Abstract
Objectives:
In other disciplines, white matter (WM) differences have been linked to cognitive impairments. This study sets out to clarify whether similar microstructural differences in WM tracts predict a person's cognitive vulnerability to the effects of total sleep deprivation (TSD).
Design:
Participants completed a simple visual-motor task both before and after 24 h of TSD. Using a median split on the percent change in accuracy from pre-TSD to post-TSD, participants were separated into susceptibility groups. A diffusion tensor MR imaging (DTI) scan was acquired from each participant, and fractional anisotropy (FA) was calculated, examined across the brain, and compared between susceptibility groups.
Setting:
University of Texas at Austin.
Participants:
Thirty-two West Point cadets (9 females, 23 males) between 19 and 25 years of age.
Results:
Participant susceptibility to TSD was correlated with lower FA values in multiple regions of white matter, including the genu of corpus callosum and ascending and longitudinal white matter pathways. Significantly higher FA values in those less vulnerable to TSD, indicating increased neural connectivity and WM organization, may moderate the cognitive effects of sleep deprivation.
Conclusions:
Differences in distributed WM pathways reflect, and may contribute to, a person's ability to function effectively when sleep deprived. The widespread nature of this effect supports previous views that TSD has a global effect on brain functioning.
Citation:
Rocklage M; Williams V; Pacheco J; Schnyer DM. TitleTitleTitle. SLEEP 2009;32(8):1100-1103.
Keywords: Sleep deprivation, magnetic resonance imaging, cerebral cortex, cognition
RESEARCH IN THE PAST 5 YEARS HAS REFLECTED INCREASING INTEREST IN UNDERSTANDING INDIVIDUAL DIFFERENCES THAT CONTRIBUTE TO VULNERABILITY to sleep deprivation. One study used regression models that included individual differences and accounted for nearly 83% of the variance in cognitive impairment due to sleep deprivation over a 14-day period.1 In contrast, models that did not include individual differences accounted for only 22% of the variance.
To further investigate individual differences, researchers have looked to the neural correlates of sleep deprivation vulnerability. Utilizing functional magnetic resonance imaging (fMRI) with a verbal working memory task, researchers found an overall decrease in both regional and global neural activation after 30 h of sleep deprivation.2 In addition, decreases in neural activity were uniquely correlated with participants' performance, such that as numbers of errors and reaction times (RTs) increased, brain activation tended to decrease. This finding indicates that it is possible to uncover neural correlates of individual differences in response to sleep deprivation.
To date, examinations of the neural correlates of sleep deprivation have been restricted to functional techniques (e.g., fMRI and electroencephalography [EEG]). However, other research domains have begun to reveal significant structural brain correlates of individual differences in cognitive abilities. One of the domains that has been studied extensively, in which changes in cognitive ability have been linked to brain structure, is aging. A recent examination of both cortical gray and white matter (WM) changes associated with aging demonstrated a significant main effect of age across both tissue types, but it was only a measure of WM that revealed significant correlations with cognitive performance.3 The WM measure utilized in this study was obtained with diffusion tensor MR imaging (DTI), in which a scalar measure of diffusion, referred to as fractional anisotropy (FA), was calculated and tested. FA is thought to reflect in part, the number of fibers, myelination, and compactness of WM tracts.4 In the aging study, reduced FA values in functionally specific regions were found to reflect lower performance on tasks of cognitive control and episodic memory. Other studies have demonstrated that even in healthy young persons, differences in the microstructure of WM tracts can be correlated with cognitive processing abilities.5 Therefore, it is possible that the ability to function cognitively under conditions of sleep deprivation could be related to differences in WM microstructure.
The current study examined whether differences in WM structure may help explain important individual differences in vulnerability to sleep deprivation. Using DTI, we indexed WM FA values of 32 West Point cadets who underwent 24 h of total sleep deprivation (TSD). A simple visual-motor control (VMC) task was used to separate participants into vulnerability groups.
METHODS
Participants
Thirty-two West Point cadets (9 female) between the ages of 19 and 25 (M = 20.8 years) participated as part of a larger study on the effects of TSD on cognition that included fMRI.6 Participants were screened to be free from prior history of neurological or psychiatric illness or current psychotropic or sleep related medication. Cadets who were unable to stay awake and perform at 75% accuracy or better on the VMC task after 24 h of TSD were excluded. Five cadets were excluded based on these criteria and another 3 were removed from the final analysis due to poor image quality. The final analyzed group consisted of 24 West Point cadets (7 female) between the ages of 19 and 25 (M = 20.8 years). The Institutional Review Board at the University of Texas at Austin and West Point Military Academy approved the study, and informed consent was obtained from all participants.
