Abstract
Objective
Dietary intake of polyunsaturated n-3 fatty acids has been associated with a reduced incidence of adverse cardiovascular events. The protective mechanisms involved are not fully understood, but may include anti-inflammatory factors. We sought to investigate the relationship between n-3 fatty acid levels in erythrocyte membranes and markers of systemic inflammation in 992 individuals with stable coronary artery disease.
Methods
Cross-sectional associations of C-reactive protein (CRP) and Interleukin-6 (Il-6) with docosahexaenoic acid (DHA) and eicosapentaenoic acid (EHA) were evaluated in multivariable linear regression models adjusted for demographics, cardiovascular risk factors, medication use, exercise capacity, body mass index, and waist-to-hip ratio.
Results
After multivariable adjustment, n-3 fatty acid levels (DHA + EPA) were inversely associated with CRP and IL-6. The inverse association of n-3 fatty acids with CRP and IL-6 was not modified by demographics, body-mass index, smoking, LDL-cholesterol, or statin use (p values for interaction > 0.1)
Conclusions
In patients with stable coronary artery disease, an independent and inverse association exists between n-3 fatty acid levels and inflammatory biomarkers. These findings suggest that inhibition of systemic inflammation may be a mechanism by which n-3 fatty acids prevent recurrent cardiovascular events.
Keywords: Omega-3, Inflammation, C-Reactive Protein, Interleukin-6, Fish Oil
INTRODUCTION
Epidemiologic studies have demonstrated a protective effect of dietary n-3 fatty acids on adverse cardiovascular events, particularly sudden cardiac death (1–4). The mechanisms underlying this protective effect are not well understood, and may include anti-inflammatory factors (5). Systemic inflammation is a key component in the development and progression of atherosclerosis, and the circulating inflammatory biomarkers C-reactive protein (CRP) and interleukin-6 (IL-6) are independent risk factors for cardiovascular disease (6,7). The n-3 fatty acids exhibit anti-inflammatory properties which have proven useful in the treatment of systemic inflammatory diseases such as rheumatoid arthritis and Crohn’s disease (8).
The relationship between blood levels of n-3 fatty acids and inflammatory biomarkers in persons with stable coronary artery disease has not previously been reported. The primary aim of this study was to examine the relationship between blood levels of two n-3 fatty acids (docosahexaenoic acid, eicosapentaenoic acid)* and two inflammatory biomarkers (CRP, IL-6). A secondary aim was to determine whether the relationship between n-3 fatty acids and inflammatory biomarkers was modified by demographics, body-mass index, smoking, LDL-Cholesterol, or statin use.
METHODS
Participants
The Heart and Soul Study is a prospective cohort study investigating the influence of psychosocial factors on cardiovascular events in outpatients with stable coronary artery disease. The enrollment process for the Heart and Soul Study has been previously described (9). Eligible participants were recruited from outpatient clinics in the San Francisco Bay Area if they met at least one of the following inclusion criteria: 1) history of myocardial infarction, 2) angiographic evidence of at least 50% stenosis by area in at least 1 coronary artery, 3) evidence of exercise-induced ischemia by treadmill electrocardiogram or stress nuclear perfusion imaging, or 4) history of coronary revascularization. Individuals were excluded if they had a history of myocardial infarction in the past 6 months, deemed themselves unable to walk 1 block, or if they were planning to move out of the local area within 3 years.
The study protocol was approved by the following Institutional Review Boards: the University of California San Francisco Committee on Human Research, the Research and Development Committee at the San Francisco VA Medical Center, the Medical Human Subjects Committee at Stanford University, the Human Subjects Committee at the VA Palo Alto Health Care System, and the Data governance Board of the Community Health Network of San Francisco. All participants provided written informed consent. Between September 2000 and December 2002, a total of 1024 participants enrolled in the study. Of these, blood samples were available for assessment of n-3 fatty acid levels from 992 participants. There were no demographic differences between those who did and did not provide blood.
Clinical Evaluation
Baseline demographics, age, sex, income, and ethnicity were determined by self-reported questionnaire. Cardiovascular co-morbidities including hypertension, diabetes, hyperlipidemia, prior myocardial infarction, stroke, prior revascularization (angioplasty or coronary artery bypass grafting), and smoking status were determined by self-reported questionnaire. Smoking status was collected as a dichotomous variable (current smoking vs. current nonsmoking) and as a continuous variable (cumulative pack years). Participants were weighed and measured without shoes. Body Mass Index (BMI) was calculated as the ratio of mass (kg) to the square of height (m2). Waist and hip circumferences were measured, standing and without clothing, using a flexible plastic measure at the end of normal expiration. Waist circumference was measured midway between the lower rib margin and iliac crest. Hip circumference was measured at the level of the greater trochanters. Waist-to-hip ratio was calculated as waist circumference divided by hip circumference (10).
All participants were instructed to bring their medication bottles to the study appointment where study personnel recorded all current medications. Medications were categorized using Epocrates Rx (San Mateo, CA), an electronic reference database that contains 3300 drugs classified by both generic and commercial product names. To determine income, participants completed a questionnaire item asking “which of the following categories best describes your total combined household income for the previous 12 months?” (<$10,000, $10,000 to $19,000, $20,000 to $29,000, $30,000 to $39,999, $40,000 to $50,000, or >$50,000).
