Abstract
Estrogen has been demonstrated to enhance the use of hippocampal-based place learning while reducing the use of striatal-based motor-response strategy (Korol et al., 2004). Previous research has focused on task acquisition and the switch from a place to motor-response navigation with training. The current paradigm allowed an examination of the interplay between these two systems by having well-trained animals switch strategies “on demand.” Female and male Sprague Dawley rats were taught a motor-response task on a plus maze. The rats were then introduced to a place task and taught to switch, by cue, from the motor-response to place strategy. Finally, the rats were trained to continuously alternate between place and motor-responses strategies. The maze configuration allowed for an analysis of cooperative choices (both strategies result in the same goal arm), competitive choices (both strategies result in different goal arms), and single strategy choices (can only use the motor-response strategy). The results indicate that sex and estrogen-related effects on navigation strategy are limited to the initial stages of learning a task. The role of sex and estrogen is diminished once the task is well learned, and presumably, the relative involvement of the hippocampal and striatal systems is established.
Keywords: estrogen, place, response, learning, spatial, memory systems, navigation
Introduction
Different neural systems mediate a range of behaviors and learning. Two such systems are the hippocampal system - known to be involved in spatial navigation (Jarrard, 1995) and the dorsal striatal system - involved in habit and motor-responses (Packard & Knowlton, 2002). These disparate systems are suggested to be parallel processors - that is, they receive the same information but only activate it when that information is pertinent to the task at hand (Poldrack, & Packard, 2003; White & McDonald, 2002). In place learning, the rat utilizes spatial cues in order to determine the whereabouts of a goal, while in motor-response learning, the goal location is based on a body movement. Exactly how these systems interact is the subject of much research (Chang & Gold, 2003; Gibson & Shettleworth, 2005; Kesner et al., 1993; Packard & McGaugh, 1996; Packard & Knowlton, 2002; Schroeder et al., 2002; White & McDonald, 2002). Evidence that the hippocampus mediates place learning (“go there”) and the striatum mediates motor-response learning (“turn right”) is demonstrated using an ambiguous T-maze alternation task (Tolman et al., 1946). Packard and McGaugh (1996) trained rats on a T-maze to turn right (east arm) for food reward. After eight and sixteen trials, a probe trial was administered, and the start location was rotated 180 degrees. The rat could use a motor-response and make a right hand turn (west arm) or use a place strategy and turn left (east arm). On the first probe trial, rats predominately used place strategies. Upon extended training, the rats predominately used a motor-response. Reversible lesions of the hippocampus biased the animals to the motor-response strategy, while striatal inactivation resulted in a place strategy preference (Packard & McGaugh, 1996). Similar effects are seen with post-training hippocampal inactivation (Schroeder et al., 2002). Additionally, post-training infusions of glutamate has been shown to enhance memory. Using the same place and motor-response strategy T-maze paradigm Packard (1999) infused glutamate into the dorsal striatum or hippocampus. Glutamate infusions into the hippocampus preserved place strategy preference from the early to the late probe trial. Additionally, when glutamate was infusioned into the striatum motor-response learning was accelerated in the early probe and maintained during the late probe trial. Chang and Gold (2003) demonstrated that with training there is increased acetylcholine in the striatum that is correlated with the switch from place to motor-response strategies. The double dissociation between the hippocampus and striatum suggests that these two systems are in competition, with the relative amount of activity in each system affecting which system will dictate the animal’s behavior. The dissociation between the hippocampus and striatum has been seen in humans (Doeller et al., 2008).
Research regarding sex differences on learning shows conflicting results, varying from no sex differences to male or female advantages (for review see Jonasson, 2005). Blokland et al. (2006) tested male and female rats on a Morris Water Maze task that could be solved by utilizing place strategies, cues, and motor-response strategies. Sex differences were not found for the acquisition of place learning or motor-response learning. However, males did have significantly lower latencies and swim distances, though there were no sex differences in swimming speed.
Similarly, reports regarding the effects of estrogen on learning and memory are also contradictory (Dohanich, 2002). Some of the conflicting results may stem from the fact that these tasks differed in the degree to which they depended upon a spatial or motor-response strategy. It has been shown that estrogen modulates strategy use during maze tasks (Korol et. al, 2004; Zurkovsky et al., 2007). Systemic injections of estradiol facilitated place learning and exacerbated motor-response learning (Korol & Kolo, 2002). Infusions of estradiol into the hippocampus enhanced place learning, but had no effect on motor-response learning (Zurkovsky et al., 2007). However, estradiol infusions into the striatum produced the opposite effect; while there was no effect on place learning, motor-response learning was impaired. Similar results have also been seen in natural cycling rats. Rats in proestrus (high estrogen levels) show a preference of place over motor-response strategies, while rats in estrus (low estrogen) show the opposite (Korol et. al, 2004).
In the past, two approaches have been used to examine strategy dominance. The first, the ambiguous T-maze alternation task, is based on one or two probe trials to uncover which strategy the animal has been using (e.g. see above Packard & McGaugh, 1996). This paradigm provides only two data points and does not allow for a continuous assessment of behavior. A second approach is based on training the rats to solve the maze using a fixed strategy (e.g. “always turn right”) and recording the rate of acquisition (e.g. Korol & Kolo, 2002). The speed of acquisition allows for inferences regarding the dominance of the competing system; this approach is limited to examining acquisition rate. While both these experimental paradigms have shown dissociation between the hippocampal “place” system and the striatal “motor-response” system, they cannot determine the degree to which these two regions interact with each other as animals perform a navigation task.
