Abstract
Study Objectives:
Narcolepsy-cataplexy has long been thought to have an autoimmune origin. Although susceptibility to narcolepsy, like many autoimmune conditions, is largely genetically determined, environmental factors are involved based on the high discordance rate (∼75%) of monozygotic twins. This study evaluated whether Streptococcus pyogenes and Helicobacter pylori infections are triggers for narcolepsy.
Design:
Retrospective, case-control.
Setting:
Sleep centers of general hospitals.
Participants:
200 patients with narcolepsy/hypocretin deficiency, with a primary focus on recent onset cases and 200 age-matched healthy controls. All patients were DQB1*0602 positive with low CSF hypocretin-1 or had clear-cut cataplexy.
Measurements and Results:
Participants were tested for markers of immune response to β hemolytic streptococcus (anti-streptolysin O [ASO]; anti DNAse B [ADB]) and Helicobacter pylori [Anti Hp IgG], two bacterial infections known to trigger autoimmunity. A general inflammatory marker, C-reactive protein (CRP), was also studied. When compared to controls, ASO and ADB titers were highest close to narcolepsy onset, and decreased with disease duration. For example, ASO ≥ 200 IU (ADB ≥ 480 IU) were found in 51% (45%) of 67 patients within 3 years of onset, compared to 19% (17%) of 67 age matched controls (OR = 4.3 [OR = 4.1], P < 0.0005) or 20% (15%) of 69 patients with long-standing disease (OR = 4.0 [OR = 4.8], P < 0.0005]. CRP (mean values) and Anti Hp IgG (% positive) did not differ from controls.
Conclusions:
Streptococcal infections are probably a significant environmental trigger for narcolepsy.
Citation:
Aran A; Nevsimalova S; Plazzi G; Hong SC; Weiner K; Zeitser J; Mignot E. Elevated anti-streptococcal antibodies in patients with recent narcolepsy onset. SLEEP 2009;32(8):979-983.
Keywords: Narcolepsy, autoimmune, post-streptococcal, Anti Streptolysin O (ASO), Anti DNAse B (ADB), helicobacter pillory
NARCOLEPSY-CATAPLEXY IS A LIFELONG, DISABLING NEUROLOGICAL DISORDER, AFFECTING 1 IN 2000. CHARACTERISTIC SYMPTOMS INCLUDE EXCESSIVE daytime sleepiness and episodes of sudden loss of muscle tone, triggered by strong emotions (cataplexy). Onset is typically during adolescence, and predisposition involves both genetic and non-genetic factors, as suggested by the low monozygotic concordance but increased familial predisposition.1 The disorder is unique because of its extremely tight association with HLA-DQB1*0602 and hypocretin cell loss, suggesting autoimmune destruction; only 5 patients in the world have been described with low CSF hypocretin-1, a marker of hypocretin cell destruction, and DQB1*0602 negativity.2 Recently, using a Genome Wide Association, we found association with polymorphisms in the T-cell receptor α (TCR) loci.3 TCR is the major receptor of HLA-peptide presentation, and plays a critical role in mediating immune responses in normal (e.g., infectious) or abnormal (e.g., autoimmune) responses.
Whereas much progress has been made toward understanding genetic predisposition in narcolepsy, little is known regarding environmental triggers. Retrospective questionnaire studies have found increased stress and decreased sleep amounts prior to narcolepsy onset.4 Case reports, describing a few unusual cases, have found sudden onset of narcolepsy 3 days after head trauma5 or various other unusual triggers (bee sting, etc). In all these cases, however, findings may be coincidental and are likely confounded by bias recall. More recently, a well-designed population based study of narcolepsy has been initiated and found increased smoking exposure in patients with narcolepsy as a risk factor6; the authors suggested the effect to be secondary to increased upper respiratory tract infections in secondary smoking. This, together with the report more than 20 years ago of increased ASO and ADB titers in a small number of narcoleptic patients regardless of disease duration,7,8 a finding that was later refuted,9 led us to reexamine the topic of infectious trigger in narcolepsy.