Fasting serum samples were used to measure triglycerides, HDL-cholesterol, and LDL-cholesterol. All participants underwent a symptom-limited graded exercise treadmill test according to a standard Bruce protocol. To achieve maximal heart rate, participants who were unable to continue the standard Bruce protocol were switched to lower settings on the treadmill and encouraged to exercise for as long as possible. Maximal exercise capacity (in metabolic equivalents) was determined at peak exercise.
n-3 Fatty Acid Assay
Levels of docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) were measured in the membranes of erythrocytes from fasting venous whole blood samples. Upon enrollment, study participants refrained from smoking for five hours and completed an overnight 12-hour fast (except for prescribed medications taken with water). The fatty acid composition of erythrocyte membranes was assessed by capillary gas chromatography using a GC2010 Gas Chromatograph (Shimadzu Corporation, Columbia, MD) equipped with a SP2560, 100-m column (Supelco, Bellefonte, PA) after generation of fatty acid methyl esters by treatment with boron trifluoride-methanol. Fatty acid methyl esters were identified by comparison with a weighed standard mixture consisting of 22 fatty acids characteristic of erythrocytes (GLC-727, Nuchek Prep, Elysian, MN). Blood levels of EPA and DHA were determined as a percentage composition of total fatty acid methyl esters. Two erythrocyte control pools were included with each batch to monitor analytical performance. Acceptable runs were those in which both controls fell within 2.5 standard deviations. The coefficient of variation for EPA+DHA was 5–6%. The laboratory measurement of fatty acids was blinded to patient characteristics.
Inflammatory Biomarker Measurements
High sensitivity C-reactive protein (CRP) was measured using the Roche Integra assay in approximately 20% of participants and, due to a change at the laboratory, the Beckman Extended Range high sensitivity CRP assay in the remaining 80% of participants. The lowest detectable CRP measurements with these assays were 0.025 mg/dL and 0.20 mg/dL respectively. Results from the two assays were highly correlated (r = 0.99 in 185 participants). IL-6 was measured using the R & D Systems Quantikine HS IL-6 Immunoassay (R & D Systems, Minneapolis, Minnesota). The inter-assay coefficient of variation for IL-6 was 6.5–9.6%. The reportable range for IL-6 using this assay is 0.43–8.87 g/dl.
Statistical Analysis
For descriptive purposes, we categorized participants a priori by tertile of n-3 fatty acid levels. Differences in baseline characteristics were compared with the use of ANOVA for continuous variables and the chi-squared test for dichotomous variables. Since CRP and IL-6 levels had a skewed distribution, they were natural log-transformed for subsequent statistical analyses. For subsequent regression models, the n-3 variable was standardized.
We used multivariable linear regression models to determine the relationship, expressed as β-coefficients, between n-3 fatty acid levels (DHA+EPA; the n-3 Index) and the inflammatory biomarkers CRP and IL-6 (continuous dependent variables). The β-coefficients were exponentiated to yield percentage changes and 95% confidence intervals. Multivariate adjustment was made for demographic characteristics as well as covariates known to influence inflammatory biomarker levels: hypertension, prior myocardial infarction (MI), diabetes, current smoking, triglyceride, LDL-cholesterol, HDL-cholesterol, statin use, aspirin use, exercise capacity, BMI, and waist-to-hip ratio. Since BMI and waist-to-hip ratio have been suggested to be more proximal determinants of systemic inflammation, these variables were added last in the sequential multivariable adjustment models (11, 12). In a parallel analysis using similar adjustment models, we also performed multivariate logistic regression to determine the association between n-3 fatty acid levels and elevated inflammatory biomarkers defined as dichotomous variables: CRP > 3mg/dl (13) and IL-6 > 5pg/ml (14). To explore potential modifiers, we tested for interaction between n-3 fatty acid levels and age, gender, BMI, smoking, LDL-Cholesterol, statin use, history of myocardial infarction, and history of revascularization in multivariable-adjusted models as described above.
Statistical analysis was performed using SAS software version 9.1 (SAS Institute Inc, Cary, NC). The authors take responsibility for the integrity of the data. All authors had full access to the data, except W.S.H. who was blinded to the clinical data. All authors have read and agree to the manuscript as written.
RESULTS
The mean erythrocyte (DHA+EPA) level for the entire cohort was 4.2% with a standard deviation of 2.0%. The baseline characteristics of the study population categorized by tertiles of n-3 fatty acid levels are shown in Table 1. Lower levels of n-3 fatty acids were significantly associated with younger age, non-white ancestry, low income, hypertension, prior myocardial infarction, diabetes, current smoking, higher body mass index, higher waist-to-hip ratio, lower exercise capacity, higher triglycerides, and lower HDL-cholesterol levels. Participants in the lowest tertile were also less likely to be taking statins.
Table 1.