A new paradigm developed in our lab (Jacobson et al., unpublished data) combines hippocampal-dependent place strategy and striatal-dependent motor-response strategies within the same training session. First, rats were taught a simple motor-response (“turn right”) task on a plus maze. Second, once proficient at the motor-response task, rats were introduced to the place task, identified by a salient cue (flashing light), and taught to find the same goal arm (east arm), regardless of their location on the maze. Rats were then trained to switch between blocks of the previously learned motor-response and newly learned place strategies. Finally, rats were trained to pseudorandomly switch between place and motor-response trials. This paradigm allows for the examination of sex differences in the acquisition of motor-response and place tasks. Importantly, this paradigm also allows for an examination of post-acquisition effects of estrogen on strategy choice.
Methods
Subjects
Eight male and ten female Sprague-Dawley, 4 months old at the beginning of the study, were trained (Harlan Sprague Dawley, Indiana). The rats were housed individually in clear Plexiglas cages (46 × 20 × 23cm) with wood chip bedding and maintained on 12/12 hour light/dark cycle. The rats were maintained at about 90 percent of their ad lib. weight during the experiment. All procedures were approved by the University of Connecticut IACUC.
Estrogen Levels
Daily lavages were conducted for one month before the beginning of the experiment. During maze training, the cycle phase of female rats was obtained twice a day, before and after training, every day through vaginal smears (Tropp et al., 2005). Vaginal secretions were extracted by mild penetration of the vaginal orifice with a water dropper filled with water and examined under a light microscope. Estrous cycle was determined by the proportion of leukocytes, nucleated epithelial cells, and cornified cells. Smears containing predominantly leukocytes (≥60%) were classified as diestrus. Smears containing predominantly nucleated epithelial cells (≥60%) and no leukocytes (≥10%) were classified as proestrus. Smears containing primarily cornified cells (≥90%) were classified as estrus. Data from irregular cycling animals were not used in the analysis. The male rats were given an anal probe with a Q-tip in parallel to the female lavage in an attempt to equate handing.
Behavioral training & Apparatus
Apparatus
A black Plexiglas runway (120.7 cm × 10.2 cm) was used for pretraining. The four arm plus maze was constructed of black Plexiglas (112.4 cm × 10.8 cm raised 15.9 cm off the table) and had four black Plexiglas perimeter runways (see Figure 1).
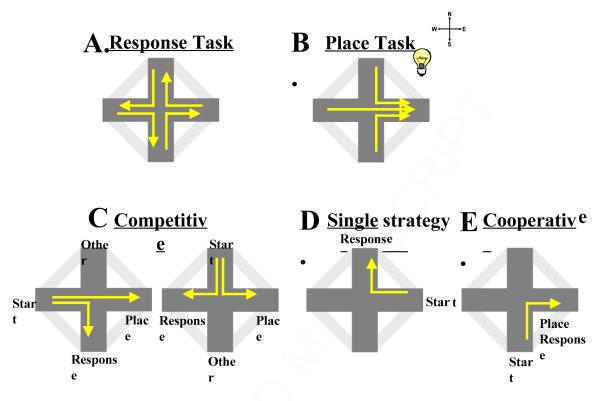
Figure 1.
Representation of task types and error configuration. The rats were taught two different strategies. (A) In the motor-response task rats were trained to make a right hand turn regardless of start arm. (B) A strobe light cued the place task where the rat learned to go to the same end arm regardless of start arm. Errors are based on task type (place/response) and the trial configuration of the start arm; each start arm is designated competitive (north and west), single strategy (east) and cooperative (south) (i.e. when designated a place trial (light on), if the rat starts at the north arm and then goes to the west arm, the rat has made a competitive place error). (C) On a competitive trial place and motor-response strategies indicated different goal locations. The rat could also make an “other choice” and disregard both place and response possibilities by going to the third arm. (D) A single strategy trial only allowed for the use of the motor-response strategy since the start arm is the place strategy goal arm. (E) On a cooperative trial both place and motor-response strategies result the same goal location.
Pretraining
Rats were trained to run back and forth on a linear runway for chocolate sprinkle rewards once a day, daily for 14 days. The daily training sessions continued up to 15 minutes on the maze or until the rat reached criterion of five trials in five minutes.
General Plus Maze Procedure
The animals were trained on a plus maze in a dimly lit room. Animals were placed on the start arm behind a transparent barrier. The trial started when the barrier was lifted and ended as soon as the rat chose an arm (forelimbs half way through the arm). Once a trial ended, the rat was blocked from re-entering the maze and guided through the perimeter runways to the next start arm (see Figure 1). The perimeter allowed the animal to return to the start arm after each trial without traversing back through the maze. Additionally this was expected to minimize stress by reduced experimenter handling of the animal (Conrad et al., 2004). When errors were made the animal was returned to the start arm to repeat the trial until it was done successfully. The rats were divided into two groups of nine rats. Each group was run every other day, six days a week. Each rat was trained until thirty-two correct trials were completed or until 25 minutes had passed.
