Abstract
Study Objectives:
Parkinson disease (PD) is the second most common neurodegenerative disorder in the United States. It is associated with motor deficits, sleep disturbances, and cognitive impairment. The pathology associated with PD and the effects of sleep deprivation impinge, in part, upon common molecular pathways suggesting that sleep loss may be particularly deleterious to the degenerating brain. Thus we investigated the long-term consequences of sleep deprivation on short-term memory using a Drosophila model of Parkinson disease.
Participants:
Transgenic strains of Drosophila melanogaster.
Design:
Using the GAL4-UAS system, human α-synuclein was expressed throughout the nervous system of adult flies. α-Synuclein expressing flies (αS flies) and the corresponding genetic background controls were sleep deprived for 12 h at age 16 days and allowed to recover undisturbed for at least 3 days. Short-term memory was evaluated using aversive phototaxis suppression. Dopaminergic systems were assessed using mRNA profiling and immunohistochemistry.
Measurments and Results:
When sleep deprived at an intermediate stage of the pathology, αS flies showed persistent short-term memory deficits that lasted ≥ 3 days. Cognitive deficits were not observed in younger αS flies nor in genetic background controls. Long-term impairments were not associated with accelerated loss of dopaminergic neurons. However mRNA expression of the dopamine receptors dDA1 and DAMB were significantly increased in sleep deprived αS flies. Blocking D1-like receptors during sleep deprivation prevented persistent short-term memory deficits. Importantly, feeding flies the polyphenolic compound curcumin blocked long-term learning deficits.
Conclusions:
These data emphasize the importance of sleep in a degenerating/reorganizing brain and shows that pathological processes induced by sleep deprivation can be dissected at the molecular and cellular level using Drosophila genetics.
Citation:
Seugnet L; Galvin JE; Suzuki Y; Gottschalk L; Shaw PJ. Persistent short-term memory defects following sleep deprivation in a drosophila model of parkinson disease. SLEEP 2009;32(8):984-992.
Keywords: Learning, Parkinson's Disease, dopamine, curcumin, short-term memory
PARKINSON DISEASE (PD) RESULTS IN THE PROGRESSIVE LOSS OF DOPAMINERGIC NEURONS AND IS ASSOCIATED WITH MOTOR DEFICITS INCLUDING bradykinesia, rigidity, and loss of postural reflexes. In addition, many PD patients develop cognitive deficits eventually leading to dementia, thus significantly increasing the personal and socioeconomic burden of the disease.1 Cognitive impairments occur both at the early and late stages of the disease, independently of depression and dementia.2 These impairments are well documented and predominantly affect prefrontal cortex and dopamine dependent executive functions including working memory, response inhibition, and behavioral flexibility.3,4 Poorer cognitive performance correlates with increased severity of the motor deficits, indicating that they may share a similar etiology.5
In addition to motor and cognitive deficits, sleep abnormalities are highly prevalent in PD (> 74% of patients).6–9 Sleep disturbances are both a primary feature of the disease and a secondary consequence of medications, tremor, rigidity, and bradykinesia. Primary sleep disturbances include excessive daytime sleepiness and REM sleep behavior disorder (RBD). RBD may predate the motor symptoms by many years,10 can predict cognitive impairment,11 and is associated with dementia.12 In addition, PD patients present narcolepsy-like symptoms that appear to be associated with a substantial loss of hypocretin neurons.13 Secondary sleep disturbances include insomnia, sleep fragmentation, restless legs syndrome, and sleep apnea.14
Multiple lines of evidence in both mammals and flies indicate important interactions between sleep and dopaminergic systems.15–22 For example, in healthy adult wild-type flies, sleep deprivation results in an increase in dopamine levels, a reduction in dopamine D1-receptor (dDA1) mRNA and learning impairments that are recovered following a brief 2-h nap.21 A similar process affecting the D2/D3 receptor has been reported in sleep deprived humans.22 Importantly, dopaminergic transmission plays a critical role in regulating sleep following environmental manipulations associated with brain plasticity.23 Although increased dopamine may facilitate plastic processes in the healthy adult, an acute change in dopamine signaling during critical windows of plasticity in dopaminergic circuits may result in long-lasting decrements in learning and other adaptive behaviors that persist when normal dopamine levels are restored.24 Together, these observations raise the possibility that sleep deprivation, by affecting the function of dopaminergic systems,21 may be particularly deleterious during degenerative processes that alter dopamine signaling such as PD. Drosophila, with the availability of models recapitulating many features of PD, and the ease of manipulating and monitoring sleep, appears well suited to address this question..
Using the GAL4-UAS system25 human α-synuclein can be expressed throughout the nervous system of the adult fly. Flies expressing α-synuclein (αS flies) reproduce key symptoms of human PD: age dependent and progressive disruption of selective dopaminergic neuronal groups, motor dysfunction responsive to dopaminergic treatment, and formation of Lewy body-like protein inclusions.26,27 To evaluate the consequences of sleep deprivation in αS flies, a functional readout of dopaminergic systems that is relevant to the deficits observed in PD is required. Since cognitive impairments appearing early in PD affect executive functioning,3 we chose to evaluate short-term memory and response inhibition in αS flies, using aversive phototaxis suppression (APS).28 Here we show that at an intermediate stage of the pathology, a single sleep deprivation challenge results in persistent short-term memory defects in αS flies. To explore possible mechanisms underlying these impairments, we evaluated dopaminergic neuron numbers using whole brain immunohistochemistry and mRNA levels of genes involved in dopamine signaling. We found that learning impairments are prevented when αS flies are administered a D1 antagonist during sleep deprivation suggesting that the deficits are due to an extended activation of D1-like receptors. Finally, we report that flies fed curcumin, a compound known to protect against the neurotoxic effects of 6-hydroxydopamine in rats29 and inhibit the formation of α-synuclein aggregates,30 prevents sleep deprivation induced impairments in short-term memory in αS flies.
METHODS
Fly Stocks, Sleep Monitoring, and Sleep Deprivation
We obtained ElavGAL4 and UAS α-synuclein flies from Mel Feany (Harvard University). ElavGAL4 and UAS α-synuclein (UAS α-syn) flies outcrossed to a w1118 stock served as genetic controls. Flies were cultured at 25°C, 50% to 60% humidity, in 12h:12h light: dark cycle, on a standard food containing yeast, dark corn syrup, molasses, dextrose, and agar. Newly eclosed female adult flies were collected from culture vials daily under CO2 anesthesia. Sleep was evaluated using the TriKinetics activity monitoring system as previously described18 (www.trikinetics.com). Flies were aged in groups of 20 to 30 individuals per vial and transferred to TriKinetics tubes at least 48 h before being sleep deprived. Flies were sleep deprived using an automated sleep deprivation apparatus that has been found to produce waking without nonspecifically activating stress responses.31 Flies were sleep deprived for 12 h, from ZT12 to ZT0, during the primary sleep period.
