Abstract
Introduction:
Despite the high prevalence of insomnia, there is little information about its incidence and risk factors. This study estimated the incidence of insomnia and examined potential risk factors in a cohort of good sleepers followed over a one-year period.
Methods.
Participants were 464 good sleepers who completed 3 postal evaluations over a one-year period (i.e., baseline, 6 months, and 12 months). Questionnaires assessed sleep, psychological and personality variables, stressful life events and coping skills, and health-related quality of life. Participants were categorized into 3 subgroups: (a) good sleepers (i.e., participants who remained good sleepers at the 3 assessments), (b) insomnia symptoms incident cases (i.e., developed insomnia symptoms either at 6- or 12-month follow-up), and (c) insomnia syndrome incident cases (i.e., developed an insomnia syndrome either at 6- or 12- month follow-up).
Results:
One-year incidence rates were 30.7% for insomnia symptoms and 7.4% for insomnia syndrome. These rates decreased to 28.8% and 3.9% for those without prior lifetime episode of insomnia. Compared to good sleepers and insomnia symptoms incident cases, insomnia syndrome incident cases presented a premorbid psychological vulnerability to insomnia, characterized by higher depressive and anxiety symptoms, lower extraversion, higher arousability, and poorer self-rated mental health at baseline. They also presented a higher level of bodily pain and a poorer general health. Five variables were associated with a new onset of an insomnia syndrome: previous episode of insomnia, positive family history of insomnia, higher arousability predisposition, poorer self-rated general health, and higher bodily pain.
Conclusion:
The one-year insomnia incidence rate was very high and several psychological and health factors were associated with new onset insomnia. Improved knowledge about the nature of these predisposing factors would be helpful to guide the development of effective public health prevention and intervention programs to promote better sleep quality.
Citation:
LeBlanc M; Mérette C; Savard J; Ivers H; Baillargeon L; Morin CM. Incidence and risk factors of insomnia in a population-based sample. SLEEP 2009;32(8):1027-1037.
Keywords: Epidemiology, insomnia, risk factors, correlates, sleep
INSOMNIA IS AMONG THE MOST PREVALENT HEALTH COMPLAINTS. APPROXIMATELY 9% OF THE GENERAL POPULATION REGULARLY SUFFER FROM INSOMNIA, and about 30% do so occasionally.1–3 Incidence rates reported in longitudinal studies vary extensively (from 3% to 20%), depending on the population studied, the time interval (e.g., 1 year versus 10 years), and the definition of insomnia used (i.e., insomnia syndrome versus symptoms). For instance, it is estimated that over a period of one year, approximately 6% of the general population develop an insomnia syndrome,4 and approximately 20% develop insomnia symptoms, with the latter figures being based on samples of older adults5 and individuals with chronic health problems.6 Furthermore, new onset of insomnia is generally more frequent among women and individuals with medical conditions, psychiatric disorders, and a perceived stressful life.4,7–9
Based on a tripartite conceptual framework widely used to explain the development of insomnia,10–12 3 types of factors are involved at different times during the course of insomnia. First, everyone is, to some degree, predisposed to develop insomnia. Second, a precipitating event is usually associated with the onset of insomnia. Third, insomnia is perpetuated over time by psychological and behavioral factors, even after precipitating factors have been controlled or eliminated. The most commonly hypothesized predisposing factors include demographic factors (e.g., aging, female gender, living alone),1,3,13 familial/hereditary conditions (a personal or family history of insomnia),14–16 psychological factors (e.g., anxiety, depression, personality traits),4,17–19 and physiological and lifestyle factors (e.g., arousability and smoking).20,21 Precipitating factors include stressful life events (e.g., divorce),9,22 as well as psychological and health-related factors (e.g., pain, mental health problems).10,23 Finally, maintaining factors include maladaptive sleep habits (e.g., excessive amounts of time spent in bed, napping, chronic medication use) and dysfunctional cognitions about sleep loss and its impact on life (e.g., worry over sleep loss).10
Most studies investigating insomnia risk factors have been either retrospective or cross-sectional, precluding unequivocal inference about the relationship between these factors and the development of insomnia. The extent to which depression and anxiety trigger insomnia or represent consequences of insomnia remains ambiguous. The few longitudinal studies of insomnia have provided informative data about incidence and risk factors, although most of those studies have focused predominantly on selected samples such as young adults,7,28 elderly adults,8,24,25 or patients attending medical practices.6,26,27 Only one longitudinal study evaluating the relation between sleep problem symptoms (i.e., insomnia and hypersomnia) and psychiatric disorders sampled the population at large.4
Few studies have used standard diagnostic criteria to define insomnia. Moreover, previous studies have used various time frames and most have not adequately operationalized their measure of incidence. For instance, most studies have not differentiated between incident cases of first episode of insomnia (no prior history of insomnia) and cases of recurrence (with past history of insomnia). Furthermore, the majority of studies included only individuals experiencing insomnia at the time of the second assessment in their incidence estimates5,6,8,29 rather than all cases emerging during the interval between baseline and follow-up assessment.7 Since insomnia may prove transient or episodic, incidence rates may have been underestimated in previous studies. The National Institutes of Health30 called for additional longitudinal studies using well-operationalized and stringent diagnostic criteria to estimate insomnia incidence and identify risk factors within the general population.
The objectives of this study were to estimate the incidence of insomnia symptoms and syndrome and to identify associated risk factors in a cohort of good sleepers sampled from the general population and followed over a one-year period.
METHODS
Study Context
Data from this study were derived from a larger epidemiological study conducted in the province of Quebec, Canada. The study began with a telephone survey to document the prevalence of insomnia and determinants of health-seeking behaviors.2 The sample was composed of French-speaking residents of the province of Quebec, aged 18 years and over. Sample selection involved 2 procedures: (1) random digit dialing method, which generates geographically stratified phone numbers; and (2) the Kish method,31 to identify the individual to be interviewed within each household. At the conclusion of the telephone interview, participants were asked if they wanted to take part in the longitudinal phase of the study, which involved completion of 7 postal evaluations over a 5-year period. The first postal evaluation was conducted one month after the telephone interview. The remaining evaluations were scheduled respectively 6, 12, 24, 36, 48, and 60 months after the first postal evaluation. Data from the first 3 postal evaluations (1-year period) were used in the present study.
Measures
Several measures were included in the questionnaires sent to the participants. These included French-Canadian versions of validated self-report measures, as well as questions developed specifically for this study, covering 4 general domains: sleep; mood and personality; coping and life events; and lifestyle and health-related quality of life. Participants received the same measures at each evaluation with the exception of the personality inventory and the coping inventory for stressful situations, which were completed only at the first evaluation. Two sleep/insomnia measures (ISI10 and PSQI35) were used to classify participants in the 3 sleep status groups and to describe the sample. All other measures were used to derive dependent variables.
