkephalin (data not shown).
Trpm5-positive cells in duodenum express uroguanylin
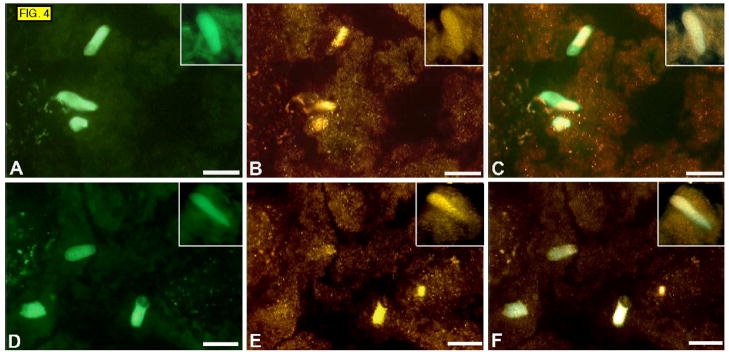
The immunolocalization of β-endorphin and Met-enkephalin to the apical part of cells suggests that the Trpm5-positive cells may be secreting their biological peptides into the lumen of the gut, rather than into the interstitium. Therefore we looked for other gut peptides, known to be secreted into the gut lumen, which might also be produced by the Trpm5-positive cells. We found that uroguanylin, a peptide thought to regulate chloride secretion, is present in almost all Trpm5-positive cells in duodenum (FIG. 4). The structurally and functionally similar peptide guanylin was not expressed with Trpm5 cells in duodenum (data not shown).
FIG. 4.
Sections of duodenum from Trpm5-GFP mice. GFP fluorescence (A and D), immunostaining with antibodies against uroguanylin (B and E), overlays (C and F). Insets show higher magnification of cells.
Mice with a disruption of the Trpm5 gene still contain Trpm5-GFP cells
We and others have shown previously that mice in which the Trpm5 gene is disrupted by homologous recombination (Trpm5−/− mice) are defective in taste responses to bitter, sweet and umami compounds.6,7 When we crossed the Trpm5−/− mice with the Trpm5-GFP transgenic line we found that Trpm5-GFP cells are still present in the taste buds of the Trpm5−/− mice in comparable numbers to those in WT mice (data not shown). Likewise, we determined that Trpm5-GFP cells are present in the intestinal tract of Trmp5−/−, Trpm5-GFP animals in the same numbers as in WT and heterozygous Trpm5+/− animals (data not shown). We conclude that the lack of the Trpm5 ion channel does not affect development of taste and enteroendocrine cells that normally express this gene.
β-endorphin release into the intestinal lumen depends on Trpm5
We hypothesized that Trpm5-expressing cells in the gut may have a chemosensory role analogous to that of taste cells in the gustatory system, i.e. detecting and responding to products of digestion within the gut lumen. However, due to their configuration in the gut mucosa as solitary cells without synaptic contact the function and signaling of these presumptive chemosensory cells may differ from the taste cells. Because β-endorphin and Met-enkephalin immunostaining was strongest in the apical (luminal) part of the Trpm5-positive cells and because of the absence of chromogranin-containing secretory granules in the cells’ basal aspect, we speculated that the Trpm5-positive cells might secrete these opioids directly into the gut lumen.
A priori it was not known which substances, if any, might stimulate intestinal Trpm5-positive cells to release β-endorphin and/or Met-enkephalin. One recent study found that T1r3 receptors were expressed in 44% of Trpm5-positive intestinal cells.7 Another report21 in dogs showed that Met-enkephalin is released into the gut lumen in response to a high fat meat meal, acidic, or hypertonic stimuli; furthermore, that β-endorphin and Met-enkephalin stimulate production of bicarbonate into the gut after such stimuli.21 In addition, β-endorphin secretion from the gut, stomach and gallbladder mucosa was stimulated by acidic pH.19,20,22 We speculated that Trpm5-positive gut cells might be involved in releasing endogenous opioids after stimulation by sweet, hypertonic, acidic and/or lipid substances. To test this possibility we monitored macronutrient-stimulated secretion of β-endorphin into the lumen of an ex vivo gut preparation from wild type vs. Trpm5 knockout (KO) mice. Because of the short length of the mouse duodenum we included a portion of jejunum in our preparation, see Materials and Methods.
