Abstract
Study Objectives:
Periodic leg movements in sleep (PLMS) are episodes of repetitive and stereotypic leg movements occurring during sleep. In adults, research indicates that PLMS affects sleep quality and duration and are associated with a shift to relatively greater sympathetic influence over cardiovascular variables. However, little research has been performed to investigate the effect of PLMS episodes on cardiac autonomic control in children. This study aimed to quantify the effect of PLMS episodes during NREM2 sleep on heart rate variability (HRV) measures of sympathovagal balance in children.
Participants:
Overnight polysomnography data from 20 children (7–12 y) referred for assessment of sleep disordered breathing (SDB) were analyzed retrospectively. Ten children with episodes of PLMS were matched for age and SDB severity with a control group of 10 children without PLMS episodes.
Results:
The LF/HF ratio was significantly higher in the PLM+ compared with both the PLM− periods from PLMS subjects (P < 0.001) and the periods from the control group (P < 0.001). However, this effect could not be parsimoniously interpreted due to the likelihood that leg movements had a direct effect on the lower frequencies. Analysis of the ratio PLM+ to PLM+ plus PLM− indicated parasympathetic inhibition during periods of periodic leg movement and the onset of individual leg movements were associated with cardiac acceleration followed by a return to pre-movement levels.
Conclusion:
This study identified vagal inhibition in association with episodes of PLMS in children. Rapid cardiac acceleration occurring concurrently with the onset of individual leg movements also suggested decreased vagal activity associated with the movements.
Citation:
Walter LM; Foster AM; Patterson RR; Anderson V; Davey MJ; Nixon GM; Trinder J; Walker AM; Horne RSC. Cardiovascular variability during periodic leg movements in sleep in children. SLEEP 2009;32(8):1093-1099.
Keywords: Periodic leg movements in sleep, heart rate variability, children, sleep
PLMS (PERIODIC LEG MOVEMENTS IN SLEEP) ARE CHARACTERIZED BY EPISODES OF REPETITIVE, HIGHLY STEREOTYPICAL LEG MOVEMENTS DURING sleep, which involve the rhythmic extension of the big toe and dorsiflexion of the ankle, occasionally accompanied by knee and hip flexion. By definition, PLMS are not the result of generalized neurological disorders, which are typically apparent during wakefulness as well as sleep.1 PLMS primarily occur during NREM sleep. In adults, the periodic leg movements are preceded by cardiac acceleration, EEG (electroencephalogram) activation in the delta band and spikes in blood pressure (BP), thought to be linked to the activity of a common brainstem system that modulates cortical and subcortical mechanisms.2–5
Heart rate variability (HRV) is commonly used to investigate autonomic control. The output of HRV analysis is typically divided into 3 spectral components, very low frequency (VLF), low frequency (LF), and high frequency (HF). Of these, the VLF spectrum is influenced by both the renin-angiotensin-aldosterone system and parasympathetic outflow.6 The LF spectrum is considered to be mediated by both the parasympathetic and sympathetic nervous systems, while the HF spectrum represents only parasympathetic activity.7,8
The spectral analysis method has been applied to adult PLMS patients by Guggisberg et al.9 Using time frequency decomposition to quantify HRV, they identified increases in the LF/HF ratio over the course of leg movements, a change which was interpreted as indicating a primary role for the sympathetic nervous system in their generation, although low frequency changes in heart rate per se are associated with the periodic leg movements, which makes this interpretation problematic.
Although the effects of PLMS have been well studied in adults, there have been limited studies in children. In children, PLMS are associated with transient arousals and sleep fragmentation, which have been hypothesized to lead to changes in daytime neurocognitive and behavioral patterns.10 However, most previous research regarding PLMS in children centers around a severe form of PLMS, periodic limb movement disorder (PLMD). This condition is found more commonly in children with neurobehavioral problems, particularly attention-deficit hyperactivity disorder (ADHD).11–14 PLMD is also commonly associated with iron deficiency and renal failure.15,16 To date, there has been no research investigating whether the characteristics of autonomic control associated with PLMS in children might be the same as the pattern seen in adults.
In the current study we aimed to determine whether episodes of periodic leg movements in 7–12 year old children that were not associated with cortical arousals or respiratory events were associated with changes in HRV, similar to those observed in adults. We hypothesized that during episodes of periodic leg movements, there would be a shift in sympathovagal balance towards sympathetic dominance.
METHODS
The Monash Medical Centre Human Ethics Committee granted ethical approval for this project. Written informed consent was obtained from parents, and verbal assent from children prior to commencement of the study, and no monetary incentive was provided for participation.