Experiment I: Motor-Response Training
During the first two training days, the rats were allowed to explore the maze freely until the reward was found. After the reward was found, the rats were guided via the perimeter runway to the next start arm and the next trial commenced. This allowed the rats to get acclimated to the maze. These two days were not used for data analysis. On the third day of training, the rats were trained to perform a motor-response task (“make a right-hand turn”). Each trial ended after the rat entered a maze arm, whether or not a correct motor-response was made. The rat was then guided via the perimeter runway to the next start arm (if an error was made, the rat was returned to the original start arm to repeat the trial until successful). Training continued until each rat reached criterion (≥ 80% correct total trials on two consecutive training days). Once the rat reached criterion for Experiment I, the rat commenced training on Experiment II, the mixed place/motor-response task (Table A).
Table A.
Experiment Procedure
| Exp. | Task | Trials | Criterion |
|---|---|---|---|
| I | Motor-Response | All Response | 2 days 80% correct |
| II | Half Motor/Half Place | Blocks of 16 Motor/16 Place trials | 2 days 80% correct on both Place/Response |
| III | Mixed Motor/Place | Interleaved Motor and Place trials | 2 days 80% correct on both Place/Response |
Sessions ended after 32 correct trials or after 25min. Rats were trained until criterion was met. All rats were trained on all three experiments.
Experiment II: Place and Motor-Response Blocks Training
In Experiment II, rats were trained on blocks of motor-response and place trials with session lasting twenty-five minutes or thirty-two correct trials, whichever came first. The sessions were broken up roughly into half place/half motor-response blocks (∼ 16 trials each). Again, during the motor-response block, rats were rewarded for making a right hand turn on the maze. During the place block, rats were trained to go to the designated place arm (east arm) regardless of the start arm. The place trials were differentiated from the motor-response trials by a flashing light. The first training day began with a block of the motor-response trials, followed by a block of place trials. This was switched on the next training day, which began with a block of place trials, followed by a block of motor-response trials. The subsequent training consisted of switching once or twice between motor-response trails and place trials. As with Experiment I, after making a correct choice, the rats were guided via the perimeter runway to the next start arm, and an incorrect response resulted in a repeat of the trial until successful. Training continued until each rat reached criterion (≥ 80% correct for both place and motor-response trials for two consecutive days) after which the rat commenced training on Experiment III, the mixed place and motor-response task (Table A).
Experiment III: Mixed Place and Motor-Response Training
In Experiment III, each rat was given a similar number of place and motor-response trials with sessions lasting twenty-five minute or thirty-two correct trials, whichever came first. However, the place and motor-response trials were interwoven with no more than three consecutive trials of one trial type. All rats were tested for eight days past criterion (≥ 80% correct on both place and motor-response trials for two consecutive days). To gather more estrous cycle data, female rat testing continued up to sixteen days past criterion.
Data Analysis
Sex differences were assessed with one-tailed student’s t-tests with the alpha level set at .05. Trial type error analysis (see below) and estrous cycle effects were assessed with a one-way ANOVA
Maze Performance Analysis
The configuration of the plus maze allowed for an examination and analysis of errors made (see Figure 1). The four arms of the maze contained a designated start arm, a goal arm, and two alternate arms. Each trial configuration was designated as cooperative, competitive, or single option, based on the strategy that could be used at the start arm. The north and west arms are designated competitive arms (Figure 1C). On a competitive arm/trial the rat could utilize either a place or motor-response strategy, resulting in different end arms (east/west for a north start arm and east/south for a west start arm). Specifically, the rat could use a place strategy, which would lead to the east arm, or a motor-response strategy and make a right hand turn (i.e. if the rat is starting on the north arm, the rat could use a place strategy, which would lead to the east arm, or use a motor-response strategy that would lead to the west arm). The east arm is designated a single strategy trial (Figure 1D). A single strategy arm/trial permits for the use of only the motor-response strategy (i.e. the rat would start on the east arm, allowing only for a right hand turn onto the north arm). The south arm is designated a cooperative arm/trial (Figure 1E). On a cooperative trial, both strategies resulted in ending in the goal arm/east arm (i.e. the rat could use a place strategy, which would lead to the east arm, or a motor-response strategy and make a right hand turn, also ending on the east arm). Therefore, errors were designated based upon task and trial configuration (i.e. when designated a place trial (light on), if the rat starts at the north arm and then goes to the west arm, the rat has made a competitive place error). Correct percentages on place trials, motor-response trials, and total trials were assessed, as well as total number of trials and latency.
Results
Experiment I: Motor-Response Task
On the first day of training, males had a higher percentage of correct trials on the motor-response task than females (t (15) = 2.35, p < .05). However, the total number of errors made per session, total number of trials run per session, and latency per trial were not affected by sex (Table B). Overall, the males required fewer days to reach criterion (t (15) = 2.90, p < .05). Once all the rats reached criterion (two days of ≥ 80% correct), there were no effects of sex (Table B) or estrous cycle (Table C) on percent choice correct, number of trials or latency.
Table B.