It has been our clinical experience that narcolepsy is increasingly recognized close to onset, whereas 10-20 years ago, the disorder was diagnosed more than 10 years after onset (median time).10 We reasoned that a possible infectious trigger would not be detectable long after onset of narcolepsy, thus explaining variable results obtained in these first studies. Indeed, we also ourselves attempted to duplicate these anti-streptococcal findings in long standing narcolepsy cases, but could not find any differences with controls (Scott Fromhertz, unpublished results). We hypothesized that if streptococcal infections were indeed a trigger for narcolepsy onset, it would be best detected in newly identified patients, many of which had recent onset.
METHODS
Subjects
We selected all recent onset patients recruited within the last 5 years and similar size groups of corresponding patients with longer disease duration, recruited during the same period in similar sleep centers. Patients were recruited from North America (USA, n = 326), Europe (Czech Republic, n = 50; Italy, n = 12) and South Korea (n = 12). Patients were positive for DQB1*0602 and had hypocretin deficiency (n = 51, with and without clear-cut cataplexy), or were DQB1*0602 positive with clear-cut cataplexy (n = 149); based on prior analysis, DQB1*0602 positive subjects with cataplexy are 98% likely to be hypocretin deficient.2 Disease duration was defined as time between first symptom and time of blood draw. The sample included 23 patients with a disease duration of less than 1 year (mean age = 12.3 ± 5.4 y, range = 5.6–27 y, 52% females); 44 patients with a disease duration of 1–3 years (mean age = 19.3 ± 13.0, range = 6–79 y, 54% females); 64 patients with a disease duration of 3 to 10 years (mean age 26.3 ± 11.4, range: 8–72.5 y, 50% females); and 69 patients with a disease duration > 10 y (mean age 46.5 ± 17.1, range 15–79 y, 54% females). Most (90.3%) were Caucasians. Each control was selected from a larger pool of subjects to match each patient for age, race and geographic region. Overall control groups were also balanced by sex with each patient group, thus mean age, range and percentage of females were identical to patient groups described above. All subjects gave written informed consent for the study, which was approved by Institutional Review Boards at all locations. The presence or absence of DQB1*0602 was determined using DQB1 exon-2 sequence-specific primers3.
Assays
As β hemolytic streptococcus and Helicobacter pylori are known triggers of autoimmunity,11,12 we measured antibodies against streptolysin O (ASO) and DNAse B (ADB) as serologic markers of post-streptococcal status and Anti Hp IgG as a marker of H. pylori infection. C-reactive protein (CRP) was used as measure of general inflammation. These markers were assessed using commercially available kits (SeraTest ASO, Remel KS, USA; Streptonase-B, Wampole Laboratories, NJ, USA; HP IgG ELISA, BioCheck Inc, CA, USA and CRP ELISA, Alpha Diagnostic International, TX, USA) according to the manufacturer's instructions.
Statistical Analysis
Data is presented as mean ± SD or %. Group comparisons were primarily made using Pearson χ2 or Student t-tests. In selected cases, multivariate analyses were used to control for possible covariates of interest (e.g., body mass index, age, gender, season, HLA). The statistical package SYSTAT (SPSS, Evanston, IL, USA) was used for these analyses.