Learning
The APS learning test was performed as previously described.28 Each fly was individually tested in a T-maze and allowed to choose between a dark and a lighted vial. Flies are phototaxic and choose the lighted vial in > 80% of the trials in the absence of reinforcer. During training, choices to the lighted vial are associated with an aversive stimulus provided by a filter paper soaked with a quinine solution placed in the vial. The test is constituted by 16 trials through the maze during which flies learn to make more frequent choices to the dark vial (photonegative choices). The performance score is the average percentage of photonegative choices made in the last block of 4 trials for all the participating flies. For each experiment, learning was evaluated by the same experimenter who was blind to genotype and condition. All flies were tested in the morning between ZT0 and ZT4. Score differences between control and experimental groups were assessed using a Student t-test or analyses of variance (ANOVA), which were followed by planned pair-wise comparisons with a Tukey correction. Phototaxis index: phototaxis was evaluated in the T-maze without filter paper. The average proportion of choices to the lighted vial during 10 trials was calculated for each individual fly. The phototaxis index (PI) is the average of the scores obtained for at least 5 flies ± SEM. Sensitivity to quinine/humidity was evaluated as in Seugnet et al. 200821: each fly was individually placed in a 14-cm transparent cylindrical tube covered with filter paper. The quinine/humidity sensitivity index (referred to as quinine sensitivity index or QSI) was determined by calculating the time in seconds that the fly spent on the dry side of the tube when the other side had been wetted with quinine, during a 5-min period.
Climbing Assay
Flies were aged in groups of 20 to 30 individuals per vial and then tested for geotaxis as described before.26 Briefly, groups of 10 flies were placed in an empty 95 × 27 mm vial. Flies were gently taped to the bottom of the vial, and the number of flies crossing an 8-cm mark after 10 s was recorded. Scorers were blind for genotype and condition. Thirty flies were assayed for each experimental condition.
Drug Treatment
Flies were raised on regular food and transferred to curcumin or melatonin containing food after eclosion and until being tested. Curcumin (0.5 mg/mL), melatonin (1.16 mg/mL), and the D1 antagonist SCH23390 (1 mg/mL) were suspended in melted regular food at 40°C. SCH23390 has been shown to inhibit activation of Drosophila D1-like receptors.32
Immunohistochemistry
Fly brains were dissected in cold PBS and fixed in 4% paraformaldehyde. Mouse anti-tyrosine hydroxylase (ImmunoStar) antibodies were used at 1:50 to detect DA neurons. Brains were mounted in Vectashield HardSet mounting medium (Vector Laboratories) and imaged using a FluoView confocal microscope (Olympus). Dopaminergic neurons were counted using a Zeiss Axiophot epifluorescence microscope. At least 10 brains were scored for each experimental condition. For α-synuclein detection, fly heads were fixed in formalin and embedded in paraffin using standard methods.27 Immunostaining on paraffin sections was performed using the avidin-biotin-peroxidase complex (ABC) system from Vector laboratories. The LB509 anti α-synuclein antibodies (Zymed) were used at a 1:5000 dilution to detect protein aggregates.
QPCR
Total RNA was isolated from groups of 20 fly heads and processed as described21 for cDNA synthesis and QPCR. Expression values for RP49 were used to normalize results between groups.
RESULTS
α-Synuclein pathology in 16- to 20-day-old αS Flies
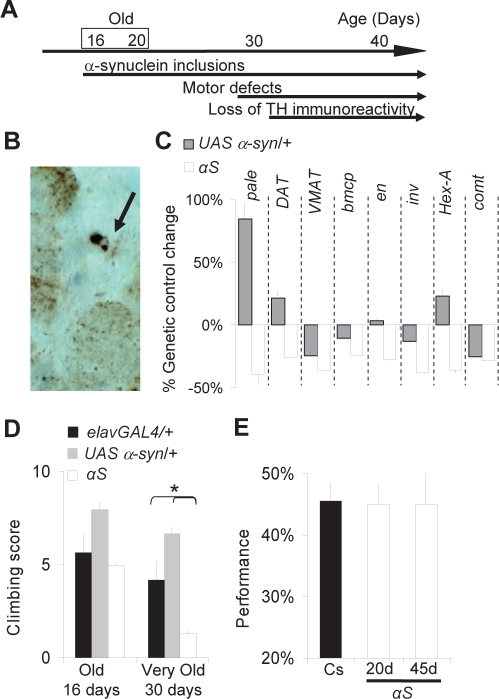
We used the pan-neuronal driver elav-GAL4 to drive expression of human α-synuclein throughout the nervous system. As previously reported,27 we observed widespread accumulation of α-synuclein inclusions in 16- to 20-day-old αS flies (Figure 1A-B). Importantly, genes associated with dopamine handling and synthesis including tyrosine hydroxylase (pale), the vesicular monoamine transporter (VMAT), and the Dopamine transporter (DAT) were reduced in 20-day-old αS flies (Figure 1C). These transcriptional changes suggest that dopaminergic transmission is disrupted by the pathology. Moreover, we found that several genes that are transcriptionally down-regulated in association with α-synuclein pathology in mammals33 were also down-regulated in 20-day-old αS flies (Figure 1C). Previous works have shown that a decrease in the ability of the flies to climb up the wall of a plastic vial is associated with the progression of the pathology and is sensitive to L-DOPA treatment.26,27 In accordance with those findings, we found that climbing ability was disrupted in 30-day-old αS flies compared to genetic controls (Figure 1D). In contrast, no climbing defects were observed in younger flies (16-day-old, Figure 1D). We then used APS to evaluate short-term memory and response inhibition in αS flies. In this operant paradigm, flies are trained individually in a T-maze to repress an instinctive attraction towards light.28 Normal performance requires flies to maintain the association between light and an aversive stimulus for ∼2 minutes.34 As shown in Figure 1E, 20-day-old and 45-day-old αS flies showed normal performance. Together, these results indicate that at 16-20 days of age αS flies display an early stage of pathogenesis, when dopamine circuits are already affected, but not to the extent that they interfere with normal behavior or short-term memory and response inhibition.
Figure 1.
PD-like pathology in flies expressing human α-synuclein. A, Time line for the progression of α-synuclein pathology in flies expressing UAS α-synuclein under the control of the elavGAL4 driver (αS flies). B, Detection of numerous α-synuclein aggregates in the brains of 20-day-old αS flies. Arrow: large Lewy-body like inclusion in a cell body. Immunohistochemistry on brain sections using anti α-synuclein antibodies. Similar results were obtained in 16 day old αS flies (not shown). C, Genes associated with α-synuclein pathology are down-regulated in 20-day-old αS flies compared to elavGAL4/+ and UAS α-syn/+ controls. Transcript levels are presented as the difference in expression between elavGAL4/+ and UAS α-syn/+ or αS divided by the expression value in elavGAL4/+ flies (% Genetic control change). QPCR data obtained with 2 independent sets of 20 whole fly heads for each condition. One of 2 replicates shown. D, No significant impairment in climbing ability is observed in 16-day-old αS flies; 30-day-old αS flies show significant motor impairments compared to age-matched elavGAL4/+ and UAS α-syn/+ controls. A main effect for age is revealed by 3 (Genotype: elavGAL4/+, UAS α-syn/+, αS) × 2 (Age: 16 vs. 30 days) ANOVA (F2,12 = 18.1, P = 0.001, *P < 0.05, planned comparison with Tukey Correction). E, APS performance in 20- and 45-day-old αS flies compared to wild-type Cs flies (n = 10 for each group).
