Abstract
Study Objectives:
We investigated if donepezil, a long-acting orally administered cholinesterase inhibitor, would reduce episodic memory deficits associated with 24 h of sleep deprivation.
Design:
Double-blind, placebo-controlled, crossover study involving 7 laboratory visits over 2 months. Participants underwent 4 functional MRI scans; 2 sessions (donepezil or placebo) followed a normal night's sleep, and 2 sessions followed a night of sleep deprivation.
Setting:
The study took place in a research laboratory.
Participants:
26 young, healthy volunteers with no history of any sleep, psychiatric, or neurologic disorders.
Interventions:
5 mg of donepezil was taken once daily for approximately 17 days.
Measurements and Results:
Subjects were scanned while performing a semantic judgment task and tested for word recognition outside the scanner 45 minutes later. Sleep deprivation increased the frequency of non-responses at encoding and impaired delayed recognition. No benefit of donepezil was evident when participants were well rested. When sleep deprived, individuals who showed greater performance decline improved with donepezil, whereas more resistant individuals did not benefit. Accompanying these behavioral effects, there was corresponding modulation of task-related activation in functionally relevant brain regions. Brain regions identified in relation to donepezil-induced alteration in non-response rates could be distinguished from regions relating to improved recognition memory. This suggests that donepezil can improve delayed recognition in sleep-deprived persons by improving attention as well as enhancing memory encoding.
Conclusions:
Donepezil reduced decline in recognition performance in individuals vulnerable to the effects of sleep deprivation. Additionally, our findings demonstrate the utility of combined fMRI–behavior evaluation in psychopharmacological studies.
Citation:
Chuah LYM; Chong DL; Chen AK; Rekshan WR; Tan JC; Zheng H; Chee MWL. Donepezil improves episodic memory in young individuals vulnerable to the effects of sleep deprivation. SLEEP 2009;32(8):999-1010.
Keywords: Sleep deprivation, cholinergic system, episodic memory, prefrontal cortex, parietal cortex, fusiform gyrus, fMRI
SLEEP AND MEMORY ARE STRONGLY INTERLINKED. SLEEP IS VITAL TO MEMORY CONSOLIDATION1–3 AND SLEEP DEPRIVATION RESULTS IN THE IMPAIRMENT OF short-term as well as long-term memory.4 In theory, the impact of sleep deprivation on memory might be reduced by directly influencing its component processes, encoding, consolidation and retrieval, or indirectly by enhancing arousal and/or attention.
The best-known countermeasures, caffeine, amphetamines and modafinil, all boost arousal and vigilant attention5 and to date, the more direct approach has not yielded much success. For example, while CX717, an ampakine, could theoretically improve memory through glutaminergic mechanisms, promising results obtained with primates6 were not similarly realized in a human study.7
Here, we evaluated the enhancement of cholinergic transmission with the long acting cholinesterase inhibitor, donepezil, as a means of ameliorating memory deficits in sleep deprivation, using a combination of behavioral and functional magnetic resonance imaging (fMRI) measures. Augmenting cholinergic transmission could benefit cognition in sleep-deprived individuals via multiple mechanisms: promoting wakefulness,8 through top-down increases in attention,9 increasing the signal-to-noise ratio of processing within visual sensory cortex,10,11 and enhancing LTP in hippocampal circuits.12 Additionally, cholinesterase inhibitors secondarily influence dopaminergic and noradrenergic neurotransmission.13 fMRI provides a noninvasive means of identifying the neuroanatomical locus of drug action that could help discern the functional relevance of these putative mechanisms.14
The neurobehavioral effects of manipulating cholinergic transmission in the context of non-sleep-deprived, healthy adults, performing tasks tapping attention and memory, have been well characterized in several functional imaging studies.15–21 In these studies, increasing cholinergic transmission generally improved attention and short-term memory whereas antagonists like scopolamine induced decline in long-term, episodic memory. Behavior-only studies using donepezil have yielded positive22 and negative23 results.