Sex Difference Summary for Student t-Tests & ANOVA Analysis
| Experiment | Male | Female |
|---|---|---|
| I: Response Task | ||
| Day 1 | ||
| % Correct | 58%(.12)* | 29%(.06)* |
| Total Errors | 13.86(3.91) | 21.1(3.67) |
| Latency | 40.6(4.80) | 42.05(2.99) |
| Total # of Trials | 33.57(2.20) | 27.9(3.81) |
| Days to Criteria | 3.0(.31)* | 4.1(.23)* |
| At Criteria | ||
| %Correct | 91%(.01) | 92%(.02) |
| Total Errors | 3.35(.52) | 3.0(.72) |
| Latency | 31.91(1.21) | 31.70(1.33) |
| Total # of Trials | 34.92(.70) | 34.72(.56) |
| II: Place & Response Task Blocks | ||
| Day One | ||
| Total % Correct | 61%(.03) | 60%(.03) |
| Place Trials % Correct | 38%(.05) | 38%(.04) |
| Response Trials % Correct | 98%(.01)* | 92%(.03)* |
| Total # of Errors | 19.0(1.96) | 18.7(1.52) |
| Place Errors on Response Trial | .00(.00) | .2(.13) |
| Response Errors on Place Trial | 11.25(1.57) | 11.3(1.02) |
| Other Arm Errors | 6.63(.88) | 6.1(.69) |
| Single Strategy Error | .125(.125)^ | .70(.33)^ |
| Cooperative Strategy Error | 1.0(.38)^ | .40(.16)^ |
| Latency | 26.64(1.57) | 29.14(1.46) |
| Total # of Trials | 48.38(1.63) | 47.1(1.24) |
| Day Two | ||
| Total % Correct | 67%(.04) | 63%(.04) |
| Place Trials % Correct | 79%(.03)^ | 69%(.05)^ |
| Response Trials % Correct | 58%(.06) | 58%(.06) |
| Latency | 31.09(1.80) | 29.22(2.26) |
| Total # of Trials | 42.75(.82) | 46.4(1.93) |
| Days to Criterion | 7(1.05) | 6.9(.75) |
| At Criteria | ||
| Total % Correct | 86%(.01) | 87%(.01) |
| Place Trials % Correct | 84%(.02) | 86%(.02) |
| Response Trials % Correct | 89%(.02) | 89%(.02) |
| Latency | 21.84(1.04)* | 25.10(1.18)* |
| Total # of Trials | 37.13(.46) | 36.75(.54) |
| III: Pseudorandom Place & Response Task | ||
| Day 1 | ||
| Latency | 22.04(2.41) | 23.94(1.27) |
| Total # of Trials | 38.0(1.46) | 40.8(.55) |
| Days to Criterion | 3.5(.38) | 3.2(.43) |
| At Criteria | ||
| Total % Correct | 90%(.01) | 90%(.01) |
| Place Trials % Correct | 90%(.01) | 90%(.01) |
| Response Trials % Correct | 90%(.01) | 90%(.01) |
| Total # of Errors | 3.79(.22) | 3.88(.27) |
| Place Errors on Response Trial | 1.82(.17) | 1.82(.16) |
| Response Errors on Place Trial | 1.79(.14) | 1.77(.16) |
| Other Arm Errors | .13(.04)* | .24(.05)* |
| Single Strategy Error | .01(.01) | .02(.01) |
| Cooperative Strategy Error | .04(.02) | .05(.02) |
| Latency | 19.43(.36)* | 22.28(1.07)* |
| Total # of Trials | 35.81(.22) | 35.24(.29) |
Note. Values given represent mean. Standard error of measurement is represented in parentheses.
p<.10.
p < .05.
Table C.
Estrous Cycle Summary of Student t-tests & ANOVA Analysis
| Experiment | Diestrus | Proestrus | Estrus | Metestrus |
|---|---|---|---|---|
| I: Response Training | ||||
| At Criteria | ||||
| %Correct | 91%(.02) | 89%(.05) | 91%(.02) | NA |
| Total Errors | 3.44(.85) | 4.33(2.03) | 3.2(.73) | NA |
| Latency | 30.89(1.94) | 29.94(1.68) | 35.07(3.30) | NA |
| II: Place & Response Training Blocks | ||||
| At Criteria | ||||
| Total % Correct | 86%(1.78) | 85%(2.02) | 88%(6.03) | 88%(4.12) |
| Place Trials % Correct | 85%(2.55) | 84%(5.81) | 89%(4.12) | 88%(2.89) |
| Response Trials % Correct | 87%(2.97) | 88%(6.94) | 88%(2.14) | 90%(2.60) |
| Total # of Errors | 5.5(.78) | 5.67(.88) | 4.5(2.5) | 4.67(1.76) |
| Place Errors on Response Trial | 2.5(.71) | 2.33(1.86) | 1.5(.50) | 1.33(.88) |
| Response Errors on Place Trial | 2.00(.46) | 3.0(1.15) | 2.0(2.0) | 3.33(.88) |
| Other Arm Errors | .75(.31) | .33(.33) | .00(.00) | .00(.00) |
| Single Strategy Error | .00(.00)* | .00(.00)* | .50(.50)* | .00(.00)* |
| Cooperative Strategy Error | .25(.16) | .00(.00) | .50(.50) | .00(.00) |
| Latency | 28.78(2.03) | 24.40(.60) | 22.62(.32) | 22.08(2.70) |
| III: Pseudorandom Place & Response Training | ||||
| At Criteria | ||||
| Total % Correct | 91%(.01) | 91%(.01) | 89%(.02) | 93%(.01) |
| Place Trials % Correct | 90%(.01) | 90%(.02) | 90%(.02) | 92%(.02) |
| Response Trials % Correct | 92%(.01) | 92%(.02) | 90%(.02) | 94%(.01) |
| Total # of Errors | 3.36(.37) | 3.44(.56) | 4.1(.68) | 2.45(.43) |
| Place Errors on Response Trial | 1.48(.23) | 1.52(.34) | 2.05(.49) | 1.15(.25) |
| Response Errors on Place Trial | 1.61(.19) | 1.76(.32) | 1.8(.45) | 1.1(.27) |
| Other Arm Errors | .16(.06) | .16(.07) | .20(.12) | .15(.11) |
| Single Strategy Error | .09(.04) | .00(.00) | .00(.00) | .00(.00) |
| Cooperative Strategy Error | .07(.04) | .00(.00) | .05(.05) | .05(.05) |
| Latency | 20.13(.86) | 21.90(2.51) | 20.44(1.00) | 19.44(.66) |
Note. Values given represent mean. Standard error of measurement is represented in parentheses.
p < .05.