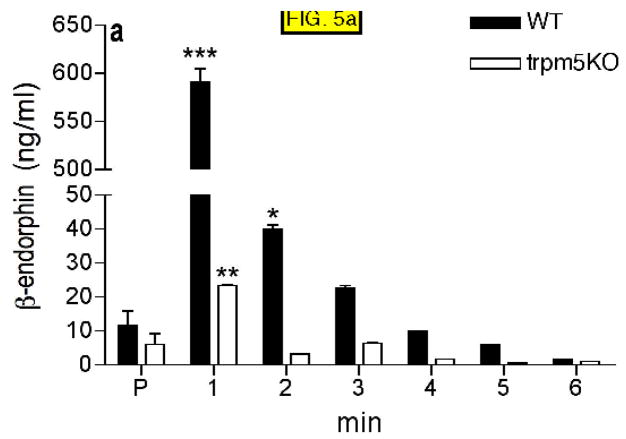
The gut preparation was perfused at a constant speed of 0.5ml/min for 5 minutes with Hanks buffered normal saline (HBSS) to establish a baseline and then the solution was changed to the test substance (hypertonic saline, acidic solutions, sugar, sucralose and a 20% lipid emulsion (Intralipid). One minute fractions were collected and β-endorphin levels determined by ELISA. The basal concentration of duodenal β-endorphin in lumen under HBSS perfusion was approximately 10 ng/ml. In gut preparations from wild type animals, intraluminal perfusion of the duodenal test segment with hypertonic saline (100mM NaCl in HBSS, 500mOsm, pH 6.0) very strongly and significantly (p<0.0.1) increased β-endorphin secretion during the first few minutes of test solution application, followed by a return to basal levels by 6 min. (FIG. 5A). In contrast, secretion of β-endorphin from the ex vivo gut preparation of Trpm5 KO animals showed only a small (but statistically significant) increase 1 minute after test solution addition, which then returned to baseline at 2 minutes and remained low throughout the course of the experiment (FIG. 5A).
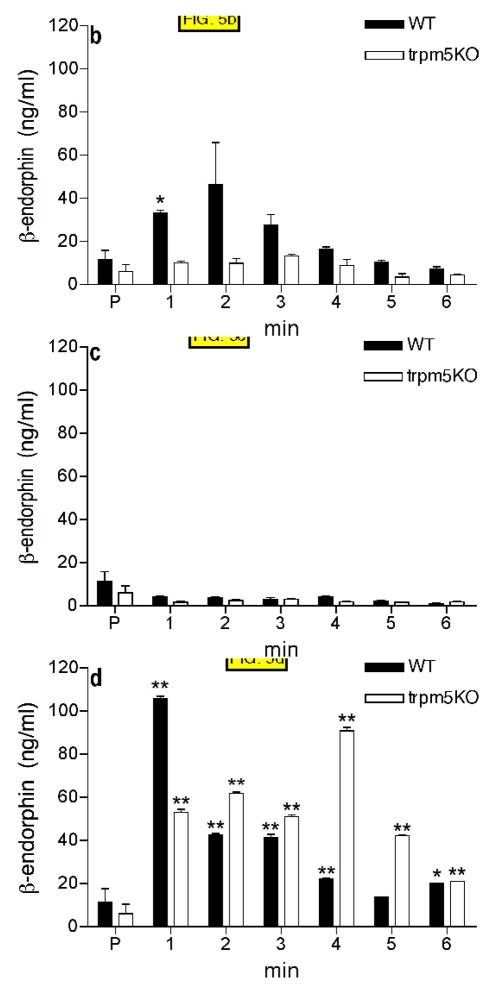
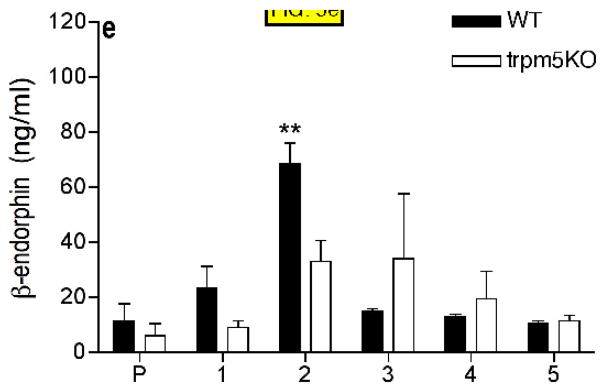
FIG. 5.
β-endorphin concentration determined by ELISA in samples collected in 1 minute intervals from duodenum-jejunum perfused with 100mM NaCl in HBSS (A), 300mM glucose in HBSS (B), 20mM sucralose in HBSS (C), 20% intralipid (D), 5% mannitol in HBSS (E). Control samples were collected after 3–5 min of perfusion with HBSS only (P). Four animals were tested in each group (see Materials and Methods). HBSS (Hanks buffered saline). * p<0.05, ** p<0.01, *** p<0.001 compared with preincubation values (P).