Reflecting the multiple mechanisms through which acetylcholine can modulate cognition across different tasks,24 the locus of drug effect(s) has varied across different studies. Drug induced modulation of neural activity has been reported in top-down control regions such as the frontal17 and parietal lobes,15,20 regions involved in visual processing such as the extrastriate visual cortex17,25 and the fusiform cortex19 as well as brain regions involved in memory encoding such as the hippocampal formation19,21 and the lateral prefrontal cortex.19
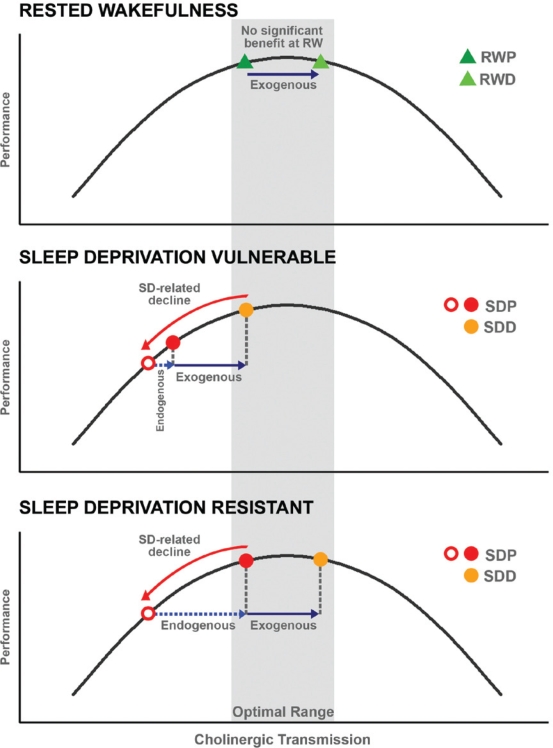
Another source of the varied results in these psychopharmacological-imaging studies is the point along the neurotransmitter signaling continuum that a subject lies. Recent studies involving cholinergic agents in elderly volunteers,15,23 sleep-deprived volunteers,25 as well as a pharmacogenetic study involving modafinil in sleep-deprived persons,26 have reinforced the notion that there exists a bell-shaped response curve to neurotransmitter augmentation.27,28 This is particularly relevant to the present study because the cognitive deficits experienced following sleep deprivation reportedly show trait-like inter-individual variation.29,30 One can reasonably postulate that inter-individual variation in reduced cholinergic drive might mediate vulnerability to sleep deprivation and that there would be a corresponding range of response to exogenous cholinergic augmentation (Supplementary Figure 1, available at www.journalsleep.org).
In this report, we evaluated two hypotheses. Firstly, in accordance with a sister study25 that evaluated visual short-term memory, we anticipated that donepezil would benefit episodic memory performance in persons vulnerable to the effects of sleep deprivation, but not otherwise. Secondly, we predicted that we would find correlation between donepezil-induced modulation of brain activation and memory improvement in left lateral prefrontal cortex, a region that participates in successful word encoding. This would be in line with the thesis that while cholinergic neurons have widespread projections in the cerebral cortex, modulation in brain activation can occur in a task-specific manner.14,31
In consideration of these goals, we conducted a double-blind, placebo-controlled, cross-over functional imaging study (Figure 1) that involved 26 healthy young adult participants. In this within-subject design, volunteers were scanned 4 times, twice following a normal night's sleep and twice after 24 hours of total sleep deprivation. During scanning, participants encoded words while they performed a semantic judgment task. A 5 mg dose of donepezil was compared to placebo. Delayed recognition memory (reflecting episodic memory), non-responses (reflecting attentional lapses), and judgment accuracy at encoding were the main behavioral measures of interest. Task-related activation associated with successfully encoded words was the main imaging variable of interest. As we studied the same subjects in both the present and previous report, we minimized the likelihood that between-subject anatomical variation would confound the localization of drug-induced fMRI signal modulation.