Sleep Measures
The Insomnia Severity Index (ISI)10 is a 7-item questionnaire assessing the nature, severity, and impact of sleep difficulties over the last month. Dimensions assessed are: severity of sleep-onset, sleep-maintenance, and early morning awakening problems; sleep satisfaction; interference of sleep difficulties with daytime functioning; noticeability of sleep problems by others; and distress caused by the sleep difficulties. A 5-point Likert scale (0 = not at all, 4 = extremely) is used to rate each items, yielding a total score ranging from 0 to 28. Scores can be classified into 4 severity categories: absence of insomnia (0–7); subthreshold insomnia (8–14); moderate insomnia (15–21); and severe insomnia (22–28). The ISI has adequate psychometric properties and is sensitive to measure treatment outcome.37 The French version of the questionnaire has good internal consistency, test-retest reliability, and convergent validity (r = 0.65 compared with sleep diary).38
The Pittsburgh Sleep Quality Index (PSQI)35 is a 19-item questionnaire evaluating sleep quality and disturbances over a one-month time interval. The first 4 items are open questions, whereas items 5 to 19 are rated on a 4-point Likert scale. Seven component scores are obtained (e.g., subjective sleep quality; sleep latency; sleep duration) and the total score, ranging from 0 to 21, is derived by adding the 7 component scores. A score > 5 suggests poor sleep quality. Psychometric properties of the original PSQI are adequate, as are those of the French version.35,38
Sleep-promoting products (i.e., prescribed and over-the-counter) utilization was assessed with the following questions: “During the past month, how many nights per week have you taken prescribed medication to help you sleep?” and “During the past month, how many nights per week have you taken over-the-counter medication (e.g., Nytol, Sominex) to help you sleep?” There was one question about use of alcohol as a sleep aid: “During the last month, how many nights per week have you used alcohol to help you sleep?”
Personal and family histories of insomnia were assessed with the following questions: “In the past, have you ever experienced insomnia a few days per week for more than one month? (yes/no),” “Is a member of your immediate family (parents, children, brothers, sisters) currently experiencing sleep difficulties? (yes/no),” and “Has a member of your immediate family (parents, children, brothers, sisters) ever experienced sleep difficulties? (yes/no).” For those answering in the affirmative, follow-up questions were asked to identify the family member and the type of sleep problem (i.e., insomnia, excessive daytime sleepiness, sleep apnea, restless legs or periodic limb movements, others). A family history of insomnia was defined as a report of at least one parent or sibling with past or current insomnia.
Psychological Measures
The Beck Depression Inventory II (BDI-II)39 contains 21 items rating depressive symptoms experienced during the past 2 weeks on a 4-point Likert scale. A total score, ranging from 0 to 63, is derived with a higher score suggesting a higher depressive symptomatology. The psychometric properties of the French version are well documented and equivalent to those of the original version.39
The Trait part of the State-Trait Anxiety Inventory (STAI-Trait)40 was used to assess anxiety as a personality trait. The STAI-Trait is composed of 20 items rated on a 4-point Likert scale (1 = not at all, 4 = a lot). Participants are asked to score how they relate to the statements in general. Total score ranges from 20 to 80. Psychometric properties of the STAI and the validated French-Canadian adaptation used in the present study are excellent.40,41
Stress and Coping Skills Measures
The Life Experience Survey (LES)42 is a 57-item (47 for general population and 10 for students) self-report measure asking respondents to indicate life events they experienced during the past year. Individuals are also asked to rate, on a 7-point scale (−3 = extremely negative, +3 = extremely positive), the perceived impact of the particular event on their life at the time of occurrence. Several scores can be derived from this scale: frequency of events in the past year (i.e., number of positive, negative, and neutral events and total number of events), intensity of positive events, intensity of negative events, and total score intensity. The LES has adequate psychometric properties, including internal consistency and test-retest reliability.42 For the purpose of the present study, only the 47 items formulated for the general population were retained. Moreover, participants were asked to report events they have experienced for the last 6 months rather than for the past year.
The Perceived Stress Scale (PSS)43 is a 14-item self-report scale measuring the degree to which situations in one’s life are appraised as stressful. Items represent feelings and thoughts that have occurred in the past month in relation to stressful situations or events. Individuals rate the frequency of each item on a 5-point Likert scale (0 = never, 4 = very often). The higher the total score, the more the person appraises life as unpredictable and uncontrollable. The PSS has adequate test-retest reliability (0.85) and internal consistency (0.80) and is correlated with a range of self-report and behavioral criteria.43 A French-Canadian version of the questionnaire was used in the present study.
The Coping Inventory for Stressful Situations (CISS)44 is a 48-item self-report measure of coping. It is divided into 3 subscales, each containing 16 items: task-oriented coping; emotion-oriented coping; and avoidance-oriented coping. CISS items exemplify different ways of coping, and respondents are asked to rate on a 5-point scale (1 = not at all, 5 = very much) how each item is representative of their own ways of coping with stress. The higher the score for a scale, the more likely the respondent is to rely on the type of coping strategies measured by the scale. The CISS has adequate properties with internal α reliabilities ranging from 0.76 (men on the emotion subscale) to 0.91 (women on the task subscale).44,45
Arousal Predisposition
The Arousal Predisposition Scale (APS)20 is a 12-item inventory that has been designed to measure arousability. Respondents are asked to report the frequency to which they experience the proposed emotion or behavior on a 5-point Likert scale (1 = never, 5 = always). A higher score reflects greater predisposition to arousal. The APS is a useful measure of individual differences in predisposition towards arousability and presents an adequate internal consistency (0.84).46
Personality
The NEO Five-Factor Inventory (NEO-FFI)47 is a 60-item questionnaire measuring 5 personality domains: neuroticism (N), extraversion (E), openness (O), agreeableness (A), and conscientiousness (C). Each factor is evaluated by 12 items rated on a 5-point Likert scale (strongly disagree to strongly agree). This 5-factor model is considered an excellent representation of key personality traits.48 The psychometric properties of the NEO-FFI in a Canadian context have been considered adequate, with internal consistency coefficients of at least 0.70 for each of the 5 subscales.49 A French-Canadian version was used in the present study.50
Health-Related Quality of Life
The SF-12 Health Survey version 251 is a short form of the SF-36, the most widely used health survey. The 12 items are rated on a 5-point Likert scale, and 8 subscale scores can be derived from the answers (physical functioning, role physical, bodily pain, general health, vitality, social functioning, role emotional, and mental health). Three subscales were used in the present study: bodily pain, general health, and mental health (as the others were more likely to be associated with the consequences rather than the risk factors of insomnia). A higher score on those subscales is associated with a better quality of life. The psychometric properties of the SF-12v2 are adequate, with reliability coefficients for the 8 subscales ranging from 0.73 to 0.87 in general population.52 A French-Canadian version was used.
Lifestyle Factors
Weekly alcohol consumption was measured with one specific item: “On the average, how many alcoholic beverages do you drink per week?” Frequency of cigarette smoking was also assessed with one question: “Do you smoke cigarette? (yes, daily; yes, occasionally; and no, never).” Lastly, frequency of physical activity practice as measured with the following item: “How many times per week do you spend more than 20 minutes exercising in your free time?” Body mass index (BMI) was calculated from participants’ self-reported height and weight.
Participants and Procedure
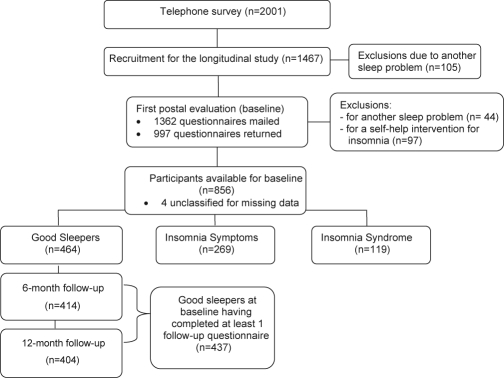
Of the 5991 persons solicited, a total of 2001 (33.4%) completed the telephone interview, and 1467 (73.3%) of them (57.5% women) agreed to take part in the longitudinal study (Figure 1). Of these, 105 (7.1%) were excluded because they reported the presence of a sleep disorder other than insomnia, the only exclusion criterion of the study. An apnea diagnosis was reported by 28 individuals (1.9%), 4 reported hypersomnia (0.3%), and 73 persons (5.0%) reported periodic limb movements or restless legs. For each postal evaluation, participants were asked to return the completed questionnaire within a 2-week period, and they were paid $25 for completing it. Reminder calls were made to maximize response rate. The first postal evaluation (baseline) was mailed to 1362 participants; 997 of them (73.2%) returned the completed questionnaire within a one-month period. Of those, 141 were excluded because they reported the presence of another sleep disorder, not reported at the telephone interview (n = 44) or received a self-help behavioral intervention for insomnia in the context of another study (n = 97).32 The remaining 852 participants were classified into one of 3 groups: 464 (54.5%) good sleepers, 269 (31.6%) with insomnia symptoms, and 119 (13.9%) with an insomnia syndrome (4 subjects could not be classified due to missing data).
