Abstract
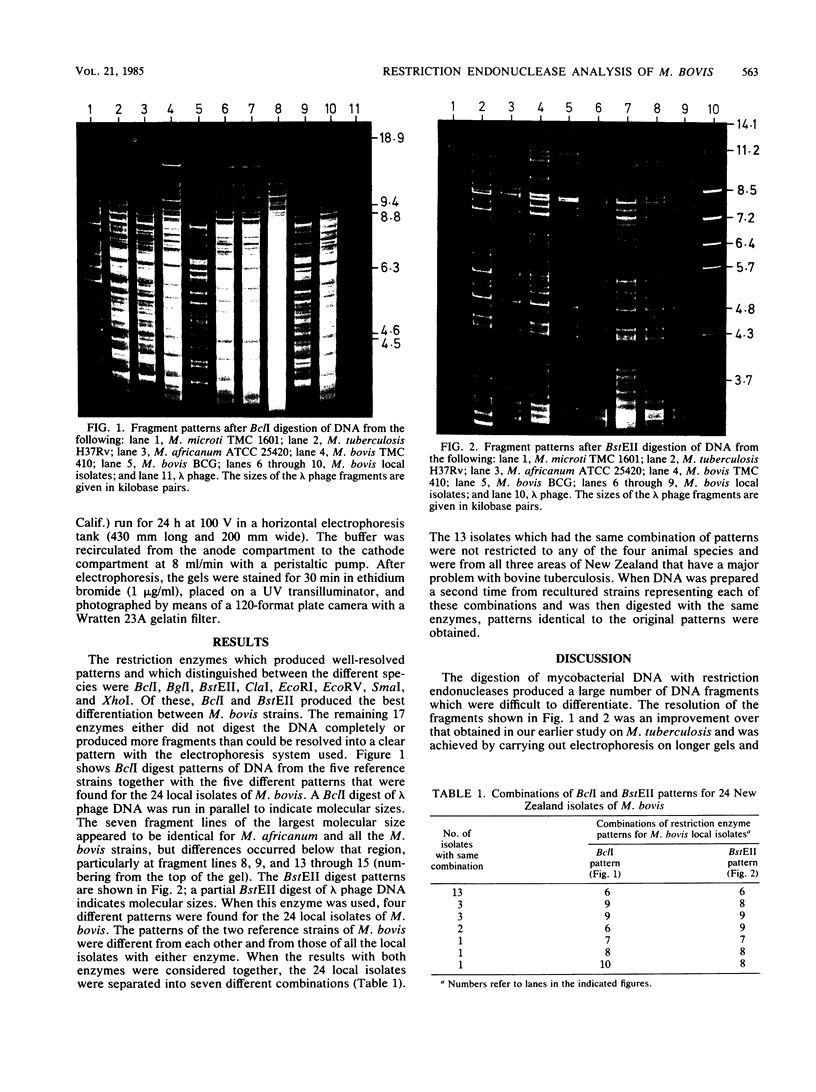
DNA preparations from 24 New Zealand isolates, two reference strains of Mycobacterium bovis, and one reference strain each of Mycobacterium microti, Mycobacterium africanum, and Mycobacterium tuberculosis were characterized by restriction endonuclease analysis. Twenty-five restriction enzymes were investigated. The clearest differences in M. bovis patterns were obtained with the enzymes BstEII and BclI. These produced four and five different patterns, respectively, for the 24 local isolates. When the results from both enzymes were considered, seven different combinations were obtained. The patterns produced for the two reference strains of M. bovis could be distinguished from each other and also from the patterns produced for the local isolates. All patterns were reproducible and are now being used for typing M. bovis isolates. With either enzyme, the patterns produced for the M. tuberculosis, M. bovis, and M. africanum strains had many features in common, but all the M. bovis patterns were clearly more similar to each other than to the M. tuberculosis patterns. The patterns produced for the M. microti strain were markedly different from those produced for the other species. Restriction endonuclease analysis is clearly a useful method for inter- and intraspecific classifications of the tuberculosis complex.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrow P. A. Aspects of the epidemiology of bovine tuberculosis in badgers and cattle. II. The development and use of a typing system for Mycobacterium bovis. J Hyg (Lond) 1981 Jun;86(3):247–257. doi: 10.1017/s0022172400068996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorvatn B., Lund V., Kristiansen B. E., Korsnes L., Spanne O., Lindqvist B. Applications of restriction endonuclease fingerprinting of chromosomal DNA of Neisseria meningitidis. J Clin Microbiol. 1984 Jun;19(6):763–765. doi: 10.1128/jcm.19.6.763-765.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins C. H., Grange J. M. The bovine tubercle bacillus. J Appl Bacteriol. 1983 Aug;55(1):13–29. doi: 10.1111/j.1365-2672.1983.tb02643.x. [DOI] [PubMed] [Google Scholar]
- Collins D. M., De Lisle G. W. DNA restriction endonuclease analysis of Mycobacterium tuberculosis and Mycobacterium bovis BCG. J Gen Microbiol. 1984 Apr;130(4):1019–1021. doi: 10.1099/00221287-130-4-1019. [DOI] [PubMed] [Google Scholar]
- Collins D. M., Ross D. E. Restriction endonuclease analysis of Campylobacter strains with particular reference to Campylobacter fetus ss. fetus. J Med Microbiol. 1984 Aug;18(1):117–124. doi: 10.1099/00222615-18-1-117. [DOI] [PubMed] [Google Scholar]
- Kaper J. B., Bradford H. B., Roberts N. C., Falkow S. Molecular epidemiology of Vibrio cholerae in the U.S. Gulf Coast. J Clin Microbiol. 1982 Jul;16(1):129–134. doi: 10.1128/jcm.16.1.129-134.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Pecq J. B., Paoletti C. A new fluorometric method for RNA and DNA determination. Anal Biochem. 1966 Oct;17(1):100–107. doi: 10.1016/0003-2697(66)90012-1. [DOI] [PubMed] [Google Scholar]
- Marshall R. B., Wilton B. E., Robinson A. J. Identification of Leptospira serovars by restriction-endonuclease analysis. J Med Microbiol. 1981 Feb;14(1):163–166. doi: 10.1099/00222615-14-1-163. [DOI] [PubMed] [Google Scholar]
- Stanford J. L., Grange J. M. The meaning and structure of species as applied to mycobacteria. Tubercle. 1974 Jun;55(2):143–152. doi: 10.1016/0041-3879(74)90008-7. [DOI] [PubMed] [Google Scholar]
- Tsukamura M. Numerical classification of 280 strains of slowly growing mycobacteria. Proposal of Mycobacterium tuberculosis series, Mycobacterium avium series, and Mycobacterium nonchromogenicum series. Microbiol Immunol. 1983;27(4):315–334. doi: 10.1111/j.1348-0421.1983.tb00591.x. [DOI] [PubMed] [Google Scholar]
- Wayne L. G. Microbiology of tubercle bacilli. Am Rev Respir Dis. 1982 Mar;125(3 Pt 2):31–41. doi: 10.1164/arrd.1982.125.3P2.31. [DOI] [PubMed] [Google Scholar]
- Wieten G., Haverkamp J., Groothuis D. G., Berwald L. G., David H. L. Classification and identification of Mycobacterium africanum by pyrolysis mass spectrometry. J Gen Microbiol. 1983 Dec;129(12):3679–3688. doi: 10.1099/00221287-129-12-3679. [DOI] [PubMed] [Google Scholar]