Behavioral
Groups of 3 West Point cadets participated in each study session. Participants were not allowed to consume alcohol 24 h prior to the study or to consume caffeine between midnight and 06:00 before the first or second day. They were instructed to engage in normal sleep-wake cycles prior to arriving in Austin for the study and the night before the actual run. Compliance on this was peer monitored. Participants arrived for MRI scanning and other cognitive tasks at 06:00 on the morning of Day 1. DTI and high-resolution T1 scans used in this analysis were performed at this session. After a full day of testing, which included afternoon physical endurance testing, an assigned monitor accompanied the cadets at all times. During the evening and night, they ate meals and engaged in both light physical and mental activities such as walking, bowling, and video/board games to keep them awake. After breakfast on Day 2, they returned for more MRI and cognitive tasks at 06:00.
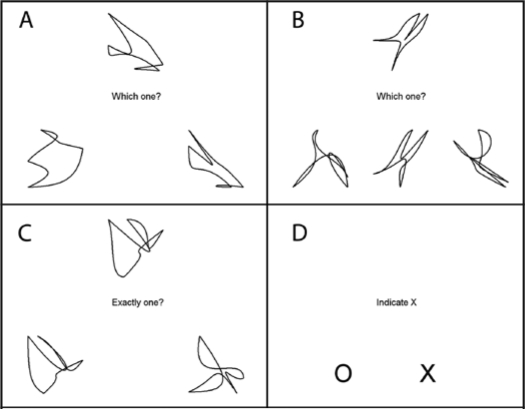
On each morning participants engaged in an fMRI protocol designed to assess neural changes associated with sleep deprivation on decision-making. The fMRI component had 4 conditions: a visual-motor task (VMC) that was used as a control task and 3 decision-making tasks. The different tasks were organized in a block design and were presented in counterbalanced orders, with the VMC task separating each decision-making task throughout a 26-min scan session. In the VMC task, participants were asked to indicate with a response button on which side of the screen an ‘X’ was presented. A total of 160 VMC trials, split into 4 runs, were completed in each experimental session, one before and one after 24 h of TSD. Performance declines on the 3 decision-making tasks did not reveal reliable WM differences using whole brain analysis. However, performance changes on the VMC task did reveal widespread significant differences, and therefore are the focus of this report (see online supplement for more detail on the additional decision making tasks and procedures).
Imaging
Two high-resolution SPGR, T1-weighted scans were collected using a 3 Tesla GE MRI equipped with an 8-channel phased array head coil (GE Medical Systems, Milwaukee, WI) with the following image parameters: TR/TE = 5.0/1.2, flip angle = 11°, slice thickness = 1.3 mm, 172 slices, FOV = 256 × 256 mm. The two T1 scans were first motion corrected and then averaged to create a single high-signal, high-contrast volume.
Whole brain DTI was also acquired for 25 directions using a dual shot echo planar imaging and a twice-refocused spin echo pulse sequence, optimized to minimize eddy current-induced distortions (GE 3T, TR/TE = 12000/71.1, B = 1000, 128 × 128 matrix, 3-mm (0 mm gap) slice thickness, 0.94 × 0.94 mm in plane resolution, 1 T2 + 25 DWI). Forty-one slices were acquired and diffusion tensors and FA values were calculated on a voxel-by-voxel basis using conventional reconstruction methods in FSL.7 Individual FA images were registered in MNI-152 standard space using FMRIB's Nonlinear Registration Tool (FNIRT), a part of the Tract Based Spatial Statistics (TBSS) software.8 This approach is based on calculating FA skeletons that ensure that each participant's WM tracts were accurately represented in both group and individual space. Whole brain group differences in WM tracts were analyzed using a permutation method with the α level set at P < 0.01 (corrected using threshold-free cluster enhancement (TFCE);9 see supplement for more details on the imaging analysis methods).