Figure 1.
Participant Flow in the Study
As the main topic of the present paper is about insomnia incidence, data are based only on individuals classified as good sleepers at baseline (n = 464). Of those, 414 completed the 6-month follow-up assessment, 404 completed the 12-month follow-up assessment, and 381 (82.1%) completed both follow-up assessments (Figure 1). Of the 437 (94.2%) participants who completed at least one of the follow-up assessments, 49 completed only the 6-month and 34 only the 12-month follow-up.
Sleep Status Groups and Insomnia Incidence
Following each evaluation participants were classified in one of 3 groups according to their sleep patterns at that particular evaluation (baseline, 6 months, and 12 months). The classification used an algorithm based on a combination of insomnia diagnostic criteria from DSM-IV-TR33 and the International Classification of Diseases, 10th Edition (ICD-10)34; and on the utilization of sleep-promoting products (prescribed and over-the-counter). For each assessment, responses from the ISI,10 the PSQI,35 and from questions on sleep-promoting medication utilization were used to determine the presence or absence of each criterion.36 The 3 groups were defined as follows.
Insomnia Syndrome
Participants in this group met all the diagnostic criteria for insomnia. They were dissatisfied with their sleep (dissatisfied [3] or very dissatisfied [4] on a 0–4 scale; item 2 of the ISI) and presented symptoms of initial, maintenance, or late insomnia ≥ 3 nights per week (assessed by the items 5a and b of the PSQI) for a minimum duration of one month. Psychological distress or daytime impairment related to sleep difficulties was also reported by those individuals (much [3] or very much [4] on 0–4 scales; items 3 and 5 of the ISI). Finally, if prescribed medication was used as a sleep-promoting agent ≥ 3 nights per week, participants were automatically classified in the insomnia syndrome group, whether or not they presented symptoms of initial, maintenance, or late insomnia.
Insomnia Symptoms
Participants classified in this group presented symptoms of initial, maintenance, or late insomnia ≥ 3 nights per week, without fulfilling all the diagnostic criteria of an insomnia syndrome (i.e., they could be satisfied with their sleep, not report distress or daytime consequences, or their sleep difficulties could last for less than one month). Also included in this group were individuals dissatisfied with their sleep quality, but without symptoms of initial, maintenance, or late insomnia. Last, participants using prescribed medication to promote sleep < 3 nights per week, or over-the-counter medication ≥ 1 night per week were automatically classified in this group.
Good Sleepers
These participants were satisfied with their sleep (very satisfied [0], satisfied [1], or neutral [2] on a 0–4 scale; item 2 of the ISI); did not report symptoms of initial, maintenance, or late insomnia; and did not use prescribed or over-the-counter medication as a sleep-promoting agent over the last month.
Definition of Incident Cases
An insomnia symptom incident case was defined as a participant classified as a good sleeper at baseline (with or without a previous episode of insomnia), who was classified in the insomnia symptoms group at either 6- or 12-month follow-up and who was never classified in the insomnia syndrome group. An insomnia syndrome incident case was defined as a participant classified as a good sleeper at baseline, who was classified in the insomnia syndrome group either at 6- or 12-month follow up. As the time frame used to evaluate the presence of insomnia symptoms or syndrome at each assessment included only the preceding one month (rather than covering the entire previous 6 or 12 months), incidence rates did not include cases that could have occurred and remitted in the intervening period.
Data Analysis
Incidence Rate Estimation
One year insomnia incidence rates were estimated from the total sample of good sleepers at baseline (n = 464). Separates estimates were computed for the subgroup of individuals with no prior lifetime history of insomnia at baseline (n = 381). Incidence rates were obtained using the actuarial method produced by the SAS LIFETEST procedure.53 For those who did not complete the 6-month but completed the 12-month follow-up, a good sleeper status was imputed in place of the missing value. Otherwise, no data were imputed to participants who did not complete follow-up.
Analyses of Risk Factors
To identify potential risk factors for insomnia, groups were compared on baseline demographics, sleep, psychological, and health measures. Between-group comparisons were performed using χ2 for categorical variables and analyses of variance (ANOVAs) and analyses of covariance (ANCOVAs) for continuous measures. ANCOVAs were conducted on psychological and health variables to control for significant group differences on baseline insomnia symptoms severity, as assessed by the ISI and the PSQI. The ANCOVA approach was justified to control for the fact that individuals with subthreshold insomnia symptomatology at baseline (i.e., not meeting criteria for symptoms or syndrome categories) might be at greater risk to develop these conditions during follow-up. Significant χ2 analyses were followed by 3 post hoc, pairwise comparisons, in 2 × 2 contingency tables.54,55 If the post hoc χ2 was higher than the Bonferroni critical value, χ2(1, 1 – α / c) = χ2(1, 1 – .05/3) = 5.73,54 this comparison was considered significant. For significant ANCOVAs, 3 a priori contrast tests (paired comparisons) were performed comparing each group to the others. To explore the strength of the relation between demographic, sleep, psychological, health variables, and the sleep status, we performed χ2 tests for linear trend56 (categorical variables), Spearman correlation (education), Pearson correlation (age), and partial correlations (psychological and health variables adjusted for baseline ISI and PSQI total scores). A backward stepwise regression was also conducted to identify the most significant risk factors in predicting insomnia syndrome incidence at follow-up. This analysis was conducted with the group of good sleepers (n = 275) and the group of insomnia syndrome incident cases (n = 32) and included variables that exhibited significant groups’ differences at baseline and ISI and PSQI total scores to control for baseline disparities on severity of insomnia symptoms. From the final model, we investigated a few interactive terms in order to verify whether the effect of a factor could depend on the level of another one.
Analyses of Precipitating Factors
ANOVAs were performed to compare groups (incident cases versus good sleepers) on life events (number of positive and negative events, total number of events, and intensity of events) experienced within the 6 months preceding the development of insomnia symptoms or syndrome, compared to the same period for good sleepers. Last, between-group comparisons were performed to explore if changes in sleep quality (steady good sleepers versus insomnia symptoms or syndrome incident cases) were associated with concomitant changes in psychological and health variables. For significant ANOVAs, multiple comparisons were conducted using Ryan-Einot-Gabriel-Welch multiple F tests (REGWF) to ensure statistically powerful comparisons while controlling α error inflation.57 Pearson correlations were then calculated to verify the strength of the relation between life events and insomnia severity. For each psychological and health-related variable, a change score (delta) was calculated. For the insomnia symptoms and syndrome incident cases, change scores were defined by the subtraction of the baseline score from the score obtained at the 6- or 12-month follow-up evaluation when the participant presented insomnia symptoms or syndrome. For steady good sleepers, change scores were computed between baseline and 12-month follow-up. Change scores were compared between groups using ANOVAs.
RESULTS
The sample included 464 adults (60.3% women). Their mean age was 44.6 years (SD = 13.8; range 18–83). Most participants had completed at least a high school degree (94%), were married or living with a partner (58.3%), and were working or studying (72%). The 27 participants who failed to complete at least one follow-up assessment after baseline were younger (mean age of 37.3; SD = 13.8) than those who completed at least one follow-up; F1, 457 = 4.16, P < 0.05. There was no difference between these subgroups on gender, marital status, occupation, education, and ISI and PSQI scores.