Subjects
Subjects were selected retrospectively from 7–12 year old children participating in a larger study (n = 26, at the time of recruitment for this study), who attended the Melbourne Children's Sleep Unit for routine assessment of sleep disordered breathing (SDB) between April 2007 and February 2008. Children with syndromes affecting limb movement, such as Duchenne muscular dystrophy and Down syndrome were not included. From the study population, 10 children exhibited PLMS. They were matched for age and SDB category with a control group of 10 children from the same cohort, who did not have PLMS. All children had an obstructive apnea-hypopnea index (OAHI) of < 5 events/h.
Recording Methods
All children underwent overnight polysomnography (PSG) using a commercially available PSG system (Series S Sleep System, Compumedics, Melbourne, Australia). Electrodes for recording electroencephalograms (EEG; C4-A1, O2-A1), left and right electroculograms (EOG), submental electromyogram (EMG) and electrocardiogram (ECG) were attached. The ECG signal was digitized at a sampling rate of 512 Hz. Leg movements were measured by EMG of the left and right anterior tibialis muscle, with 2 surface electrodes placed longitudinally over the muscle on each leg. Thoracic and abdominal breathing movements (Resp-ez piezoelectric sensor, EPM Systems, Midlothian, VA, USA), oxygen saturation (Biox 3700e Pulse Oximeter, Ohmeda, Louisville, CO, USA), transcutaneous carbon dioxide (TINA TCM3, Radiometer, Copenhagen, Denmark), nasal pressure, and oronasal airflow were also recorded. Following the PSG study, data were transferred via European data format to data analysis software (LabChart 6, ADInstruments, Sydney, Australia) for HRV analysis.
Data Analysis
Sleep and arousals were scored from the EEG, EOG, and chin EMG channels in 30-s epochs according to standard criteria.17,18 Episodes of PLMS were identified according to the published standards devised by the World Association of Sleep Medicine in collaboration with a Task Force from the International Restless Legs Syndrome Study Group.19 The frequency of leg movements was represented as the periodic leg movement index (PLMI; number/h of total sleep time). Episodes of PLMS were defined as leg movements with an amplitude increase of 8 μV above the baseline value, a duration of 0.5–10 s, a period length between 2 consecutive movements of 5–90 s, and a minimum of 4 consecutive movements.19 The subject average frequency of leg movements during leg movement periods was 0.038 (SD = 0.016) Hz with a positively skewed distribution over subjects. A leg movement associated with the breath that ended an apnea or an hypopnea, or that introduced a significant transitory improvement in respiration during these respiratory events, such that the breath and the leg movement overlapped, or the offset of the earlier event preceded the onset of the later by < 0.5 s, (regardless of which was first), was not included as a PLMS.19 The duration of the leg movement episode was not necessarily as long as the HRV period of analysis, although the start of each were aligned.
In the subjects with PLMS, HRV was determined during stable stage 2 NREM sleep over 5-min periods during PLMS episodes (PLM+; 31 periods in total) and during 5-min periods without periodic leg movements (PLM−; 31 periods in total). HRV was also analyzed in control subjects over 5-min periods during stable stage 2 NREM sleep (31 periods in total). Stable sleep refers to full 5-min periods during which the sleep stage did not change from NREM2. All periods that were selected for analyses were free of movement artefact on the EEG signal, respiratory events, or cortical arousals. Measures of HRV were performed in both the time and frequency domains, according to published criteria.20
Time domain analysis
Overall HRV was determined using the standard deviation of the NN (normal R-R) intervals (SDNN; ms). High frequency variability was determined using the square root of the mean of the squared differences between adjacent NN intervals (RMSSD; ms) and the number of pairs of adjacent NN intervals that differed in length by more than 50 ms (NN50). Because of the length of the HRV periods, time domain measures reflecting LF activity were not able to be performed.
Frequency Domain Analysis
For each 5-min period, the power spectral density was computed with a 1024-point fast Fourier transform algorithm using a Welsh window function with ½ overlap and a frequency resolution of 0.001 Hz. Power density was calculated for VLF (< 0.04 Hz), LF (0.04–0.15 Hz), and HF (0.15–0.4 Hz) bands. Total power represents the total power in the spectrum for the current analysis region (≤ 0.4 Hz). The LF/HF ratio was also determined as a measure of sympathovagal balance. To remove the impact of large individual differences in total spectral variance, an additional ratio of PLM+ to PLM+ plus PLM− for each of the spectral components was calculated. This ratio was used rather than normalized LF and HF values as the normalized values are mathematically equivalent to the LF/HF ratio and do not add information over and above that measure.
Beat-to-Beat Analysis
To further characterize cardiac activity associated with PLMS, NN intervals were calculated for 5 heart beats before and 10 heart beats after the onset of each leg movement. The NN intervals were then standardized by subtracting the mean of the 4 NN intervals before the onset of the periodic leg movement from all of the data points (before and after the leg movement). The values were then averaged over leg movements for the 4 NN intervals before and the 9 NN intervals after the leg movements.