RESULTS
Behavioral
To index decline in cognitive performance caused by sleep deprivation, a percent change score was calculated for the VMC task ((Day 2 VMC accuracy - Day 1 VMC accuracy) / Day 1 VMC accuracy). Participants were divided, using a median split, into those who were more vulnerable (VUL, n = 12, M = −6.5%) and those who were less vulnerable (NON, n = 12, M = −1.5%) to TSD. There were no differences in age, χ 2 (5, N = 24) = 8.70, P > 0.05, or gender distribution, χ2 (1, N = 24) = 0.202, P > 0.05, across either of the 2 groups. In addition, there were no differences between groups in baseline performance on Day 1, (mean proportion correct VUL = 1.0 (SE = 0.002), or mean NON = 1.0 (SE = 0.001), F1,23 < 1).
WM Differences
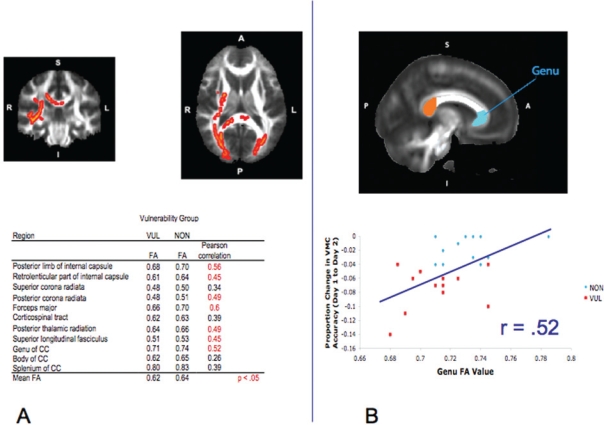
A whole brain comparison of FA values that contrasted NON > VUL indicated multiple WM pathways where FA values were significantly greater for the NON group relative to the VUL group. There were no significant pathways where VUL demonstrated greater FA values than NON (Figure 1a). A percent change score was also calculated separately for each of the 3 decision-making tasks used in the fMRI study (excluding missed trials) using the same method as with the VMC task. When vulnerability groups were defined, based on performance declines on these “higher order” decision tasks, none of these whole brain comparisons of FA values showed any significant differences.
Figure 1.
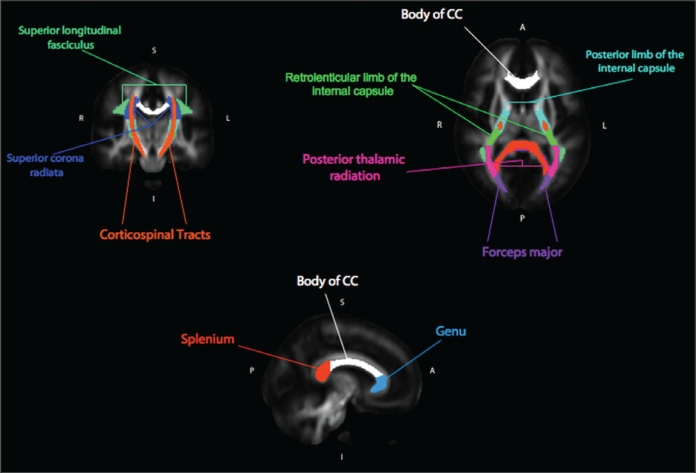
A) Across the top row, from left to right, is one coronal and one axial brain slice from the whole brain Tract Based Spatial Statistics (TBSS) results, highlighting those regions that showed those less vulnerable to sleep deprivation (NON) greater than those more vulnerable to sleep deprivation (VUL) in fractional anisotropy (FA) differences (P < 0.01, corrected). For purposes of visualization, significant portions of the tested white matter skeleton were expanded to fill more of the corresponding white matter tract. Table of mean FA values for the extracted pathways of interest and their correlations with percent change in visual-motor control (VMC) accuracy between Day 1 and Day 2. B) From top to bottom is one sagittal slice showing the 3 regions of the corpus callosum (CC) that were analyzed for FA differences. Graph showing the correlation between the FA values extracted from the genu of the CC and the proportion change in VMC accuracy between Day 1 and Day 2.
