Abstract
Study Objectives:
To evaluate the short-term efficacy and safety of electroacupuncture for the treatment of primary insomnia.
Design:
Randomized, single-blind, placebo-controlled, parallel-group.
Setting:
A university-based sleep clinic.
Participants:
Community sample of 60 Chinese adult volunteers who report having insomnia 3 or more nights per week, whose symptoms meet the DSM-IV criteria for primary insomnia for at least 3 months, and who have an Insomnia Severity Index total score of at least 15. Participants were screened with polysomnography and the Structured Clinical Interview for the DSM-IV prior to randomization.
Intervention:
Electroacupuncture at Yintang (EX-HN3), Baihui (GV20), bilateral ear Shenmen, Sishencong (EX-HN1), and Anmian (EX) 3 times per week for 3 weeks or placebo acupuncture using Streitberger needles at the same points.
Measurements and Results:
Self-reported questionnaires, 1-week sleep diaries, and 3-day actigraphy were collected at baseline and 1 week after treatment. The Insomnia Severity Index was used as the primary outcome measure. Both groups showed significant improvement compared with the pretreatment baseline. One-way analysis of covariance adjusted for baseline scores showed that there were significantly greater improvements in sleep efficiency by sleep diary and actigraphy in the electroacupuncture group. However, no significant between-group differences were observed in the Insomnia Severity Index and other outcome measures. The proportions of subjects having less than 30 minutes of wake after sleep onset and a sleep efficiency of at least 85% at the posttreatment visit were significantly higher in the electroacupuncture group. All adverse events were mild in severity.
Conclusion:
We found a slight advantage of electroacupuncture over placebo acupuncture in the short-term treatment of primary insomnia. Because of some limitations of the current study, further studies are necessary to verify the effectiveness of acupuncture for insomnia.
Citation:
Yeung WF; Chung KF; Zhang SP; Yap TG; Law ACK. Electroacupuncture for primary insomnia: a randomized controlled trial. SLEEP 2009;32(8):1039-1047.
Keywords: Acupuncture, electroacupuncture, insomnia, randomized controlled trial, sleep, traditional Chinese medicine
INSOMNIA IS A FREQUENT CLINICAL COMPLAINT. THE PREVALENCE OF INSOMNIA SYMPTOMS ACCOMPANIED BY DAYTIME CONSEQUENCES RANGES FROM 9% to 15% for the general population, whereas the prevalence of primary insomnia ranges from 2% to 4%.1 Insomnia may lead to fatigue, irritability, and impaired daytime functioning. It is associated with reduced quality of life, an increased risk of having a motor vehicle crash, impaired job performance, and absenteeism.2,3 Early diagnosis and treatment of insomnia is important, since untreated insomnia is associated with accidents and functional impairments, as well as the development of anxiety and depressive disorders.4
Although effective pharmacologic treatments for insomnia are available, their uses are limited by concerns regarding long-term efficacy and the potential for abuse, dependence, and adverse effects.5 Psychological and behavioral therapies have empirical evidence for insomnia,6 but they have remained largely underutilized because of the time-intensive nature and requirement of significant training for effective implementation.7 Faced with the limitations of the currently available treatments, the rate of complementary and alternative medicine use in treating insomnia increased from 20.6% in 1990 to 26.4% in 1997 in the general population of the United States.8 Among the complementary treatment modalities, acupuncture has been one of the most popular and safest procedures.9 To treat illnesses, the acupuncturist inserts fine needles at special points on the body, called acupoints, according to the traditional Chinese medicine theory. Electroacupuncture, which stimulates acupoints by electric currents via inserted acupuncture needles, is often a preferred form of treatment in research settings. Compared with manual acupuncture, electroacupuncture is more readily controllable and easier to standardize, it allows stronger and more continuous stimulation with less pain and tissue damage, it is less time consuming to apply, and its effects are more rapid and longer lasting. It is considered to be more effective than manual acupuncture.10
There have been multiple anecdotal reports and clinical studies on the use of acupuncture for the treatment of insomnia. We recently conducted a systematic review of traditional needle acupuncture for insomnia and found that acupuncture might be a promising treatment modality for insomnia.11 However, the majority of the studies were limited by poor research designs, such as problems in imprecise enrollment criteria, randomization, allocation concealment, blinding, and insufficient safety data. Furthermore, parameters of electroacupuncture were often not stated, and acupoint selection, duration and frequency of acupuncture treatment, and number of acupuncture sessions varied greatly among studies.11 Nevertheless, in 2 higher-quality trials reviewed, traditional needle acupuncture was more efficacious in improving sleep than was sleep-hygiene counseling in pregnant women.12 It is also more effective than sham acupuncture in treating poststroke insomnia.13 From our previous review, we found that the most commonly used acupoints in traditional needle acupuncture treatment for insomnia were Baihui (GV20), Shenting (GV24), Sishencong (EX-HN1), Shenmen (HT7), Neiguan (PC6), and Sanyinjiao (SP6), and the number of acupoints selected ranged from 4 to 16. On average, the course of acupuncture treatment for insomnia lasted for 22 days and ranged from 2 to 56 days.
Based on the TCM literature, most acupoints are not limited to treating 1 condition.14 For example, Baihui (GV20) has been used for to treat insomnia and headache. There has been limited research on the mechanistic aspects of individual acupoints in humans. Previous studies have shown that needling at Sishencong (EX-HN1) and Neiguan (PC6) can reduce heart rate, suggesting a sympathoinhibitory effect.15,16 Another study has shown that acupuncture increases nocturnal melatonin secretion and is associated with sleep improvement, though the exact acupoints used were not mentioned.17
To the best of our knowledge, there has been no randomized placebo-controlled study on the efficacy and safety of acupuncture for the treatment of primary insomnia. We therefore conducted a randomized controlled trial to examine the effects of electroacupuncture and noninvasive placebo acupuncture in treating primary insomnia. Our hypothesis was that electroacupuncture would be superior to placebo acupuncture in the short-term treatment of primary insomnia.