Experiment II: Place & Motor-Response Task Blocks Task
The first training day began with a block of motor-response trials followed by a block of place trials. Introducing the place task was confusing for the animals, bringing their performance close to chance (Table B), with no sex effects on percent correct (t (16) = -.07, p > .10). Day two of training began with a block of place trials followed by a block of motor-response trials. Females tended to make more errors than males during the place trial block (t (16) = -1.518, p = . 067, Figure 2). There was a carry over effect, due to an increased use of previously learned motor-response strategy during competitive place type trials and failure to switch to the newly learned place strategy (t (16) = 1.86, p < .05, Figure 1 & 2). There were no other sex differences (Table B). Following the place trial block, the motor-response trial block was reintroduced, and again the rats had a carry over effect since both sexes had difficulty switching back to the motor-response strategy (combined male-female data, comparing percentage of correct motor-response trials day one to day two, t (34) = 8.37, p <.0001). After day two of Experiment II a carry over effect was not looked for in the following training sessions. The data analysis focused on a general incapability to switch between tasks regardless of the previous trial type.
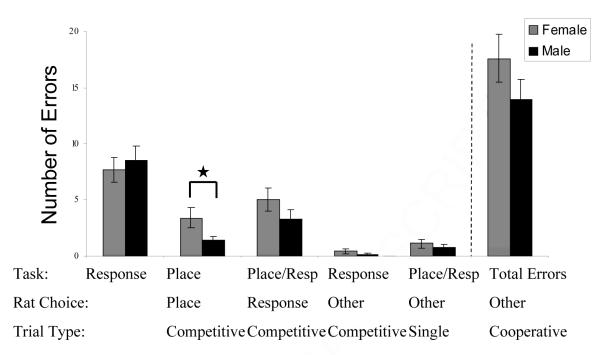
Figure 2.
Experiment II, day two error analysis. In Experiment II, the rats were taught to use a place strategy for half of the session. The first half of the day one session consisted of motor-response trials. The second half of the session consisted of place trials. Depicted are the data from the following day (day 2), where the first half of the session consisted of place trials and the second half motor-response trials. In the first half of the session (place trials) females continued to (incorrectly) use motor-response strategies more than the males. In the second half of the session (response trials), the males and females both incorrectly used place trials to solve the task. Rats that were trained successfully on Experiment I were subsequently trained in Experiment II.
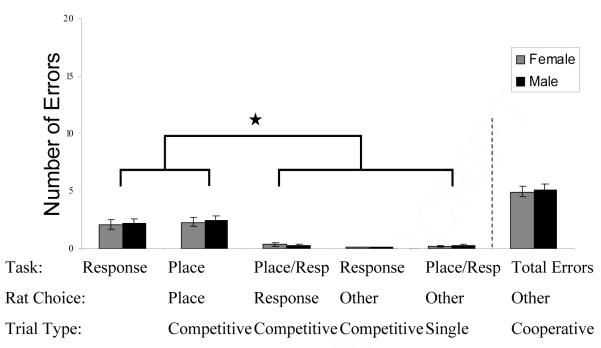
Once at criterion, sex did not have an effect on correct responses, although males ran faster than females (t (31) = -2.067, p < .05, Table B). Notably, although the rats were at asymptotic performance at criterion, they continued to show a distinct pattern of errors on the maze. The rats made more incorrect strategy errors on competitive than other types of trials (combined male-female data, F(4, 175) = 32.05, p < .000; Figure 3). A post hoc analysis showed more competitive place or competitive response type errors than on single, cooperative, and choosing the “other” arm type errors (all Tukey p < .0001). Despite the errors on competitive trials, an analysis of the female data did not show any effect of the estrous cycle on any type of error (all p > .10) or latency ( F (3,12) = 2.20, p > .10) (Table C).
Figure 3.
Experiment II, at criterion. Rats made more incorrect strategy errors on competitive arms than other types of trials. Specifically, competitive trials where the rat could utilize either strategy, place or motor-response, had more errors than other trials types that utilized both strategies (cooperative trials), only the motor-response strategy (single trial) or lastly a competitive arm where neither place or motor-response strategies would lead to a goal arm (competitive “other”). Rats that were trained successfully on Experiment I were subsequently trained in Experiment II.