RESULTS
β Hemolytic Streptococcus
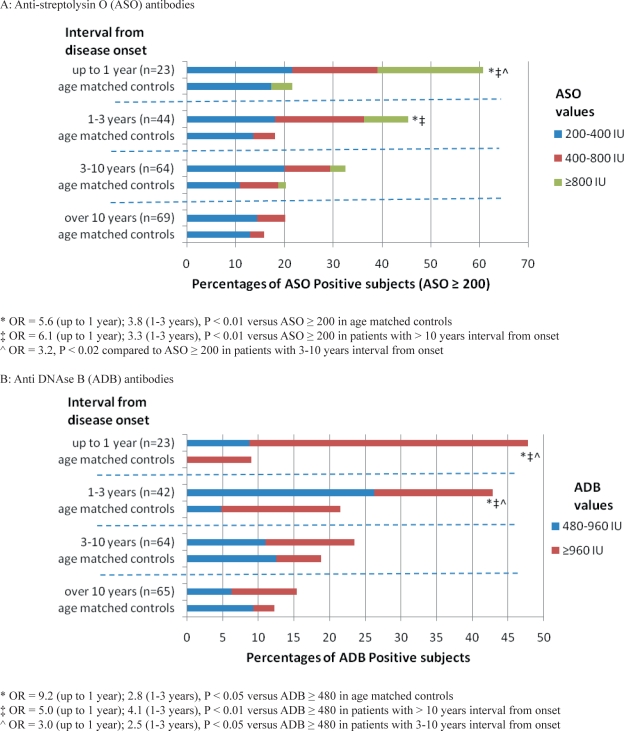
Titers of antistreptococcal antibodies were higher in patients with narcolepsy (n = 200) versus age-matched, healthy controls (n = 200) for both ASO (ASO ≥ 200 IU in 34.5% vs. 18.5%, OR = 2.3, P = 0.0003) and ADB (ADB ≥ 480 IU in 28% vs. 16%, OR = 2.0, P = 0.005), in the overall sample (which was purposely enriched in recent onset cases). Further stratification by disease duration revealed higher titers only in cases with onset within 3 years, when compared to controls. Similarly, we found that recent onset patients had significantly higher titers than subjects with longstanding disease (Figure 1, Table 1). No difference in season of blood draw (evenly distributed across 12 months and the 4 seasons) was noted across different groups of patients and with age-matched controls (4- and 12-way χ2). Further, although % ASO ≥ 200 was slightly higher in March to June included (in controls only), it was not significantly so. Similarly, the percentage of ADB ≥ 480 in controls was slightly higher in March to August included, but not significantly.
Figure 1A and B.
Anti-Streptococcal Antibodies in Patients with Narcolepsy and Age Matched Controls
A: Anti-streptolysin O (ASO) antibodies
* OR = 5.6 (up to 1 year); 3.8 (1-3 years), P < 0.01 versus ASO ≥ 200 in age matched controls
‡ OR = 6.1 (up to 1 year); 3.3 (1-3 years), P < 0.01 versus ASO ≥ 200 in patients with > 10 years interval from onset
∧ OR = 3.2, P < 0.02 compared to ASO ≥ 200 in patients with 3-10 years interval from onset
B: Anti DNAse B (ADB) antibodies
* OR = 9.2 (up to 1 year); 2.8 (1-3 years), P < 0.05 versus ADB ≥ 480 in age matched controls
‡ OR = 5.0 (up to 1 year); 4.1 (1-3 years), P < 0.01 versus ADB ≥ 480 in patients with > 10 years interval from onset
∧ OR = 3.0 (up to 1 year); 2.5 (1-3 years), P < 0.05 versus ADB ≥ 480 in patients with 3-10 years interval from onset
Table 1.
Combination of Anti-Streptolysin O (ASO) ≥ 200 IU and Anti DNAse B (ADB) ≥ 480 IU in Patients with Narcolepsy and Age-Matched Controls
| Interval from disease onset | ASO and ADB | ASO or ADB |
|---|---|---|
| Up to 1 year (n = 23) | 43% 1,2 | 65% 1,2 |
| Controls | 4.5% | 26% |
| 1-3 years (n = 42) | 33% 3,4 | 57% 3,4 |
| Controls | 9.5% | 30% |
| 3-10 years (n = 64) | 16% | 40% |
| Controls | 11% | 28% |
| ≥ 10 years (n = 65) | 6% | 29% |
| Controls | 3% | 25% |
1 OR = 16.1 (ASO and ADB); 5.3 (ASO or ADB), P < 0.01 versus age matched controls
2 OR = 11.7 (ASO and ADB); 4.5 (ASO or ADB), P < 0.01 versus patients > 10 years from onset
3 OR = 4.8 (ASO and ADB); 3.2 (ASO or ADB), P < 0.05 versus age matched controls
4 OR = 7.6 (ASO and ADB); 4.5 (ASO or ADB), P < 0.01 versus patients > 10 years from onset
HLA
All patients were DQB1*0602 positive per inclusion criteria. Of 200 controls, 28.5% were HLA positive, as expected from a largely Caucasian sample. The percentage of HLA positive subjects was similar in all 4 subgroups of age-stratified controls (26%, 34%, 22%, and 31%) as matched to patients with < 1 y, 1–3 y, 3–10 y, and > 10 y of disease duration. Further, percentage of ASO ≥ 200 did not differ between DQB1*0602 positive and negative controls (22% versus 17%, OR = 1.4, P = 0.32), although ADB ≥ 480 was found in 26% of the HLA positive controls compared to 12% of HLA negative controls (OR = 2.6, P = 0.02, independent of season and age). The difference in % ADB between recent onset patients and age-matched controls was however still as significant when controlled for HLA status. New onset patients still had significantly higher % ADB ≥ 480 when compared to aged matched HLA positive controls (OR = 4.8, P = 0.002).