Baseline characteristics of participants by tertile of erythrocyte n-3 fatty acid levels (DHA + EPA)
| Tertile (EPA+DHA) (%)±SD | I | II | III | P value (ANOVA or chi2) |
|---|---|---|---|---|
| 2.0 ± 0.4 | 3.6 ± 0.4 | 6.4 ± 2.0 | ||
| N=327 | N=328 | N=337 | ||
| Age | 64±11 | 67±10 | 69±10 | <.0001 |
| Male (%) | 83 | 80 | 82 | 0.77 |
| Ethnicity (%) | <.0001 | |||
| Hispanic | 14 | 9 | 3 | |
| White | 59 | 58 | 65 | |
| Black | 19 | 22 | 8 | |
| Asian | 5 | 9 | 21 | |
| Other | 4 | 3 | 3 | |
| Income < $20000/yr (%) | 60 | 48 | 37 | <.0001 |
| Medical History (%) | ||||
| Hypertension | 74 | 75 | 64 | 0.002 |
| MI | 60 | 53 | 49 | 0.01 |
| CHF | 19 | 20 | 14 | 0.08 |
| Stroke | 14 | 14 | 14 | 0.97 |
| Diabetes | 33 | 27 | 20 | 0.0006 |
| CABG | 33 | 41 | 34 | 0.10 |
| Angioplasty | 37 | 42 | 39 | 0.37 |
| Current smoking | 100(31) | 74(23) | 23(7) | <.0001 |
| Smoking (pack years) | 8±5 | 8±4 | 6±4 | <.0001 |
| Body mass index (kg/m2) | 28±6 | 29±6 | 28±5 | 0.02 |
| Waist to hip ratio | 0.96±.08 | 0.96±.08 | 0.94±.07 | 0.02 |
| Medication Use (%) | ||||
| Beta-blocker | 62 | 55 | 55 | 0.09 |
| Statin | 56 | 66 | 69 | 0.001 |
| ACE-inhibitor | 47 | 53 | 53 | 0.16 |
| Aspirin | 77 | 80 | 73 | 0.09 |
| HDL-Cholesterol (mg/dL) | 43±14 | 46±14 | 47±14 | 0.001 |
| Triglycerides (mg/dL) | 192±190 | 125±74 | 108±62 | <.0001 |
| LDL-Cholesterol (mg/dL) | 106±38 | 106±31 | 101±33 | 0.13 |
| Exercise Capacity (Mets) | 7±3 | 7±3 | 8±4 | <.0001 |
In multivariable-adjusted linear regression models with both n-3 levels and inflammatory biomarkers entered as continuous variables, there was an inverse association between n-3 fatty acid levels and the inflammatory biomarkers CRP and IL-6 (table 2). After sequential adjustment for demographics, cardiovascular risk factors, medication use, exercise capacity, body mass index, and waist-to-hip ratio, n-3 fatty acid levels remained inversely associated with CRP (10% decrease in CRP per standard deviation increase in DHA+EPA; 95% CI = 3–17%) and IL-6 (7% decrease in IL-6 per standard deviation increase in DHA+EPA; 95% CI = 3–11%).
Table 2.
Percentage change in inflammatory biomarkers per standard deviation decrease (2.0%) in n-3 fatty acid levels (DHA+EPA) with sequential adjustment for potential confounding variables
| OUTCOME | Model 1* | Model 2+ | Model 3++ | Model 4$ |
|---|---|---|---|---|
| Percentage change in CRP (95% CI) | 21 (25, 15) | 16 (21, 9) | 11 (17, 3) | 10 (17, 3) |
| Percentage change in IL-6 (95% CI) | 14 (17, 10) | 11 (15, 7) | 8 (11, 3) | 7 (11, 3) |
Adjusted for Age, Gender, Ethnicity, Income
Adjusted for all variables in Model 1 + Hypertension, Diabetes, Myocardial Infarction, Current Smoking, Triglyceride, LDL-Cholesterol, HDL-Cholesterol, Medication Use (Statins, Aspirin)
Adjusted for all variables in Model 2 + Exercise Capacity
Adjusted for all variables in Model 3 + BMI, Waist-to-hip ratio
In multivariable-adjusted logistic regression models with elevated CRP (> 3mg/dl) and elevated IL-6 (>5pg/dl) entered as dichotomous variables, we again found an inverse association between n-3 fatty acid levels and both inflammatory biomarkers (table 3). After sequential adjustment for demographics, cardiovascular risk factors, medication use, exercise capacity, body mass index, and waist-to-hip ratio, n-3 fatty acid levels remained inversely associated with elevated CRP (OR per standard deviation decrease in DHA+EPA = 1.4; 95% CI = 1.1–1.7) and elevated IL-6 (OR per standard deviation decrease in DHA+EPA = 1.3; 95% CI=1.0–1.7).
Table 3.