Experiment III: Mixed Place & Motor-Response Task
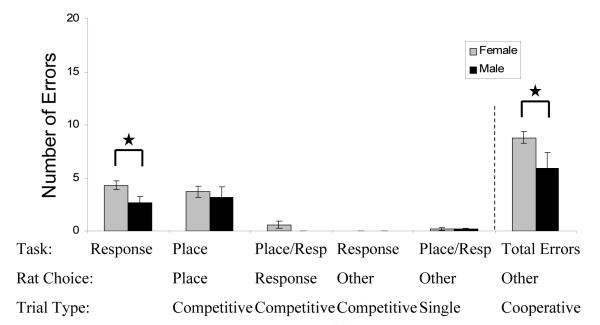
On the first day of the mixed motor-response/place task, males performed better than females. Males had more correct trials overall (t (16) = 2.25, p < .05); this was primarily due to better performance on motor-response trials (t (16) = 2.67, p < .01, Figure 4 and Table B). Females made more errors on motor-response trials - specifically, females made more errors by incorrectly utilizing the place strategy on the motor-response task (Figure 5). There was no difference in latency or days to criterion (Table B).
Figure 4.
Percent correct for Experiment III, day one. These sessions consisted of mixed place and motor-response trials. Overall the males had a higher percent of correct trials. This was mostly due to the fact that males performed better than females on the motor-response trials. Rats that were trained successfully on Experiment II were subsequently trained in Experiment III.
Figure 5.
Error analysis for Experiment III, day one. Females made more overall errors than males. Additionally, females made more errors by incorrectly using place strategies on motor-response trials. Interestingly, there were no differences between incorrectly using motor-response strategies on place trials. Rats that were trained successfully on Experiment II were subsequently trained in Experiment III.
Once at criterion, males were faster than females (t (155) = -2.34, p < .05). The rats continued to make errors while at asymptomatic performance, however, the trial configuration error analysis showed no sex differences (all trials p >.10) except on competitive “other” trials (t (178) = 1.78, p <.05). As in Experiment II, the combined data showed the same pattern of errors (F (4, 1075) = 179.73, p < .0001). A post hoc analysis showed more competitive place or motor-response type errors than on single, cooperative, and choosing the “other” arm type errors (all Tukey p< . 0001). Also, once at criterion there were no effects of the estrous cycle on performance or latency (Table C).
Discussion
The current series of experiments allowed for an examination of both acquisition and post-acquisition performance in a number of navigation tasks. The first was a motor-response task similar to that used by Korol and Kolo (2002). This was followed by the addition of a second method of reaching the goal, using a place strategy. The degree to which adding this second strategy disrupted the previously learned motor-response strategy was measured. The third experiment used a task that demanded a constant shift in navigation strategy. This allowed for the analysis of the continuous interplay between the place system and motor-response system.
Ten naturally cycling female rats were tested across the estrous cycle. The repeated testing of the animals at criterion allowed for a within-animal analysis, and this was ideal for determining post-acquisitional effects of the cycle. Given the rapid acquisition of the tasks (3-7 days), the current design had little power to detect estrous cycle effects on acquisition. Conversely, the design allowed for a determination of sex differences both during acquisition and post-acquisition.
The results indicated that sex-related effects on navigation strategy were limited to the initial response to novel task requirements. Once the tasks were acquired and presumably the relative involvement of the hippocampus and striatal systems established, no sex or estrous cycle-related effects were found. These findings are consistent with previous findings in our lab which demonstrated initial sex differences in anxiety, exploration, and cue utilization; however, these effects are diminished with repeated exposure to the environment (Tropp & Markus, 2001).
Learning the Motor-Response Task
In Experiment I, rats were trained on a motor-response task. Packard and McGaugh (1996) demonstrated that rats are initially disposed to use spatial strategies. Thus the motor-response task training required the animals to inhibit their place bias. The current results indicate that initially males were better than females at learning the motor-response task. However, with continuous training, both sexes showed equal asymptotic performance.
Korol and colleagues (Korol, 2004; Korol & Kolo, 2002; Korol et al., 2004; McElroy & Korol, 2005) found that estrogen-treated rats had a harder time than oil-treated animals when they had to acquire this motor-response strategy over the course of a single day. In the current study, no such affect was found at criteria. Taken together these data indicate that the estrogen effects are limited to the acquisition of a navigation task.
Introducing the Place Task & Mixed Trials
By the end of Experiment I, the rats had successfully learned the motor-response task, and the effect of adding a second strategy (place) was examined. Experiment II required switching back and forth between the newly learned place task and previously learned motor-response task. This task was harder and required twice as much training to reach criterion as Experiment I. After learning this task, rats were trained to switch between place and motor-response strategies many times during the same session.
On the first day of Experiment II, all groups showed only chance performance on the place trials. On the following training day, both groups showed above chance performance during the block of place trials, and sex differences and a carry over effect were found. The female rats were utilizing the old and incorrect (at the time) motor-response strategy and did not switch over to utilizing the place strategy as well as the males. Following the place trials, the rats were given a block of motor-response trials, and both males and females were equally able to switch back to the previous strategy (although performance for both was significantly impaired, again a carry over effect, note Table B). On the first day of Experiment III, again females made more errors than males. This was mainly due to females making more place strategy errors on motor-response trials. Beyond the initial day of training, both males and females acquired the task equally well. Again, once at asymptomatic performance, sex no longer mitigated the type of errors made, nor the total amount of errors.
At criterion for Experiments II and III, the males ran faster than the females, in agreement with previous data (Blokland et al., 2006). Additionally, once at asymptomatic performance, estrogen did not modulate cognitive strategies. Unlike previous acquisition studies, once at criterion, rats in proestrus did not demonstrate improved performance on place trials, and rats in estrus were not better on motor-response trials. These findings are in contrast with previous research on acquisition where high levels of circulating estrogen potentiated place learning and exacerbated motor-response learning (Davis et al., 2005; Korol, 2004; Korol & Kolo, 2002; Korol et al., 2004; McElroy & Korol, 2005).