Visual examination of the VMC vulnerability analysis revealed greater effects in the right hemisphere (Figure 1a). To assess the reliability of this apparent finding, as well as to probe the results for a linear relationship between FA and VMC change, mean FA values were extracted from each individual's FA skeleton map. This was accomplished by using masks defined by the Johns Hopkins University (JHU) DTI-based white matter atlases available in FSL (see supplement for more procedural detail and Figure 2 in the supplement for the corresponding WM pathways). FA values were tested in a 2 × 2 × 11 repeated measures ANOVA with hemisphere and region as within-subjects factors and group as a between-subjects factor. The results revealed a significant main effect of group (F1, 22 = 16.93, p < 0.001), hemisphere (F1, 22 = 67.70, p < 0.001), region (F10, 22 = 628.10, p < 0.001), and a hemisphere by region interaction (F10, 22 = 6.10, p < 0.001). This latter interaction revealed that some posterior regions showed a right > left laterality in FA values, something that has been found in past studies.10 Most critically, there was no hemisphere by group interaction and no region by group interaction, thereby indicating that the visually apparent laterality was not a reliable effect. To test whether total WM volume, age, or sex could be contributing factors to these results, each variable was entered separately as a covariate in the ANOVA analysis. Using total WM volume as a covariate, the effect of group remained highly significant (F1, 21 = 15.12, P < 0.001), with no main effect of WM volume (F < 1). The same basic finding was true for both age (main effect of group, F1, 21 = 30.10, P < 0.001) and sex (main effect of group, F1, 21 = 15.87, P < 0.001). Of the age and sex variables, only age showed an additional main effect (F1, 21 = 9.77, P < 0.01), reflecting increasing FA values with increasing age.
FA values from those regions identified as significant by the TBSS analysis were tested for a linear relationship with the VMC percent change score. Nearly all regions showed a significant linear relationship (see Figure 1a), but given the ceiling effect for performance of the NON group, this relationship is likely driven primarily by the VUL group (see Figure 1b for the correlation results for the genu of the corpus callosum (CC)).
DISCUSSION
The current study evaluated whether differences in WM are associated with cognitive vulnerability to TSD over a 24-h period. FA, an index of WM organization, was significantly correlated with an individual's vulnerability to TSD as measured by change in performance on a simple visual-motor task. Specifically, higher FA values in the posterior limb and retrolenticular part of the internal capsule, posterior corona radiata, forceps major, posterior thalamic radiation, superior longitudinal fasciculus, and the genu of the corpus callosum all seem to be associated with decreased vulnerability to TSD. These results were not reflected in changes associated with TSD across any of the “higher order” decision-making tasks, in which no relationship between performance and FA were found. In general, FA values have been found to be higher in regions with restricted diffusion—regions with highly organized structure—and lower in regions with less organized structure. Moreover, it has been postulated that FA may reflect the degree of myelination and/or fiber bundle density.4 NON participants had higher FA values and thus potentially greater WM organization and degree of connectivity. Therefore, it is possible that NON participants were able to more easily recruit additional neural resources or better coordinate activity between regions, allowing them to remain effectively engaged in the visual-motor task (see Drummond for a formulation of the compensatory hypothesis).11
The VMC task is a relatively simple visual-motor task with a response timeout of 3.5 s; if participants are going to respond at all, they will do so within the time limit and will likely respond correctly. Therefore, changes in the VMC task associated with sleep deprivation probably reflect brief “attentional lapses.”12 Investigators have argued that the impact of sleep deprivation on attention takes place through multiple interacting brain systems, including “bottom-up” pressures from subcortical/brain stem regions modulating sleep-wake cycles and “top-down” efforts to remain alert in the face of sleep deprivation.12,13 It is possible that increased cross-regional connectivity, as revealed by greater FA values, may help lessen these lapses by aiding top-down efforts to remain alert. It is not surprising that the differences in WM associated with vulnerability would be nonspecific to location; the diffuseness of these WM differences supports findings that an overall brain difference, rather than differences in more select regions, is likely to be associated with vulnerability to sleep deprivation.2,13 This view is supported by the lack of sleep deprivation vulnerability findings for the decision-making tasks, which would likely require coordination of multiple cognitive/neural processes that may have differed between individuals (e.g., cognitive control, working memory capacity, processing speed, or ability to effectively learn novel decision-making tasks). Therefore, as argued by other researchers,12 cognitive vulnerability to sleep deprivation may be best captured by a lower level task that emphasizes primary attention. Our findings demonstrate for the first time that disruptions in lower level attention associated with sleep deprivation can be tied to differences in brain structure.