Sleep Loss in αS Flies Produces Persistent Deficits in Short-Term Memory
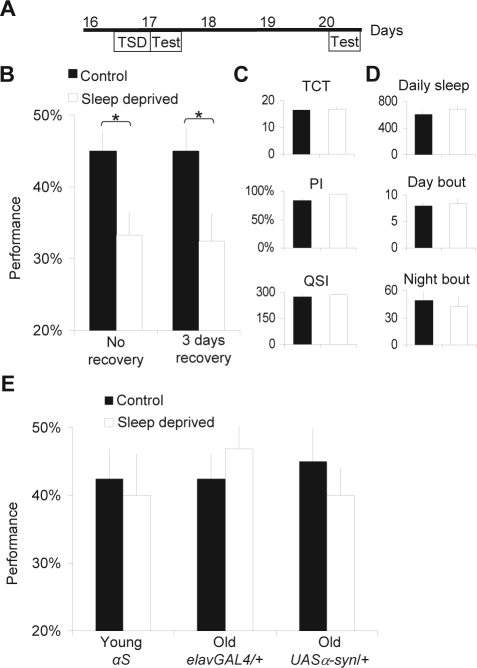
To determine whether sleep loss would have long-term consequences on learning, 16-day-old αS flies were sleep deprived for 12 h and allowed to recover unperturbed for 3-14 days (Figure 2A). As expected from previous work,21 learning was impaired immediately following sleep deprivation (Figure 2B, No recovery). Surprisingly, flies that were allowed to sleep unperturbed for 3 days following 12 h of sleep deprivation continued to display a significant reduction in performance (Figure 2B, 3 days recovery). Subsequent follow-up studies indicated that learning impairments could still be observed 14 days after the initial sleep deprivation challenge (data not shown). Flies that had been deprived at 16 days of age and allowed to recover for 3 days completed the 16 trials (TCT) in the same amount of time as control flies indicating that they did not suffer from long-term changes in motivation or locomotor activity (Figure 2C). Similarly, sleep deprivation did not alter the phototaxis index (PI), nor the sensitivity to quinine (QSI), indicating that the learning deficits were not due to changes in sensory thresholds (Figure 2C). Moreover, sleep deprivation did not result in long-term modifications to either sleep time or sleep architecture, indicating that baseline sleep could not account for the persistent learning impairments (Figure 2D). As shown in Figure 2E, long-term learning impairments were specific to αS flies and were not observed in aged-matched genetic control flies that underwent the same treatment. Finally, we showed that when αS flies were sleep deprived at a pre-symptomatic younger age (7 days old), no persistent learning impairments were observed (Figure 2E).
Figure 2.
Long term learning impairments in old α-synuclein expressing flies. A, Sleep deprivation and test schedule: 16-day-old flies were sleep deprived (TSD, Total Sleep Deprivation) for 12 h (ZT12 to ZT0) and tested for learning (Test) either immediately after sleep deprivation or after being allowed to recover 3 days unperturbed before being tested. Control siblings were left untreated during the same period. All flies were put in TriKinetics tubes for sleep monitoring. B, Learning in sleep deprived αS flies (n = 10) is significantly impaired immediately after sleep deprivation (“No recovery,” left) and after 3 days of recovery (right) compared to untreated age-matched control (n = 10 for each group,*t-test P < 0.05). C, Control metrics: time to complete the learning test (TCT, in minutes), phototaxis index (PI), and Quinine sensitivity index (QSI, in seconds) are similar in sleep deprived flies with 3 days of recovery and untreated age-matched control flies. D, Total daily sleep (min), and average sleep bout duration (min) during both the day and night are similar in sleep deprived and untreated control flies. E, Sleep deprivation in 7-day-old (Young) αS flies or in 20-day-old (Old) flies bearing either elavGAL4 or UAS α-syn alone does not produce long-term performance deficits.
Dopaminergic Neurons and Dopamine Receptors in Sleep Deprived αS Flies
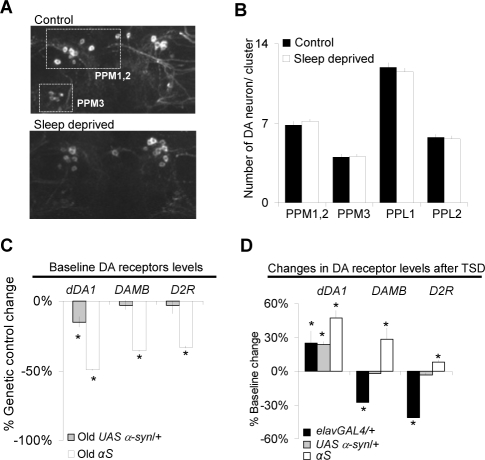
Next we evaluated potential mechanisms that might account for the persistent learning impairments in sleep deprived αS flies. Whole brain tyrosine-hydroxylase immunohistochemistry was used to evaluate the number and morphology of dopaminergic neurons in control and sleep deprived flies after 0, 5, and 10 days of recovery. As seen in Figure 3A the number of dopaminergic neurons, their morphology, and their staining intensity was similar between control and sleep deprived flies 5 and 10 days following sleep deprivation (see also supplementary Figure S1 for additional examples). Quantification of the images is shown in Figure 3B for 5 identified clusters of dopamine neurons, including the most vulnerable to α-synuclein neurodegeneration27,35; no changes were observed between previously sleep deprived flies and their controls (see supplementary Table S1 at www.journalsleep.org for quantification at 0 and 10 day recovery time points). Given that learning impairments following 12 h of sleep deprivation are associated with an acute decrease in transcript levels of dopamine receptors in young Canton-s (Cs) flies, we evaluated dopamine receptor mRNA in αS flies using QPCR.21 We began by evaluating the expression levels of the two D1-like receptors dDA1 and DAMB, and the D2-like receptor D2R under baseline conditions in the absence of sleep deprivation.
Figure 3.
Long term changes in DA signaling. A-B, Long term impairments in αS flies are not associated with an accelerated loss of dopaminergic neurons. Untreated age-matched controls (control) are compared to sleep deprived flies allowed 3-5 days of recovery (sleep deprived). A, Representative dopaminergic neuronal clusters (PPM1,2 and PPM3) in old αS flies,whole-mount immunostaining with anti-TH antibodies). B, Number of dopaminergic neurons included in the PPM1,2, PPM3, PPL1 and PPL2 clusters. Data represents the average obtained from 12 independent clusters. No difference is observed between control and sleep deprived flies allowed 5 days of recovery. C, Under baseline conditions old αS flies show reduced DA receptor mRNA levels compared to their genetic background controls elavGAL4/+ and UAS α-syn/+. Gene expression is presented as % change from elavGAL4/+ control levels (% Genetic control change). One of 2 replicates shown, 20 whole heads used for each condition. (*P < 0.05 one-sample t-test). D, Dopamine receptor mRNA levels are increased after 3 days of recovery following total sleep deprivation (TSD) in αS flies. Gene expression is presented as % change from untreated controls (% Baseline change). One of 2 replicates shown. (*P < 0.05 one-sample t-test).