The only other study to examine the effects of estrogen on acquisition and post-acquisition performance was by Frye (1995). In one experiment from this study rats were trained on the MWM until criterion, given a 6 week break, and then ovariectomized. After this, diestrus and estrus phases were “mimicked” by estrogen treatment. When the rats were retested, rats in estrus demonstrated impaired performance on the familiar maze compared to rats in diestrus and ovariectomized controls. However, the effects were minimal and only existed on the initial trial of the day. These data demonstrate that the effects of estrogen, if any, are minimal once the task is well learned (Frye, 1995). To what degree Frye’s findings are specific to using estrogen replacement, the water maze, or the relearning of a task is unclear.
Behavioral Flexibility
Studies exploring multiple memory systems suggest that the prefrontal cortex is important in behavioral flexibility (i.e. switching between/within tasks) (Ragozzino et al., 1999; Rich & Shapiro, 2007). Thus, Ragozzino et al. (1999) showed that the prefrontal cortex is necessary for the behavioral flexibility between different strategies (i.e. place followed by motor-response or motor-response followed by place) but isn’t required for the switching within strategies (i.e. place reversal learning or motor-response reversal learning). However, the contribution of the prefrontal cortex in strategy switching is diminished with extensive training (Rich & Shapiro, 2007). A similar study in humans using fMRI (Doeller et al., 2008) looked at hippocampal and striatal activity during a simulated version of the MWM, as well as prefrontal cortex activity. Activation of the ventromedial prefrontal cortex was highest when both the hippocampus and striatum were highly active. Ventromedial prefrontal cortex activation was lowest when only the hippocampus or striatum was active. Taken together these two studies suggest that even though the prefrontal cortex is involved in behavioral flexibility, the intensity of the activation drives which system is utilized.
Conclusion
Multiple memory systems have been a topic of recent interest. Several studies have elucidated how different neural systems interact with each other during learning (Packard & McGaugh, 1996; Poldrack, & Packard, 2003; White & McDonald, 2002). The maze paradigm used in the current study was designed to do the following: 1) analyze how the hippocampus and striatum interact during strategy selection, and 2) analyze how sex and estrous cycle affect navigation choice. Place and motor-response trials were used to presumably engage (respectively) the hippocampal and striatal systems (Packard & McGaugh, 1996; Packard & Knowlton, 2002; Poldrack, & Packard, 2003; Schroeder et al., 2002; White & McDonald, 2002; Kesner et al., 1993). The analysis of trial configuration type (Figures 2, 3 & 5) demonstrates that trials requiring the place and motor-response navigation system to work competitively resulted in more errors. Conversely, trials that allowed these systems to work cooperatively to solve a task resulted in almost no errors. This error pattern was retained through criterion (See Table B III), and even extensively trained animals found the constant switching between tasks difficult, as shown by their continuing ∼10% errors, mostly on the competitive trial configuration. Dissociations studies, such as that of Packard & McGaugh (1996) and Kesner et al. (1993), used lesions to demonstrate that place and motor-response learning is contingent upon the hippocampus and striatum, respectively. Taken together with previous research (Kesner, et al., 1993; Packard & McGaugh, 1996; White & McDonald, 2002), our data reaffirms that hippocampus and striatum are active in parallel and compete with each other to influence behavioral output. Our maze paradigm suggests that when the hippocampus and striatum are simultaneously engaged during place and motor-response learning, respectively, there is a competition between these two systems which results in an increase in performance errors. Specifically, when an error was made during a competitive trial, the rat most often made a competing system error and rarely a neutral error (i.e. on a competitive place trial, an error would result in the incorrect use of a motor-response strategy). This suggests that when one system was not being utilized (for example, place), the other system (motor-response) was readily available.
Importantly, this pattern of errors at criterion was not affected by sex or estrous cycle. Taken together with previous research, these data indicate an estrogen role only during the initial organization of a navigation behavior. Once the relative contributions of the hippocampus and striatum have been established, they become independent from the effects of naturally circulating hormones.