Perfusion of the gut preparation with 300mM glucose in normal saline (approximately 600mOsm) stimulated β-endorphin release from wild type, but not Trpm5 KO animals (FIG. 5B). In contrast, 20mM sucralose in normal saline had no stimulatory effect on β-endorphin release from gut in either genotype (FIG. 5C). 20% intralipid stimulated strong release of β-endorphin from intestines of both wild type and Trpm5 KO animals, although release from the Trpm5 KO animals appeared delayed (FIG. 5D). Stimulation with acidic saline (pH~ 4.5, 300mOsm) also stimulated β-endorphin release from gut in both WT and Trpm5KO animals (data not shown). Low pH inhibits the Trpm5 channel, 32 therefore acidic treatment would likely produce the same results in WT and Trpm5 mice.
From the above experiments we conclude that the Trpm5 channel is required for β-endorphin release in response to hypertonic stimuli and glucose, but is not required for β-endorphin release in response to lipid or acid stimuli. Because there was no response to sucralose we infer that osmolarity was the major stimulus in response to 300mM glucose. To test this hypothesis we examined responses to solutions of mannitol as a simple hypertonic stimulus. 5% mannitol in saline (580mOsm) stimulated β-endorphin release that was significantly higher in WT animals than in Trpm5−/− animals (FIG. 5E).
To limit variability in our experimental procedures and to control for effects of physical stimulation we tested various conditions of mild intestinal stretching and bolus enlargement of intestines by increasing the perfusion rate of HBSS, and/or temporarily clamping the ends of intestines and increasing the internal intestinal volume. There was no increase in β-endorphin levels in perfusate under any of these conditions; rather there was a steady decline in its concentration in time(data not shown).
We next asked whether under the conditions that stimulate β-endorphin release into the gut lumen, there is also an increase in serum β-endorphin levels. We chose the stimulation with high salt concentration. We found that serum beta endorphin levels did not change within 5 minutes after salt stimulation of intestine in either WT or Trpm5−/− animals (data not shown).
Discussion
In taste cells the Trpm5 ion channel is indispensable for propagating chemosensory signals to the nervous system. Its main role appears in assisting membrane depolarization in a final step in signal transmission from activation of G-protein coupled receptors. Mice deficient in Trpm5 are severely affected in their ability to sense sweet, bitter and umami compounds, tastants that all activate GPCRs.6,7
In this article we describe solitary chemosensory cells in mouse duodenum that express POMC and process it into endogenous opioids β-endorphin and Met-enkephalin. These same cells also express uroguanylin. All three peptides were known to be present in the intestinal lumen, but that they share a common cellular source in mouse duodenum was not known. We show here that these three peptides are expressed together with Trpm5 in specific cells in mouse duodenum that differ from typical enteroendocrine and enterochromaffin cells in that they lack chromogranin rich secretory granules in their basal portion. The immunostaining for the opioid peptides and uroguanylin within these cells is strongest in the apical portion suggesting that these peptides may be released predominantly into the intestinal lumen. Sato and Miyoshi47 observed membrane-bound electron-dense granules in the submicrovillous and intermicrovillous zone in tuft cells in the rat submandibular gland. They suggested that the tuft cells, as well as brush cells in gallbladder, may be chemosensory and engage in secretory activity. This may also be the mechanism by which Trpm5-expressing cells secrete their peptides into the gut lumen. Trpm5 cells in mouse very closely resemble brush cells, a conclusion which is in agreement with other reports.6,7,48
We found that β-endorphin is secreted into the gut lumen in response to various stimuli, particularly hypertonic stimuli. Using Trpm5 null mice we determined that Trpm5 is key to hypertonic-stimulated release of β-endorphin from gut. Many of the stimuli delivered to the duodenum by the incoming stomach contents may trigger β-endorphin release. Stomach contents of high osmolarity are likely to exist with most diets, and are likely to be among the major stimuli. As shown in Fig. 5, high osmolarity and high salt appear to stimulate the secretion of β-endorphin in Trpm5-dependent fashion. Trpm5−/− mice were much less responsive to such stimuli than were WT animals. Low pH also stimulated β-endorphin release; however, this secretion was not dependent on Trpm5. The Trpm5 channel itself is blocked by low pH, so it may not be involved in this regulation, or conversely its blockage by low pH would have an effect similar to the absence of Trpm5. Lipids also stimulated β-endorphin release but this was independent of Trpm5. Our experiment with 300mM glucose showed positive response and dependence on Trpm5. However, whether glucose itself or high osmolarity was the main stimulus cannot be discerned from the data. Recent publications suggest that T1r3 (a GPCR subunit of taste receptors for sweet and umami) is expressed on a subset of Trpm5 cells in the gut. 7 Whether T1r3-containing receptors play a role in chemosensory signaling in Trpm5 cells remains to be determined; our experiment with sucralose suggests that a receptor similar to the sweet taste receptor is not involved. Therefore high glucose may have acted through other receptors or glucose transporters or, as our data with mannitol and high salt suggest, the main stimulus may be the high osmolarity. Osmoreceptors were suggested to exist in duodenum, as hyperosmolar mannitol was reported to increase duodenal motility.33