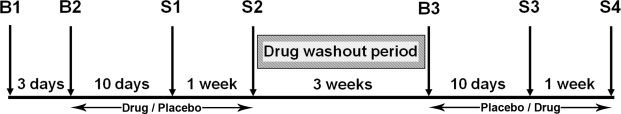
Figure 1.
A schematic of the study timeline. B1, B2, and B3 denote briefing and screening sessions while S1, S2, S3, and S4 denote scanning sessions. The drug (placebo, donepezil) and state (rested wakefulness, sleep deprivation) conditions were counterbalanced across individuals.
METHODS
Participants
Forty-three healthy young adults were recruited through web-based advertisements and were screened by questionnaire and an interview. Prospective participants had to: (1) be right-handed, (2) be between 18 and 35 years of age, (3) have habitual good sleeping habits (sleeping ≥ 6.5 h each night for the past month), (4) have no history of sleep disorder, (5) have no history of any psychiatric or neurologic disorders, (6) have normal color vision, (7) drink < 3 cups of coffee a day, (8) not smoke cigarettes, and (9) score ≤ 22 on a modified Morningness-Eveningness scale.32 Only 12.2% of our potential subjects were excluded on the basis of this criteria (estimated based on 500 individuals who completed our internet-based questionnaire; 1.2% were definitely morning types and the remaining 11% were moderately morning types). Filtering of subjects on the basis of chronotype was intended to reduce this as a source of variance in our study. As recently shown, chronotype can influence fMRI findings.33 The sleeping habits of qualifying participants were monitored using wrist actigraphy and only those who had good sleep habits (i.e., they usually slept no later than 01:00 and woke no later than 09:00) were eligible for brain imaging. All participants were screened for color blindness, and cleared blood and urine biochemistry tests prior to enrollment.
The final sample consisted of 26 participants (13 males, mean age = 22.19 years, SD = 1.02 years). Fifteen did not complete the study: 4 were medically unsuitable; 3 were unsuitable for MR scanning (claustrophobia, large braces); 4 experienced gastrointestinal side-effects; and 4 were unable to maintain a regular sleep-wake schedule. Technical error caused the loss of data for another subject, and one subject was rejected because of an extremely low rate of correct rejects during delayed recognition (range = 0 to 3.29%).
Participants were financially compensated for their time.
Design and Procedure
The experimental protocol was approved by the Singapore General Hospital IRB. This double-blind, placebo-controlled, crossover study involved 7 visits to the laboratory over a period of about 2 months (Figure 1). The experiment was conducted minutes after experiments that evaluated visual short-term memory and visual perception.25
Three of the visits were briefing sessions. The 4 remaining sessions were test/scanning sessions. Two of these test sessions were conducted following a night of normal sleep (or rested wakefulness) and the remaining 2 sessions followed a night of sleep deprivation. Scanning always took place at fixed times for both rested wakefulness and sleep deprivation sessions. The order of the scanning sessions (rested wakefulness followed by sleep deprivation and vice versa) was counterbalanced across all subjects. A pharmacist who was not involved in data collection or analysis dispensed the tablets (5 mg donepezil/matched placebo) in a counterbalanced manner across all subjects. Compliance to the drug schedule was checked daily by telephone and further verified with tablet counts that were conducted before every scanning session.
During Briefing Session 1 (B1), participants were provided with detailed information about the aims and requirements of the study and gave informed consent before undergoing a physical examination and blood tests.
Briefing Session 2 (B2) took place approximately 3 days after B1 and involved subjects who passed the medical and lab test screens. Participants' sleeping patterns were verified by sleep diary and wrist actigraphy. At the end of this session, participants were given tablets (either 5 mg donepezil or placebo) with instructions to take one a day at 09:00 and to record this. Participants took the tablets daily for an average of 10 days prior to their first scan session and continued taking the same tablets for another week until the end of the second scanning session. There was a 3-week washout period between each drug phase.