Insomnia Incidence
Of the 464 good sleepers at baseline, 61.8% remained good sleepers over the one-year period; 30.7% (n = 67 at 6 months and n = 63 at 12 months) developed insomnia symptoms; and 7.3% (n = 11 at 6 months and n = 21 at 12 months) developed an insomnia syndrome. For good sleepers with no prior lifetime episode of insomnia (n = 381), incidence rates for insomnia symptoms and syndrome were 28.8% (n = 22 at 6 months and n = 26 at 12 months) and 3.9% (n = 6 at 6 months and n = 8 at 12 months), respectively.
Insomnia Risk Factors
Risk Factors
Table 1 presents demographic characteristics of good sleepers and insomnia symptoms and syndrome incident cases. Groups were not significantly different on any of these variables. Baseline sleep, psychological and health variables are presented in Table 2. Groups were significantly different on ISI and PSQI total scores, with non-incident cases exhibiting significantly lower scores for both ISI and PSQI, compared to incident cases of insomnia symptoms and syndrome. Insomnia symptoms and syndrome incident cases also significantly differed on baseline ISI scores but not on PSQI scores. Incident syndrome cases also presented higher rates of previous episode of insomnia and family history of insomnia than incident symptoms cases and good sleepers. Groups were significantly different on measures of depression (BDI-II), the trait-anxiety (STAI), and arousability (APS). Based on Cohen's criteria (1988), the magnitude of partial correlations between psychological variables and insomnia severity were labeled as “small,” ranging from 0.05 (NEO-FFI conscientiousness subscale) to 0.15 (BDI-II). Regarding health variables, insomnia syndrome incident cases presented significantly lower scores, suggesting a poorer functioning than insomnia symptoms incident cases and good sleepers on the SF-12v2 general health, bodily pain, and mental health subscales. There was also a significant between-group difference on cigarette smoking, with incident syndrome cases smoking less frequently than incident symptoms cases. The magnitude of partial correlation between health variables and insomnia severity were also found to be small, ranging from 0.07 (BMI) to 0.16 (bodily pain).
Table 1.
Demographic Characteristics of Good Sleepers and Insomnia Symptoms and Syndrome Incident Cases
| Demographics | Good Sleepers (n = 275) | Incident Cases |
|||||
|---|---|---|---|---|---|---|---|
| Insomnia Symptoms (n = 130) | Insomnia Syndrome (n = 32) | ||||||
| % (n) | % (n) | % (n) | χ2 | P | Pa,b | rs | |
| Gender (women) | 55.11 (151) | 60.46 (78) | 75.00 (24) | 5.06 | 0.08 | 0.03 | |
| Marital Status | |||||||
| Single/divorced/widowed | 35.66 (97) | 42.64 (55) | 40.63 (13) | 1.90 | 0.39 | 0.24 | |
| Married /common-law relationship | 64.34 (15) | 57.36 (74) | 59.38 (19) | ||||
| Family Income | |||||||
| $60,000 and less | 69.11 (179) | 68.59 (83) | 64.52 (20) | 0.27 | 0.87 | 0.67 | |
| $60,001 and over | 30.88 (80) | 31.40 (38) | 35.48 (11) | ||||
| Occupation | |||||||
| Working/student | 79.93 (215) | 79.84 (99) | 74.19 (23) | 0.57 | 0.75 | 0.59 | |
| Non working/retired | 20.07 (47) | 20.16 (25) | 25.80 (8) | ||||
| Work Schedule | |||||||
| Daytime | 87.03 (161) | 83.91 (73) | 81.82 (8) | 2.46 | 0.87 | ||
| Evening/Night/Rotating | 12.97 (24) | 16.09 (14) | 18.18 (4) | ||||
| Education | |||||||
| Primary or less | 3.33 (9) | 5.47 (7) | 10.00 (3) | 12.80 | 0.05 | 0.06 | |
| High school | 49.63 (134) | 39.06 (50) | 50.00 (15) | ||||
| Junior college | 23.06 (62) | 18.75 (24) | 10.00 (3) | ||||
| University | 24.07 (65) | 36.72 (47) | 30.00 (9) | ||||
| M (SD) | M (SD) | M (SD) | F | P | R | ||
| Age | 42.36 (13.76) | 43.91 (14.3) | 44.52 (12.4) | 0.76 | 0.47 | 0.06 | |
P values for chi-squares for trend.
Given that work schedule included more than 2 categories, χ2 for trend was not computed. rs = Spearman correlation. R = Pearson correlation
Table 2.
Baseline Evaluation of Sleep, Psychological and Health Variables for Good Sleepers and Insomnia Symptoms and Syndrome Incident Cases
| Baseline Evaluation | Good Sleepers (n = 275) | Incident Cases |
||||
|---|---|---|---|---|---|---|
| Insomnia Symptoms (n = 130) | Insomnia Syndrome (n = 32) | |||||
| Sleep Variables | % (n) | % (n) | % (n) | χ2 | P | P1 |
| Previous episode of insomnia (yes) | 12.36a (34) | 20.77a (27) | 53.13b (17) | 33.56 | 0.00 | 0.00 |
| Familial history of insomnia (yes) | 27.64a (76) | 33.85a (44) | 59.38b (19) | 13.67 | 0.00 | 0.00 |
| M (SE) | M (SE) | M (SE) | F | P | ||
| Insomnia Severity Index | 3.04a (2.67) | 4.37b (3.26) | 5.72c (4.31) | 17.14 | 0.00 | |
| Pittsburgh Sleep Quality Index | 3.20a (1.77) | 3.92b (1.75) | 4.50b (1.97) | 12.57 | 0.00 | |
| Psychological Variables2 | M (SE) | M (SE) | M (SE) | F | P | rp |
| Beck Depression Inventory II | 5.31a (0.32) | 5.73a (0.46) | 8.45b (0.94) | 4.97 | 0.01 | 0.15 |
| State-Trait-Anxiety Inventory –Trait | 36.60a (0.46) | 37.25a (0.67) | 40.40b (1.36) | 3.48 | 0.03 | 0.13 |
| Arousal Predisposition Scale | 29.23a (0.38) | 30.13a (0.55) | 32.88b (1.12) | 4.91 | 0.01 | 0.15 |
| Perceived Stress Scale | 21.13 (0.37) | 21.62 (0.54) | 23.39 (1.09) | 1.92 | 0.15 | 0.10 |
| Coping Inventory for Stressful Situations | ||||||
| Task-Oriented Coping | 55.46 (0.59) | 56.10 (0.85) | 56.51 (1.73) | 0.29 | 0.75 | 0.03 |
| Emotion-Oriented Coping | 36.96 (0.61) | 37.97 (0.89) | 40.43 (1.80) | 1.78 | 0.17 | 0.09 |
| Avoidance-Oriented Coping | 45.23 (0.68) | 44.20 (0.98) | 44.16 (1.99) | 0.42 | 0.66 | −0.05 |
| NEO Five-Factor Inventory | ||||||
| Neuroticism | 15.05 (0.44) | 15.71 (0.63) | 17.77 (1.28) | 2.07 | 0.13 | 0.10 |
| Extraversion | 29.91a (0.38) | 28.70 (0.55)a,b | 27.41b (1.11) | 3.16 | 0.04 | −0.12 |
| Openness | 25.96 (0.36) | 27.11 (0.52) | 27.29 (1.05) | 1.98 | 0.14 | 0.10 |
| Agreeableness | 34.90 (0.31) | 34.22 (0.44) | 33.70 (0.90) | 1.27 | 0.28 | −0.08 |
| Conscientiousness | 37.10 (0.35) | 36.70 (0.50) | 36.37 (1.02) | 0.36 | 0.70 | −0.05 |
| Health Variables2 | ||||||
| SF-12v2 Health Survey | ||||||
| General Health | 76.11a (1.00) | 76.15a (1.45) | 66.87b (3.01) | 4.40 | 0.01 | −0.14 |
| Bodily Pain | 89.14a (1.13) | 88.36a (1.64) | 76.98b (3.30) | 5.94 | 0.00 | −0.16 |
| Mental Health | 74.19a (0.78) | 73.30a (1.13) | 66.61b (2.30) | 4.78 | 0.01 | −0.15 |
| Body Mass Index | 25.09 (0.31) | 25.85 (0.44) | 25.39 (0.90) | 0.99 | 0.37 | 0.07 |
| Alcohol Consumption (glasses/week) | 3.34 (0.29) | 3.24 (0.41) | 1.80 (0.84) | 1.49 | 0.23 | −0.08 |
| Cigarette Smoking3 | 2.46a,b (0.05) | 2.56a (0.75) | 2.15b (0.15) | 3.07 | 0.05 | −0.12 |
| Physical Activity Practice3 | 3.75 (0.12) | 3.38 (0.18) | 4.11 (0.36) | 2.36 | 0.10 | −0.11 |
P value for χ2 for trend.