CRP
CRP values were not more elevated in patients close to disease onset ( ≤ 3 years, n = 67, mean value = 17 ± 32) compared to age matched controls (n = 67, mean value = 19 ± 42). Narcoleptic subjects with longer disease duration ( > 3 y), however, had significantly higher CRP levels (n = 133, mean value = 42 ± 44) compared to age matched controls (n = 133, mean value = 27 ± 38), a difference that disappeared when controlled for BMI in this group (data not shown). Increased CRP in long standing narcolepsy was thus a reflection of secondary obesity (increased BMI) in longstanding disease, as previously reported,13 and not inflammation at onset.
Helicobacter pylori
Among 200 narcolepsy patients, 9.5% of narcolepsy patients were positive for antibodies against H. pylori (Anti Hp IgG > 20 IU/mL), as were 10.5% of controls (n = 200) suggesting no role for this bacteria in the pathogenesis of narcolepsy and strengthening the specific role of Streptococcus.
DISCUSSION
Streptococcal infections are usually benign and self-limited. Invasive diseases and post-infectious immune mediated sequelae can, however, occur. In rheumatic fever, probably the most widely recognized post-streptococcal autoimmune disease, molecular mimicry between streptococcal and selected cardiac antigens triggers cardiac inflammation, with resulting valvular damage.11 Importantly, post-streptococcal diseases have also been linked to brain autoimmune diseases, most notably Sydenham chorea, and more controversially encephalitis lethargica,14 obsessive-compulsive disorder, and tics.15 In this context, the presence of streptococcal infections may initiate or catalyze an autoimmune response against hypocretin cells in narcolepsy. Interestingly, although narcolepsy is associated with HLA and TCRA polymorphisms, direct proof for autoimmunity is still lacking.16,17 In fact, onset is not associated with a detectable inflammatory process, as exemplified by the measures of CRP in this study or neuroimaging studies around disease onset.18 The lack of detectable inflammation at onset is unlike most other autoimmune conditions, suggesting a highly specific, organ-targeted process. We suggest that selected streptococcal infections may lead to the destruction of hypocretin neurons via molecular mimicry or superantigen interactions with the HLA-TCR complex. Alternatively, these infections could simply make it permissive for other, more specific factors to trigger narcolepsy, for example by increasing blood brain permeability or simply reactivating the immune system nonspecifically.
Table 2 compares our results with those obtained in well-established post-streptococcal disorders. Only large studies, or those including controls of the same geographic origin, were included. We found anti-streptococcal antibodies in 65% of patients within 1 year of onset, a rate slightly lower than found in rheumatic fever, and similar to that reported in isolated Sydenham chorea. The similarity with isolated Sydenham chorea is not surprising, considering the relatively long interval between infection and diagnosis/onset in both cases. Unlike tics and obsessive-compulsive disorder, increased ASO in narcolepsy clearly correlated with disease onset, thus strongly suggesting a pathophysiological link.
Table 2.