Association of n-3 fatty acid levels with elevated CRP and IL-6 after sequential adjustment for potential confounding variables expressed as odds ratios and 95% confidence intervals per standard deviation (2.0%) decrease in DHA+EPA
| Model 1* | Model 2+ | Model 3++ | Model 4$ | |
|---|---|---|---|---|
| Odds Ratio (95% CI) | Odds Ratio (95% CI) | Odds Ratio (95% CI) | Odds Ratio (95% CI) | |
| Elevated CRP (>3 mg/dl) | 1.5 (1.3–1.8) | 1.4 (1.2–1.7) | 1.3 (1.1–1.6) | 1.4 (1.1–1.7) |
| Elevated IL-6 (>5pg/ml) | 1.5 (1.2–1.9) | 1.5 (1.2–1.9) | 1.3 (1.0–1.7) | 1.3 (1.0–1.7) |
Adjusted for Age, Gender, Ethnicity, Income
Adjusted for all variables in Model 1 + Hypertension, Diabetes, Myocardial Infarction, Current Smoking, Triglyceride, LDL-Cholesterol, HDL-Cholesterol, Medication Use (Statins, Aspirin)
Adjusted for all variables in Model 2 + Exercise Capacity
Adjusted for all variables in Model 3 + BMI, Waist-to-hip ratio
Lower n-3 fatty acid levels were associated with increased CRP and IL-6 in both users and nonusers of statins. We found no evidence that the association between n-3 fatty acids and inflammatory markers varied by demographics, body-mass index, LDL-Cholesterol, smoking, statin use, history of myocardial infarction, or history of revascularization (all p values for interactions in multivariable-adjusted models > 0.1)
DISCUSSION
In a large cross-sectional study of patients with stable coronary artery disease, we found that blood levels of n-3 fatty acids were inversely associated with biomarkers of systemic inflammation. This inverse association persisted after multivariate adjustment for potential confounding variables, and did not appear to be modified by demographics or statin use. These findings raise the possibility that reduced inflammation may be a mechanism through which n-3 fatty acids benefit patients with coronary heart disease.
Wide-ranging cardioprotective effects of n-3 fatty acids have been observed in multiple epidemiologic studies, including three large randomized controlled trials (15–17). On this basis, the American Heart Association endorses the use of n-3 fatty acids for secondary prevention of cardiovascular events in persons with documented coronary artery disease (18). The underlying mechanisms remain incompletely understood, but may include anti-hypertensive, anti-platelet, anti-arrhythmic, anti-inflammatory, and triglyceride-lowering effects (5).
Prior observational studies have generated conflicting data regarding the effect of dietary n-3 fatty acid intake on inflammatory biomarkers. Pischon et al. found that intake of EPA and DHA was inversely associated with plasma levels of the soluble tumor necrosis factor receptors 1 and 2. However, the inverse relationship between n-3 intake and CRP and IL-6 was not statistically significant (19). The latter relationship was, however, significant in the Nurses’ Health Study (20). More recently, Ohsawa et al found a significant inverse association between dietary n-3 intake and CRP levels in male smokers, confirming previous observations by Niu et al in elderly Japanese individuals (21,22). In these and other studies, n-3 fatty acid intake was quantified on the basis of dietary questionnaires. Along with the limitations of self-reported dietary intake, no information was available regarding differential absorption and metabolism of n-3 fatty acids.
Prospective, randomized controlled trials of n-3 fatty acid supplementation to reduce circulating markers of inflammation have likewise produced mixed results. In neither patients with type 2 diabetes mellitus nor previous myocardial infarction did n-3 supplementation lower CRP levels (23,24). Yusof et al. reported similar results for healthy middle-aged men (25). On the other hand, pure EPA reduced CRP in Japanese subjects with metabolic syndrome (26), and combined EPA and DHA were effective in obese women (27) and in women on hormone replacement therapy (28).
We used gas chromatography to directly measure n-3 fatty acids levels in blood. To date, few studies have examined the relationship between n-3 fatty acid levels and circulating biomarkers of inflammation. In a study of healthy individuals in Tuscany aged 20–98 years, Ferrucci et al found no significant relationship between total n-3 fatty acid levels and CRP (29). By contrast, in a study of 269 patients referred for coronary angiography, there was an inverse correlation between CRP and DHA levels (30). However, several important confounding factors including exercise capacity and blood pressure were excluded from the multivariate analysis. The present study establishes an independent inverse relationship between n-3 fatty acid levels and the inflammatory biomarkers CRP and IL-6 in a large cross-sectional study of individuals with stable coronary artery disease. Further epidemiologic studies are needed to quantify the magnitude of this potential anti-inflammatory effect, and to evaluate the role of inflammation in the clinical efficacy of n-3 fatty acids for secondary prevention.
The findings of the present study demonstrate a cross-sectional inverse association and therefore cannot establish causality. However, since mammals lack the enzymes necessary to synthesize n-3 fatty acids de novo, the primary determinants of blood levels are dietary intake, with genetic and other dietary factors playing as yet undefined roles (31). Although it is possible that inflammation could lead to decreased absorption or accelerated degradation of n-3 fatty acids, this explanation is biologically less plausible than the possibility that n-3 fatty acids reduce inflammation as observed in some randomized intervention trials.
Several well-studied biochemical pathways support the putative anti-inflammatory effects of n-3 fatty acids. First, through enrichment of membrane phospholipids, EPA competes with arachidonic acid as a substrate for the production of cycloxygenase- and lipoxygenase-derived eicosanoids (32). Functionally, the inflammatory mediators formed from EPA are less potent than those formed from arachidonic acid, resulting in an overall anti-inflammatory effect (33). Second, a novel group of anti-inflammatory mediators, the E-series resolvins, are also generated from EPA by the action of cycloxygenase-2 (34). Third, n-3 fatty acids have direct inhibitory effects on adhesion molecule expression and chemotaxis (35). Finally, as suggested by the present study, n-3 fatty acids can inhibit the production of inflammatory cytokines (36). Whether this effect is mediated through the anti-inflammatory mechanisms described above, or through novel direct transcriptional effects, is not known. Further in vitro studies are needed to elucidate the mechanism of inflammatory cytokine suppression by n-3 fatty acids. Evidence from such studies suggests that DHA can interfere with IL-1 signaling to prevent activation of nuclear factor kappa-b, a major mediator of inflammation (37).