Psychological and health variables were adjusted for baseline ISI and PSQI total scores (ANCOVAs).
Those ordinal variables are treated as continuous variables ranging from 1 (never) to 3 (every day) for cigarette smoking and from 1 (never) to 7 (every day) for physical activity practice. rp = Partial correlation. Note: Means with different subscripts (a, b, c) are significantly different.
A backward stepwise regression was conducted to identify the most significant risk factors in predicting new insomnia syndrome cases, taking into account the presence of other factors. This analysis included variables for which significant group differences were obtained at baseline (i.e., previous episode and family history of insomnia, BDI-II, STAI-trait, APS, the NEO-FFI extraversion subscale, the SF-12v2 general health, bodily pain, and mental health subscales, and cigarette smoking); ISI and PSQI total scores were also included to control for baseline differences on severity of insomnia symptoms. A total of 301 observations (missing n listwise = 6 cases or 2.0%) were submitted to the analysis (270 good sleepers and 31 insomnia syndrome incident cases; the insomnia symptoms incidence group were excluded from this analysis). Results indicated that 5 variables remained significantly associated with the incidence of new cases of an insomnia syndrome: previous episode of insomnia (odds ratio [OR] = 5.42), family history of insomnia (OR = 2.96), arousal predisposition (OR = 1.12), and the SF-12v2 general health (OR = 0.97) and bodily pain subscales (OR = 0.98) (Table 3). Thus, individuals who previously experienced insomnia were 5.42 times more likely to develop insomnia than those who had no prior history of insomnia. Individuals with a family history of insomnia were 2.96 times more likely to develop insomnia than those without a family history of insomnia. Each increase of one point on the arousal predisposition scale increases 1.12 times the risk of being an incident cases. On the other hand, each increase (i.e., improvement) of one point on the score obtained to the SF-12v2 general health and bodily pain subscales was associated with, respectively, a 3% and 2% decrease in the risk of being an incident case.
Table 3.
Backward Stepwise Regression Results for the Incidence of Insomnia Syndrome versus Steady Good Sleepers (N = 301)
| Predictor | Odds Ratio Point Estimate | 95% Confidence Limits |
Wald χ2 | P | |
|---|---|---|---|---|---|
| Previous episode of insomnia | 5.41 | 2.15 | 13.61 | 12.90 | 0.00 |
| Family history of insomnia | 2.96 | 1.20 | 7.30 | 5.57 | 0.02 |
| Arousal Predisposition Scale (APS) | 1.12 | 1.47 | 1.21 | 10.42 | 0.00 |
| SF-12v2 Health Survey | |||||
| General Health | 0.97 | 0.95 | 0.99 | 5.09 | 0.02 |
| Bodily Pain | 0.98 | 0.96 | 0.99 | 4.67 | 0.03 |
From the final model, we investigated 6 potential interaction terms: (a) previous episode of insomnia × activation predisposition, (b) previous episode of insomnia × general health, (c) previous episode of insomnia × bodily pain, (d) family history of insomnia × activation predisposition, (e) family history of insomnia × bodily pain, and (f) family history of insomnia × general health. Two of them were significant when added to the final model: previous episode of insomnia × activation predisposition (P < 0.01) and family history of insomnia × bodily pain (P < 0.05). Thus, for participants who presented lower scores on the arousal predisposition scale (i.e., scores ≤ 29, which was the median), the contribution of a previous episode of insomnia was less important (OR = 1.07) than for those who presented higher scores ( > 29) (OR = 13.25). For individuals with higher bodily pain (i.e., SF-12v2 bodily pain scores < 100, which is the median), the contribution of a family history of insomnia was less important (OR = 2.00) than for those with less bodily pain (i.e., SF-12v2 bodily pain scores = 100) (OR = 9.17).
Precipitating Factors
Table 4 presents data on life events experienced within the 6 months preceding the onset of new insomnia symptoms or syndrome. Results showed significant group differences regarding the total number of events (F2, 428 = 3.67, P < 0.05), the number of negative events (F2, 428 = 6.34, P < 0.05), and the event intensity (F2, 428 = 4.20, P < 0.05) during the 6 months preceding new onset of insomnia symptoms and syndrome. Insomnia syndrome incident cases experienced significantly more events, more negative events, and higher intensity of events than good sleepers. Insomnia symptoms incident cases did not significantly differ from syndrome regarding those 3 variables, but differed from good sleepers regarding the number of negative events experienced.
Table 4.
Life Events Experienced Within the Six Months Preceding the Onset of Insomnia Symptoms and Syndrome
| Life Experience Survey (LES) | Good Sleepers1 (n = 275) | Incident Cases |
||||
|---|---|---|---|---|---|---|
| Insomnia Symptoms (n = 130) | Insomnia Syndrome (n = 32) | F | P | r | ||
| Number of events | 2.00a (2.33) | 2.46a,b (2.74) | 3.16b (3.14) | 3.67 | 0.03 | 0.13 |
| Number of negative events | 0.99a (1.53) | 1.45b (2.02) | 2.03b (2.56) | 6.34 | 0.00 | 0.17 |
| Number of positive events | 0.81 (1.29) | 0.83 (1.36) | 0.94 (1.39) | 0.13 | 0.88 | 0.02 |
| Event intensity (positive + negative) | 3.36a (4.30) | 4.32a,b (5.47) | 5.66b (5.56) | 4.20 | 0.02 | 0.14 |
For good sleepers, life events experienced within the 6 months preceding T3 are presented. r = Pearson correlations. Note: Means with different subscripts (a,b) are significantly different.
Between-group comparisons were performed to explore if changes in sleep quality (good sleepers versus insomnia symptoms and syndrome incident cases) were associated with concomitant changes on psychological and health variables (Table 5). Regarding psychological variables, change scores were significantly different between groups for the BDI-II (F2, 406 = 5.02, P < 0.01), the STAI-trait (F2, 404 = 10.57, P < 0.01), and PSS scores (F2, 407 = 3.01, P = 0.05). For those 3 measures, incident syndrome cases presented a larger increase (worsening) of their scores, compared to incident symptoms cases and good sleepers. Regarding health-related variables, groups were significantly different on the SF-12v2 mental-health subscale only (F2, 411 = 5.46, P < 0.01), with incident syndrome cases presenting a greater worsening of their mental health functioning, compared to incident symptoms cases and good sleepers. Pearson correlations between psychological and health-related variables and insomnia severity were small, ranging from 0.01 (physical activity practice) to 0.22 (STAI-trait).