Narcolepsy in Comparison with Established Post-Streptococcal Diseases
| Disorder | Interval to Symptoms | Increased ASO titers*‡ | Increased ADB titers*‡ | References |
|---|---|---|---|---|
| Rheumatic fever | P 68% (n = 786) | |||
| arthritis/ carditis | 3–8 weeks | P 68% (n = 100) | P 69% (n = 100) | 25 |
| Isolated Sydenham | P 33% (n = 60) | 26 | ||
| chorea | 1–8 months | P 75% (n = 71) | P 10% (n = 71) | 27 |
| Post-streptococcal | P 48% (n = 79) | P 68% (n = 73) | ||
| glomerulonephritis | 1–2 weeks | C 11% (n = 57) | C 13% (n = 53) | 28 |
| P 78% (n = 37) | P 65% (n = 37) | 29 | ||
| C 11% (2321) | C 8% (2321) | |||
| Narcolepsy | Weeks to | P 51% (n = 67) | P 45% (n = 65) | This study |
| months? | C 19% (n = 67) | C 17% (n = 65) |
Interval from infection to symptom onset and anti-streptococcal antibody status at diagnosis is reported. % ASO and ADB positive in patients (P), versus healthy controls (C).
*Of note, in rheumatic fever and post-streptococcal glomerulonephritis, evidence of preceding streptococcal infection is part of established diagnostic criteria, causing an obvious inclusion biased toward higher titers.
‡ ASO and ADB positivity cut-offs were upper limit normal 20% (ULN-20, where 20% controls had higher titers), or, more questionably, as determined by the authors if no controls were included.
A limitation of this study was our inability to demonstrate an actual streptococcal infection and subsequent increasing antibacterial titers. We attempted to culture Streptococcus in 10 early onset cases, but could not recover positive cultures. This is not surprising, as even in rheumatic fever, cultures are usually (90%) negative even though it occurs only a few weeks after the reported infection. More intriguingly was the fact that anti-streptococcal titers were still elevated in a group of narcoleptic patients collected 1–3 years after onset. In uncomplicated infections, anti-streptococcal antibodies are reported to increase after 2 weeks, to peak at 2–4 months, and decrease thereafter.19 The long-lasting antibody response in narcolepsy may thus reflect the special genetic background of these subjects and/or a sustained narcolepsy-related immune reaction. A similar pattern of slow ASO titer decrease lasting several years, with moderately increased titers up to 3 years from diagnosis, was found in acute rheumatic fever,20 despite supervised administration of penicillin, every 3 weeks after diagnosis. The HLA-DQB1*0602 specific background could also be involved. This particular subtype protects against septic shock due to streptococcal infection,21 suggesting better immune response against this bacteria. Further, we found slightly higher titers of ADB in controls with DQB1*0602, suggesting a more sustained response to streptococcus. As DQB1*0602 is protective against rheumatic fever,22 it is also possible that different HLA haplotypes dictate phenotype expression of various post-streptococcal syndromes.
As the prevalence of anti-streptococcal antibodies varies significantly across populations, additional replications are needed in other settings and ethnic groups. If confirmed, our results will have implications for the prevention, diagnosis and treatment of patients with new onset narcolepsy, especially when cataplexy has not yet developed (most cases develop cataplexy within one year of sleepiness onset). Indeed, diagnosis of these cases is difficult, and it is unclear how the MSLT or CSF hypocretin-1 predicts narcolepsy so close to the onset. In contrast, the sensitivity and specificity of HLA positivity in combination with anti-streptococcal antibodies should be highest the closest to the onset (Table 3). The current treatment of narcolepsy is symptomatic, with a controversial immunomodulation trial with IVIG in patients diagnosed within 6 months of onset.23 Whether such cases should be treated with antibiotics as currently done in rheumatic fever is open for debate.
Table 3.
Sensitivity and specificity of DQB1*0602 positivity in combination with anti-streptolysin O (ASO) ≥ 200 IU and /or anti DNAse B (ADB) ≥ 480 IU
| Interval from onset of symptoms | ASO and HLA positive | ASO or ADB and HLA positive | ASO and ADB and HLA positive |
|---|---|---|---|
| Up to 1 year (n = 23) | Sensitivity 61% | Sensitivity 65% | Sensitivity 43% |
| Specificity 96% | Specificity 91% | Specificity 100% | |
| 1-3 years (n = 42) | Sensitivity 45% | Sensitivity 57% | Sensitivity 33% |
| Specificity 92% | Specificity 86% | Specificity 95% |
DISCLOSURE STATEMENT
This was not and industry supported study. Dr. Mignot has consulted for Jazz, Actelion, and Cephalon; is on the advisory board of Eli Lilly and Actelion; has participated in speaking engagements for Roche; and owns stock in ResMed. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
We are indebted to all the participants of the study, most notably narcoleptic patients and thank James B. Dale (Memphis Medical Center, Veterans Administration Medical Center) for helpful discussions. This study was supported primarily by the National Institutes of Neurological Disease and Stroke grant P50 NS23724. And also by research project MSM 0021620849 (Ministry of Education, Czech Republic). E. Mignot is an HHMI supported investigator.