The inhibitors of 3-hydroxyl-3-methylglutaryl coenzyme A (statins) are widely prescribed for the prevention and treatment of coronary artery disease. In addition to their lipid-lowering effects, statins exhibit a range of pleiotropic effects that may account for their clinical efficacy. The anti-inflammatory properties of statins are well described and are the focus of large ongoing clinical trials (38). In the present study, we found no evidence that use of statins modifies the association of n-3 fatty acid levels with CRP and IL-6. It is therefore possible that combination therapy with n-3 fatty acids and statins could produce a greater anti-inflammatory response than statins alone. However, this is speculative, and although the JELIS trial results are consistent with this possibility (13), properly designed randomized clinical studies would be needed to test this hypothesis.
Strengths of the present study include the direct chromatographic measurement of n-3 fatty acid levels and the evaluation of multiple potential confounding variables including clinical risk factors, medication use, exercise capacity, body mass index, and waist-to-hip ratio. However, several limitations should be considered in the interpretation of our results. First, our study population was predominantly male, and therefore the results cannot automatically be extrapolated to females. Second, we did not collect detailed dietary information with which to estimate n-3 fatty acid intake or supplementation. However, given the substantial individual variability in absorption and metabolism, circulating blood levels of fatty acids may be more relevant to health than dietary intake (39). Third, since all study participants were recruited from clinics in the San Francisco Bay Area, the association between n-3 fatty acid levels and inflammation may not generalize to other geographic areas, to other groups of patients, or to the general population. Fourth, our assessment of comorbidities based on self-reported questionnaire may be subject to recall bias resulting in an overestimate of true prevalence. Finally, despite biologic plausibility, our cross-sectional findings cannot establish a causal inverse relationship between n-3 fatty acid levels and inflammatory biomarkers.
In conclusion, we found an independent inverse association between n-3 fatty acid levels and the circulating inflammatory biomarkers CRP and IL-6 in a large cross-sectional study of patients with stable coronary artery disease. Future studies are needed to further explore the anti-inflammatory effects of n-3 fatty acids, and to investigate the potential benefits of this effect in therapeutic trials. Measurement of n-3 fatty acids levels in blood may provide an objective target of therapy in such studies.
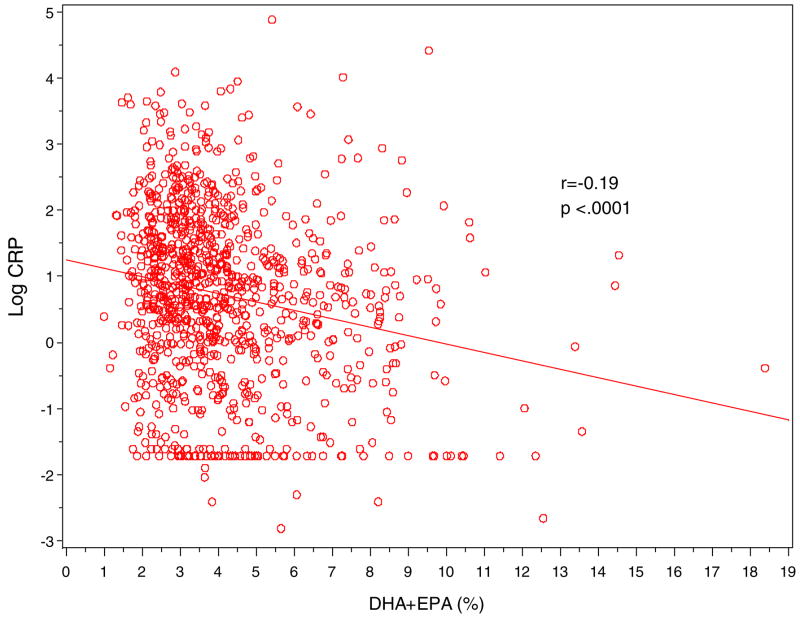
Figure 1.
Scatter Plot of log-CRP (y-axis) against erythrocyte n-3 fatty acid levels (DHA+EPA in %; x-axis)
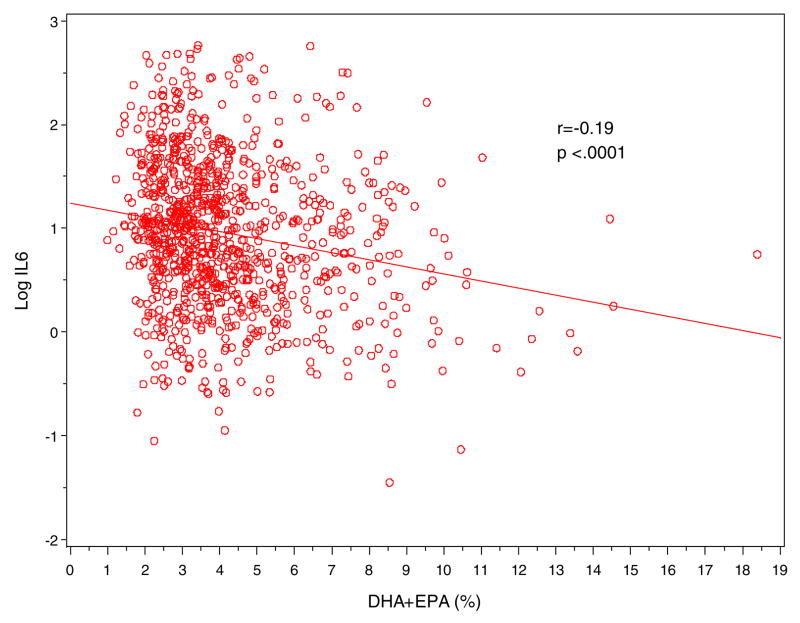
Figure 2.