The concentration of β-endorphin in the gut lumen is several orders of magnitude higher than in plasma. Taking into account the length of the gastrointestinal tract the gut’s total production of β-endorphin vastly surpasses that of other organs. This is similar to other bioactive substances such as serotonin and melatonin, production of which in the gut is much greater than in the nervous system.49
Our results are consistent with other reports that β-endorphin and Met-enkephalin are produced in the gut and secreted into the gastric, duodenal and jejunal lumina, and into the gallbladder.19–22 It is unknown at this point what may be the major target of the opioids in the gut, however there are cells and nerve endings with opioid receptors in the vicinity that can be reached by opioids. Small peptides, either produced by gut or resulting from food breakdown have been shown to cross the gut barrier,50 and were also shown experimentally to cross a monolayer of Caco cells.51 Peptide hormones from 5–166 amino acids (including Leu-enkephalin LHRH, insulin, calcitonin, and interferon beta), introduced into the intestinal lumen were effective in crossing the intestinal epithelium and entering the bloodstream. 55–59
This suggests that small peptides such as β-endorphin and Met-enkephalin in the gut would be able to reach at least submucosal nerve endings and act upon their expressed receptors. The half-life of β-endorphin in the gut has not been determined, but in blood it is around 5 minutes.52 Given the relatively large concentration of opioids in the gut it is possible that this time is sufficient for these molecules to reach receptors in the mucosa and submucosa. Other target cells with opioid receptors that could be reached by food-borne peptides and opioids are inflammatory cells in the gut’s mucosa and submucosa. Specific binding sites for opioids were recently localized to the basal portion of villous and crypt cells of porcine intestinal epithelium, enterocytes, cell lines and tumors derived from intestinal mucosa.53,60,61 These binding sites may represent opioid receptors capable of modulating active electrolyte transport.53
Serum β-endorphin and Met-enkephalin were reported to stimulate bicarbonate release into the duodenum.34,35 Uroguanylin, a trypsin resistant hormone from the natriuretic family, known to act on guanylyl-cyclase receptors also increases bicarbonate secretion into the gut.23–25 Therefore it is conceivable that both endogenous opioids and uroguanylin function in concert to normalize the intestinal content to more physiological parameters. However, the opioids and uroguanylin may have net opposite effects on intestinal motility. Opiate compounds have well known inhibitory effects on intestinal motility,9,13–15 whereas activation of guanylyl-cyclase receptors by overproduction of guanylin, uroguanylin or by bacterial endotoxins leads to secretory diarrhea.24,36 Production of both hormones may ensure adequate buffering of the intestinal content with optimal secretion and motility and thus work together to fine tune the digestive process.
Whether endogenous opioids from the gut in humans can under certain physiological or pathological conditions penetrate into deeper structures, reach nerve plexi and/or the systemic circulation, and exert their known effects on sense of well being, metabolism and energy balance remains to be determined. In our short term assay we have found no evidence of gut opioids entering the systemic circulation in adult animals. However, the situation may be physiologically different in very young organisms; it is well known that neonates and infants have the capacity to enter an analgesic state after consuming certain diets, particularly sugars.37–39 The time window when this effect can be observed correlates well with the time when gut is permeable to molecules as large as IgG from mother’s milk.40–43
In some pathological situations, such as the leaky gut syndrome,54 gut-derived opioids could also cross the gut barrier similarly to small peptides or toxins. Whether these opioids would play any role in gut inflammation and/or other pathologies is currently unknown. Recent studies have shown that opioid receptors are involved in controlling gut inflammation and irritable bowel syndrome (IBS),44,45 and that opioid receptor agonists have protective and perhaps analgesic effects in chronic colitis.46 Intestinal inflammation also induces a significant increase in the intestinal but not in the central levels of opioid receptor proteins.62 It is interesting to speculate that endogenous opioids in the gut lumen may have a local analgesic effect under physiological conditions, blocking awareness of the digestive process. Knowing what controls secretion of endogenous opioids in the gut may help in designing diets and interventions that would help in the prevention or treatment of these gut disorders. Conversely, lack of sufficient production of endogenous opioids may explain greater susceptibility to colitis and IBS. Whether our Trpm5−/− mice are more susceptible to colitis remains to be determined. So far we have found no histological or functional signs related to colitis in Trpm5−/− animals maintained under regular housing conditions and on regular rodent chow. However, β-endorphin is produced in and released into the gut of the Trpm5−/− animals, albeit its regulation is impaired and may be diminished.