Table 5.
Change Scores (Δ) Obtained for Psychological and Health Variables for Good Sleepers and Insomnia Symptoms and Syndrome Incident Cases
| Good Sleepers1 (n = 275) | Incident Cases |
|||||
|---|---|---|---|---|---|---|
| Insomnia Symptoms1 (n = 130) | Insomnia Syndrome1 (n = 32) | |||||
| M (SE) | M (SE) | M (SE) | F | P | r | |
| Changes in Psychological Variables | ||||||
| Δ Beck Depression Inventory II (BDI-II)2 | 0.55a (4.28) | −0.02a (5.05) | 2.44b (8.49) | 5.02 | 0.01 | 0.13 |
| Δ State-Trait-Anxiety Inventory–Trait (STAI)2 | −3.40a (5.83) | −1.82a (6.73) | 1.83b (8.53) | 10.57 | 0.00 | 0.22 |
| Δ Arousal Predisposition Scale (APS)2 | −0.70 (4.17) | −0.59 (4.63) | 0.41 (4.09) | 0.94 | 0.39 | 0.06 |
| Δ Perceived Stress Scale (PSS)2 | −0.98a (5.70) | −0.60a (5.88) | 1.75b (1.37) | 3.01 | 0.05 | 0.10 |
| Changes in Health Variables | ||||||
| SF-12v2 Health Survey 3 | ||||||
| Δ General Health | −0.88 (15.80) | −0.66 (15.55) | −6.55 (20.64) | 1.83 | 0.16 | −0.06 |
| Δ Bodily Pain | 1.91 (20.18) | 1.15 (22.84) | −3.13 (25.20) | 0.78 | 0.46 | −0.05 |
| Δ Mental Health | −0.45a (14.62) | −4.13a (14.38) | −8.20b (18.13) | 5.46 | 0.01 | −0.16 |
| Δ Body Mass Index2 | −0.01 (2.38) | 0.19 (2.55) | 1.20 (2.80) | 2.98 | 0.05 | 0.11 |
| Δ Alcohol Consumption (number of glasses/week)2 | −0.21 (2.96) | 0.41 (3.14) | 0.36 (1.27) | 2.02 | 0.13 | 0.09 |
| Δ Cigarette Smoking4 | 0.00 (0.33) | 0.05 (0.35) | 0.44 (0.08) | 0.88 | 0.42 | 0.08 |
| Δ Physical Activity Practice4 | −0.13 (1.64) | −0.09 (1.44) | −0.28 (1.85) | 0.19 | 0.82 | −0.01 |
For good sleepers, change scores were computed between T1 and T3. For insomnia symptoms and syndrome incident cases, change scores were computed between baseline and T2 for those who developed difficulties at 6-month follow-up and between baseline and 12-month follow-up for those who developed difficulties at 12-month follow-up.
For those variables, an increment (positive delta) indicates a poorer functioning and a decrement (negative delta) indicates a better functioning.
For the SF-12 subscales, an increment (positive delta) indicates a better functioning and a decrement (negative delta) indicates a poorer functioning.
for those variables, a negative delta indicates a decrement of the event frequency and a positive delta indicates an increment of the event frequency. Note: Means with different subscripts are significantly different on the contrast test.
DISCUSSION
This study revealed high incidence rates for both insomnia symptoms and syndrome, rates that remained elevated even after controlling for prior history of insomnia. The most important risk factors associated with new onset insomnia syndrome included both psychological (i.e., higher arousal predisposition and anxiety and depressive symptoms) and health-related variables. Negative life events and higher intensity of events were also temporally associated with new onset insomnia syndrome.
This is the first study documenting the incidence of insomnia symptoms in a general population; the rate observed was higher than those reported in previous studies of elderly adults (20%)5 and individuals with chronic health problems (21%).6 The incidence rate of insomnia syndrome was similar to those obtained in previous studies with comparable sample and similar criteria (6.2%)4 or more liberal ones (9.1%).58
Incidence rates of insomnia symptoms (28.8%) and syndrome (3.9%) were very high even among individuals with no prior history of insomnia. Only one study has differentiated incidence rates for the overall sample (13%) and for individuals with no prior history of insomnia (8.7%), based on a sample of young adults.7 Unlike most studies,5,6,8,29 Breslau and colleagues included in their incidence estimates all individuals who had developed insomnia during the 3.5 year follow-up interval, not only those meeting insomnia criteria at the time of the follow-up. Since the present study evaluated participants' sleep patterns twice within a year and added the incident cases over the two periods, we expected our rates to be higher than those based on a point estimate, and comparable to those reported by Breslau et al.7 Despite this similarity with the latter study, the lower incidence rates in the present study may be explained by the use of a shorter follow-up interval and the use of a more stringent definition of insomnia. Moreover, because the time frame used in the present study included only the previous one month period, rather than the entire 6- and 12-month intervals, this procedure may have produced lower incidence rates as it did not include participants that may have developed insomnia and subsequently remitted within the follow-up intervals.
An interesting result was the premorbid psychological vulnerability observed among incident cases of insomnia syndrome, revealing higher depressive and anxiety symptomatology, arousability, and a lower mental health functioning compared to good sleepers and incident cases of insomnia symptoms. These preexisting characteristics also worsened prior to or concomitant with the development of sleep difficulties. Such changes over time would suggest that such psychological variables can play both a predisposing as well as a precipitating role in the development of insomnia.
The present study provides new evidence supporting the longstanding hypothesis that increased arousal is a predisposing factor to insomnia.20,60 Insomnia syndrome incident cases obtained higher scores on the arousal predisposition measure relative to good sleepers, and this characteristic did not change with the onset of insomnia, confirming its predisposing character. These results expand on previous cross-sectional studies reporting higher levels of psychological and physiological arousal in individuals with insomnia compared to good sleepers.23,61–64
Regarding life events, stress, and coping skills, insomnia syndrome incident cases experienced more life events and more negative events than good sleepers prior to the onset of insomnia. There was no group difference regarding coping skills and stress perception at baseline, but, compared to the two other groups, the insomnia syndrome incident cases presented a larger increment of their stress perception concomitant with development of sleep difficulties. This change in stress perception could reflect either a response to the experience of stressful life events or a response to the onset of sleep difficulties. Even if we did not observe between-group difference on coping skills, these findings corroborate the model suggesting that stress perception, activation, and insomnia are strongly related.23
None of the psychological and health related factors targeted in this study differed between good sleepers and insomnia symptoms incident cases. This finding is surprising and suggests that these targeted variables may be important risk factors for severe and chronic insomnia but not for milder and more transient sleep difficulties. Alternatively, it might also suggest that insomnia symptoms do not necessarily lead to an insomnia disorder.65
While insomnia can be a diagnostic entity unto itself, it can also be symptomatic of another disorder. Given that comorbid insomnia represent the largest single group of insomnia diagnoses seen in epidemiological studies,66 it is probable that several individuals in the incident group developed insomnia in association with another physical or mental health problem. The findings that general health and pain were associated with new onset of insomnia would support this hypothesis. However, the questions asked and the method used to assess participants' physical and mental health precluded reliable differentiation between primary insomnia and insomnia comorbid to mental or medical disorder. Given the frequent occurrence of comorbid insomnia and major depression, a more thorough evaluation would be critical in future studies to optimize the interpretation and integration of the results.