METHODS
Design
This was a randomized, single-blind, parallel-group study designed to compare the effects of electroacupuncture and placebo acupuncture. Major assessments were at baseline and 1 week after treatment. We followed the CONSORT and STRICTA recommendations in designing and reporting the controlled trial.18\?\.19
Subjects
Participants were recruited via advertisements in local newspapers. The inclusion criteria of this study were (1) ethnic Chinese; (2) aged 18 to 65 years; (3) insomnia complaint 3 or more nights per week; (4) diagnosis of primary insomnia according to the DSM-IV criteria20 for at least 3 months; and (5) total score of Insomnia Severity Index (ISI) (the primary outcome measure) was at least 15, indicating moderate severity of insomnia.21
Subjects were excluded if they (1) were taking herbal remedies, over-the-counter medications, or psychotropic drugs that were intended for the treatment of insomnia within the last 2 weeks prior to baseline or during the study; (2) were diagnosed to with a concurrent major depressive disorder, generalized anxiety disorder, panic disorder, manic or hypomanic episode, substance use disorder besides caffeine or nicotine use, organic mental disorder, schizophrenia, or any other psychotic disorder based on the Structured Clinical Interview for the DSM-IV (SCID);22,23 (3) had received any acupuncture treatment during the previous 12 months prior to the baseline time point; (4) had an apnea-hypopnea index of 10 or greater or a periodic limb movement index with arousal of 15 or greater, as detected during overnight polysomnography; (5) had an infection or abscess close to the site of the selected acupoints; (6) were pregnant, breast-feeding, or a woman of childbearing potential not using adequate contraception; (7) had valvular heart defects or bleeding disorders or were taking anticoagulant drugs; (8) had a significant risk of suicide; (9) had any serious physical illness; or (10) had participated in any clinical trial within the 3 months prior to baseline. No subjects were paid for participation; however, the electroacupuncture treatment, which costs US$20-30 per session in Hong Kong, was provided free of charge.
Our sample size estimation was based on changes in ISI score. A clinically significant treatment effect was defined as an at least 3-point difference in ISI total score between electroacupuncture and placebo acupuncture. We expected that the active treatment would outperform the placebo treatment by 1 point on at least 2 of the 3 severity items of the ISI and at least 1 of the 4 impairment items. Analysis of covariance (ANCOVA) was used to examine changes in outcome measures between groups.24 Based on previous work,21 a sample size of 24 in each group would have a power of 80% to detect a 3-point difference in the ISI total score between the 2 groups at an α level of 0.05.25 Allowing a 20% attrition rate, we estimated that this study would require a sample size of 30 in each group.
The study was reviewed and approved by the local institutional review board. Subjects showing an interest in participating in the study were assessed via telephone or email about their sleep, medical, and psychiatric history. Potential subjects participated in a comprehensive face-to-face interview to obtain a more detailed history, including the SCID. Written informed consent was obtained from all participants prior to the in-person interview. Laboratory-based overnight polysomnography (Alice 4 Diagnostics System, Respironics, Atlanta, GA) was arranged to rule out specific sleep disorders. During polysomnography, electroencephalogram (C3/A2 and C4/A1), electrooculogram, and submental and bilateral anterior tibialis electromyogram were recorded using surface electrodes. Airflow was measured by thermistors, respiratory movements by impedance plethysmography, and arterial oxygen saturation by pulse oximetry. The electrocardiogram was also assessed. Sleep variables were analyzed according to the standard Rechtschaffen and Kales criteria26 by a registered polysomnographic technologist.
Eligible subjects completed a 1-week sleep diary and a 3-day actigraphy recording in the week prior to a scheduled baseline visit. At the baseline visit, the subjects returned the completed sleep records and were asked to fill out a set of self-reported questionnaires. The subjects were then randomly assigned to either electroacupuncture or placebo acupuncture in a 1:1 ratio by an independent administrator using a computer-generated list of numbers27 and received their first treatment on the same day. At the 1-week posttreatment visit, the subjects returned the 1-week sleep diary and 3-day actigraph recording for the previous week and completed the same set of questionnaires.
The subjects were instructed that they would be randomly assigned to traditional acupuncture or acupuncture-like placebo treatment. Due to the nature of the intervention, it was not possible to blind the only acupuncturist in this study. The questionnaires, sleep diaries, and actigraphy results were analyzed by independent research assistants who were blinded to group allocation.
Intervention
Electroacupuncture
Subjects assigned to electroacupuncture were needled at Yintang (EX-HN3) and Baihui (GV20) and bilateral Ear Shenmen, Sishencong (EX-HN1), and Anmian (EX) using disposable acupuncture needles. The acupoint selection was based on our systematic review11 and expert opinion. The acupoints on the head and ears were treated using 0.25 × 25-mm needles and 0.20 × 25-mm needles (Tai Chi, China), respectively. De qi—an irradiating feeling considered to be indicative of effective needling—was achieved if possible. Surgical tapes or hair pins were used to secure the needles. An electric stimulator (CEFAR Acus II, Lund, Sweden) was connected to the needles and delivered a constant-current, 0.45-ms, square-wave, brief-pulse stimulus of 4-Hz frequency to the subjects. The needles were left for 30 minutes and then removed. Subjects were treated 3 times per week for 3 consecutive weeks in a quiet treatment room. The thrice-weekly treatment schedule, but not a more frequent or daily treatment, was selected to enhance treatment adherence, and the 3-week treatment duration was chosen to examine the short-term effect of acupuncture. The acupuncture was performed by a licensed acupuncturist who had 2 years of experience in providing needle acupuncture.
Placebo Acupuncture
Subjects assigned to the placebo group were treated at the same acupoints using placebo needles.28 The blunt needle was not fixed inside the copper handle. When its tip touched the skin, a pricking sensation was felt by the subject, thereby simulating the puncturing of the skin. The needle moved inside the handle and appeared to be shortened. A previous study showed that the credibility of placebo needles is high, particularly in acupuncture-naïve subjects.29 Similar to the technique used in the electroacupuncture group, the needles were held by surgical tape or hair pins and connected to the same electric stimulator but with zero frequency and amplitude. The subjects were told that the electric stimulator was set at a fixed level and were advised to inform the acupuncturist if they felt the impulse was too strong. The acupuncturist, setting, treatment frequency, and duration of the treatment course were the same as in the electroacupuncture group.