As seen in Figure 3C, non-sleep deprived 20-day-old αS flies displayed decreased mRNA levels for all 3 dopamine receptors compared to the elavGAL4/+ and UAS α-syn/+ genetic background controls (Figure 3C, results expressed as % change from levels observed in elavGAL4/+). These results indicate that, under baseline conditions, αS flies are able to obtain normal performance despite a reduction in dopamine receptor expression. These transcriptional changes in dopamine receptor expression, combined with those observed for dopamine handling and synthesis genes (Figure 1C), further confirm that dopamine systems are undergoing changes in 20-day-old αS flies. Although sleep deprivation results in an acute down-regulation of dDA1 expression in young Cs flies,21 mRNA levels for dDA1 and DAMB were significantly increased in sleep deprived αS flies compared to their non-sleep deprived age-matched controls after 3 days of recovery (Figure 3D). Note that the data presented in Figure 3C represent relative differences between genotypes, while the data presented in Figure 3D are expressed as a percentage of non-sleep deprived age-matched siblings. In the later comparison, a relative increase in expression level in one genotype in response to sleep loss does not mean that the absolute level of the gene is higher than another genotype. Caution must therefore be used when interpreting the relative changes in gene expression between these two data sets. Although we observed a modest increase in dDA1 levels in both genetic background controls following sleep deprivation, the change was to lesser extent than in the αS flies. However, the expression of the other D1-like receptor, DAMB, was either unchanged or decreased in the genetic controls after sleep deprivation (Figure 3D). We did not observe consistent changes in genes involved in dopamine synthesis and handling following sleep deprivation in αS flies compared to controls (data not shown). Thus the persistent short-term memory deficits in sleep deprived αS flies were associated with changes in dopamine receptor expression.
Curcumin Protects αS flies from Persistent Impairments
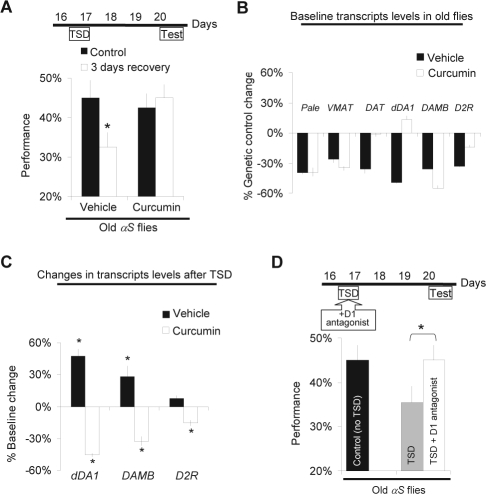
The data presented above indicates that learning may be used as a functional assay to rapidly evaluate potential therapeutic interventions. With this in mind, we fed αS flies melatonin or curcumin throughout their adult life. Curcumin is a natural polyphenolic antioxidant that has been shown to inhibit the formation of α-synuclein aggregates30 and to protect against 6-hydroxydopamine induced loss of tyrosine hydroxylase cells in rats.29 Melatonin has neuroprotective effects on dopaminergic neurons in both mammalian and Drosophila models of PD.36–38 As before, flies were sleep deprived at 16 day of age and tested with APS 3 days later (Figure 4A). Interestingly, long-term impairments were successfully prevented when αS flies were treated with curcumin throughout their lives (Figure 4A). However, similar treatment with melatonin did not protect flies from the long-term learning deficits induced by sleep deprivation (data not shown). As above, feeding flies curcumin did not change control metrics for APS (Table1), indicating that the treatment did not simply optimize motoric ability or sensory thresholds.
Figure 4.
Pharmacological treatments preventing sleep deprivation induced long term impairments in αS flies. A, Sleep deprivation and test schedule (top). αS flies were tested after 3 days of recovery following 12 h of total sleep deprivation (TSD) and compared to untreated age-matched siblings (Control). Flies fed curcumin did not show long-term performance impairments after sleep deprivation (bottom graph, right). N = 10 for each condition. A 2 (Drug: curcumin vs. vehicle) × 2 (Condition: 3 days recovery vs. control) ANOVA show main effect for Drug (F2,36 = 4.38, P = 0.04, *planned comparison with Tukey correction P < 0.05). B, Feeding αS flies curcumin (curcumin, white) changes DAT and dDA1 mRNA levels compared to flies fed vehicle (vehicle, black). Gene expression represented as % change from elavGAL4/+ controls fed vehicle. One of 2 replicates shown. C, DA receptor mRNA levels are decreased after 3 days of recovery following sleep deprivation in flies maintained on curcumin (white) and are increased in controls maintained on vehicle (black). Gene expression represented as % change from untreated controls (% Baseline change). One of 2 replicates shown. (*P < 0.05 one-sample t-test). D, Blocking D1 receptor activation during sleep deprivation prevents long term performance deficits. Top scheme: sleep deprivation and test schedule. Old αS flies were fed the D1 antagonist SCH-23390 (1 mg/mL) or vehicle during total sleep deprivation (TSD), then transferred to regular food until being tested for learning 3 days later. Performance in flies fed the D1 antagonist during sleep deprivation (TSD+ D1 antagonist, n = 10) was significantly improved compared to flies sleep deprived on vehicle (TSD, n = 12). The score of age-matched control αS flies is shown for comparison (n = 10) (*t-test, P < 0.05).
Table 1.
Control Metrics after Pharmacological Treatment
| Condition | TCT | PI | QSI |
|---|---|---|---|
| mean ± sem | mean ± sem | mean ± sem | |
| vehicle | 13.4 ± 0.5 | 74% ± 7% | 291 ± 04 |
| curcumin | 14.1 ± 1.1 | 84% ± 6% | 281 ± 09 |
| D1 antagonist | 12.4 ± 0.6 | 82% ± 4% | 287 ± 04 |
Data obtained with sleep deprived αS flies allowed to recover 3 days undisturbed.
TCT: Time to complete test in minutes; PI: Phototaxic index (n=5); QSI: quinine sensitivity index (n=5).
To evaluate potential mechanisms for the protection provided by curcumin, we used QPCR to examine the transcriptional expression of genes involved in dopamine synthesis and handling in flies treated with curcumin, compared to vehicle controls. As shown in Figure 4B, Pale, VMAT, and the dopamine receptors DAMB and D2R were all down-regulated compared to genetic controls in non-sleep deprived 20-day-old αS flies treated with curcumin, similar to flies fed vehicle. On the other hand, DAT and dDA1 mRNA levels were no longer down-regulated in curcumin treated αS flies. Importantly, we found that curcumin treatment prevented sleep deprivation from increasing the levels of dopamine receptors (Figure 4C). Instead, we observed a decrease in the expression of all 3 dopamine receptors in curcumin treated flies (Figure 4C). As above, caution must be used when evaluating relative expression levels between the experiments described in 4B and 4C. Thus, curcumin treatment appears to affect dopamine receptor expression both under baseline conditions and after sleep deprivation.
Sleep Deprivation Induced Impairments Require D1 Receptor Activation
Our recent data suggest that sleep deprivation results in learning impairments and that these deficits are mediated, in part, through the dDA1 receptor.21 If extended activation of dDA1 in αS flies contributes to the persistent learning deficits, then it should be possible to prevent long-term impairments in short-term memory and response inhibition by blocking D1-like receptors during sleep loss. Thus, 16-day-old αS flies were administered the D1 antagonist SCH23390 during sleep deprivation and allowed to recover unperturbed in the absence of the antagonist for 3 days (Figure 4D). As seen in Figure 4D, blocking D1-like receptor activation during sleep deprivation protected flies from the long-term cognitive impairments normally associated with sleep loss in these conditions. No changes in the control metrics were observed in flies treated with the D1 antagonist indicating that the drug did not produce long-term changes in motivation or sensory thresholds (Table 1).