REFERENCES
- 1.Dauvilliers Y, Maret S, Bassetti C, et al. A monozygotic twin pair discordant for narcolepsy and CSF hypocretin-1. Neurology. 2004;62:2137–8. doi: 10.1212/wnl.62.11.2137. [DOI] [PubMed] [Google Scholar]
- 2.Bourgin P, Zeitzer JM, Mignot E. CSF hypocretin-1 assessment in sleep and neurological disorders. Lancet Neurol. 2008;7:649–62. doi: 10.1016/S1474-4422(08)70140-6. [DOI] [PubMed] [Google Scholar]
- 3.Hallmayer J, Faraco J, Lin L, et al. Narcolepsy is strongly associated with the T-cell receptor alpha locus. Nat Genet. 2009;41:708–11. doi: 10.1038/ng.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Orellana C, Villemin E, Tafti M, Carlander B, Besset A, Billiard M. Life events in the year preceding the onset of narcolepsy. Sleep. 1994;17:S50–3. doi: 10.1093/sleep/17.suppl_8.s50. [DOI] [PubMed] [Google Scholar]
- 5.Ebrahim IO, Peacock KW, Williams AJ. Posttraumatic narcolepsy--two case reports and a mini review. J Clin Sleep Med. 2005;1:153–6. [PubMed] [Google Scholar]
- 6.Ton TG, Longstreth WT, Jr, Koepsell T. Active and passive smoking and risk of narcolepsy in people with HLA DQB1*0602: a population-based case-control study. Neuroepidemiology. 2009;32:114–21. doi: 10.1159/000177037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Billiard M, Laaberki M, Reygrobellet C, Seignalet J, Brissaud L, Besset A. Elevated antibodies to streptococcal antigens in narcoleptic subjects. Sleep Res. 1989;18:201. [Google Scholar]
- 8.Montplaisir J, Poirer G, Lapierre O, Montplaisir S. Streptococcal antibodies in narcolepsy and idiopathic hypersomnia. Sleep Res. 1989;18:271. [Google Scholar]
- 9.Mueller-Eckhardt G, Meier-Ewart K, Schiefer HG. Is there an infectious origin of narcolepsy? Lancet. 1990;335:424. doi: 10.1016/0140-6736(90)90270-f. [DOI] [PubMed] [Google Scholar]
- 10.Morrish E, King MA, Smith IE, Shneerson JM. Factors associated with a delay in the diagnosis of narcolepsy. Sleep Med. 2004;5:37–41. doi: 10.1016/j.sleep.2003.06.002. [DOI] [PubMed] [Google Scholar]
- 11.Cunningham MW. Pathogenesis of group A streptococcal infections. Clin Microbiol Rev. 2000;13:470–511. doi: 10.1128/cmr.13.3.470-511.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Franchini M, Veneri D. Helicobacter pylori-associated immune thrombocytopenia. Platelets. 2006;17:71–7. doi: 10.1080/09537100500438057. [DOI] [PubMed] [Google Scholar]
- 13.Arnulf I, Lin L, Zhang J, et al. CSF versus serum leptin in narcolepsy: is there an effect of hypocretin deficiency? Sleep. 2006;29:1017–24. doi: 10.1093/sleep/29.8.1017. [DOI] [PubMed] [Google Scholar]
- 14.Vincent A. Encephalitis lethargica: part of a spectrum of post-streptococcal autoimmune diseases? Brain. 2004;127:2–3. doi: 10.1093/brain/awh063. [DOI] [PubMed] [Google Scholar]
- 15.Dale RC. Post-streptococcal autoimmune disorders of the central nervous system. Dev Med Child Neurol. 2005;47:785–91. doi: 10.1017/S0012162205001647. [DOI] [PubMed] [Google Scholar]