Measures
The ISI is a scale assessing the perceived severity of insomnia symptoms and the associated functional impairment, whereas the Pittsburgh Sleep Quality Index (PSQI) is designed to measure general sleep disturbances. Both questionnaires are self-rating scales with higher scores suggestive of increasing severity of sleep disturbance.21,30 In this study, the first question of the ISI was phrased to assess insomnia severity in the past week, whereas the PSQI was used to evaluate sleep disturbances in the past month. The 1-week daily sleep diary inquired about bedtime and rising time, from which total time in bed (TIB) was calculated.31 Subjects were also advised to estimate sleep-onset latency (SOL), wake time after sleep onset (WASO), and total sleep time (TST). They also rated their sleep quality using a 4-point scale (very good, fairly good, fairly bad, and very bad). Sleep efficiency (SE) was calculated as (TST/TIB × 100%).
Because movement correlates with wakefulness and lack of movement with sleep,32 wrist actigraphy was used to objectively estimate sleep. Actigraphs are watch-like devices that record physical movement by means of an accelerometer-microprocessor link. In this study, actigraphs (Octagonal Basic Motionlogger, Ambulatory Monitoring, Inc., Ardsley, NY) were worn 24 hours per day on the nondominant wrist for 3 days prior to baseline and posttreatment study visits. The subjects were asked to press the actigraphy event marker to indicate “lights out.” We used Zero-Crossing Mode for quantification of wrist movement and ACTION-W 2.0 software to estimate SOL, WASO, TST, and SE using 1-minute epoch.
The Hospital Anxiety and Depression Scale (HADS)33 was used to assess the severity of depression and anxiety in the past week; higher scores are suggestive of more severe symptoms. The Sheehan Disability Index (SDI)34 was used to assess occupational, family, and social functioning of the subjects during the previous month; a higher score is indicative of more severe functional impairment. The Credibility of Treatment Rating Scale (CTRS)35 was used to assess subjects' “perceived logic of the treatment,” “confidence in the treatment to alleviate your complaint,” “confidence in recommending the treatment to your friends who have similar complaints,” and “likelihood that the treatment would alleviate your other complaints.” The CTRS was completed by the subjects after the second and ninth treatment sessions; a lower score is suggestive of greater confidence toward the received treatment.
All questionnaires were presented in the Chinese language. We used the Chinese versions of PSQI and HADS, known to be reliable and valid measures.36,37 The ISI, SDI, and CTRS were translated into Chinese using a 2-phase technique.38,39 The validity of the translation was examined in groups of medical students and psychiatric patients. We found that the Chinese and English versions of the scales were highly similar in interpretation. The Pearson correlation coefficient of the total score of the Chinese and English version of the ISI was 0.98. The 1-week test-retest reliability of the Chinese version of the ISI was 0.95.
Statistical Analyses
All the statistical analyses were performed using SPSS version 15.0. Baseline differences between the electroacupuncture and placebo acupuncture groups were examined using either the unpaired t-test or χ2 test. The last observation carried forward method was used to handle missing data, with the last post-baseline data obtained carried as the endpoint value. The outcome measures at the posttreatment visit were compared with the measures obtained at baseline using the paired t-test. A oneway ANCOVA with baseline measure as covariate was used to investigate differences between the electroacupuncture and placebo acupuncture groups at posttreatment. Treatment impact was estimated by standardized within-group and between-group effect size.40 The within-group effect size was calculated as the difference between pretreatment and posttreatment means divided by the pooled standard deviation, whereas the between-group effect size was computed as the between-group difference in posttreatment means divided by the pooled standard deviation. The clinical significance of the interventions was estimated by the proportion of participants who reached sleep-diary-recorded SOL or WASO of 30 minutes or less and a SE of at least 85%. Either the χ2 or Fisher exact test was used to test for group differences in the proportion of subjects who reached clinically significant improvement. We used stepwise multiple linear regression to examine the predictors of outcome. Sleep-diary–derived SE change from baseline to posttreatment was selected as the dependent variable, and demographic, clinical, sleep, and treatment-related data were chosen as possible predictors. SE was selected as the dependent variable because SE is a recognized summary index of insomnia.
RESULTS
Participants
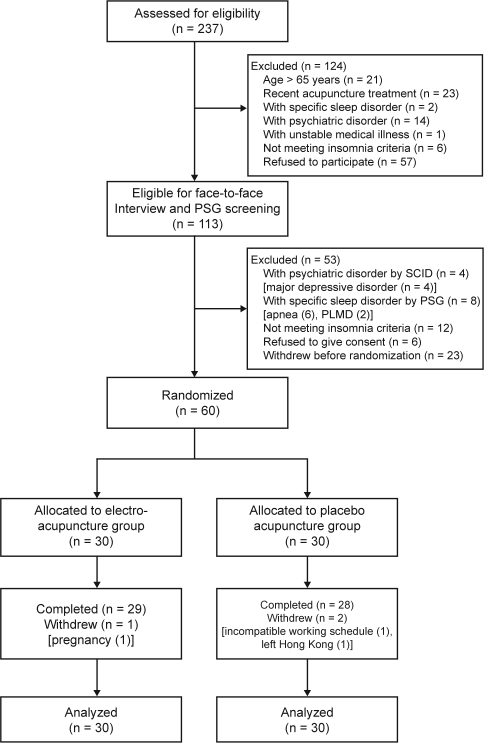
Two hundred and thirty-seven adults were assessed for eligibility, of whom 113 were screened in person and 60 were randomly assigned to electroacupuncture or placebo acupuncture (Figure 1). Table 1 presents the subject characteristics. There were no significant differences in demographic and clinical features between the randomly assigned subjects (n = 60) and the subjects who withdrew before randomization (n = 23) (All P values > 0.05). Participants had a mean age of 48.0 years and had had insomnia for an average of 9.3 years. Forty-six participants (77%) were women. About 60% of the subjects had used hypnotics, and the same percentage had previously taken Chinese herbal medicine for sleep. The most frequent insomnia complaint according to the ISI responses was “difficulty staying asleep,” with 58 participants (97%) having moderate, severe, or very severe problems. The next most frequent complaints were “problem waking up too early” (90.0%) and “difficulty falling asleep” (77%). The baseline characteristics were balanced between the 2 groups, except for the ISI score, which was significantly higher in the electroacupuncture group (Table 1).
Figure 1.
Participant flowchart. PSG refers to polysomnography; SCID, Structured Clinical Interview for DSM-IV
Table 1.