Statistical Analysis
Statistical analyses were performed using SPSS for Windows, version 15.0 (SPSS Inc., Chicago, IL, USA). Average values for each subject were analyzed. Data were first tested for normality and equal variance and determined not to be normally distributed. Therefore, nonparametric analyses were used. Mann-Whitney U tests (U) were used to analyze the difference between groups with and without PLMS (control) for demographic and polysomnographic variables. Kruskal-Wallis (H) and post hoc Mann-Whitney (U) analyses were used to compare HRV measures across the three conditions, PLM+ periods and PLM− periods in the PLMS group and PLM− periods in the control group. The Wilcoxon rank sum test (W) was used to analyze PLM+ to PLM+ plus PLM− ratios. The Friedman test (χr2) with Dunn post test were used to compare the means of the NN intervals prior to the onset of the periodic leg movements to the NN intervals following the onset of the periodic leg movements. Data are presented as median and range. P values < 0.05 were considered significant.
RESULTS
Demographic and polysomnographic data for the groups are presented in Tables 1 and 2 respectively. With the exception of the periodic leg movement index (PLMI) there were no significant differences in any of the demographic, sleep or respiratory characteristics between the PLMS group subjects and the control subjects. The upper range of the obstructive apnea-hypopnea index (4.5/h) scored during this study was slightly higher than would be expected in normal children.21 However, only 4 of the 20 children in the study were classified as having mild SDB (OAHI of 1 to 5), and the remaining 16 were classified as primary snorers (PS, OAHI < 1). The number of arousals scored was higher than the range observed in normal children (< 10/h is normal).21 In 35.5% of the PLM+ periods, the duration of the periodic leg movements was less than the 5-min HRV period.
Table 1.
Demographic Data for Subjects with and without PLMS
| Demographics | Subjects with PLMS | Subjects without PLMS (controls) | P |
|---|---|---|---|
| Age (years) | 9.8 (7.7–12.3) | 10.5 (7.2–11.5) | 0.82 |
| Height (cm) | 137 (128–168) | 140 (125–157) | 0.62 |
| Weight (kg) | 32.1 (24.9–69) | 36.4 (23.1–54.9) | 0.63 |
| BMI (kg/m2) | 16.7 (14.5–24.4) | 18.5 (14.4–27) | 0.57 |
Data are expressed as median (range). BMI refers to body mass index. Statistical significance determined between groups by Mann-Whitney U test. P < 0.05 considered significant. n = 10 per group.
Table 2.
Polysomnography Indices for Subjects with PLMS and for Subjects without PLMS Periods
| Polysomnography indices | Subjects with PLMS | Subjects without PLMS (controls) | P |
|---|---|---|---|
| TST | 418.8 (361.5–483.5) | 382 (331–450) | 0.24 |
| Sleep efficiency (%) | 83.1 (79–93.1) | 80.0 (69–92.2) | 0.46 |
| Sleep stage (% TST) | |||
| Stage 1 | 9.0 (7–13.2) | 10.4 (4.4–14.3) | 0.24 |
| Stage 2 | 53.1 (42.8–61.0) | 50.8 (43.2–58.7) | 0.46 |
| Stage 3 | 5.2 (2.4–8.6) | 5.0 (4.3–6.1) | 0.96 |
| Stage 4 | 13.9 (11.8–21.5) | 18.7 (10.5–27.1) | 0.15 |
| REM | 16.6 (11.4–26.1) | 13.3 (11.1–19.7) | 0.20 |
| PLMI (/h TST) | 3.2 (0.7– 13.3) | 0 | |
| PLMS episodes/subject | 2 (1–10) | 0 | |
| Total leg movements | 10.5 (4–57) | 0 | |
| OAHI (/h) | 0.6 (0–4.2) | 0.3 (0–4.5) | 0.51 |
| AI (/h) | 11.5 (9.6–14.9) | 11.1 (4.8–17.6) | 0.90 |
| SpO2 nadir (%) | 94.5 (87–97) | 96 (84–97) | 0.40 |
| Mean heart rate (bpm) | 76 (61–100) | 75 (58–84) | 0.70 |
Data are expressed as median (range). TST refers to total sleep time; REM, rapid eye movement sleep; PLMI, periodic leg movement index; OAHI, obstructive apnea-hypopnea index; AI, arousal index; SpO2, oxygen saturation. Statistical significance determined between groups by Mann-Whitney U test. P < 0.05 considered significant. n = 10 per group
Time Domain Measures of HRV
Time domain measures of HRV during stable stage 2 NREM sleep for the 3 conditions are presented in Table 3. There were no significant differences between the mean NN interval, SDNN, RMSSD, or NN50 during PLM+ and PLM− periods for the PLMS subjects, or PLM− periods from control subjects.