The present study examined only a limited number of potential risk factors for insomnia. It is likely that other candidates (e.g., genetic factors) represent equally important risk factor and should be investigated in future studies. Moreover, some of the factors analyzed are prone to a recall bias (life events, family history and past episode of insomnia). The use of prospective measures in future studies would help to minimize the recall bias.
Despite some limitations, the present study is innovative on a number of levels. It is the first longitudinal study to have followed a cohort of adult good sleepers drawn from the population at large. Furthermore, our study employed standard diagnostic criteria of insomnia, and well-defined, operationalized incidence measures. Improved knowledge of the incidence and risk factors of insomnia could guide the development of effective public health prevention and intervention programs to promote better sleep quality.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Morin has received research support from Sanofi-Aventis and Organon; has consulted for Sanofi-Aventis, Sepracor, Actelion, Neurocrine, Eli Lilly, and Pfizer; and is on the speakers bureau for Takeda and Sanofi-Aventis. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
This research was supported by a Canadian Institutes of Health Research grant (#42504).
REFERENCES
- 1.Ancoli-Israel S, Roth T. Characteristics of insomnia in the United States: Results of the 1991 National Sleep foundation survey. Sleep. 1999;22:347–53. [PubMed] [Google Scholar]
- 2.Morin CM, LeBlanc M, Daley M, et al. Epidemiology of insomnia: prevalence, self-help treatments and consultations initiated, and determinants of help-seeking behaviors. Sleep Med. 2006;7:123–30. doi: 10.1016/j.sleep.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 3.Ohayon MM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med Rev. 2002;6:97–111. doi: 10.1053/smrv.2002.0186. [DOI] [PubMed] [Google Scholar]
- 4.Ford DE, Kamerow DB. Epidemiologic study of sleep disturbances and psychiatric disorders: An opportunity for prevention? JAMA. 1989;262:1479–84. doi: 10.1001/jama.262.11.1479. [DOI] [PubMed] [Google Scholar]
- 5.Quan SF, Katz R, Olson J, et al. Factors associated with incidence and persistence of symptoms of disturbed sleep in elderly cohort: The cardiovascular health study. Am J Med Sci. 2005;329:163–72. doi: 10.1097/00000441-200504000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Katz DA, McHorney CA. Clinical correlates of insomnia in patients with chronic illness. Arch Intern Med. 1998;158:1099–107. doi: 10.1001/archinte.158.10.1099. [DOI] [PubMed] [Google Scholar]
- 7.Breslau N, Roth T, Rosenthal L, et al. Sleep disturbance and psychiatric disorders: A longitudinal epidemiological study of young adults. Biol Psychiatry. 1996;39:411–18. doi: 10.1016/0006-3223(95)00188-3. [DOI] [PubMed] [Google Scholar]
- 8.Foley DJ, Monjan AA, Izmirlian G, et al. Incidence and remission of insomnia among elderly adults in a biracial cohort. Sleep. 1999;22:S373–78. [PubMed] [Google Scholar]
- 9.Healy ES, Kales A, Monroe LJ, et al. Onset of insomnia: Role of life-stress events. Psychosom Med. 1981;43:439–51. doi: 10.1097/00006842-198110000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Morin CM. Insomnia: Psychological assessment and management. New York: The Guilford Press; 1993. [Google Scholar]
- 11.Spielman AJ. Assessment of insomnia. Clin Psychol Rev. 1986;6:11–25. [Google Scholar]
- 12.Spielman AJ, Glovinsky P. The varied nature of insomnia. In: Hauri P, editor. Cases studies in insomnia. New York, NY: Plenum Press; 1991. pp. 1–15. [Google Scholar]
- 13.Mellinger GD, Balter MB, Uhlenhuth EH. Insomnia and its treatment: Prevalence and correlates. Arch Gen Psychiatry. 1985;42:225–32. doi: 10.1001/archpsyc.1985.01790260019002. [DOI] [PubMed] [Google Scholar]
- 14.Bastien CH, Morin CM. Familial incidence of insomnia. J Sleep Res. 2000;9:49–54. doi: 10.1046/j.1365-2869.2000.00182.x. [DOI] [PubMed] [Google Scholar]
- 15.Dauvilliers Y, Morin CM, Cervena K, et al. Family studies in insomnia. J Psychosom Res. 2005;58:271–78. doi: 10.1016/j.jpsychores.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 16.Hauri PJ, Olmstead EM. Childhood-onset insomnia. Sleep. 1980;3:59–65. doi: 10.1093/sleep/3.1.59. [DOI] [PubMed] [Google Scholar]
- 17.Edinger JD, Stout AL, Hoelscher TJ. Cluster analysis of insomniacs' MMPI profiles: Relation of subtypes to sleep history and treatment outcome. Psychosom Med. 1988;50:77–87. doi: 10.1097/00006842-198801000-00010. [DOI] [PubMed] [Google Scholar]
- 18.Kales A, Caldwell AB, Soldatos C, et al. Biopsychobehavioral correlates of insomnia II. Pattern specificity and consistency with the Minnesota Multiphasic Personality Inventory. Psychosom Med. 1983;45:341–56. doi: 10.1097/00006842-198308000-00008. [DOI] [PubMed] [Google Scholar]
- 19.Taylor DJ, Lichstein KL, Durrence H, et al. Epidemiology of insomnia, depression, and anxiety. Sleep. 2005;28:1457–64. doi: 10.1093/sleep/28.11.1457. [DOI] [PubMed] [Google Scholar]
- 20.Coren S. Prediction of insomnia from arousability predisposition score: scale development and cross-validation. Behav Res Ther. 1988;26:415–20. doi: 10.1016/0005-7967(88)90076-9. [DOI] [PubMed] [Google Scholar]
- 21.Phillips BA, Danner FK. Cigarette smoking and sleep disturbance. Arch Intern Med. 1995;10:734–37. [PubMed] [Google Scholar]
- 22.Bastien CH, Vallières A, Morin CM. Precipitating factors of insomnia. Behav Sleep Med. 2004;2:50–62. doi: 10.1207/s15402010bsm0201_5. [DOI] [PubMed] [Google Scholar]
- 23.Morin CM, Rodrigue S, Ivers H. Role of stress, arousal, and coping skills in primary insomnia. Psychosom Med. 2003;65:259–67. doi: 10.1097/01.psy.0000030391.09558.a3. [DOI] [PubMed] [Google Scholar]
- 24.Brabbins CJ, Dewey ME, Copeland JRM, et al. Insomnia in the elderly: prevalence, gender differences and relationships with morbidity and mortality. Int J Geriatr Psychiatry. 1993;8:473–80. [Google Scholar]
- 25.Ganguli M, Reynolds CF, Gilby JE. Prevalence and persistence of sleep complaints in a rural older community sample: The MoVIES Project. J Am Geriatr Soc. 1996;44:778–84. doi: 10.1111/j.1532-5415.1996.tb03733.x. [DOI] [PubMed] [Google Scholar]
- 26.Hohagen F, Rink K, Kappler C, et al. Prevalence and treatment of insomnia in general practice. Eur Arch Psychiatry Clin Neurosci. 1993;242:329–36. doi: 10.1007/BF02190245. [DOI] [PubMed] [Google Scholar]
- 27.Simon GE, Vonkorff M. Prevalence, burden, and treatment of insomnia in primary care. Am J Psychiatry. 1997;154:1417–23. doi: 10.1176/ajp.154.10.1417. [DOI] [PubMed] [Google Scholar]
- 28.Vollrath M, Wicki W, Angst J. The Zurich study VIII. Insomnia: Association with depression, anxiety, somatic syndromes, and course of insomnia. Eur Arch Psychiatry Neurol Sci. 1989;239:113–24. doi: 10.1007/BF01759584. [DOI] [PubMed] [Google Scholar]
- 29.Roberts RE, Shema SJ, Kaplan GA. Prospective data on sleep complaints and associated risk factors in an older cohort. Psychosom Med. 1999;61:188–96. doi: 10.1097/00006842-199903000-00011. [DOI] [PubMed] [Google Scholar]
- 30.National Institutes of Health (NIH) NIH State-of-the-science conference statement: Manifestations and management of chronic insomnia in adults. 2005 http://consensus.nih.gov/2005/2005InsomniaSOS026html.htm.