Demographic and Clinical Characteristics of the Sample
| Variables | Electro-acupuncture (n = 30) | Placebo acupuncture (n = 30) | Total (n = 60) | χ2 t valuea | P Value |
|---|---|---|---|---|---|
| Age, y | 48.3 ± 9.5 | 47.8 ± 8.6 | 48.0 ± 9.0 | 0.23 | 0.82 |
| Sex, male/female | 8/22 | 6/24 | 14/46 | 0.37 | 0.54 |
| Education attainment, y | 14.8 ± 3.0 | 14.3 ± 3.4 | 14.5 ± 3.2 | 0.60 | 0.55 |
| Marital status | 0.31 | 0.86 | |||
| Never married | 10 (33.3) | 9 (30.0) | 19 (31.7) | ||
| Married/cohabiting | 16 (53.3) | 18 (60.0) | 34 (56.7) | ||
| Divorced/widowed | 4 (13.3) | 3 (10.0) | 7 (11.7) | ||
| Occupation | 4.62 | 0.33 | |||
| Professional or associate professional | 10 (33.3) | 5 (16.7) | 15 (25.0) | ||
| Skilled or semiskilled worker | 9 (30.0) | 11 (36.7) | 20 (33.3) | ||
| Unskilled worker | 6 (20.0) | 5 (16.7) | 11 (18.3) | ||
| Retired | 4 (13.3) | 4 (13.3) | 8 (13.3) | ||
| Unemployed/housework | 1 (3.3) | 5 (16.7) | 6 (10.0) | ||
| Insomnia duration, y | 7.7 (8.1) | 10.8 (16.7) | 9.3 (8.9) | 1.36 | 0.18 |
| Previous treatment for insomnia | |||||
| Western medication | 20 (66.7) | 16 (53.3) | 36 (60.0) | 1.17 | 0.29 |
| Psychological treatment | 3 (10.0) | 1 (3.3) | 4 (6.7) | 1.07 | 0.30 |
| OTC drug | 14 (46.7) | 17 (56.7) | 31 (51.7) | 0.60 | 0.44 |
| Chinese herbal medicine | 18 (60.0) | 18 (60.0) | 36 (60.0) | 0.00 | 1.00 |
| Otherb | 9 (30.0) | 6 (20.0) | 15 (25.0) | 0.89 | 0.37 |
| Coffee use ≥ 1 cup/d | 11 (36.7) | 6 (20.0) | 17 (28.3) | 2.05 | 0.15 |
| Alcohol use ≥ 3 times/wk | 3 (10.0) | 2 (6.7) | 5 (8.3) | 0.22 | 0.64 |
| Chronic medical illness | 6 (20.0) | 2 (6.7) | 8 (13.3) | 2.31 | 0.13 |
| ISI total score | 18.8 ± 2.8 | 17.4 ± 2.5 | 18.1 ± 2.8 | 2.07 | 0.04 |
| PSQI total score | 12.0 ± 2.8 | 11.9 ± 2.1 | 12.0 ± 2.4 | 0.21 | 0.83 |
| HADS score | |||||
| Anxiety | 7.5 ± 4.2 | 7.0 ± 3.3 | 7.2 ± 3.8 | 0.47 | 0.64 |
| Depression | 6.3 ± 3.8 | 5.9 ± 3.0 | 6.1 ± 3.4 | 0.49 | 0.63 |
| Polysomnography at screening visit | |||||
| TST | 382.8 ± 71.1 | 370.4 ± 57.3 | 376.6 ± 64.3 | 0.74 | 0.46 |
| SOL | 31.4 ± 25.6 | 40.7 ± 42.2 | 36.1 ± 34.2 | 1.05 | 0.30 |
| WASO | 66.9 ± 46.4 | 80.5 ± 53.0 | 73.7 ± 49.8 | 1.06 | 0.29 |
| SE | 78.0 ± 14.5 | 75.9 ± 12.1 | 77.0 ± 13.2 | 0.62 | 0.54 |
Data are presented as mean ± SD or number (%).
HADS, Hospital Anxiety and Depression Scale; ISI, Insomnia Severity Index; OTC, over-the-counter; PSQI, Pittsburgh Sleep Quality Index; SE, sleep efficiency; SOL, sleep-onset latency; TST, total sleep time; WASO, wake after sleep onset.
Comparison between electroacupuncture and placebo acupuncture by χ2 or unpaired t-test.
Others, including health and dietary products, yoga, massage, and hypnosis.
Three subjects (5%) dropped out during the 4-week study period, 1 from the electroacupuncture group (3.3%) during the first week after baseline and 2 from the placebo acupuncture group (6.7%) during the first and second weeks after baseline (Figure 1). There were no missing data in the remaining 57 subjects who completed the study. None of the subjects withdrew due to adverse events. The dropout rates between the 2 groups were not significantly different (P = 1.0, Fisher exact test).
Efficacy
Subjective Sleep Measures
Summary data for the electroacupuncture and placebo acupuncture groups are presented in Table 2. One-way ANCOVA adjusted for baseline scores showed that there was no significant between-group difference in ISI total score, the primary outcome measure; PSQI total score; and sleep-diary–derived SOL, TST, WASO, and sleep quality at 1 week after treatment. The only between-group difference was in sleep-diary–derived SE (F1,57 = 10.83, P = 0.002), with greater improvement in the electroacupuncture group from baseline to posttreatment.
Table 2.