- 16.Jackson MW, Reed JH, Smith AJ, Gordon TP. An autoantibody in narcolepsy disrupts colonic migrating motor complexes. J Neurosci. 2008;28:13303–9. doi: 10.1523/JNEUROSCI.4489-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Black JL., 3rd. Narcolepsy: a review of evidence for autoimmune diathesis. Int Rev Psychiatry. 2005;17:461–9. doi: 10.1080/02646830500381492. [DOI] [PubMed] [Google Scholar]
- 18.Hecht M, Lin L, Kushida CA, et al. Report of a case of immunosuppression with prednisone in an 8-year-old boy with an acute onset of hypocretin-deficiency narcolepsy. Sleep. 2003;26:809–10. doi: 10.1093/sleep/26.7.809. [DOI] [PubMed] [Google Scholar]
- 19.Kaplan EL, Ferrieri P, Wannamaker LW. Comparison of the antibody response to streptococcal cellular and extracellular antigens in acute pharyngitis. J Pediatr. 1974;84:21–8. doi: 10.1016/s0022-3476(74)80548-2. [DOI] [PubMed] [Google Scholar]
- 20.Machado CS, Ortiz K, Martins Ade L, Martins RS, Machado NC. [Antistreptolysin O titer profile in acute rheumatic fever diagnosis] J Pediatr (Rio J) 2001;77:105–11. doi: 10.2223/jped.185. [DOI] [PubMed] [Google Scholar]
- 21.Kotb M, Norrby-Teglund A, McGeer A, et al. An immunogenetic and molecular basis for differences in outcomes of invasive group A streptococcal infections. Nat Med. 2002;8:1398–404. doi: 10.1038/nm1202-800. [DOI] [PubMed] [Google Scholar]
- 22.Stanevicha V, Eglite J, Zavadska D, Sochnevs A, Shantere R, Gardovska D. HLA class II DR and DQ genotypes and haplotypes associated with rheumatic fever among a clinically homogeneous patient population of Latvian children. Arthritis Res Ther. 2007;9:R58. doi: 10.1186/ar2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Plazzi G, Poli F, Franceschini C, et al. Intravenous high-dose immunoglobulin treatment in recent onset childhood narcolepsy with cataplexy. J Neurol. 2008;255:1549–54. doi: 10.1007/s00415-008-0983-7. [DOI] [PubMed] [Google Scholar]
- 24.da Silva CH Pediatric Committee--Sao Paulo Pediatric Rheumatology Society. Rheumatic fever: a multicenter study in the state of Sao Paulo. Rev Hosp Clin Fac Med Sao Paulo. 1999;54:85–90. doi: 10.1590/s0041-87811999000300004. [DOI] [PubMed] [Google Scholar]
- 25.Nair PM, Philip E, Bahuleyan CG, Thomas M, Shanmugham JS, Suguna Bai NS. The first attack of acute rheumatic fever in childhood--clinical and laboratory profile. Indian Pediatr. 1990;27:241–6. [PubMed] [Google Scholar]
- 26.Kulkarni ML, Anees S. Sydenham's chorea. Indian Pediatr. 1996;33:112–5. [PubMed] [Google Scholar]
- 27.Zomorrodi A, Wald ER. Sydenham's chorea in western Pennsylvania. Pediatrics. 2006;117:e675–9. doi: 10.1542/peds.2005-1573. [DOI] [PubMed] [Google Scholar]
- 28.Parra G, Rodriguez-Iturbe B, Batsford S, et al. Antibody to streptococcal zymogen in the serum of patients with acute glomerulonephritis: a multicentric study. Kidney Int. 1998;54:509–17. doi: 10.1046/j.1523-1755.1998.00012.x. [DOI] [PubMed] [Google Scholar]
- 29.Blyth CC, Robertson PW, Rosenberg AR. Post-streptococcal glomerulonephritis in Sydney: a 16-year retrospective review. J Paediatr Child Health. 2007;43:446–50. doi: 10.1111/j.1440-1754.2007.01109.x. [DOI] [PubMed] [Google Scholar]