Scatter Plot of log-IL-6 (y-axis) against erythrocyte n-3 fatty acid levels (DHA+EPA in %; x-axis)
Acknowledgments
FUNDING SOURCES
The Heart and Soul study was supported by the Department of Veterans Affairs (Epidemiology Merit Review Program), the National Heart, Lung and Blood Institute (R01 HL079235), the Robert Wood Johnson Foundation (Generalist Physician Faculty Scholars Program), the American Federation for Aging Research (Paul Beeson Faculty Scholars in Aging Research Program), the Ischemia Research and Education Foundation, and the Nancy Kirwan Heart Research Fund. Ramin Farzaneh-Far MD is supported by an American Heart Association Fellow-to-Faculty Transition Award.
Footnotes
The term “n-3 fatty acids” in this paper refers to docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA). Alpha-linoleic acid, a vegetable oil-derived n-3 fatty acid was not studied.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Breslow JL. N-3 Fatty acids and cardiovascular disease. Am J Clin Nutr. 2006;83(S):1477S–1482S. doi: 10.1093/ajcn/83.6.1477S. [DOI] [PubMed] [Google Scholar]
- 2.Lee JH, O’Keefe JH, Lavie CJ, Marchioli R, Harris WS. Omega-3 Fatty Acids for Cardioprotection. Mayo Clin Proc. 2008;83(3):324–332. doi: 10.4065/83.3.324. [DOI] [PubMed] [Google Scholar]
- 3.Harris WS, Poston WC, Haddock CK. Tissue n-3 and n-6 fatty acids and risk for coronary heart disease events. Atherosclerosis. 2007;193(1):1–10. doi: 10.1016/j.atherosclerosis.2007.03.018. [DOI] [PubMed] [Google Scholar]
- 4.Albert CM, Campos H, Stampfer MJ, Ridker PM, Manson JE, Willett WC, Ma J. Blood levels of long chain n-3 fatty acids and the risk of sudden death. N Engl J Med. 2002;346:1113–1118. doi: 10.1056/NEJMoa012918. [DOI] [PubMed] [Google Scholar]
- 5.Harris WS, Miller M, Tighe AP, Davidson MH, Schaefer EJ. Omega-3 fatty acids and coronary heart disease risk: clinical and mechanistic perspectives. Atherosclerosis. 2008;197(1):12–24. doi: 10.1016/j.atherosclerosis.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 6.Tsimikas S, Willerson JT, Ridker PM. C-reactive protein and other emerging blood biomarkers to optimize risk stratification of vulnerable patients. J Am Coll Cardiol. 2006;47(8S):C19–C31. doi: 10.1016/j.jacc.2005.10.066. [DOI] [PubMed] [Google Scholar]
- 7.Blake GJ, Ridker PM. C-reactive protein and other inflammatory risk markers in acute coronary syndromes. J Am Coll Cardiol. 2003;41(4S):37S–42S. doi: 10.1016/s0735-1097(02)02953-4. [DOI] [PubMed] [Google Scholar]
- 8.Calder PC. N-3 Polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am J Clin Nutr. 2006;83(S):1505S–1519S. doi: 10.1093/ajcn/83.6.1505S. [DOI] [PubMed] [Google Scholar]
- 9.Ruo B, Rumsfeld JS, Hlatky MA, Liu H, Browner WS, Whooley MA. Depressive symptoms and health-related quality of life: the Heart and Soul Study. JAMA. 2003;290:215–221. doi: 10.1001/jama.290.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dagenais GR, Yi Q, Mann JF, Bosch J, Pogue J, Yusuf S. Prognostic impact of body weight and abdominal obesity in women and men with cardiovascular disease. Am Heart J. 2005;149(1):54–60. doi: 10.1016/j.ahj.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 11.Yudkin JS, Kumari M, Humphries SE, Mohamed-Ali V. Inflammation, obesity, stress and coronary heart disease: is interleukin-6 the link? Atherosclerosis. 2000;148(2):209–14. doi: 10.1016/s0021-9150(99)00463-3. [DOI] [PubMed] [Google Scholar]
- 12.Zuliani G, Volpato S, Galvani M, Ble A, Bandinelli S, Corsi AM, Lauretani F, Maggio M, Guralnik JM, Fellin R, Ferrucci L. Elevated C-reactive protein levels and metabolic syndrome in the elderly: The role of central obesity: Data from the Inchianti study. Atherosclerosis. 2008 doi: 10.1016/j.atherosclerosis.2008.07.038. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Casas JP, Shah T, Hingorani AD, Danesh J, Pepys MB. C-reactive protein and coronary heart disease: a critical review. J Intern Med. 2008;264(4):295–314. doi: 10.1111/j.1365-2796.2008.02015.x. [DOI] [PubMed] [Google Scholar]