Subjective Sleep Measures at Baseline and 1-week Posttreatment
| Electroacupuncture |
Placebo Acupuncture |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Paired t-test P value | Within-group effect size | Mean | SD | Paired t-test P value | Within-group effect size | ANCOVA P valuea | Between-group effect size | |
| ISI total score | ||||||||||
| Baseline | 18.8 | 2.8 | 17.4 | 2.5 | ||||||
| Posttreatment | 12.9 | 5.6 | < 0.001 | 1.33 | 13.8 | 3.5 | < 0.001 | 1.18 | 0.12 | 0.19 |
| PSQI total score | ||||||||||
| Baseline | 12.0 | 2.8 | 11.9 | 2.1 | ||||||
| Posttreatment | 9.9 | 3.2 | < 0.001 | 0.70 | 9.7 | 2.6 | < 0.001 | 0.93 | 0.86 | 0.07 |
| Sleep diary | ||||||||||
| SOL in min | ||||||||||
| Baseline | 50.2 | 66.2 | 45.7 | 37.1 | ||||||
| Posttreatment | 34.4 | 33.7 | 0.04 | 0.30 | 36.9 | 26.8 | 0.05 | 0.27 | 0.29 | 0.08 |
| TST in min | ||||||||||
| Baseline | 292.9 | 80.6 | 307.9 | 61.3 | ||||||
| Posttreatment | 329.5 | 62.3 | 0.003 | 0.51 | 332.5 | 51.8 | 0.004 | 0.43 | 0.63 | 0.05 |
| WASO in min | ||||||||||
| Baseline | 79.0 | 60.7 | 87.4 | 52.8 | ||||||
| Posttreatment | 55.5 | 72.8 | 0.10 | 0.35 | 89.1 | 82.9 | 0.91 | 0.02 | 0.13 | 0.43 |
| SE in % | ||||||||||
| Baseline | 69.8 | 17.9 | 70.2 | 12.9 | ||||||
| Post-treatment | 81.2 | 10.7 | < 0.001 | 0.77 | 73.7 | 12.7 | 0.06 | 0.27 | 0.002 | 0.64 |
| Sleep qualityb | ||||||||||
| Baseline | 2.83 | 0.5 | 2.73 | 0.4 | ||||||
| Posttreatment | 2.42 | 0.5 | 0.001 | 0.82 | 2.39 | 0.4 | 0.001 | 0.85 | 0.96 | 0.07 |
ISI refers to Insomnia Severity Index; PSQI, Pittsburgh Sleep Quality Index; SE, sleep efficiency; SOL, sleep-onset latency; TST, total sleep time; WASO, wake after sleep onset.
One-way analysis of covariance (ANCOVA) using baseline sleep measures as covariates.
A lower score represents better sleep quality.
We assessed the clinical significance of our results by computing the respective proportion of subjects in each group who had improved from having an SOL greater than 30 minutes, a WASO of greater than 30 minutes, and a SE of less than 85% at baseline to an SOL of 30 minutes or less, a WASO of 30 minutes or less, and a SE of 85% or greater 1 week after treatment. The proportions of participants who had reached a SOL of 30 minutes or less at 1 week after treatment were 7 of 18 for the electroacupuncture group (38.9%) and 3 of 17 for the placebo acupuncture group (17.6%). There was no significant difference between groups on this criterion of improvement (P = 0.26, Fisher exact test). The proportions of participants achieving a WASO of 30 minutes or less at the posttreatment visit were 8 of 22 for the electroacupuncture group (36.4%) and 1 of 28 for the placebo acupuncture group (3.6%), whereas the proportions achieving a posttreatment SE of at least 85% were 10 of 23 for the electroacupuncture group (43.5%) and 1 of 26 for the placebo acupuncture group (3.8%). The electroacupuncture group significantly differed from the placebo acupuncture group by the criterion using WASO (P = 0.007) and SE (P = 0.001).
The standardized within-group and between-group effect sizes are presented in Table 2. The within-group effect size for the subjective sleep measures from baseline to 1 week after treatment ranged from 0.30 to 1.33 in the electroacupuncture group; for the placebo acupuncture group, it ranged from 0.02 to 1.18. The between-group effect size at posttreatment varied from 0.05 to 0.64.
Among all demographic, clinical, sleep, and treatment-related variables, only baseline SE, baseline PSQI score, and treatment group were significantly related to SE change from baseline to posttreatment assessment. Stepwise multiple regression revealed that baseline SE and treatment group were significant predictors of SE change (F2,57 = 28.3, P = 0.0001). Baseline SE (β = −0.51, P = 0.0001) accounted for 40% of the variance of SE change, with higher baseline SE predictive of less improvement in SE. Treatment group (β = 7.6, P = 0.002) contributed 10% of the variance, with electroacupuncture treatment associated with greater SE improvement.
Actigraphic Estimates of Sleep
Table 3 presents the summary data for actigraphy measures. One-way ANCOVA adjusted for baseline score showed that there was a significant between-group difference in actigraphy derived SE at posttreatment (F1,57 = 4.55, P = 0.04), with greater improvement for the electroacupuncture group from baseline to 1 week after treatment. No other significant between-group differences between the electroacupuncture and placebo acupuncture groups were found.
Table 3.
Actigraphy Measures of Sleep at Baseline and 1-week Posttreatment
| Electroacupuncture |
Placebo Acupuncture |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Paired t-test P value | Within-group effect siae | Mean | SD | Paired t-test P value | Within-group effect siae | ANCOVA P valuea | Between-group effect size | |
| Actigraphy | ||||||||||
| SOL, min | ||||||||||
| Baseline | 21.6 | 23.1 | 15.8 | 13.0 | ||||||
| Posttreatment | 12.2 | 15.6 | 0.03 | 0.48 | 14.1 | 11.7 | 0.45 | 0.14 | 0.25 | 0.14 |
| TST, min | ||||||||||
| Baseline | 408.4 | 64.5 | 422.0 | 51.9 | ||||||
| Posttreatment | 412.3 | 53.4 | 0.63 | 0.07 | 417.8 | 47.1 | 0.72 | 0.08 | 0.97 | 0.11 |
| WASO, min | ||||||||||
| Baseline | 28.7 | 32.9 | 19.2 | 19.1 | ||||||
| Posttreatment | 17.0 | 20.1 | 0.01 | 0.43 | 19.7 | 18.9 | 0.89 | 0.03 | 0.10 | 0.14 |
| SE, % | ||||||||||
| Baseline | 89.4 | 8.8 | 92.6 | 4.6 | ||||||
| Posttreatment | 93.2 | 6.5 | 0.003 | 0.49 | 92.4 | 4.8 | 0.77 | 0.04 | 0.04 | 0.14 |
Abbreviations: sleep efficiency; SOL, sleep-onset latency; TST, total sleep time; WASO, wake after sleep onset.
One-way analysis of covariance (ANCOVA) using baseline sleep measures as covariates.
Other Clinical Outcomes
Table 4 presents the data for HADS and SDI. One-way ANCOVA adjusted for baseline scores showed that there were no significant between-group differences in HADS and SDI scores at 1 week after treatment. In addition, there were no significant group differences in posttreatment CTRS scores after adjusting the initial scores.
Table 4.