In conclusion, we have identified solitary chemosensory cells in mouse duodenum that co-express β-endorphin, Met-enkephalin, uroguanylin and Trpm5. We further determined that these cells are likely to secrete the bioactive peptides into the intestinal lumen in response to dietary factors and that the release of the opioid peptides requires the Trpm5 ion channel.
Supplementary Material
Acknowledgments
This study was supported in part by NIH grants DC007399 and DK073248 to B.M. and DC03055 to R.F.M.
Abbreviations
- Trpm5
Transient receptor potential cation channel, subfamily M, member 5
- GFP
Green fluorescent protein
- HBSS
Hanks buffered standard saline
- GLP-1
glucagon-like peptide-1
- POMC
Proopiomelanocortin
Footnotes
Conflict of interest statement: R.F.M. has a personal financial interest in the form of stock ownership in the Redpoint Bio company, receives consulting fees from the Redpoint Bio company, and is an inventor on patents and patent applications which have been licensed to the Redpoint Bio company.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rindi G, Leiter AB, Kopin AS, Bordi C, Solcia E. The “normal” endocrine cell of the gut: changing concepts and new evidences. Ann NY Acad Sci. 2004;1014:1–12. doi: 10.1196/annals.1294.001. Review. [DOI] [PubMed] [Google Scholar]
- 2.Strader AD, Woods SC. Gastrointestinal hormones and food intake. Gastroenterology. 2005;128:175–91. doi: 10.1053/j.gastro.2004.10.043. Review. [DOI] [PubMed] [Google Scholar]
- 3.Cummings DE, Overduin J. Gastrointestinal regulation of food intake. J Clin Invest. 2007;117:13–23. doi: 10.1172/JCI30227. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang Y, Hoon MA, Chandrashekar J, Mueller KL, Cook B, Wu D, Zuker CS, Ryba NJ. Coding of sweet, bitter, and umami tastes: different receptor cells sharing similar signaling pathways. Cell. 2003;112:293–301. doi: 10.1016/s0092-8674(03)00071-0. [DOI] [PubMed] [Google Scholar]
- 5.Damak S, Rong M, Yasumatsu K, Kokrashvili Z, Pérez CA, Shigemura N, et al. Trpm5 Null Mice Respond to Bitter, Sweet, and Umami Compounds. Chemical Senses. 2006;31:253–264. doi: 10.1093/chemse/bjj027. [DOI] [PubMed] [Google Scholar]
- 6.Kaske S, Krasteva G, Konig P, Kummer W, Hofmann T, Gudermann T, Chubanov V. TRPM5, a taste-signaling transient receptor potential ion-channel, is a ubiquitous signaling component in chemosensory cells. BMC Neurosci. 2007 Jul 4;8:49. doi: 10.1186/1471-2202-8-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bezençon C, le Coutre J, Damak S. Taste-signaling proteins are coexpressed in solitary intestinal epithelial cells. Chem Senses. 2007;32:41–9. doi: 10.1093/chemse/bjl034. Trpm5 cells secrete opioids. [DOI] [PubMed] [Google Scholar]
- 8.Lin W, Ogura T, Margolskee RF, Finger TE, Restrepo D. TRPM5-expressing solitary chemosensory cells respond to odorous irritants. J Neurophysiol. 2008;99:1451–60. doi: 10.1152/jn.01195.2007. [DOI] [PubMed] [Google Scholar]
- 9.Bodnar RJ, Klein GE. Endogenous opiates and behavior: 2005. Peptides. 2006;27:3391–478. doi: 10.1016/j.peptides.2006.07.011. Review. [DOI] [PubMed] [Google Scholar]
- 10.Parton LE, Ye CP, Coppari R, Enriori PJ, Choi B, Zhang C-Y, Xu C, Vianna CR, et al. Glucose sensing by POMC neurons regulates glucose homeostasis and is impaired in obesity. Nature. 2007;449:228–232. doi: 10.1038/nature06098. [DOI] [PubMed] [Google Scholar]
- 11.Appleyard SM, Hayward M, Young JI, Butler AA, Cone RD, Rubinstein M, Low MJ. A role for the endogenous opioid beta-endorphin in energy homeostasis. Endocrinology. 2003;144:1753–1760. doi: 10.1210/en.2002-221096. [DOI] [PubMed] [Google Scholar]
- 12.Coll AP, Farooqi S, Challis BG, Yeo GSH, O’Rahilly S. Proopiomelanocortin and energy balance: Insights from human and murine genetics. J Clin Endocrin Metab. 2004;89:2557–2562. doi: 10.1210/jc.2004-0428. [DOI] [PubMed] [Google Scholar]