Table 3.
Time Domain Measures of Heart Rate Variability During Stable Stage 2 NREM Sleep for Periods with (PLM+) and without (PLM−) Periodic Leg Movement in PLMS Subjects and PLM− Periods in Control Subjects
| Subjects with PLMS |
Subjects without PLMS Controls | PLM+ vs PLM− P | PLM+ vs controls P | PLM− vs controls P | ||
|---|---|---|---|---|---|---|
| PLM+ | PLM− | |||||
| Mean NN interval (ms) | 789 (750–829) | 816 (723–907) | 797 (746–856) | 0.35 | 0.76 | 0.76 |
| SDNN (ms) | 72 (56–105) | 53 (33–100) | 55 (39–83) | 0.28 | 0.36 | 0.89 |
| RMSSD (ms) | 59 (41–85) | 47 (33–103) | 56 (36–89) | 0.80 | 0.97 | 0.83 |
| NN50 | 85 (59–160) | 90 (29–199) | 134 (58–186) | 0.85 | 0.70 | 0.76 |
Data are expressed as median (interquartile range) SDNN refers to standard deviation of the NN intervals; RMSSD, square root of the mean of the squared differences between adjacent NN intervals; NN50, number of pairs of adjacent NN intervals that differ in length by more than 50 ms. n = 10 per group.
Power Spectral Analysis of HRV
The power spectral analysis of HR intervals is reported in Table 4. No significant difference in absolute power existed between the 3 conditions for total power, VLF power, LF power, or HF power. There was a significant difference between groups for the LF/HF ratio (H = 14.5, P = 0.001), the ratio being higher in the PLM+ periods for the PLMS subjects compared with the PLM− periods from both the PLMS subjects (U = 9.0, P = 0.001) and the control subjects (U = 3.0, P = 0.001).
Table 4.
Spectral Analysis of Heart Rate Variability During Stable Stage 2 NREM Sleep for Periods with (PLM+) and without (PLM−) Periodic Leg Movement in PLMS Subjects and PLM− Periods in Control Subjects
| Subjects with PLMS |
Subjects without PLMS controls | PLM+ vs PLM− P | PLM+ vs controls P | PLM− vs controls P | ||
|---|---|---|---|---|---|---|
| PLM+ | PLM− | |||||
| Total Power (ms2) | 4415 (2999–13210) | 3152 (981–9003) | 3368 (1448–7181) | 0.32 | 0.51 | 0.83 |
| VLF Power (ms2) | 1494 (1046–1552) | 737 (157–1552) | 733 (288–1384) | 0.05 | 0.10 | 0.76 |
| LF Power (ms2) | 1362 (799–4636) | 617 (263–1988) | 813 (433–1749) | 0.19 | 0.32 | 0.89 |
| HF Power (ms2) | 1088 (773–2797) | 1242 (428–4982) | 1708 (626–3696) | 0.93 | 0.70 | 0.76 |
| LF/HF | 1.13 (0.86–1.95) | 0.54 (0.30–0.73) | 0.61 (0.29–0.65) | 0.001 | 0.001 | 0.63 |
Data are expressed as median (interquartile range) VLF refers to power in very low frequency; LF, low frequency; HF, high frequency. P < 0.05 considered significant.
A noticeable aspect of these data was that there were large individual differences in total power, such that subjects did not contribute equally to absolute values, potentially biasing the data (see range values in Table 4). As a consequence we calculated the quantity PLM+ divided by PLM+ plus PLM− for each PLMS subject, for each variable, and tested the hypothesis that the calculated value was different to the null hypothesis value of 0.5 (i.e., PLM+ = PLM−). The results are presented in Table 5. There was no significant difference in total power (W = 29, P = 0.16); however, the VLF and LF ratios were significantly higher than 0.5 (W = 53, P = 0.004 and W = 55, P = 0.002, respectively), while the HF component ratio was significantly lower (W = 41, P = 0.04).
Table 5.
Median Values of PLMS Subjects Ratios of PLM+ to PLM+ Plus PLM− Periods for Total, VLF, LF, and HF Power, Compared to the Theoretical Median of 0.5
| PLM+ : (PLM+) + (PLM−) | P | |
|---|---|---|
| Total Power (ms2) | 0.5003 (0.4145–0.7869) | 0.16 |
| VLF Power (ms2) | 0.7476 (0.6396–0.8781) | 0.004 |
| LF Power (ms2) | 0.5974 (0.4911–0.8186) | 0.002 |
| HF Power (ms2) | 0.4821 (0.2800–0.6342) | 0.04 |
Data are expressed as medians (interquartile range). VLF refers to power in very low frequency; LF, low frequency; HF, high frequency. P < 0.05 considered significant.