Other Clinical Outcomes Measures at Baseline and 1-week Posttreatment
| Electroacupuncture |
Placebo Acupuncture |
||||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Paired t-test P value | Mean | SD | Paired t-test P value | ANCOVA P value a | |
| HADS | |||||||
| Anxiety score | |||||||
| Baseline | 7.5 | 4.2 | 7.0 | 3.3 | |||
| Posttreatment | 6.3 | 4.3 | 0.001 | 5.8 | 3.8 | 0.005 | 0.90 |
| Depression score | |||||||
| Baseline | 6.3 | 3.8 | 5.9 | 3.0 | |||
| Posttreatment | 4.8 | 3.3 | 0.001 | 5.3 | 3.8 | 0.29 | 0.19 |
| SDI | |||||||
| Work | |||||||
| Baseline | 4.3 | 2.9 | 3.6 | 2.3 | |||
| Posttreatment | 3.2 | 2.1 | 0.009 | 2.3 | 2.0 | 0.003 | 0.18 |
| Social | |||||||
| Baseline | 3.6 | 2.4 | 2.6 | 2.2 | |||
| Posttreatment | 2.8 | 2.0 | 0.11 | 2.5 | 1.8 | 0.74 | 0.98 |
| Family | |||||||
| Baseline | 3.2 | 2.2 | 1.9 | 2.0 | |||
| Posttreatment | 2.8 | 2.0 | 0.28 | 2.3 | 1.7 | 0.27 | 0.88 |
Abbreviations: HADS, Hospital Anxiety and Depression Scale; SDI, Sheehan Disability Index.
One-way analysis of covariance (ANCOVA) using baseline sleep measures as covariates.
Adverse Events
Both electroacupuncture and placebo acupuncture were well tolerated. Two subjects in the electroacupuncture group developed a headache, one subject developed hand numbness, and one developed a hematoma at the site of acupuncture; for the placebo acupuncture group, adverse events reported were headache (n = 1), hand numbness (n = 1), and worsening of insomnia (n = 1). All adverse events were mild in severity.
Discussion
To the best of our knowledge, this is the first study of the use of acupuncture for the treatment of primary insomnia using a well-documented screening process, randomization, placebo acupuncture needles, validated subjective scales, and objective measures. Compared with noninvasive placebo acupuncture, electroacupuncture showed statistically significant improvements in subjective and objective measures of SE. The proportions of subjects achieving sleep-diary–derived WASO of 30 minutes or less and a SE of at least 85% after treatment were significantly higher in the electroacupuncture group. However, there were no between-group differences in ISI total score, the primary outcome measure, and other secondary measures at the posttreatment visit. In terms of safety and treatment compliance, the 3-week thrice-weekly course of electroacupuncture and placebo acupuncture was well tolerated and accepted by most participants.
Although traditional needle acupuncture has previously been reported to be more effective than benzodiazepines, sleep-hygiene counseling, and sham acupuncture in the acute treatment of insomnia, the previous studies were generally of low methodologic quality.11 In addition, none of the studies examined subjects with primary insomnia or used noninvasive placebo acupuncture as comparison; hence, it is difficult to compare our data with the previous findings.
The tentatively positive effects seen in the present study should be treated with caution. Our subjects were recruited through newspaper advertisements and agreed to participate in this time-intensive study, which could suggest that these subjects had high expectations in the effectiveness of acupuncture, thereby overoptimizing the responses in both the acupuncture and placebo acupuncture groups. It is important to reexamine the efficacy of acupuncture in unselected “real-world” patients with insomnia. We found that baseline SE accounted for 40% of the variance in SE change, whereas electroacupuncture treatment contributed only 10% of the variance. The finding suggests that regression to the mean could be a possible factor for the sleep improvement in both groups. Measures to minimize the statistical problem in future studies are needed.41,42
Complementary and alternative medicine are frequently used by individuals suffering from insomnia;43 however, much less is known about the effectiveness and safety of such therapies. The uses of herbs, vitamins, and supplements for the treatment of insomnia are limited by insufficient scientific evidence and safety issues.44 Our data suggest that electroacupuncture can be considered as a safe, well-tolerated, and potentially useful nonpharmacologic intervention for primary insomnia. Although our results found that the efficacy of electroacupuncture was less impressive than that of pharmacotherapy and cognitive behavioral therapy,45,46 the moderate between-group effect sizes achieved for WASO and SE by sleep diaries suggest that acupuncture for the treatment of insomnia is worthy of future studies. Improved efficacy might be achieved by increasing session frequency and having individually tailored acupuncture regimens. Moreover, it would be worthwhile to explore whether combination treatment strategies, such as acupuncture and cognitive behavioral therapy, could produce synergistic therapeutic effects.
There are several methodologic limitations in our study. The severity of insomnia, as measured by actigraphy before treatment, was relatively mild; hence, there was limited room available for improvement, especially in the placebo acupuncture group. The apparent superiority of electroacupuncture over placebo acupuncture in the improvement of actigraphy measures should therefore be interpreted with caution, despite the between-group differences identified by ANCOVA with baseline measures as covariates. Another concern was that the actigraphy data were obtained for only 3 consecutive days in the present study. Although a consensus report on the research assessment of insomnia recommends a minimum of 3 days of actigraphy,47 we considered that 3 days may be insufficient to include data on variations between weekday and weekend schedules. There have been limited studies examining the difference in weekdays and weekend sleep-wake patterns in subjects with insomnia. A sleep-diary study in healthy adults has shown that SOL, WASO, and SE are similar between weekdays and weekends—although, in this study, TIB was, on average, 28 minutes longer on weekends.48 It is noteworthy that there was a discrepancy between the subjects' sleep-diary and actigraphy measures in the present study. Previous studies have found a low correlation between the 2 sleep measures, with actigraphy consistently producing better estimates of sleep.49,50 One explanation is that actigraphy relies on movement to identify periods of waking. Because a number of our subjects might have been lying awake without moving, their waking periods would therefore not have been detected. The discrepancy between sleep diary and actigraphy suggested that our study would have benefited by the use of additional objective measures, such as polysomnography. Another study limitation was that only a few subjects had baseline actigraphy-derived SOL of greater than 30 minutes, WASO or greater than 30 minutes, and SE of less than 85%; hence, the clinical significance of treatment for the actigraphy data could not be analyzed.