- 31.Kish L. Survey sampling. New York: John Wiley and Sons Inc; 1965. [Google Scholar]
- 32.Morin CM, Beaulieu-Bonneau S, LeBlanc M, et al. Self-help treatment for insomnia: a randomized controlled trial. Sleep. 2005;28:1319–27. doi: 10.1093/sleep/28.10.1319. [DOI] [PubMed] [Google Scholar]
- 33.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington (DC): American Psychiatric Press; 2000. text revision. [Google Scholar]
- 34.World Health Organization [WHO] The ICD-10 classification of mental and behavioral disorder: diagnostic criteria for research (10th revision) Geneva: World Health Organization; 1992. [Google Scholar]
- 35.Buysse DJ, Reynolds CF, Monk TH, et al. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 36.LeBlanc M, Beaulieu-Bonneau S, Mérette C, Savard J, Ivers H, Morin CM. Psychological and health-related quality of life factors associated with insomnia in a population-based sample. J Psychosom Res. 2007;63:157–166. doi: 10.1016/j.jpsychores.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 37.Bastien CH, Vallières A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2:297–307. doi: 10.1016/s1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- 38.Blais FC, Gendron L, Mimeault V, et al. Évaluation de l'insomnie: Validation de trois questionnaires. Encephale. XXIII:447–53. [PubMed] [Google Scholar]
- 39.Beck AT, Steer RA, Brown GK. Manuel de l'inventaire de dépression de Beck. 2ème édition. Toronto, ON, Canada: The Psychological Corporation; 1996. [Google Scholar]
- 40.Spielberger CD. Manual for the State-Trait Anxiety Inventory (STAI) Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- 41.Gauthier J, Bouchard S. Adaptation canadienne-française de la forme révisée du State-Trait Anxiety Inventory de Spielberger. Can J Behav Sci. 1993;25:559–78. [Google Scholar]
- 42.Sarason IG, Johnson JH, Siegel JM. Assessing the impact of life change: development of the life experience survey. J Consult Clin Psychol. 1978;46:932–46. doi: 10.1037//0022-006x.46.5.932. [DOI] [PubMed] [Google Scholar]
- 43.Cohen S, Karmack T, Mermelstein R. A global measure of perceived stress. J Health Behav. 1983;24:386–96. [PubMed] [Google Scholar]
- 44.Endler NS, Parker JD. Stress and anxiety: Conceptual and assessment issues. Stress Med. 1990;6:243–48. [Google Scholar]
- 45.Endler NS, Parker JD. Multidimensional assessment of coping: a critical evaluation. J Pers Soc Psychol. 1990;58:844–54. doi: 10.1037//0022-3514.58.5.844. [DOI] [PubMed] [Google Scholar]
- 46.Coren S, Mah KB. Prediction of physiological arousability: a validation of the Arousal Predisposition Scale. Behav Res Ther. 1993;31:215–19. doi: 10.1016/0005-7967(93)90076-7. [DOI] [PubMed] [Google Scholar]
- 47.Costa PT, Jr, McCrae RR. Revised NEO Personality Inventory (NEO-PI-R) and NEO Five-Factor Inventory (NEO-FFI) professional manual. Odessa, Fl: Psychological Assessment Resources Inc; 1992. [Google Scholar]
- 48.Digman JM. Personality structure: Emergence of the Five-Factor Model. Annu Rev Psychol. 1990;41:417–40. [Google Scholar]
- 49.Holden RR, Fekken GC. The NEO Five-Factor Inventory in a Canadian context: Psychometric properties for a sample of university women. Pers Individ Dif. 1994;17:441–44. [Google Scholar]
- 50.Sabourin S, Lussier Y. Traduction française de l'inventaire de personnalité NEO-FFI [A French translation of the NEO-FFI] Unpublished manuscript. 1992 [Google Scholar]
- 51.Ware JE, Kosinski M, Keller SD. A 12-item short-form health survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–33. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 52.Ware JE, Kosinski M, Turner-Bowker DM, et al. How to score version 2 of the SF-12 health survey. Lincoln: QualityMetric Incorporated; 2002. [Google Scholar]
- 53.SAS Institute. SAS/STAT 9.1 User's Guide, Volumes 1-7. Cary (NC): SAS Institute; 2004. [Google Scholar]
- 54.Gardner RC. Psychological statistics using SPSS for windows. Upper Saddle River, NJ: Prentice Hall; 2000. [Google Scholar]
- 55.Macdonald PLG, Robert C. Type I Error Rate Comparisons of Post Hoc Procedures for I j Chi-Square Tables. Educ Psychol Meas. 2000;60:735–54. [Google Scholar]
- 56.Rosner B. Fundamentals of biostatistics. 6th ed. Belmont, CA: Thomson-Brooks/Cole; 2006. [Google Scholar]
- 57.Kirk RE. Procedures for the behavioral sciences. Pacific Grove, CA: Brooks/Cole Publishing Company; 1995. Experimental design. [Google Scholar]
- 58.Janson C, Lindberg E, Gislason T, et al. Insomnia in men: A 10-year prospective population based study. Sleep. 2001;24:425–30. doi: 10.1093/sleep/24.4.425. [DOI] [PubMed] [Google Scholar]
- 59.Morgan K, Clarke D. Risk factors for late-life insomnia in a representative general practice sample. Br J Gen Pract. 1997;47:166–69. [PMC free article] [PubMed] [Google Scholar]
- 60.Bonnet MH, Arand DL. Hyperarousal and insomnia. Sleep Med Rev. 1997;1:97–108. doi: 10.1016/s1087-0792(97)90012-5. [DOI] [PubMed] [Google Scholar]
- 61.Freedman RR, Sattler HL. Physiological and psychological factors in sleep-onset insomnia. J Abnorm Psychol. 1982;91:380–89. doi: 10.1037//0021-843x.91.5.380. [DOI] [PubMed] [Google Scholar]
- 62.Gross RT, Borkovec TD. Effects of a cognitive intrusion manipulation on the sleep-onset latency of good sleepers. Behav Ther. 1982;13:112–16. [Google Scholar]
- 63.Harvey AG. Pre-sleep cognitive activity: a comparison of sleep onset insomniacs and good sleepers. Br J Clin Psychother. 2000;39:275–86. doi: 10.1348/014466500163284. [DOI] [PubMed] [Google Scholar]
- 64.Jacobs GD, Benson H, Friedman R. Home-based central nervous system assessment of a multifactor behavioral intervention for chronic sleep-onset insomnia. Behav Ther. 1993;24:159–74. [Google Scholar]
- 65.Morin CM, Bélanger L, LeBlanc M, et al. The natural history of insomnia. A population-based 3-year longitudinal study. Arch Intern Med. 2009;169:447–53. doi: 10.1001/archinternmed.2008.610. [DOI] [PubMed] [Google Scholar]
- 66.Ohayon MM. Prevalence of DSM-IV diagnostic criteria of insomnia: distinguishing between insomnia related to mental disorders from sleep disorders. J Psychiatr Res. 1997;31:333–46. doi: 10.1016/s0022-3956(97)00002-2. [DOI] [PubMed] [Google Scholar]