DISCUSSION
The pathogenesis of PD is multifactorial and is influenced by a complex interaction between behavioral, environmental, and genetic factors.39 Interestingly, cognitive impairments and sleep deficits may precede motor symptoms in PD patients by several years, making their evaluation particularly well suited for early detection and the assessment of therapeutic interventions.40 Using a well characterized Drosophila model of PD,27,41 we showed that a single challenge of sleep deprivation that occurs prior to the onset of motor symptoms results in deficits in short-term memory and response inhibition that persist for at least 3 days. In addition, we demonstrated that pathology associated with the expression of α-synuclein could be prevented by administering the polyphenolic compound curcumin. Together these data suggest that sleep may play an important role in the degenerating/reorganizing brain and that therapeutic interventions can be quickly evaluated in a genetic model organism using a relevant functional assay—short-term memory.
Given that synuclein pathology is known to affect many brain circuits,13,42,43 we chose to express human α-synuclein throughout the brain using a pan-neuronal driver. However, dopaminergic neurons are known to be negatively affected in humans, rodents and flies. Dopamine is also known to promote synaptic plasticity, long-term potentiation, and memory consolidation.44 Recent evidence obtained from PD patients and in rats with 6-hydroxydopamine-lesions suggests that the loss of dopamine is associated with aberrant forms of plasticity.45 Moreover, patients with PD also exhibit disruptions in executive functions and disturbed sleep.4,14 Thus we evaluated dopaminergic pathways to determine the extent to which the fly could be used to model the effects of sleep deprivation on pathology found upon α-synuclein expression.
At 20 days of age, αS flies showed widespread α-synuclein aggregates and reduced expression of several genes associated with dopamine synthesis and handing. However, in the absence of sleep loss, αS flies showed no overt signs of behavioral deficits as measured by changes in climbing ability, locomotor activity, sleep, or short-term memory. Together, these results suggest that αS flies compensate for emerging deficits to maintain normal behavioral output. Indeed, performance in the APS requires dopamine,21 and while brain dopamine levels decline substantially with age,46,47 50-day-old wild-type flies continue to learn.48 Moreover, compensatory mechanisms are known to preserve the function of degenerating dopaminergic systems in the presymptomatic phase of human PD.49–51 As a consequence of these compensations, parkinsonian symptoms only appear after a substantial degeneration of dopaminergic systems has occurred. As with mammals, Drosophila display evidence of plastic changes at both the synaptic and structural levels that can be induced in adults.52,53 Thus, we hypothesize that during the early stages of αS pathology, the brain may be in a transition state requiring significant plastic changes and that these changes are highly vulnerable to sleep loss.
The long-term deficits in short-term memory observed in αS flies following sleep deprivation stand in marked contrast to the rapid recovery of performance seen in both mature wild-type flies and age-matched genetic controls. However, the long-term learning deficits are similar to what is observed when flies are prevented from sleeping on their first full day of adult life when brain plasticity is high. Sleep loss in both the developing fly, and in 16-day-old αS flies resulted in an increase in dDA1 mRNA expression but no evidence of disruption in dopaminergic neurons as measured by tyrosine-hydroxylase immunohistochemistry (Seugnet et al., in preparation). Moreover, administering a D1 antagonist during sleep deprivation is able to prevent long-lasting learning deficits in both the developing fly and in flies expressing α-synuclein. Together these results suggest that sleep loss durably modifies components downstream of dopaminergic neurons. These data are consistent with recent reports showing that Lewy body diseases result in pathologies postsynaptic to dopaminergic neurons54 and with the long-standing observation that dopaminergic neurons are not the only neurons affected in PD.13,42,43 The cellular and molecular consequences of sleep deprivation thus add another layer of complexity in α-synuclein pathology. The Drosophila genetic tool-kit should open new ways to characterize and dissect the interactions between the effects of sleep disruption and the neurodegenerative processes associated with PD.
An important question, that is not easily resolved, is whether the learning impairments induced by sleep deprivation advance the progression of αS pathology or reveal a new αS phenotype. Old αS flies at 45 days of age obtain normal learning scores similar to that found in young flies. These data imply that sleep deprivation may not simply advance the progression of αS pathology as measured by memory. However, as mentioned above, we cannot exclude the possibility that learning is maintained in older flies due to compensatory processes. Could the learning impairments represent a new αS phenotype? In mammals, pharmacologically altering dopamine during critical windows of plasticity in dopaminergic circuits results in long-lasting deficits.24,55 Moreover, elevated cytoplasmic dopamine is a main factor underlying the early stage of αS mediated neurodegeneration in Drosophila primary neuronal cultures.56 Thus it is possible that sleep deprivation, by altering dopamine levels21 during a specific stage of degeneration, has uncovered a previously unrecognized vulnerability in αS flies. A final possibility is that the short-term memory deficits induced by sleep deprivation are not specific to αS and reflect a general vulnerability of a degenerating brain. That is, sleep deprivation may result in long-term learning impairments in any fly expressing neurodegenerative disease genes. Distinguishing between these possibilities is not trivial and thus is beyond the scope of the current investigation. Nonetheless, our data suggest that studies of this type have the potential to reveal new insights into the relationship between sleep and neurodegenerative diseases.
Does sleep deprivation have consequences in human patients with PD? Although, a single sleep deprivation challenge has been reported to improve motor symptoms in PD patients,57,58 a more recent study concluded that there was no significant beneficial effect of either partial or total sleep deprivation.59 Comparisons between studies are complicated by differences in the evaluation of motor symptoms, patient selection, and medication regimen.59 Moreover, not all studies have controlled for depression, a condition that is improved by sleep deprivation. In contrast to the effects of sleep deprivation, several studies suggest that sleep itself might be beneficial for PD symptoms.60–62 That is, a significant proportion of PD patients display improvement in motor symptoms during the first hour after awakening from a night of sleep.60–62 However, a recent report indicates that patients in the early stages of PD do not display the consolidation of procedural memory that typically occurs following a night of sleep.63 Thus, pathology associated with PD and the effects of sleep deprivation might impinge, at least in part, upon molecular pathways underlying components of synaptic plasticity. Given the complexity of these issues further work is clearly warranted to determine which symptoms are modified by sleep deprivation in human patients with PD.