Despite these limitations, the present study provides important data on the treatment of primary insomnia by using acupuncture. We found a slight advantage of electroacupuncture over noninvasive placebo acupuncture. Because of the small difference and some shortcomings of the current study, the benefits of acupuncture for insomnia are still uncertain. Further studies with improved methodologic design are warranted to accurately determine the efficacy of acupuncture for the treatment of insomnia.
DISCLOSURE STATEMENT
This was not and industry supported study. Dr. Chung has received research support from AstraZeneca. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors are grateful to the staff of the Hong Kong Professional Teachers' Union and Prof. Leung Wing-Nang, as well as Mr. Chan Ying-Pan of Hong Kong PTU TCM clinic, for their support and assistance. We also extend special thanks to the participants of the study.
Trial registration: ClinicalTrials.gov NCT00839592.
REFERENCES
- 1.Ohayon MM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med Rev. 2002;6:97–111. doi: 10.1053/smrv.2002.0186. [DOI] [PubMed] [Google Scholar]
- 2.Leger D, Massuel MA, Metlaine A. Professional correlates of insomnia. Sleep. 2006;29:171–8. [PubMed] [Google Scholar]
- 3.Walsh JK. Clinical and socioeconomic correlates of insomnia. J Clin Psychiatry. 2004;65(Suppl 8):13–9. [PubMed] [Google Scholar]
- 4.Neckelmann D, Mykletun A, Dahl AA. Chronic insomnia as a risk factor for developing anxiety and depression. Sleep. 2007;30:873–80. doi: 10.1093/sleep/30.7.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Institutes of Health. National Institutes of Health State of the Science Conference Statement on manifestations and management of chronic insomnia in adults, June 13-15, 2005. Sleep. 2005;28:1049–57. doi: 10.1093/sleep/28.9.1049. [DOI] [PubMed] [Google Scholar]
- 6.Morin CM, Bootzin RR, Buysse DJ, Edinger JD, Espie CA, Lichstein KL. Psychological and behavioral treatment of insomnia: update of the recent evidence (1998-2004) Sleep. 2006;29:1398–414. doi: 10.1093/sleep/29.11.1398. [DOI] [PubMed] [Google Scholar]
- 7.Krystal AD. The changing perspective on chronic insomnia management. J Clin Psychiatry. 2004;65(Suppl 8):20–5. [PubMed] [Google Scholar]
- 8.Eisenberg DM, Davis RB, Ettner SL, et al. Trends in alternative medicine use in the United States, 1990-1997: results of a follow-up national survey. JAMA. 1998;280:1569–75. doi: 10.1001/jama.280.18.1569. [DOI] [PubMed] [Google Scholar]
- 9.Kaptchuk TJ. Acupuncture: theory, efficacy, and practice. Ann Intern Med. 2002;136:374–83. doi: 10.7326/0003-4819-136-5-200203050-00010. [DOI] [PubMed] [Google Scholar]
- 10.Mayor DF, editor. Electroacupuncture: a practical manual and resource. Edinburgh: Churchill Livingstone/Elsevier; 2007. [Google Scholar]
- 11.Yeung WF, Chung KF, Leung YK, Zhang SP, Law AC. Traditional needle acupuncture treatment for insomnia: A systematic review of randomized controlled trials. Sleep Med (in press) doi: 10.1016/j.sleep.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 12.Kim YS, Lee SH, Jung WS, et al. Intradermal acupuncture on shen-men and nei-kuan acupoints in patients with insomnia after stroke. Am J Chin Med. 2004;32:771–8. doi: 10.1142/S0192415X04002399. [DOI] [PubMed] [Google Scholar]
- 13.da Silva JB, Nakamura MU, Cordeiro JA, Kulay LJ. Acupuncture for insomnia in pregnancy - a prospective, quasi-randomised, controlled study. Acupunct Med. 2005;23:47–51. doi: 10.1136/aim.23.2.47. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y, editor. Xinbian shiyong shuxue xue. Beijing: Zhongguo yi yao ke ji chu ban she; 1999. (in Chinese) [Google Scholar]
- 15.Abad-Alegria F, Pomaron C, Aznar C, Munoz C, Adelantado S. Objective assessment of the sympatholytic action of the Nei-Kuan acupoint. Am J Chin Med. 2001;29:201–10. doi: 10.1142/S0192415X0100023X. [DOI] [PubMed] [Google Scholar]
- 16.Wang JD, Kuo TB, Yang CC. An alternative method to enhance vagal activities and suppress sympathetic activities in humans. Auton Neurosci. 2002;100:90–5. doi: 10.1016/s1566-0702(02)00150-9. [DOI] [PubMed] [Google Scholar]
- 17.Spence DW, Kayumov L, Chen A, et al. Acupuncture increases nocturnal melatonin secretion and reduces insomnia and anxiety: a preliminary report. J Neuropsychiatry Clin Neurosci. 2004;16:19–28. doi: 10.1176/jnp.16.1.19. [DOI] [PubMed] [Google Scholar]
- 18.Boutron I, Moher D, Altman DG, Schulz KF, Ravaud P. Extending the CONSORT statement to randomized trials of nonpharmacologic treatment: explanation and elaboration. Ann Intern Med. 2008;148:295–309. doi: 10.7326/0003-4819-148-4-200802190-00008. [DOI] [PubMed] [Google Scholar]
- 19.MacPherson H, White A, Cummings M, Jobst K, Rose K, Niemtzow R. Standards for reporting interventions in controlled trials of acupuncture: The STRICTA recommendations. Standards for Reporting Interventions in Controlled Trails of Acupuncture. Acupunct Med. 2002;20:22–5. doi: 10.1136/aim.20.1.22. [DOI] [PubMed] [Google Scholar]
- 20.American Psychiatric Association. Diagnostic and statistical manual of mental disorders: (DSM-IV) 4th Edition. Washington: DC: American Psychiatric Association; 1994. [Google Scholar]
- 21.Bastien CH, Vallieres A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2:297–307. doi: 10.1016/s1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- 22.Spitzer RL, Williams JB, Gibbon M, First MB. The Structured Clinical Interview for DSM-III-R (SCID). I: History, rationale, and description. Arch Gen Psychiatry. 1992;49:624–9. doi: 10.1001/archpsyc.1992.01820080032005. [DOI] [PubMed] [Google Scholar]
- 23.So EK, Leung CM, Chung D, Liu Z, Fong S. The Chinese-bilingual SCID-I/P Project: Stage 1 - reliability for mood disorders and schizophrenia. Hong Kong J Psychiatry. 2003;13:7–18. [Google Scholar]
- 24.Vickers AJ, Altman DG. Statistics notes: Analysing controlled trials with baseline and follow up measurements. BMJ. 2001;323:1123–4. doi: 10.1136/bmj.323.7321.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Borm GF, Fransen J, Lemmens WA. A simple sample size formula for analysis of covariance in randomized clinical trials. J Clin Epidemiol. 2007;60:1234–8. doi: 10.1016/j.jclinepi.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 26.Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring system of sleep stages in human subjects. Los Angeles: UCLA Brain Information Service/Brain Research Institute; 1968. [Google Scholar]
- 27.Simon S. A simple approach for randomisation [Internet] BMJ. 1999 Sep 17; Available from: http://www.bmj.com/cgi/eletters/319/7211/703#4642.