- 13.Vaccarino AL, Kastin AJ. Endogenous opiates: 2000. Peptides. 2001;22:2257–2328. doi: 10.1016/s0196-9781(01)00566-6. [DOI] [PubMed] [Google Scholar]
- 14.Holzer P. Opioids and opioid receptors in the enteric nervous system: from a problem in opioid analgesia to a possible new prokinetic therapy in humans. Neurosci Lett. 2004;6(361):192–5. doi: 10.1016/j.neulet.2003.12.004. Review. [DOI] [PubMed] [Google Scholar]
- 15.Sandhu BK, Phillips AD, Milla PJ. Opiate agonists and enterotoxin. Biochem Soc Trans. 1984:205–208. doi: 10.1042/bst0120205. [DOI] [PubMed] [Google Scholar]
- 16.Smith TW, Hughes J, Kosterlitz HW, Sosa RP. Enkephalins: isolation, distribution and function. In: Kosterlitz HW, editor. Opiates and Endogenous Opiate Peptides. Elsevier/North-Holland Biomedical Press; Amsterdam: 1986. pp. 57–62. [Google Scholar]
- 17.Tari A, Miyachi Y, Sumii K, Haruma K, Yoshihara M, Kajiyama G, Miyoshi A. Beta-endorphin-like immunoreactivity in normal mucosa, muscle layer, adenocarcinoma, and polyp of the colon. Dig Dis Sci. 1988;33:429–34. doi: 10.1007/BF01536027. [DOI] [PubMed] [Google Scholar]
- 18.Lolova IS, Davidoff MS, Itzev DE. Histological and immunocytochemical data on the differentiation of intestinal endocrine cells in human fetus. Acta Physiol Pharmacol Bulg. 1998;23:61–71. [PubMed] [Google Scholar]
- 19.Matsumura M, Saito S, Fujino M. Effects of solution of low pH and taurocholate on release of beta-endorphin-like immunoreactivity from human duodenal mucosa in vitro. Regul Pept. 1982;3:173–81. doi: 10.1016/0167-0115(82)90123-9. [DOI] [PubMed] [Google Scholar]
- 20.Matsumura M, Wada H, Saito S. Effect of solution of low pH on release of beta-endorphin-like immunoreactivity and ACTH-like reactivity from human gastric antral mucosa in vitro. Gastroenterol Jpn. 1983;18:210–215. doi: 10.1007/BF02774962. [DOI] [PubMed] [Google Scholar]
- 21.Money SR, Petroianu A, Gintzler AR, Jaffe BM. Meal-stimulated release of Methionie-enkephalin into the canine jejunal lumen. J Clin Invest. 1988;81:822–825. doi: 10.1172/JCI113390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shimizu I, Matsumura M, Hirota M, Fujii T, Shima K. Beta-endorphin in the human gallbladder. Regul Pept. 1986;16:331–338. doi: 10.1016/0167-0115(86)90033-9. [DOI] [PubMed] [Google Scholar]
- 23.Nakazato M. Guanylin family: new intestinal peptides regulating electrolyte and water homeostasis. J Gastroenterol. 2001;36:219–25. doi: 10.1007/s005350170106. Review. [DOI] [PubMed] [Google Scholar]
- 24.Forte LR., Jr Uroguanylin and guanylin peptides: pharmacology and experimental therapeutics. Pharmacol Ther. 2004;104:137–62. doi: 10.1016/j.pharmthera.2004.08.007. Review. [DOI] [PubMed] [Google Scholar]
- 25.Sindiu A, Schlatter E. Cellular effects of guanylin and uroguanylin. J Am Soc Nephrol. 2006;17:607–16. doi: 10.1681/ASN.2005080818. Review. [DOI] [PubMed] [Google Scholar]
- 26.Clapp TR, Medler KF, Damak S, Margolskee RF, Kinnamon SC. Mouse taste cells with G protein-coupled taste receptors lack voltage-gated calcium channels and SNAP-25. BMC Biol. 2006;4:7. doi: 10.1186/1741-7007-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sbarbati A, Osculati F. A new fate for old cells: brush cells and related elements. J Anat. 2005;206:349–58. doi: 10.1111/j.1469-7580.2005.00403.x. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sbarbati A, Osculati F. The taste cell-related diffuse chemosensory system. Prog Neurobiol. 2005;75:295–307. doi: 10.1016/j.pneurobio.2005.03.001. Review. [DOI] [PubMed] [Google Scholar]
- 29.Morroni M, Cangiotti AM, Cinti S. Brush cells in the human duodenojejunal junction: an ultrastructural study. J Anat. 2007;211:125–31. doi: 10.1111/j.1469-7580.2007.00738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Orwoll ES, Kendall JW. Beta-endorphin and adrenocorticotropin in extrapituitary sites: gastrointestinal tract. Endocrinology. 1980;107:438–42. doi: 10.1210/endo-107-2-438. [DOI] [PubMed] [Google Scholar]