Acknowledgements
We would like to thank Chris Cleaver for his contribution to the paradigm, Greg Peters for help with lavaging, and David Morrow for help with lavaging and data input.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Blokland A, Rutten K, Prickaerts J. Analysis of spatial orientation strategies of male and female wistar rats in a morris water escape task. Behav Brain Res. 2006;171:216–224. doi: 10.1016/j.bbr.2006.03.033. [DOI] [PubMed] [Google Scholar]
- Chang Q, Gold PE. Switching memory systems during learning: Changes in patterns of brain acetylcholine release in the hippocampus and striatum in rats. J Neurosci. 2003;23(7):3001–3005. doi: 10.1523/JNEUROSCI.23-07-03001.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad CD, Jackson JL, Wieczorek L, Baran SE, Harman JS, Wright RL, Korol DL. Acute stress impairs spatial memory in male but not female rats: Influence of estrous cycle. Pharmacol Biochem Behav. 2004;78:569–579. doi: 10.1016/j.pbb.2004.04.025. [DOI] [PubMed] [Google Scholar]
- Davis DM, Jacobson TK, Aliakbari S, Mizumori SJY. Differential effects of estrogen on hippocampal and striatal dependent learning. Neurobiol Learn Mem. 2005;84:132–137. doi: 10.1016/j.nlm.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Doeller CF, King JA, Burgess N. Parallel striatal and hippocampal systems for landmarks and boundaries in spatial memory. Proc Natl Acad Sci USA. 2008;105(15):5915–5920. doi: 10.1073/pnas.0801489105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohanich GP. Hormones brain and behavior. Academic; San Diego: 2002. Gonadal steroids, learning and memory; pp. 265–327. [Google Scholar]
- Frye CA. Estrus-associated decrements in a water maze task are limited to acquisition. Physiol Behav. 1995;57(1):5–14. doi: 10.1016/0031-9384(94)00197-d. [DOI] [PubMed] [Google Scholar]
- Gibson BM, Shettleworth SJ. Place versus response learning revisited: Tests of blocking on the radial arm maze. Behav Neurosci. 2005;119(2):567–586. doi: 10.1037/0735-7044.119.2.567. [DOI] [PubMed] [Google Scholar]
- Jarrard LE. What does the hippocampus really do? Behav Brain Res. 1995;71(12):1–10. doi: 10.1016/0166-4328(95)00034-8. [DOI] [PubMed] [Google Scholar]
- Jonasson Z. Meta-analysis of sex differences in rodent models of learning and memory: A review of behavioral and biological data. Neurosci Biobehav Rev. 2005;28:811–825. doi: 10.1016/j.neubiorev.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Kesner RP, Bolland BL, Dakis M. Memory for spatial locations, motor responses, and objects: Triple dissociation among the hippocampus, caudate nucleus, and extrastriate visual cortex. Exp Brain Res. 1993;93(3):462–470. doi: 10.1007/BF00229361. [DOI] [PubMed] [Google Scholar]
- Korol DL. Role of estrogen in balancing contributions from multiple memory systems. Neurobiol Learn Mem. 2004;82:309–323. doi: 10.1016/j.nlm.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Korol DL, Kolo LL. Estrogen-induced changes in place and response learning in young adult female rats. Behav Neurosci. 2002;116(3):411–420. doi: 10.1037//0735-7044.116.3.411. [DOI] [PubMed] [Google Scholar]
- Korol DL, Malin EL, Borden KA, Busby RA, Couper-Leo J. Shifts in preferred learning strategy across the estrous cycle in female rats. Horm Behav. 2004;45:330–338. doi: 10.1016/j.yhbeh.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Packard MG. Glutamate infused posttraining into the hippocampus or caudate-putamen differentially strengthens place and response learning. Proc Natl Acad Sci USA. 1999;96(22):12881–12886. doi: 10.1073/pnas.96.22.12881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard MG, Knowlton BJ. Learning and memory functions of the basal ganglia. Annu Rev Neurosci. 2000;25:563–593. doi: 10.1146/annurev.neuro.25.112701.142937. [DOI] [PubMed] [Google Scholar]
- Packard MG, McGaugh JL. Inactivation of hippocampus or caudate nucleus with lidocaine differentially affects expression of place and response learning. Neurobiol Learn Mem. 1996;65:65–72. doi: 10.1006/nlme.1996.0007. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Packard MG. Competition among multiple memory systems: Converging evidence from animal and human brain studies. Neuropsychology. 2003;41:245–251. doi: 10.1016/s0028-3932(02)00157-4. [DOI] [PubMed] [Google Scholar]
- Ragozzino ME, Detrick S, Kesner RP. Involvement of the prelimbic-infralimbic areas of the rodent prefrontal cortex in behavioral flexibility for place and response learning. J Neurosci. 1999;19(11):4585–4594. doi: 10.1523/JNEUROSCI.19-11-04585.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich EL, Shapiro ML. Prelimibic/infralimbic inactivation impairs memory for multiple task switches, but not flexible selection of familiar tasks. J Neurosci. 2007;27(17):4747–4755. doi: 10.1523/JNEUROSCI.0369-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder JP, Wingard JC, Packard MG. Post-training reversible inactivation of hippocampus reveals interference between memory systems. Hippocampus. 2002;12:280–284. doi: 10.1002/hipo.10024. [DOI] [PubMed] [Google Scholar]
- Tropp J, Figueiredo CM, Markus EJ. Stability of hippocampal place cell activity across the rat estrous cycle. Hippocampus. 2005;15(2):154–165. doi: 10.1002/hipo.20042. [DOI] [PubMed] [Google Scholar]
- Tropp J, Markus EJ. Sex differences in the dynamics of cue utilization and exploratory behavior. Behav Brain Res. 2001;19(2):143–154. doi: 10.1016/s0166-4328(00)00345-4. [DOI] [PubMed] [Google Scholar]
- Tolman EC, Ritchie BF, Kalish D. Studies in spatial learning. II. Place learning versus response learning. J Exp Psychol Gen. 1946;36(3):221–229. doi: 10.1037/h0060262. [DOI] [PubMed] [Google Scholar]
- White NM, McDonald RJ. Multiple parallel memory systems in the brain of the rat. Neurobiol Learn Mem. 2002;77:125–184. doi: 10.1006/nlme.2001.4008. [DOI] [PubMed] [Google Scholar]
- Zurkovsky L, Brown SL, Boyd SE, Fell JA, Korol DL. Estrogen modulates learning in female rats by acting directly at distinct memory systems. Neuroscience. 2007;144:26–37. doi: 10.1016/j.neuroscience.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]