A number of drawbacks to the current study should be noted. First is the skewed gender ratio and relatively restricted age range. Though there were no vulnerability group differences between the male and female participants, external validity would be bolstered with a larger sample of females and a broader range of ages. Secondly, it should be noted that prior sleep history was not collected for the participants, and sleep deprivation runs were not counterbalanced between sleep deprivation and rested conditions. Despite this, the contribution to the current results would likely be minimal, as West Point cadets live a highly regimented lifestyle that would result in similar pre-experimental sleep histories, and all subjects performed at 100% accuracy on the VMC task; thus there was no room for additional practice effects that could be introduced by the fixed run order. Nevertheless, future replications of this finding that include a more complete age and sex range as well as a more thorough assessment of prior sleep history are critically needed.
From the present study, we conclude that it is possible that more extensive development of cortical communication pathways contribute to a person's ability to function effectively when sleep deprived. This development reflected in the FA values likely continues through the lifespan of our participants, but the source of individual differences in development remains unknown. Further research will be required to clarify the effect greater WM connectivity has on cognitive and neural functioning under conditions of sleep deprivation.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
We thank Dasa Zeithamova for construction of the task that contained the VMC condition. Also, we thank the team at West Point led by Michael Matthews and Cadet Kim DeFiori who arranged participation by West Point cadets. We also thank all members of the Sleep Deprivation team at UT Austin for their assistance in the planning and implementation of the study - Steve Kornguth (Core PI), Ed Coyle, Rebecca Steinberg, and Terisha Thomas. Finally, we thank Rosa Schnyer and Todd Maddox for comments on the manuscript.
The authors are pleased to acknowledge the support of the Army, grant #W911NF-07-2-0023, through The Center for Strategic and Innovative Technologies at The University of Texas at Austin for the research described here in this report.
REFERENCES
- 1.Van Dongen HPA, Baynard MD, Maislin MS, Dinges DF. Systematic interindividual differences in neurobehavioral impairment from sleep loss: evidence of trait-like differential vulnerability. Sleep. 2004;27:423–33. [PubMed] [Google Scholar]
- 2.Mu Q, Nahas Z, Johnson KA, et al. Decreased cortical response to verbal working memory following sleep deprivation. Sleep. 2005;28:55–67. doi: 10.1093/sleep/28.1.55. [DOI] [PubMed] [Google Scholar]
- 3.Ziegler DA, Olivier P, Salat DH, Prince K, Connally E, Corkin S. Cognition in healthy aging is related to regional white matter integrity, but not cortical thickness. Neurobiol Aging. doi: 10.1016/j.neurobiolaging.2008.10.015. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beaulieu C. The basis of anisotropic water diffusion in the nervous system - a technical review. NMR Biomed. 2002;15:435–55. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- 5.Tuch DS, Salat DH, Wisco JJ, Zaleta AK, Hevelone ND, Rosas HD. Choice reaction time performance correlates with diffusion anisotropy in white matter pathways supporting visuospatial attention. Proc Natl Acad Sci U S A. 2005;102:12212–17. doi: 10.1073/pnas.0407259102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schnyer D, Zeithamova D, Williams V. Decision-making under conditions of sleep deprivation: cognitive and neural consequences. Mil Psychol. 2009;21:36–45. [Google Scholar]
- 7.Behrens TE, Woolrich MW, Jenkinson M, et al. Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magn Reson Med. 2004;50:1077–88. doi: 10.1002/mrm.10609. [DOI] [PubMed] [Google Scholar]
- 8.Smith SM, Jenkinson M, Johansen-Berg H, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 9.Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44:83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- 10.Barrick TR, Lawes IN, Mackay CE, Clark CA. White matter pathway asymmetry underlies functional lateralization. Cereb Cortex. 2007;17:591–98. doi: 10.1093/cercor/bhk004. [DOI] [PubMed] [Google Scholar]
- 11.Drummond SPA, Brown GG, Gillin JC, Stricker JL, Wong EC, Buxton RB. Altered brain response to verbal learning following sleep deprivation. Nature. 2000;403:655–57. doi: 10.1038/35001068. [DOI] [PubMed] [Google Scholar]
- 12.Lim J, Dinges DF. Sleep deprivation and vigilant attention. Ann N Y Acad Sci. 2008;1129:305–22. doi: 10.1196/annals.1417.002. [DOI] [PubMed] [Google Scholar]
- 13.Chee MW, Tan JC, Zheng H, Parimal S, et al. Lapsing during sleep deprivation is associated with distributed changes in brain activation. J Neurosci. 2008;28:5519–28. doi: 10.1523/JNEUROSCI.0733-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]