- 31.O’Donohue TL, Dorsa DM. The opiomelanotropinergic neuronal and endocrine systems. Peptides. 1982;3:353–95. doi: 10.1016/0196-9781(82)90098-5. Review. [DOI] [PubMed] [Google Scholar]
- 32.Liu D, Zhang Z, Liman ER. Extracellular acid block and acid-enhanced inactivation of the Ca2+-activated cation channel TRPM5 involve residues in the S3–S4 and S5–S6 extracellular domains. J Biol Chem. 2005;280:20691–9. doi: 10.1074/jbc.M414072200. [DOI] [PubMed] [Google Scholar]
- 33.Lin H, Elashoff JD, Kwok GM, Gu Y-G, Meyer JH. Stimulation of duodenal motility by hyperosmolar mannitol depends on local osmoreceptor control. Am J Physiol. 1994;266:G940–G943. doi: 10.1152/ajpgi.1994.266.5.G940. [DOI] [PubMed] [Google Scholar]
- 34.Flemström G, Kivilaakso E, Briden S, Nylander O, Jedstedt G. Gastroduodenal bicarbonate secretion in mucosal protection. Possible role of vasoactive intestinal peptide and opiates. Dig Dis Sci. 1985;30:63S–68S. doi: 10.1007/BF01309387. [DOI] [PubMed] [Google Scholar]
- 35.Flemstrom G, Jedstedt G, Nylander O. Beta-Endorphin and enkephalins stimulate duodenal mucosal alkaline secretion in the rat in vivo. Gastroenterology. 1986;90:368–72. doi: 10.1016/0016-5085(86)90934-0. [DOI] [PubMed] [Google Scholar]
- 36.Forte LR. Guanylin regulatory peptides: structures, biological activities mediated by cyclic GMP and pathobiology. Regul Pept. 1999;81:25–39. doi: 10.1016/s0167-0115(99)00033-6. Review. [DOI] [PubMed] [Google Scholar]
- 37.Anseloni VC, Ren K, Dubner R, Ennis M. A brainstem substrate for analgesia elicited by intraoral sucrose. Neuroscience. 2005;133:231–43. doi: 10.1016/j.neuroscience.2005.01.055. [DOI] [PubMed] [Google Scholar]
- 38.Anseloni VC, Weng HR, Terayama R, Letizia D, Davis BJ, Ren K, Dubner R, Ennis M. Age-dependency of analgesia elicited by intraoral sucrose in acute and persistent pain models. Pain. 2002;97:93–103. doi: 10.1016/s0304-3959(02)00010-6. [DOI] [PubMed] [Google Scholar]
- 39.Okan F, Coban A, Ince Z, Yapici Z, Can G. Analgesia in preterm newborns: the comparative effects of sucrose and glucose. Eur J Pediatr. 2007;166:1017–24. doi: 10.1007/s00431-006-0373-z. [DOI] [PubMed] [Google Scholar]
- 40.Vukavic T. Intestinal absorption of IgA in the newborn. J Pediatr Gastroenterol Nutr. 1983;2:248–51. [PubMed] [Google Scholar]
- 41.Vukaviu T. Timing of the gut closure. J Pediatr Gastroenterol Nutr. 1984;3:700–3. doi: 10.1097/00005176-198411000-00011. [DOI] [PubMed] [Google Scholar]
- 42.Catassi C, Bonucci A, Coppa GV, Carlucci A, Giorgi PL. Intestinal permeability changes during the first month: effect of natural versus artificial feeding. J Pediatr Gastroenterol Nutr. 1995;21:383–6. doi: 10.1097/00005176-199511000-00003. [DOI] [PubMed] [Google Scholar]
- 43.Van de Perre P. Transfer of antibody via mother’s milk. Vaccine. 2003;21:3374–6. doi: 10.1016/s0264-410x(03)00336-0. Review. [DOI] [PubMed] [Google Scholar]
- 44.Callahan MJ. Irritable bowel syndrome neuropharmacology. A review of approved and investigational compounds. J Clin Gastroenterol. 2002;35:S58–67. doi: 10.1097/00004836-200207001-00011. Review. [DOI] [PubMed] [Google Scholar]
- 45.Philippe D, Chakass D, Thuru X, Zerbib P, Tsicopoulos A, Geboes K, Bulois P, et al. Mu opioid receptor expression is increased in inflammatory bowel diseases: implications for homeostatic intestinal inflammation. Gut. 2006;55:815–23. doi: 10.1136/gut.2005.080887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Verma-Gandhu M, Verdu EF, Bercik P, Blennerhassett PA, Al-Mutawaly N, Ghia JE, Collins SM. Visceral pain perception is determined by the duration of colitis and associated neuropeptide expression in the mouse. Gut. 2007;56:358–64. doi: 10.1136/gut.2006.100016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sato A, Miyoshi S. Fine structure of tuft cells of the main excretory duct epithelium in the rat submandibular gland. Anat Rec. 1997;248:325–331. doi: 10.1002/(SICI)1097-0185(199707)248:3<325::AID-AR4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 48.Bezençon C, Fürholz A, Raymond F, Mansourian R, Métairon S, Le Coutre J, Damak SJ. Murine intestinal cells expressing Trpm5 are mostly brush cells and express markers of neuronal and inflammatory cells. Comp Neurol. 2008;509:514–525. doi: 10.1002/cne.21768. [DOI] [PubMed] [Google Scholar]