Briefing Session 3 (B3), post washout, took place about 3 weeks following the second scanning session. Participants collected tablets and their compliance with the sleep schedule was verified. During the second drug phase, subjects took either donepezil or placebo daily for an average of 10 days prior to the third scan and continued taking the same tablets daily for a week until their fourth scan.
Rested Wakefulness Sessions
Subjects arrived at the lab at approximately 07:45. Adequate sleep was verified by checking the volunteer's sleep diary and wrist actigraph. Compliance to the medication schedule was checked. Subjects were questioned on their health and well-being and for any possible side effects from the tablets. Intake of other medications was also documented. All participants verified that they had not smoked, consumed any medications, stimulants, alcohol or caffeine for at least 24 h prior to scanning. Subjects also completed a 10-min trial on the psychomotor vigilance task (PVT).34,35
Participants in this study also took part in the experiment that evaluated visual short-term memory and visual perception. After that experiment was completed, within the same session, volunteers were taken out of the scanner and took a 10-min break before participating in the current study. Prior to re-entering the scanner, they practiced the word classification (encoding) task. Subjects were informed that there would be a delayed recognition component but were instructed to focus on classifying the words. Self-ratings of sleepiness were taken using the Karolinska Sleepiness Scale (KSS) prior to scanning, after each in-scanner run and prior to the recognition component. Scanning for this task took place at approximately 09:00.
Sleep Deprivation Sessions
Subjects were required to report to the laboratory no later than 19:00 on the test night. Sleep habits, compliance with tablet intake, intake of other medications, possible side effects, and general health were checked as described for the rested wakefulness session. Participants were monitored throughout the night and were only allowed to engage in non-strenuous activities such as reading, working on a computer and conversing. Every hour from 20:00 to 05:00, participants also completed a 10-min PVT trial as described earlier. Subjects verified that they had not taken any medications, stimulants, alcohol and caffeine for 24 h prior to scanning. Practice trials on the in-scanner task and the KSS were administered in the same manner as described for the rested wakefulness session. Scanning for the episodic memory task took place at approximately 06:30, around the circadian nadir for most persons. While this time is not identical to the one used during rested wakefulness scans, most of the effects of interest in this study accrue from extended wakefulness rather than circadian phase. Further, carefully conducted studies have shown than the difference in sustained attention performance between 06:00 and 09:00 after a night of sleep deprivation are small.36 Finally, we chose this time because most vehicular accidents following SLEEP DEPRIVATION occur most frequently at 02:00 and 06:00.37
Experimental Task
Participants were scanned while performing a semantic judgment task in an event-related fMRI experiment. Recognition memory was tested out of scanner 45 min after completion of scanning.
The test set comprised 720 English, concrete nouns obtained from the MRC Psycholinguistic Database (http://www.psy.uwa.edu.au/mrcdatabase/uwa_mrc.htm). Ninety target words were used at each testing session. There were equal numbers of low, medium, and high frequency words. These words were matched for concreteness, familiarity, and imageability across all 4 scanning sessions. An additional set of 90 new words was used as foils during the delayed recognition component of the task. Each word was used only once throughout the experiment. Experience with this task and with this word database in our laboratory has been previously reported.38–40
At encoding, participants decided if a presented word depicted a living or non-living entity, and responded by pressing a “yes” or “no” button. Each word appeared for a maximum of 2 s and was replaced with a fixation cross immediately after a response. Stimulus onset asynchronies (the time interval between the onset of successive stimuli) of 2.5, 5, or 7.5 s were used. Participants had up to 2.5 s to respond on each trial. There were 2 encoding runs, and each encoding run involved the acquisition of 101 functional scans. Ninety different words were presented across the 2 runs, with 45 words in each run. Performance was continuously monitored, and participants were verbally prompted if they failed to respond to 2 consecutive words.