In order to determine if the high-frequency power density was being influenced by periods of aberrant respiration, values for respiratory frequency were calculated for subjects with PLMS and the control subjects without PLMS. Values were within the HF range of 0.15 to 0.40 Hz and not different between conditions (0.33 ± 0.02 Hz for PLM+, 0.34 ± 0.01 Hz for PLM−, and 0.35 ± 0.02 Hz for controls). Further, as reported in the methods, we calculated the frequency of leg movements within PLM+ periods. The subject mean value was 0.038 (SD = 0.016) Hz and the distribution over subjects was positively skewed.
Change in NN Interval Before and After Leg Movements
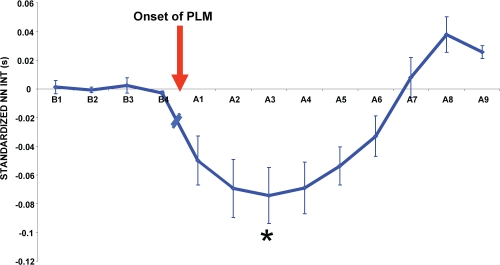
The mean standardized NN interval change for 5 heart beats immediately before and 10 heart beats following the onset of the periodic leg movement is shown in Figure 1. Following the onset of the periodic leg movement, there was a pronounced cardiac acceleration for the first 3 beats, followed by a slow return to the pre-periodic leg movement values. There was a significant difference between the mean of the NN intervals prior to the onset of the periodic leg movement and the post-onset NN intervals (χr2 = 42.9, P < 0.0001).
Figure 1.
Distribution of changes in mean NN intervals for each heartbeat before (Bn) and after (An) onset of periodic leg movements. Each point represents the mean duration of each NN interval minus the mean of the values prior to the onset of the leg movement (n = 10; mean ± SEM). *represents the NN interval that is significantly different (P < 0.05) than the mean of the NN intervals before the onset of the periodic leg movement.
DISCUSSION
The current study analyzed HRV associated with episodes of PLMS during stable, stage 2 NREM sleep. This stage constitutes approximately 50% of the total sleep time in children of this age and is the stage when most periodic leg movements occur. The results failed to identify a difference between PLMS and control subjects or between PLM+ and PLM− periods in the PLMS subjects for either time domain or absolute spectral analysis values. However, the LF/HF ratio significantly increased. Two aspects of the data displayed in Table 4 were of note and influenced interpretation of the data. First, as is common with HRV analysis, there were large individual differences in absolute power and thus in the magnitude of individual differences between conditions. The significant effect observed in the LF/HF ratio is in part because the ratio eliminates these individual differences in absolute power. Consistent with this the ratio PLM+/PLM+ plus PLM− identified significantly higher VLF and LF activity and lower HF activity in the PLM+ periods as compared to the PLM− periods for PLMS subjects.
The second important aspect of the data shown in Table 4 was that there was a large, although nonsignificant increase in total spectral power in the PLM+ condition that was confined to the VLF and LF bands. Nevertheless, this effect would clearly have contributed to the significant increase in the LF/HF ratio. While this might be interpreted as a shift in sympathovagal balance, there is potentially a critical confound in the data. Greater HR variability in the PLM+ periods could occur as a direct effect of leg movements on HR. Consistent with this are the observations that HR showed a transitory increase in association with leg movements, and that the average frequency of leg movements (0.038 Hz) was in the VLF and LF range. Thus, the differences between PLM+ and PLM− periods in VLF, LF, and LF/HR ratio cannot be parsimoniously interpreted as a shift in sympathovagal balance.
In contrast to LF activity, the lower HF activity in the PLM+ periods is most clearly interpreted as a reduction in vagal influence. This is primarily because HF (> 0.15 Hz) would be unlikely to be directly influenced by leg movements and because, if leg movements did have an influence, they would act to increase, not decrease, HF activity. Similarly, as noted above, the onset of the periodic leg movements were associated with a transient, but pronounced cardiac acceleration which, given its latency, was most probably induced by vagal deactivation.
The current data in children are empirically very similar to reports in adults. Sforza et al.22 compared PLM+ and PLM− periods in PLMS subjects and found that during periods of PLM+, measures reflecting sympathovagal balance showed increases in sympathetic dominance, while absolute LF power and total power (but not HF power) increased. More recently, Guggisberg et al.9 reported the same pattern of results in response to individual leg movements. In both papers, the results were interpreted as indicating increases in sympathetic activation in response to leg movements during sleep. In our view this interpretation is likely to be incorrect, for three reasons: First, the normalized LF and HF measures reported in these studies indicate sympathovagal balance and are not independent measures of LF and HF activity. Second, in both studies total power increased during PLM+ periods, masking any specific effects on the components. In all likelihood, as is likely in the current data, the increase in total power reflected a direct effect of leg movements on HRV. Third, the LF component cannot, in any case, be interpreted as uniquely indicating sympathetic activity.23–25 Thus all studies are in agreement in identifying a shift in low-frequency activity but were unable to determine the cause of the effect. However, the current study did identify a reduction in HF activity that likely reflects reduced vagal activity.