- 14.Danesh J, Kaptoge S, Mann AG, Sarwar N, Wood A, Angleman SB, Wensley F, Higgins JP, Lennon L, Eiriksdottir G, Rumley A, Whincup PH, Lowe GD, Gudnason V. Long-term interleukin-6 levels and subsequent risk of coronary heart disease: two new prospective studies and a systematic review. PLoS Med. 2008;5(4):e78. doi: 10.1371/journal.pmed.0050078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burr ML, Fehily AM, Gilbert JF, Rogers S, Holliday RM, Sweetnam PM, Elwood PC, Deadman NM. Effects of changes in fat, fish, and fibre intakes on death and myocardial reinfarction: diet and reinfarction trial (DART) Lancet. 1989;2(8666):757–761. doi: 10.1016/s0140-6736(89)90828-3. [DOI] [PubMed] [Google Scholar]
- 16.Marchioli R, Barzi F, Bomba E, Chieffo C, Di Gregorio D, Di Mascio R, Franzosi MG, Geraci E, Levantesi G, Maggioni AP, Mantini L, Marfisi RM, Mastrogiuseppe G, Mininni N, Nicolosi GL, Santini M, Schweiger C, Tavazzi L, Tognoni G, Tucci C, Valagussa F GISSI-Prevenzione Investigators. Early protection against sudden death by n-3 polyunsaturated fatty acids after myocardial infarction: time-course analysis of the results of the Gruppo Italiano per lo Studio della Sopravvivenza nell Infarcto Miocardico (GISSI)-Prevenzione. Circulation. 2002;105(16):1897–1903. doi: 10.1161/01.cir.0000014682.14181.f2. [DOI] [PubMed] [Google Scholar]
- 17.Yokoyama M, Origasa H, Matsuzaki M, Matsuzawa Y, Saito Y, Ishikawa Y, Oikawa S, Sasaki J, Hishida H, Itakura H, Kita T, Kitabatake A, Nakaya N, Sakata T, Shimada K, Shirato K Japan EPA lipid intervention study (JELIS) Investigators. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomized open-label, blinded endpoint analysis. Lancet. 2007;369(9567):1090–1098. doi: 10.1016/S0140-6736(07)60527-3. [DOI] [PubMed] [Google Scholar]
- 18.Kris-Etherton PM, Harris WS, Appel LJ. American Heart Association Nutrition Committee. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation. 2002;106(21):2747–2757. doi: 10.1161/01.cir.0000038493.65177.94. [DOI] [PubMed] [Google Scholar]
- 19.Pischon T, Hankinson SE, Hotamisligil GS, Rifai N, Willett WC, Timm EB. Habitual Dietary Intake of n-3 and n-6 Fatty Acids in Relation to Inflammatory Markers Among US Men and Women. Circulation. 2003;108:155–160. doi: 10.1161/01.CIR.0000079224.46084.C2. [DOI] [PubMed] [Google Scholar]
- 20.Lopez-Garcia E, Schulze MB, Manson JE, Meigs JB, Albert CM, Rifai N, Willett WC, Hu FB. Consumption of (n-3) Fatty Acids is Related to Plasma Biomarkers of Inflammation and Endothelial Activation in Women. J Nutr. 2004;134:1806–1811. doi: 10.1093/jn/134.7.1806. [DOI] [PubMed] [Google Scholar]
- 21.Ohsawa M, Itai K, Onoda T, Tanno K, Sasaki S, Nakamura M, Ogawa A, Sakata K, Kawamura K, Kuribayashi T, Yoshida Y, Okayama A. Dietary intake of n-3 polyunsaturated fatty acids is inversely associated with CRP levels, especially among male smokers. Atherosclerosis. 2008 doi: 10.1016/j.atherosclerosis.2008.01.008. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 22.Niu K, Hozawa A, Kuriyama S, Ohmori-Matsuda K, Shimazu T, Nakaya N, Fujita K, Tsuji I, Nagatomi R. Dietary long-chain n-3 fatty acids of marine origin and serum C-reactive protein concentrations are associated in a population with a rich diet in marine products. Am J Clin Nutr. 2006;84(1):223–229. doi: 10.1093/ajcn/84.1.223. [DOI] [PubMed] [Google Scholar]
- 23.Mori TA, Woodman RJ, Burke V, Puddey IB, Croft KD, Beilin LJ. Effect of eicosapentaenoic acid and docosahexaenoic acid on oxidative stress and inflammatory markers in treated-hypertensive type 2 diabetic subjects. Free Radic Biol Med. 2003;35(7):772–781. doi: 10.1016/s0891-5849(03)00407-6. [DOI] [PubMed] [Google Scholar]
- 24.Madsen T, Christensen JH, Schmidt EB. C-reactive protein and n-3 fatty acids in patients with a previous myocardial infarction: a placebo-controlled randomized study. Eur J Nutr. 2007;46(7):428–30. doi: 10.1007/s00394-007-0673-8. [DOI] [PubMed] [Google Scholar]
- 25.Yusof HM, Miles EA, Calder P. Influence of very long chain n-3 fatty acids on plasma markers of inflammation in middle-aged men. Prostaglandins Leukot Essent Fatty Acids. 2008;78(3):219–228. doi: 10.1016/j.plefa.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 26.Satoh N, Shimatsu A, Kotani K, Sakane N, Yamada K, Suganami T, Kuzuya H, Ogawa Y. Purified eicosapentaenoic acid reduces small dense LDL, remnant lipoprotein particles, and C-reactive protein in metabolic syndrome. Diabetes Care. 2007;30(1):144–146. doi: 10.2337/dc06-1179. [DOI] [PubMed] [Google Scholar]