The delayed recognition component of the task was conducted outside the scanner approximately 45 min after encoding. Participants were shown 90 target words and 90 foils and asked to respond according to 3 response categories: (1) confident that the word was old (seen previously during encoding), (2) thought the word was old, but not confident, and (3) that the word was new (not seen previously during encoding).41,42
Imaging Procedure
Stimuli were projected onto a screen using a LCD projector (Epson EMP 7250, Epson Corp, Japan) and viewed through a rearview mirror. Participants responded using a button box held in the right hand. A bite bar and foam padding were used to reduce head motion. Images were acquired on a 3T Siemens Allegra system (Siemens, Erlangen, Germany). A single-shot gradient-echo, EPI sequence was used (TR: 2500 ms; TE: 30 ms; flip angle 90°; FOV: 192 × 192 mm; Matrix: 64 × 64). Thirty-two oblique axial slices (3-mm thick; 0.3-mm inter-slice gap) approximately parallel to the AC-PC line were acquired. High-resolution T1 coplanar anatomical images were also obtained. For the purpose of image display on Talairach space, further 3D high-resolution anatomical reference images were acquired using a T1-weighted MPRAGE sequence.
Data Analysis
Behavioral data
Number of non-responses, accuracy of classification and response time were measured during encoding (Table 1). Accuracy of classification was assessed on trials where subjects made a valid response (reaction time ≥ 100 ms). Reaction time was averaged across all valid trials.
Table 1.
Classification Accuracy During Encoding According to State, Drug and Recognition Success (N = 26)
| Classification Accuracy (%) |
|||||
|---|---|---|---|---|---|
| Non-responses (%) | Overall | HC Hits | LC Hits | Miss | |
| Placebo | |||||
| RWP | 1.41 (2.05) | 88.45 (0.06) | 88.93 (6.46) | 93.57 (7.73) | 86.68 (15.26) |
| SDP | 9.62 (9.60) | 91.03 (6.80) | 92.82 (5.52) | 88.82 (11.54) | 83.33 (20.66) |
| Donepezil | |||||
| RWD | 2.22 (3.85) | 91.81 (6.85) | 92.68 (5.30) | 88.79 (22.71) | 87.11 (17.16) |
| SDD | 8.50 (12.09) | 86.78 (9.03) | 87.83 (8.38) | 81.58 (23.56) | 87.41 (15.00) |
Percentage of non-responses was computed as a percentage of all trials during encoding (i.e., 90). Trials on which subjects made advances (i.e., RT < 100 ms) were also classified as non-responses. Overall classification accuracy was computed as a percentage of trials to which subjects made a valid response during encoding (i.e., RT ≥ 100 ms). Classification accuracy for hits and misses was computed as a percentage of trials to which subjects made valid responses of that category during recognition. Standard deviations are reported in parentheses.
RWP: rested wakefulness placebo; RWD: rested wakefulness donepezil; SDP: sleep deprivation placebo; SDD: sleep deprivation donepezil; HC: high confidence; LC: low confidence.
Delayed recognition was indexed using corrected recognition (Hit Rate - False Alarm Rate).41,43,44 Only trials responded to during encoding were analyzed for delayed recognition. All trials with response times < 100 ms were considered invalid. Corrected recognition was determined using only high confidence responses.41,42 Such responses discriminated previously presented words from foils (P < 0.001 for all test sessions) whereas with low confidence responses, there were more false alarms than hits in all 4 conditions (Table 2). A', a nonparametric measure of recognition memory45 is another commonly used measure of delayed recognition. The 2 measures correlated highly within each test session (r = 0.96 to 0.99), and we arbitrarily used the former metric.
Table 2.