The Sforza22 and the Guggisberg9 studies included in their analyses periods of periodic leg movements associated with arousals. We were careful to exclude periodic leg movements associated with arousals, to distinguish ANS activity specifically associated with PLMS. Our results also suggested that the influence of leg movements did not extend beyond the PLM+ periods. Thus, we did not find significant differences in the HRV measures between the PLM− periods in the subjects with PLMS and the control subjects without PLMS, reinforcing the view that the differences observed during PLM+ periods were associated with the PLMS themselves. However, it should be noted that the ratio PLM+ to PLM+ plus PLM− could not be applied to comparisons between PLM− periods for PLMS subjects and control subjects, and thus this test was relatively insensitive.
The time course of HR changes associated with the onset of the periodic leg movement that we observed in children concurred with results from a similar study in adults by Sforza et al.,26 which also compared the distribution of NN intervals around the onset of the periodic leg movements. An initial tachycardia was observed both when the periodic leg movement was and was not followed by an arousal, but was significantly greater when the periodic leg movement was associated with an arousal. As cardiac acceleration occurred in approximately the first 2 to 3 ms following the onset of the leg movement, it would be feasible to assume that it was the result of an abrupt decrease in parasympathetic activity. This assumption is supported by previous research which determined that when small body movements occur during sleep, the parasympathetic system mediates HR variations via the muscle-heart reflex, initiating rapid vagal withdrawal.27–30
Studies by Ferrillo et al4 and Ferri et al5 have demonstrated that HR began to increase in the seconds preceding the onset of the leg movements; whereas in the current study, cardiac acceleration was not observed until the onset of movement. The difference in these results may reflect the fact that the current study analyzed PLMS only during NREM2 sleep and considered only those periodic leg movements that were not associated with arousals or respiratory events. Consistent with this interpretation, the Ferrillo and Ferri studies analyzed the temporal relationship between PLMS and EEG activity and demonstrated that, as well as increased HR, there was also increased delta EEG activity prior to the onset of the periodic leg movements. Thus, further research into the interaction of the cortical and sub-cortical mechanisms associated with PLMS in children is needed.
While a limitation of this study is that it was a retrospective analysis performed on data collected from children being assessed for suspected SDB, all of the children in the study had an OAHI of less than 5 per hour and were diagnosed as either primary snorers or mild SDB. Thus, the HRV data from the control children in the current study fall within the age-appropriate range published by Goto et al.31 These authors analyzed 60 healthy children aged from 3-15 years to determine normal values for time and frequency domain measures of HRV and correlations between the measures. As the controls in the current study were matched with the experimental subjects for both age and importantly SDB severity, it could be assumed that the degree of SDB diagnosed in the children used for this study has had a similar effect on the cardiovascular control of all subjects and was not different from that observed in the normal pediatric population.
A second limitation of this study was that the number of subjects who had PLMS and the number of leg movements for each subject was low. Only one of the children met the criteria for PLMD requiring possible clinical intervention. A diagnosis of PLMD in children requires a PLMI ≥ 5, evident clinical sleep disturbance for age, and inability to account for the leg movements by a primary neurological disorder or medication.32 There is some evidence of an increased prevalence of PLMD among children with attention deficit hyperactivity disorder (ADHD),13,14 but the prevalence of PLMD in the general pediatric population remains unknown.33 While the authors acknowledge that the power of this study was low, PLMS are relatively uncommon in children. To date there have been no studies investigating the short-term impact of PLMS on the cardiovascular system in children, let alone the long term; therefore, this study represents an important beginning into this area of pediatric sleep research. Future research is required in children with PLMS from a pediatric population without SDB to determine the impact of this condition on sleep
This study has demonstrated that children had reduced vagal activity during periods when leg movements were present during sleep; there was weaker evidence that such an effect was not present during periods when leg movements were not present in the PLMS children. The current study did not provide data relevant to assessing sympathovagal balance because of the likelihood that leg movements had direct effects on HRV, confounding any interpretation in terms of autonomic balance. Finally, this study has demonstrated that the onset of the individual leg movements, which together form PLMS, are associated with a rapidly occurring tachycardia, suggesting parasympathetic inhibition. PLMS in children remains an under-investigated field in sleep research, and this study has demonstrated significant changes in autonomic activity that occur in conjunction with periodic leg movements that warrant further research.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
This work was performed at the Ritchie Centre for Baby Health Research, Monash Institute of Medical Research, Monash University, Melbourne, Australia and the Melbourne Children's Sleep Unit, Monash Medical Centre, Melbourne, Australia.