- 27.Browning LM, Krebs JD, Moore CS, Mishra GD, O’Connell MA, Jebb SA. The impact of long chain n-3 polyunsaturated fatty acid supplementation on inflammation, insulin sensitivity and CVD risk in a group of overweight women with an inflammatory phenotype. Diabetes Obes Metab. 2007;9(1):70–80. doi: 10.1111/j.1463-1326.2006.00576.x. [DOI] [PubMed] [Google Scholar]
- 28.Ciubotaru I, Lee YS, Wander RC. Dietary fish oil decreases C-reactive protein, interleukin-6, and triacylglycerol to HDL-cholesterol ratio in postmenopausal women on HRT. J Nutr Biochem. 2003;14(9):513–521. doi: 10.1016/s0955-2863(03)00101-3. [DOI] [PubMed] [Google Scholar]
- 29.Ferrucci L, Cherubini A, Bandinelli S, Bartali B, Corsi A, Lauretani f, Martin A, Andres-Lacueva C, Senin U, Guralnik JM. Relationship of Plasma Polyunsaturated Fatty Acids to Circulating Inflammatory Markers. J Clin Endocrinol Metab. 2006;91:439–446. doi: 10.1210/jc.2005-1303. [DOI] [PubMed] [Google Scholar]
- 30.Madsen T, Skou HA, Hansen VE, Fog L, Christensen JH, Toft E, Schmidt EB. C-Reactive Protein, Dietary n-3 Fatty Acids, and the Extent of Coronary Artery Disease. Am J Cardiol. 2001;88:1139–1142. doi: 10.1016/s0002-9149(01)02049-5. [DOI] [PubMed] [Google Scholar]
- 31.Block RC, Harris WS, Pottala JV. Determinants of Blood Cell Omega-3 Fatty Acid Content. Open Biomarkers J. 2008;1:1–6. doi: 10.2174/1875318300801010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Endres S, Ghorbani R, Kelley VE, Georgilis K, Lonnemann G, van der Meer JW, Cannon JG, Rogers TS, Klempner MS, Weber PC. The effect of dietary supplementation with n-3 polyunsaturated fatty acids on the synthesis of interleukin-1 and tumor necrosis factor by mononuclear cells. N Engl J Med. 1989;320:265–271. doi: 10.1056/NEJM198902023200501. [DOI] [PubMed] [Google Scholar]
- 33.Goldman DW, Pickett WC, Goetzl EJ. Human neutrophil chemotactic and degranulating activities of leukotriene B5 (LTB5) derived from eicosapentaenoic acid. Biochem Biophys Res Commun. 1983;117:282–288. doi: 10.1016/0006-291x(83)91572-3. [DOI] [PubMed] [Google Scholar]
- 34.Serhan CN, Hong S, Gronert K, Colgan SP, Devchand PR, Mirick G, Moussignac RL. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter pro-inflammation signals. J Exp Med. 2002;196:1025–1037. doi: 10.1084/jem.20020760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sperling RI, Benincaso AI, Knoell CT, Larkin JK, Austen KF, Robinson DR. Dietary n-3 polyunsaturated fatty acids inhibit phosphoinositide formation and chemotaxis in neutrophils. J Clin Invest. 1993;91:651–660. doi: 10.1172/JCI116245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Caughey GE, Mantzioris E, Gibson RA, Cleland LG, James MJ. The effect on human tumor necrosis factor α and interleukin 1β production of diets enriched in n-3 fatty acids from vegetable oil or fish oil. Am J Clin Nutr. 1996;63:116–122. doi: 10.1093/ajcn/63.1.116. [DOI] [PubMed] [Google Scholar]
- 37.Massaro M, Habib A, Lubrano L, Del Turco S, Lazzerinin G, Bourcier T, Weksler BB, De Caterina R. The omega-3 fatty acid docosahexaenoate attenuates endothelial cycloxygenase-2 induction through both NADP(H) oxidase and PKC inhibition. PNAS. 2006;103:15184–15189. doi: 10.1073/pnas.0510086103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ridker PM, Fonseca FA, Genest J, Gotto AM, Kastelein JJ, Khurmi NS, Koenig W, Libby P, Lorenzatti AJ, Nordestgaard BG, Shepherd J, Willerson JT, Glynn RJ JUPITER Trial Study Group. Baseline characteristics of participants in the JUPITER trial, a randomized placebo-controlled primary prevention trial of statin therapy among individuals with low low-density lipoprotein cholesterol and elevated high-sensitivity C-reactive protein. Am J Cardiol. 2007;100(11):1659–1664. doi: 10.1016/j.amjcard.2007.09.072. [DOI] [PubMed] [Google Scholar]
- 39.Sun Q, Ma J, Campos H, Rexrode KM, Albert CM, Mozaffarian D, Hu FB. Blood concentrations of individual long-chain n-3 fatty acids and risk of nonfatal myocardial infarction. Am J Clin Nutr. 2008;88:216–223. doi: 10.1093/ajcn/88.1.216. [DOI] [PMC free article] [PubMed] [Google Scholar]