Although previous studies have shown that curcumin can ameliorate biochemical modifications associated with Parkinson disease, our results may be the first to demonstrate neuroprotection as measured by a cognitive behavior. While further work will be necessary to understand the effect of curcumin on α-synuclein pathology, the mRNA profiling of dopamine receptors presented here suggests that curcumin may alter the progression of the pathology. In addition, curcumin modified the progression of gene expression changes following sleep deprivation. Given the changes observed, it is likely that curcumin ameliorates a global change in synuclein pathology rather than simply protecting αS flies from specific effects of sleep deprivation. Indeed, curcumin has been reported block α-synuclein aggregation and enhance disaggregation of preformed α-synuclein aggregates in cellular models30 and does not prevent the immediate learning deficits induced by sleep deprivation in wild-type flies. In contrast to curcumin, melatonin failed to prevent long term learning impairments in αS flies. Melatonin has been shown to protect flies against rotenone induced dopaminergic cell loss, a model of sporadic PD, at the dose used in this study.37 However, its effectiveness in α-synuclein expressing flies has not been reported before. In addition, while melatonin has been shown to be neuroprotective in some instances in human and mammalian systems, it has been ineffective in other cases.38,64,65
A concern with all sleep deprivation studies that evaluate learning and memory is whether the method used to keep the animal awake can negatively affect cognitive behavior independently from sleep loss. Studies from our lab have shown that learning deficits following sleep deprivation are not caused by nonspecific effects of the mechanical stimulation used to keep the animal awake or stress.21 For example, the mechanical stimulus produced by the sleep nullifying apparatus (SNAP) does not produce learning deficits in the absence of sleep loss21 and does not activate stress response genes.31 Similarly, learning is disrupted by sleep fragmentation and episodes of spontaneous wakefulness in the absence of mechanical intervention.21 In addition, sleep deprivation in αS flies was not associated with long-term changes in the time to complete the task, sensory thresholds (phototaxis and quinine sensitivity), α-synuclein expression (not shown), or sleep. Moreover, when αS flies are sleep deprived during the early stages of degeneration (7 days old) short-term memory quickly returned to baseline indicating that the impairments in older flies are most likely due to the state of degeneration and not potential nonspecific effects of the apparatus. Finally, the administration of melatonin, an agent commonly used to protect against the effects of various stressors in flies, was not able to prevent long-term learning impairments. Together these results indicate that the persistent deficits in short-term memory and response inhibition in αS flies are most likely due to sleep loss and not confounding variables.
Previous studies have shown that the fly can be used to model neurodegenerative diseases.41,66,67 With few exceptions,68 behavioral tasks that are known to be modified in these disease states have not been evaluated in fly models. We show here that short-term memory can be evaluated in Drosophila models of neurodegeneration and that it is disrupted by acute sleep loss. It should be noted that several studies have evaluated sleep in animal models of PD with mixed results.69–71 Together these findings emphasize that sleep loss may be particularly deleterious to the degenerating brain and provide future directions for the genetic and molecular dissection of the consequences of sleep deprivation in PD.
DISCLOSURE STATEMENT
This was not and industry supported study. Dr. Shaw participated in a speaking engagement for Takeda. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
We thank Matthew Thimgan for helpful comments. This study was funded in part by 1 R01 NS051305-01A1, 5 K07 AG21164-02 the McDonnell Center for Cellular and Molecular Neurobiology, The American Parkinson's Association, and the NIH Neuroscience Blueprint Core Grant,#NS057105).
REFERENCES
- 1.Bosboom JL, Stoffers D, Wolters E. Cognitive dysfunction and dementia in Parkinson's disease. J Neural Transm. 2004;111:1303–15. doi: 10.1007/s00702-004-0168-1. [DOI] [PubMed] [Google Scholar]
- 2.Owen AM, James M, Leigh PN, et al. Fronto-striatal cognitive deficits at different stages of Parkinson's disease. Brain. 1992;115:1727–51. doi: 10.1093/brain/115.6.1727. [DOI] [PubMed] [Google Scholar]
- 3.Foltynie T, Goldberg TE, Lewis SG, et al. Planning ability in Parkinson's disease is influenced by the COMT val158met polymorphism. Mov Disord. 2004;19:885–91. doi: 10.1002/mds.20118. [DOI] [PubMed] [Google Scholar]
- 4.Galvin JE. Cognitive change in Parkinson disease. Alzheimer Dis Assoc Disord. 2006;20:302–10. doi: 10.1097/01.wad.0000213858.27731.f8. [DOI] [PubMed] [Google Scholar]
- 5.Green J, McDonald WM, Vitek JL, et al. Cognitive impairments in advanced PD without dementia. Neurology. 2002;59:1320–4. doi: 10.1212/01.wnl.0000031426.21683.e2. [DOI] [PubMed] [Google Scholar]
- 6.Schapira AH. Excessive daytime sleepiness in Parkinson's disease. Neurology. 2004;63(8 Suppl 3):S24–7. doi: 10.1212/wnl.63.8_suppl_3.s24. [DOI] [PubMed] [Google Scholar]
- 7.Pal PK, Thennarasu K, Fleming J, Schulzer M, Brown T, Calne SM. Nocturnal sleep disturbances and daytime dysfunction in patients with Parkinson's disease and in their caregivers. Parkinsonism Relat Disord. 2004;10:157–68. doi: 10.1016/j.parkreldis.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 8.Bliwise DL, Watts RL, Watts N, Rye DB, Irbe D, Hughes M. Disruptive nocturnal behavior in Parkinson's disease and Alzheimer's disease. J Geriatr Psychiatry Neurol. 1995;8:107–10. doi: 10.1177/089198879500800206. [DOI] [PubMed] [Google Scholar]
- 9.Rye DB. Excessive daytime sleepiness and unintended sleep in Parkinson's disease. Curr Neurol Neurosci Rep. 2006;6:169–76. doi: 10.1007/s11910-996-0041-8. [DOI] [PubMed] [Google Scholar]
- 10.Boeve BF, Silber MH, Ferman TJ, et al. REM sleep behavior disorder and degenerative dementia: an association likely reflecting Lewy body disease. Neurology. 1998;51:363–70. doi: 10.1212/wnl.51.2.363. [DOI] [PubMed] [Google Scholar]