This project was funded by NHMRC Project Number 384142
REFERENCES
- 1.Atlas Task Force. Recording and Scoring Leg Movements. Sleep. 1993;16:748–59. [PubMed] [Google Scholar]
- 2.Montplaisir J, Boucher S, Poirier G, Lavigne G, Lapierre O, Lesperance P. Clinical, polysomnographic, and genetic characteristics of restless legs syndrome: a study of 133 patients diagnosed with new standard criteria. Mov Disord. 1997;12:61–5. doi: 10.1002/mds.870120111. [DOI] [PubMed] [Google Scholar]
- 3.Pennestri MH, Montplaisir J, Colombo R, Lavigne G, Lanfranchi PA. Nocturnal blood pressure changes in patients with restless legs syndrome. Neurology. 2007;68:1213–8. doi: 10.1212/01.wnl.0000259036.89411.52. [DOI] [PubMed] [Google Scholar]
- 4.Ferrillo F, Beelke M, Canovaro P, et al. Changes in cerebral and autonomic activity heralding periodic limb movements in sleep. Sleep Med. 2004;5:407–12. doi: 10.1016/j.sleep.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 5.Ferri R, Zucconi M, Rundo F, Spruyt K, Manconi M, Ferini-Strambi L. Heart rate and spectral EEG changes accompanying periodic and non-periodic leg movements during sleep. Clin Neurophysiol. 2007;118:438–48. doi: 10.1016/j.clinph.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 6.Taylor JA, Carr DL, Myers CW, Eckberg DL. Mechanisms underlying very-low-frequency RR-interval oscillations in humans. Circulation. 1998;98:547–55. doi: 10.1161/01.cir.98.6.547. [DOI] [PubMed] [Google Scholar]
- 7.Hayano J, Sakakibara Y, Yamada A, et al. Accuracy of assessment of cardiac vagal tone by heart rate variability in normal subjects. Am J Cardiol. 1991;67:199–204. doi: 10.1016/0002-9149(91)90445-q. [DOI] [PubMed] [Google Scholar]
- 8.Malliani A, Pagani M, Lombardi F, Cerutti S. Cardiovascular neural regulation explored in the frequency domain. Circulation. 1991;84:482–92. doi: 10.1161/01.cir.84.2.482. [DOI] [PubMed] [Google Scholar]
- 9.Guggisberg AG, Hess CW, Mathis J. The significance of the sympathetic nervous system in the pathophysiology of periodic leg movements in sleep. Sleep. 2007;30:755–66. doi: 10.1093/sleep/30.6.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O'Brien LM, Ivanenko A, Crabtree VM, et al. Sleep disturbances in children with attention deficit hyperactivity disorder. Pediatr Res. 2003;54:237–43. doi: 10.1203/01.PDR.0000072333.11711.9A. [DOI] [PubMed] [Google Scholar]
- 11.Chervin RD, Hedger KM. Clinical prediction of periodic leg movements during sleep in children. Sleep Med. 2001;2:501–10. doi: 10.1016/s1389-9457(01)00069-7. [DOI] [PubMed] [Google Scholar]
- 12.Crabtree VM, Ivanenko A, O'Brien LM, Gozal D. Periodic limb movement disorder of sleep in children. J Sleep Res. 2003;12:73–81. doi: 10.1046/j.1365-2869.2003.00332.x. [DOI] [PubMed] [Google Scholar]
- 13.Picchietti DL, England SJ, Walters AS, Willis K, Verrico T. Periodic limb movement disorder and restless legs syndrome in children with attention-deficit hyperactivity disorder. J Child Neurol. 1998;13:588–94. doi: 10.1177/088307389801301202. [DOI] [PubMed] [Google Scholar]
- 14.Picchietti DL, Underwood DJ, Farris WA, et al. Further studies on periodic limb movement disorder and restless legs syndrome in children with attention-deficit hyperactivity disorder. Mov Disord. 1999;14:1000–7. doi: 10.1002/1531-8257(199911)14:6<1000::aid-mds1014>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 15.Simakajornboon N, Gozal D, Vlasic V, Mack C, Sharon D, McGinley BM. Periodic limb movements in sleep and iron status in children. Sleep. 2003;26:735–8. doi: 10.1093/sleep/26.6.735. [DOI] [PubMed] [Google Scholar]
- 16.Hanly PJ, Gabor JY, Chan C, Pierratos A. Daytime sleepiness in patients with CRF: impact of nocturnal hemodialysis. Am J Kidney Dis. 2003;41:403–10. doi: 10.1053/ajkd.2003.50066. [DOI] [PubMed] [Google Scholar]
- 17.Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring systems for sleep stages of human subjects. Washington DC: U.S. Government Printing Office; 1968. [Google Scholar]