- 28.Streitberger K, Kleinhenz J. Introducing a placebo needle into acupuncture research. Lancet. 1998;352:364–5. doi: 10.1016/S0140-6736(97)10471-8. [DOI] [PubMed] [Google Scholar]
- 29.White P, Lewith G, Hopwood V, Prescott P. The placebo needle, is it a valid and convincing placebo for use in acupuncture trials? A randomised, single-blind, cross-over pilot trial. Pain. 2003;106:401–9. doi: 10.1016/j.pain.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 30.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 31.Spielman A, Glovinsky P. The diagnostic interview and differential diagnosis for complaints of insomnia. In: Pressman M, Orr W, editors. Understanding sleep: The evaluation and treatment of sleep disorders. Washington: DC: American Psychological Association Press; 1997. pp. 125–60. [Google Scholar]
- 32.Lichstein KL, Stone KC, Donaldson J, et al. Actigraphy validation with insomnia. Sleep. 2006;29:232–9. [PubMed] [Google Scholar]
- 33.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–70. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 34.Leon AC, Olfson M, Portera L, Farber L, Sheehan DV. Assessing psychiatric impairment in primary care with the Sheehan Disability Scale. Int J Psychiatry Med. 1997;27:93–105. doi: 10.2190/T8EM-C8YH-373N-1UWD. [DOI] [PubMed] [Google Scholar]
- 35.Vincent C. Credibility assessment in trials of acupuncture. Complement Med Res. 1990;4:8–11. [Google Scholar]
- 36.Chung KF, Tang MK. Subjective sleep disturbance and its correlates in middle-aged Hong Kong Chinese women. Maturitas. 2006;53:396–404. doi: 10.1016/j.maturitas.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 37.Leung CM, Wing YK, Kwong PK, Lo A, Shum K. Validation of the Chinese-Cantonese version of the Hospital Anxiety and Depression Scale and comparison with the Hamilton Rating Scale of Depression. Acta Psychiatr Scand. 1999;100:456–61. doi: 10.1111/j.1600-0447.1999.tb10897.x. [DOI] [PubMed] [Google Scholar]
- 38.Sperber A. Translation and validation of study instruments for cross-cultural research. Gastroenterology. 2004;126:S124–8. doi: 10.1053/j.gastro.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 39.Sperber A, DeVellis R, Boehlecke B. Cross-cultural translation: methodology and validation. J Cross-Cult Psychol. 1994;25:501–24. [Google Scholar]
- 40.Cohen J. A power primer. Psycho Bull. 1992;112:155–9. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- 41.Yudkin PL, Stratton IM. How to deal with regression to the mean in intervention studies. Lancet. 1996;347:241–3. doi: 10.1016/s0140-6736(96)90410-9. [DOI] [PubMed] [Google Scholar]
- 42.Barnett AG, van der Pols JC, Dobson AJ. Regression to the mean: what it is and how to deal with it. Int J Epidemiol. 2005;34:215–20. doi: 10.1093/ije/dyh299. [DOI] [PubMed] [Google Scholar]
- 43.Pearson NJ, Johnson LL, Nahin RL. Insomnia, trouble sleeping, and complementary and alternative medicine: Analysis of the 2002 National Health Interview Survey data. Arch Intern Med. 2006;166:1775–82. doi: 10.1001/archinte.166.16.1775. [DOI] [PubMed] [Google Scholar]
- 44.Meolie AL, Rosen C, Kristo D, et al. Oral nonprescription treatment for insomnia: an evaluation of products with limited evidence. J Clin Sleep Med. 2005;1:173–87. [PubMed] [Google Scholar]
- 45.Belanger L, Vallieres A, Ivers H, Moreau V, Lavigne G, Morin CM. Meta-analysis of sleep changes in control groups of insomnia treatment trials. J Sleep Res. 2007;16:77–84. doi: 10.1111/j.1365-2869.2007.00566.x. [DOI] [PubMed] [Google Scholar]
- 46.Smith MT, Perlis ML, Park A, et al. Comparative meta-analysis of pharmacotherapy and behavior therapy for persistent insomnia. Am J Psychiatry. 2002;159:5–11. doi: 10.1176/appi.ajp.159.1.5. [DOI] [PubMed] [Google Scholar]
- 47.Buysse DJ, Ancoli-Israel S, Edinger JD, Lichstein KL, Morin CM. Recommendations for a standard research assessment of insomnia. Sleep. 2006;29:1155–73. doi: 10.1093/sleep/29.9.1155. [DOI] [PubMed] [Google Scholar]
- 48.Monk TH, Buysse DJ, Rose LR, Hall JA, Kupfer DJ. The sleep of healthy people - a diary study. Chronobiol Int. 2000;17:49–60. doi: 10.1081/cbi-100101031. [DOI] [PubMed] [Google Scholar]
- 49.Wilson KG, Watson ST, Currie SR. Daily diary and ambulatory activity monitoring of sleep in patients with insomnia associated with chronic musculoskeletal pain. Pain. 1998;75:75–84. doi: 10.1016/S0304-3959(97)00207-8. [DOI] [PubMed] [Google Scholar]
- 50.Nelson J, Harvey AG. The differential functions of imagery and verbal thought in insomnia. J Abnorm Psychol. 2002;111:665–9. [PubMed] [Google Scholar]