- 11.Vendette M, Gagnon JF, Decary A, et al. REM sleep behavior disorder predicts cognitive impairment in Parkinson disease without dementia. Neurology. 2007;69:1843–9. doi: 10.1212/01.wnl.0000278114.14096.74. [DOI] [PubMed] [Google Scholar]
- 12.Marion MH, Qurashi M, Marshall G, Foster O. Is REM sleep behaviour disorder (RBD) a risk factor of dementia in idiopathic Parkinson's disease? J Neurol. 2008;255:192–6. doi: 10.1007/s00415-008-0629-9. [DOI] [PubMed] [Google Scholar]
- 13.Thannickal TC, Lai YY, Siegel JM. Hypocretin (orexin) cell loss in Parkinson's disease. Brain. 2007;130:1586–95. doi: 10.1093/brain/awm097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Friedman JH, Chou KL. Sleep and fatigue in Parkinson's disease. Parkinsonism Relat Disord. 2004;10(Suppl 1):S27–35. doi: 10.1016/j.parkreldis.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 15.Dzirasa K, Ribeiro S, Costa R, et al. Dopaminergic control of sleep-wake states. J Neurosci. 2006;26(41):10577–89. doi: 10.1523/JNEUROSCI.1767-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kume K, Kume S, Park SK, Hirsh J, Jackson FR. Dopamine is a regulator of arousal in the fruit fly. J Neurosci. 2005;25:7377–84. doi: 10.1523/JNEUROSCI.2048-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andretic R, van Swinderen B, Greenspan RJ. Dopaminergic modulation of arousal in Drosophila. Curr Biol. 2005;15:1165–75. doi: 10.1016/j.cub.2005.05.025. [DOI] [PubMed] [Google Scholar]
- 18.Shaw PJ, Cirelli C, Greenspan RJ, Tononi G. Correlates of sleep and waking in Drosophila melanogaster. Science. 2000;287:1834–7. doi: 10.1126/science.287.5459.1834. [DOI] [PubMed] [Google Scholar]
- 19.Cirelli C, Gutierrez CM, Tononi G. Extensive and divergent effects of sleep and wakefulness on brain gene expression. Neuron. 2004;41:35–43. doi: 10.1016/s0896-6273(03)00814-6. [DOI] [PubMed] [Google Scholar]
- 20.Greenspan RJ, Tononi G, Cirelli C, Shaw PJ. Sleep and the fruit fly. Trends Neurosci. 2001;24:142–5. doi: 10.1016/s0166-2236(00)01719-7. [DOI] [PubMed] [Google Scholar]
- 21.Seugnet L, Suzuki Y, Vine L, Gottschalk L, Shaw PJ. D1 Receptor Activation in the Mushroom Bodies Rescues Sleep-Loss-Induced Learning Impairments in Drosophila. Curr Biol. 2008;18:1110–7. doi: 10.1016/j.cub.2008.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Volkow ND, Wang GJ, Telang F, et al. Sleep deprivation decreases binding of [11C]raclopride to dopamine D2/D3 receptors in the human brain. J Neurosci. 2008;28:8454–61. doi: 10.1523/JNEUROSCI.1443-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ganguly-Fitzgerald I, Donlea J, Shaw PJ. Waking experience affects sleep need in Drosophila. Science. 2006;313:1775–81. doi: 10.1126/science.1130408. [DOI] [PubMed] [Google Scholar]
- 24.Friedman E, Wang HY. Prenatal cocaine exposure alters signal transduction in the brain D1 dopamine receptor system. Ann N Y Acad Sci. 1998;846:238–47. [PubMed] [Google Scholar]
- 25.Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118(2):401–15. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- 26.Pendleton RG, Parvez F, Sayed M, Hillman R. Effects of pharmacological agents upon a transgenic model of Parkinson's disease in Drosophila melanogaster. J Pharmacol Exp Ther. 2002;300:91–6. doi: 10.1124/jpet.300.1.91. [DOI] [PubMed] [Google Scholar]
- 27.Feany MB, Bender WW. A Drosophila model of Parkinson's disease. Nature. 2000;404:394–8. doi: 10.1038/35006074. [DOI] [PubMed] [Google Scholar]
- 28.Le Bourg E, Buecher C. Learned suppression of photopositive tendencies in Drosophila melanogaster. Anim Learn Behav. 2002;30:330–41. doi: 10.3758/bf03195958. [DOI] [PubMed] [Google Scholar]
- 29.Zbarsky V, Datla KP, Parkar S, Rai DK, Aruoma OI, Dexter DT. Neuroprotective properties of the natural phenolic antioxidants curcumin and naringenin but not quercetin and fisetin in a 6-OHDA model of Parkinson's disease. Free Radic Res. 2005;39:1119–25. doi: 10.1080/10715760500233113. [DOI] [PubMed] [Google Scholar]
- 30.Pandey N, Strider J, Nolan WC, Yan SX, Galvin JE. Curcumin inhibits aggregation of alpha-synuclein. Acta Neuropathol. 2008;115:479–89. doi: 10.1007/s00401-007-0332-4. [DOI] [PubMed] [Google Scholar]
- 31.Shaw PJ, Tononi G, Greenspan RJ, Robinson DF. Stress response genes protect against lethal effects of sleep deprivation in Drosophila. Nature. 2002;417:287–91. doi: 10.1038/417287a. [DOI] [PubMed] [Google Scholar]
- 32.Feng G, Hannan F, Reale V, et al. Cloning and functional characterization of a novel dopamine receptor from Drosophila melanogaster. J Neurosci. 1996;16:3925–33. doi: 10.1523/JNEUROSCI.16-12-03925.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller RM, Federoff HJ. Microarrays in Parkinson's disease: a systematic approach. NeuroRx. 2006;3:319–26. doi: 10.1016/j.nurx.2006.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seugnet L, Suzuki Y, Stidd R, Shaw PJ. Aversive Phototaxic Suppression: evaluation of a short-term memory assay in Drosophila melanogaster. Genes Brain Behav. 2009 Feb 11; doi: 10.1111/j.1601-183X.2009.00483.x. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Auluck PK, Bonini NM. Pharmacological prevention of Parkinson disease in Drosophila. Nat Med. 2002;8:1185–6. doi: 10.1038/nm1102-1185. [DOI] [PubMed] [Google Scholar]
- 36.Dabbeni-Sala F, Di Santo S, Franceschini D, Skaper SD, Giusti P. Melatonin protects against 6-OHDA-induced neurotoxicity in rats: a role for mitochondrial complex I activity. FASEB J. 2001;15:164–70. doi: 10.1096/fj.00-0129com. [DOI] [PubMed] [Google Scholar]
- 37.Coulom H, Birman S. Chronic exposure to rotenone models sporadic Parkinson's disease in Drosophila melanogaster. J Neurosci. 2004;24:10993–8. doi: 10.1523/JNEUROSCI.2993-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ma J, Shaw VE, Mitrofanis J. Does melatonin help save dopaminergic cells in MPTP-treated mice? Parkinsonism Relat Disord. 2008 Sep 13; doi: 10.1016/j.parkreldis.2008.07.008. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 39.Benmoyal-Segal L, Soreq H. Gene-environment interactions in sporadic Parkinson's disease. J Neurochem. 2006;97:1740–55. doi: 10.1111/j.1471-4159.2006.03937.x. [DOI] [PubMed] [Google Scholar]
- 40.Pfeiffer RF. Non-motor parkinsonism. Parkinsonism Relat Disord. 2007;13(Suppl 3):S211–20. doi: 10.1016/S1353-8020(08)70004-X. [DOI] [PubMed] [Google Scholar]
- 41.Bonini NM, Fortini ME. Human neurodegenerative disease modeling using Drosophila. Annu Rev Neurosci. 2003;26:627–56. doi: 10.1146/annurev.neuro.26.041002.131425. [DOI] [PubMed] [Google Scholar]
- 42.Lang AE, Lozano AM. Parkinson's disease. First of two parts. N Engl J Med. 1998;339:1044–53. doi: 10.1056/NEJM199810083391506. [DOI] [PubMed] [Google Scholar]