- 18.ADSA. EEG arousals scoring rules and examples: a preliminary report from the sleep disorders atlas task force of the American sleep disorders association. Sleep. 1992;15:173–84. [PubMed] [Google Scholar]
- 19.Zucconi M, Ferri R, Allen R, et al. The official World Association of Sleep Medicine (WASM) standards for recording and scoring periodic leg movements in sleep (PLMS) and wakefulness (PLMW) developed in collaboration with a task force from the International Restless Legs Syndrome Study Group (IRLSSG) Sleep Med. 2006;7:175–83. doi: 10.1016/j.sleep.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 20.Electrophysiology Task Force of the European Society of Cardiology and The North American Society of Pacing and Electrophysiology. Heart Rate Variability, Standards of measurement, physiological interpretation, and clinical use. Eur Heart J. 1996;17:354–81. [PubMed] [Google Scholar]
- 21.Traeger N, Schultz B, Pollock AN, Mason T, Marcus CL, Arens R. Polysomnographic values in children 2-9 years old: additional data and review of the literature. Pediatr Pulmonol. 2005;40:22–30. doi: 10.1002/ppul.20236. [DOI] [PubMed] [Google Scholar]
- 22.Sforza E, Pichot V, Barthelemy JC, Haba-Rubio J, Roche F. Cardiovascular variability during periodic leg movements: a spectral analysis approach. Clin Neurophysiol. 2005;116:1096–104. doi: 10.1016/j.clinph.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 23.Trinder J. Cardiac activity and sympathovagal balance during sleep. Sleep Med Clin. 2007;2:199–208. [Google Scholar]
- 24.Berntson GG, Bigger JT, Jr, Eckberg DL, et al. Heart rate variability: origins, methods, and interpretive caveats. Psychophysiology. 1997;34:623–48. doi: 10.1111/j.1469-8986.1997.tb02140.x. [DOI] [PubMed] [Google Scholar]
- 25.Zhong Y, Wang H, Ju KH, Jan KM, Chon KH. Nonlinear analysis of the separate contributions of autonomic nervous systems to heart rate variability using principal dynamic modes. IEEE Trans Biomed Eng. 2004;51:255–62. doi: 10.1109/TBME.2003.820401. [DOI] [PubMed] [Google Scholar]
- 26.Sforza E, Nicolas A, Lavigne G, Gosselin A, Petit D, Montplaisir J. EEG and cardiac activation during periodic leg movements in sleep: support for a hierarchy of arousal responses. Neurology. 1999;52:786–91. doi: 10.1212/wnl.52.4.786. [DOI] [PubMed] [Google Scholar]
- 27.Fagraeus L, Linnarsson D. Autonomic origin of heart rate fluctuations at the onset of muscular exercise. J Appl Physiol. 1976;40:679–82. doi: 10.1152/jappl.1976.40.5.679. [DOI] [PubMed] [Google Scholar]
- 28.Ewing DJ, Neilson JM, Travis P. New method for assessing cardiac parasympathetic activity using 24 hour electrocardiograms. Br Heart J. 1984;52:396–402. doi: 10.1136/hrt.52.4.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hollander AP, Bouman LN. Cardiac acceleration in man elicited by a muscle-heart reflex. J Appl Physiol. 1975;38:272–8. doi: 10.1152/jappl.1975.38.2.272. [DOI] [PubMed] [Google Scholar]
- 30.Silvestri R, Lasco A, Marabello L, et al. Wake and sleep cardiovascular reflex tests and GH profiles in diabetic patients. Acta Diabetol Lat. 1989;26:237–44. doi: 10.1007/BF02581390. [DOI] [PubMed] [Google Scholar]
- 31.Goto M, Nagashima M, Baba R, et al. Analysis of heart rate variabiliy demonstrates effects of development on vagal modulation of heart rate in healthy children. J Pediatr. 1997;130:725–9. doi: 10.1016/s0022-3476(97)80013-3. [DOI] [PubMed] [Google Scholar]
- 32.American Academy of Sleep Medicine. Diagnostic and coding manual. 2nd ed. Westchester, IL: American Academy of Sleep Medicine; 2005. International classification of sleep disorders. [Google Scholar]
- 33.Simakajornboon N. Periodic limb movement disorder in children. Paediatr Respir Rev. 2006;7(Suppl 1):S55–7. doi: 10.1016/j.prrv.2006.04.175. [DOI] [PubMed] [Google Scholar]