- 49.Bubenik GA. Thirty four years since the discovery of gastrointestinal melatonin. J Physiol Pharmacol. 2008;(Suppl 2):33–51. [PubMed] [Google Scholar]
- 50.Shimizu M, Son DO. Food-derived peptides and intestinal functions. Curr Pharm Des. 2007;13:885–95. doi: 10.2174/138161207780414287. [DOI] [PubMed] [Google Scholar]
- 51.Iwan M, Jarmoaowska B, Bielikowicz K, Kostyra E, Kostyra H, Kaczmarski M. Transport of micro-opioid receptor agonists and antagonist peptides across Caco-2 monolayer. Peptides. 2008;29:1042–7. doi: 10.1016/j.peptides.2008.01.018. [DOI] [PubMed] [Google Scholar]
- 52.Pezalla PD, Lis M, Seidah NG, Chrétien M. Lipotropin, melanotropin and endorphin: in vivo catabolism and entry into cerebrospinal fluid. Can J Neurol Sci. 1978;5:183–8. doi: 10.1017/s0317167100024537. [DOI] [PubMed] [Google Scholar]
- 53.Quito FL, Seybold VS, Brown DR. Opiate binding sites in mucosa of pig small intestine. Life Sci. 1991;49:PL219–22. doi: 10.1016/0024-3205(91)90297-o. [DOI] [PubMed] [Google Scholar]
- 54.Vaarala O, Atkinson MA, Neu J. The “perfect storm” for type 1 diabetes: the complex interplay between intestinal microbiota, gut permeability, and mucosal immunity. Diabetes. 2008;57:2555–62. doi: 10.2337/db08-0331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee HJ, Amidon GL. The effect of enzyme inhibitor and absorption site following [D-ala2, D-leu5]enkephalin oral administration in rats. Biopharm Drug Dispos. 2002;23:131–41. doi: 10.1002/bdd.302. [DOI] [PubMed] [Google Scholar]
- 56.Roberts PR, Burney JD, Black KW, Zaloga GP. Effect of Chain Length on Absorption of Biologically Active Peptides from the Gastrointestinal Tract. Digestion. 1999;60:332–337. doi: 10.1159/000007679. [DOI] [PubMed] [Google Scholar]
- 57.Karsdal MA, Byrjalsen I, Riis BJ, Christiansen C. Optimizing bioavailability of oral administration of small peptides through pharmacokinetic and pharmacodynamic parameters: the effect of water and timing of meal intake on oral delivery of Salmon Calcitonin. BMC Clin Pharmacol. 2008;9(8):5. doi: 10.1186/1472-6904-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Builders PF, Kunle OO, Okpaku LC, Builders MI, Attama AA, Adikwu MU. Preparation and evaluation of mucinated sodium alginate microparticles for oral delivery of insulin. Eur J Pharm Biopharm. 2008;70:777–83. doi: 10.1016/j.ejpb.2008.06.021. [DOI] [PubMed] [Google Scholar]
- 59.Kamei N, Morishita M, Chiba H, Kavimandan NJ, Peppas NA, Takayama K. Complexation hydrogels for intestinal delivery of interferon beta and calcitonin. J Control Release. 2008 Nov 27; doi: 10.1016/j.jconrel.2008.11.014. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nano JL, Fournel S, Rampal P. Characterization of delta-opioid receptors and effect of enkephalins on IRD 98 rat epithelial intestinal cell line. Pflugers Arch. 2000;439:547–54. doi: 10.1007/s004249900160. [DOI] [PubMed] [Google Scholar]
- 61.Nylund G, Pettersson A, Bengtsson C, Khorram-Manesh A, Nordgren S, Delbro DS. Functional expression of mu-opioid receptors in the human colon cancer cell line, HT-29, and their localization in human colon. Dig Dis Sci. 2008;53:461–6. doi: 10.1007/s10620-007-9897-y. [DOI] [PubMed] [Google Scholar]
- 62.Pol O, Palacio JR, Margarita M, Puig MM. The Expression of delta- and kappa-Opioid Receptor Is Enhanced during Intestinal Inflammation in Mice. JPET. 2003;306:455–462. doi: 10.1124/jpet.103.049346. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.