- 43.Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 44.Lemon N, Manahan-Vaughan D. Dopamine D1/D5 receptors gate the acquisition of novel information through hippocampal long-term potentiation and long-term depression. J Neurosci. 2006;26:7723–9. doi: 10.1523/JNEUROSCI.1454-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Battaglia F, Ghilardi MF, Quartarone A, Bagnato S, Girlanda P, Hallett M. Impaired long-term potentiation-like plasticity of the trigeminal blink reflex circuit in Parkinson's disease. Mov Disord. 2006;21:2230–3. doi: 10.1002/mds.21138. [DOI] [PubMed] [Google Scholar]
- 46.Imai Y, Gehrke S, Wang HQ, et al. Phosphorylation of 4E-BP by LRRK2 affects the maintenance of dopaminergic neurons in Drosophila. EMBO J. 2008;27:2432–43. doi: 10.1038/emboj.2008.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Neckameyer WS, Woodrome S, Holt B, Mayer A. Dopamine and senescence in Drosophila melanogaster. Neurobiol Aging. 2000;21:145–52. doi: 10.1016/s0197-4580(99)00109-8. [DOI] [PubMed] [Google Scholar]
- 48.Le Bourg E. Effects of aging on learned suppression of photopositive tendencies in Drosophila melanogaster. Neurobiol Aging. 2004;25:1241–52. doi: 10.1016/j.neurobiolaging.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 49.Helmich RC, de Lange FP, Bloem BR, Toni I. Cerebral compensation during motor imagery in Parkinson's disease. Neuropsychologia. 2007;45:2201–15. doi: 10.1016/j.neuropsychologia.2007.02.024. [DOI] [PubMed] [Google Scholar]
- 50.Bezard E, Gross CE, Brotchie JM. Presymptomatic compensation in Parkinson's disease is not dopamine-mediated. Trends Neurosci. 2003;26:215–21. doi: 10.1016/S0166-2236(03)00038-9. [DOI] [PubMed] [Google Scholar]
- 51.Zigmond MJ, Abercrombie ED, Berger TW, Grace AA, Stricker EM. Compensations after lesions of central dopaminergic neurons: some clinical and basic implications. Trends Neurosci. 1990;13:290–6. doi: 10.1016/0166-2236(90)90112-n. [DOI] [PubMed] [Google Scholar]
- 52.Martin-Pena A, Acebes A, Rodriguez JR, et al. Age-independent synaptogenesis by phosphoinositide 3 kinase. J Neurosci. 2006;26:10199–208. doi: 10.1523/JNEUROSCI.1223-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Heisenberg M, Heusipp M, Wanke C. Structural plasticity in the Drosophila brain. J Neurosci. 1995;15(3 Pt 1):1951–60. doi: 10.1523/JNEUROSCI.15-03-01951.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kramer ML, Schulz-Schaeffer WJ. Presynaptic alpha-synuclein aggregates, not Lewy bodies, cause neurodegeneration in dementia with Lewy bodies. J Neurosci. 2007;27:1405–10. doi: 10.1523/JNEUROSCI.4564-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Miller JC, Friedhoff AJ. Prenatal neurotransmitter programming of postnatal receptor function. Prog Brain Res. 1988;73:509–22. doi: 10.1016/S0079-6123(08)60523-3. [DOI] [PubMed] [Google Scholar]
- 56.Park SS, Schulz EM, Lee D. Disruption of dopamine homeostasis underlies selective neurodegeneration mediated by alpha-synuclein. Eur J Neurosci. 2007;26:3104–12. doi: 10.1111/j.1460-9568.2007.05929.x. [DOI] [PubMed] [Google Scholar]
- 57.Bertolucci PH, Andrade LA, Lima JG, Carlini EA. Total sleep deprivation and Parkinson disease. Arq Neuropsiquiatr. 1987;45:224–30. doi: 10.1590/s0004-282x1987000300002. [DOI] [PubMed] [Google Scholar]
- 58.Reist C, Sokolski KN, Chen CC, Coskinas E, Demet EM. The effect of sleep deprivation on motor impairment and retinal adaptation in Parkinson's disease. Prog Neuropsychopharmacol Biol Psychiatry. 1995;19:445–54. doi: 10.1016/0278-5846(95)00025-q. [DOI] [PubMed] [Google Scholar]
- 59.Hogl B, Peralta C, Wetter TC, Gershanik O, Trenkwalder C. Effect of sleep deprivation on motor performance in patients with Parkinson's disease. Mov Disord. 2001;16:616–21. doi: 10.1002/mds.1138. [DOI] [PubMed] [Google Scholar]
- 60.Hogl BE, Gomez-Arevalo G, Garcia S, et al. A clinical, pharmacologic, and polysomnographic study of sleep benefit in Parkinson's disease. Neurology. 1998;50:1332–9. doi: 10.1212/wnl.50.5.1332. [DOI] [PubMed] [Google Scholar]
- 61.Merello M, Hughes A, Colosimo C, Hoffman M, Starkstein S, Leiguarda R. Sleep benefit in Parkinson's disease. Mov Disord. 1997;12:506–8. doi: 10.1002/mds.870120405. [DOI] [PubMed] [Google Scholar]
- 62.Bateman DE, Levett K, Marsden CD. Sleep benefit in Parkinson's disease. J Neurol Neurosurg Psychiatry. 1999;67:384–5. doi: 10.1136/jnnp.67.3.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Marinelli L, Crupi D, Di Rocco A, et al. Learning and consolidation of visuo-motor adaptation in Parkinson's disease. Parkinsonism Relat Disord. 2009;15:6–11. doi: 10.1016/j.parkreldis.2008.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Morgan WW, Nelson JF. Chronic administration of pharmacological levels of melatonin does not ameliorate the MPTP-induced degeneration of the nigrostriatal pathway. Brain Res. 2001;921:115–21. doi: 10.1016/s0006-8993(01)03106-7. [DOI] [PubMed] [Google Scholar]
- 65.Zisapel N. Melatonin-dopamine interactions: from basic neurochemistry to a clinical setting. Cell Mol Neurobiol. 2001;21:605–16. doi: 10.1023/A:1015187601628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Marsh JL, Thompson LM. Drosophila in the study of neurodegenerative disease. Neuron. 2006;52:169–78. doi: 10.1016/j.neuron.2006.09.025. [DOI] [PubMed] [Google Scholar]
- 67.Muqit MM, Feany MB. Modelling neurodegenerative diseases in Drosophila: a fruitful approach? Nat Rev Neurosci. 2002;3:237–43. doi: 10.1038/nrn751. [DOI] [PubMed] [Google Scholar]
- 68.McBride SM, Choi CH, Wang Y, et al. Pharmacological rescue of synaptic plasticity, courtship behavior, and mushroom body defects in a Drosophila model of fragile X syndrome. Neuron. 2005;45:753–64. doi: 10.1016/j.neuron.2005.01.038. [DOI] [PubMed] [Google Scholar]
- 69.Garcia-Garcia F, Ponce S, Brown R, Cussen V, Krueger JM. Sleep disturbances in the rotenone animal model of Parkinson disease. Brain Res. 2005;1042:160–8. doi: 10.1016/j.brainres.2005.02.036. [DOI] [PubMed] [Google Scholar]
- 70.Monaca C, Laloux C, Jacquesson JM, et al. Vigilance states in a parkinsonian model, the MPTP mouse. Eur J Neurosci. 2004;20:2474–8. doi: 10.1111/j.1460-9568.2004.03694.x. [DOI] [PubMed] [Google Scholar]
- 71.Almirall H, Pigarev I, de la Calzada MD, Pigareva M, Herrero MT, Sagales T. Nocturnal sleep structure and temperature slope in MPTP treated monkeys. J Neural Transm. 1999;106:1125–34. doi: 10.1007/s007020050228. [DOI] [PubMed] [Google Scholar]