Delayed Recognition Performance According to State, Drug and Recognition Success (N = 26)
| Delayed Recognition (%) |
|||||||
|---|---|---|---|---|---|---|---|
| Targets |
Lures |
CR | |||||
| Hhits | Lhits | Miss | HFA | LFA | Crej | ||
| Placebo | |||||||
| RWP | 75.26 (11.14) | 9.14 (9.99) | 15.60 (10.36) | 12.17 (12.16) | 21.17 (18.43) | 66.66 (22.71) | 0.63 (0.16) |
| SDP | 64.68 (18.44) | 15.05 (14.21) | 20.27 (16.07) | 15.99 (12.57) | 24.52 (19.61) | 59.48 (22.44) | 0.49 (0.19) |
| Donepezil | |||||||
| RWD | 75.34 (13.13) | 10.33 (11.72) | 14.33 (11.81) | 12.02 (10.86) | 22.70 (21.29) | 65.28 (26.13) | 0.63 (0.17) |
| SDD | 68.55 (17.65) | 12.50 (12.25) | 18.94 (15.91) | 15.88 (9.22) | 24.22 (19.95) | 59.89 (22.46) | 0.53 (0.17) |
Ninety targets and 90 lures were present at delayed recognition. However, only words that were responded to during encoding were treated as valid trials. Performance in each condition was computed as a percentage of the number of valid trials. Standard deviations are reported in parentheses.
RWP: rested wakefulness placebo; RWD: rested wakefulness donepezil; SDP: sleep deprivation placebo; SDD: sleep deprivation donepezil; Hhits: high confidence hits; Lhits: low confidence hits; Crej: correct rejections; NR: non-responses; HFA: high confidence false alarms; LFA: low confidence false alarms; CR: corrected recognition (high confidence hit rate – high confidence false alarm rate).
The effects of state and drug on behavior (encoding and recognition) were explored using 2 (state: rested wakefulness, sleep deprivation) by 2 (drug: placebo, donepezil) repeated-measures ANOVA conducted using SPSS 13.0 (SPSS, Chicago, IL). (RWP: rested wakefulness placebo; RWD: rested wakefulness donepezil; SDP: sleep deprivation placebo; SDD: sleep deprivation donepezil). To determine if vulnerability to sleep deprivation affected response to donepezil, participants were divided into 2 groups (median split), according to how their performance declined following sleep deprivation when unmedicated (i.e., SDP-RWP). The ANOVA described above was repeated with vulnerability as a between-subjects factor. This analysis was performed separately for non-responses and correctly recognized words so that it would be possible to determine how lapses of attention and episodic memory might be affected differently by sleep deprivation.
Imaging Data
Functional images were preprocessed and analyzed using Brain Voyager QX version 1.10.3 (Brain Innovation, Maastricht, The Netherlands). Data preprocessing included correcting for inter-slice timing differences using trilinear sinc interpolation, linear trend removal, and temporal high-pass filtering of period 83 s to remove low frequency non-linear drifts of 3 or fewer cycles per run. Three-dimensional rigid-body motion correction across runs was performed using the first image of the second functional run as the reference image. Spatial smoothing was performed using an 8-mm Gaussian kernel (full-width at half-maximum). Functional slices were co-registered to the MPRAGE anatomical volume and transformed into Talairach space.
Group statistical maps were obtained using a 2-level, multi-subject general linear model (GLM) approach. At the first level, the stimulus-related BOLD response was estimated for every individual using a finite-impulse-response (FIR) model with 35 predictors, 7 predictors for each of 5 conditions. The first 4 conditions (RWP, RWD, SDP, SDD) included only valid, high confidence hit trials. This was to avoid taking into consideration trials where a volunteer could have been asleep. The last condition contained all other events (i.e., low confidence hits, misses, and non-response trials).
To account for baseline drifts across runs and between experimental sessions, z-transformation of the signal time-courses for each run was performed. The obtained β values for each predictor in every individual served as the input for a second-level, random effects analysis.
Task-relevant regions were identified from voxels that showed significant signal change relative to baseline, for both RWP and RWD conditions (conjunction of 2 t-contrasts). Voxel-wise β values were subjected to a 2 (state) by 2 (drug), within-subject ANOVA to identify regions that showed main effects of state, drug and their interaction. These analyses involved time-points around the empirically determined peak of activation (7.5 and 10 s).
To control for type I error, voxels were processed using an iterative cluster size thresholding procedure46 that considered the spatial smoothness of functional imaging data when generating activation maps based on a corrected cluster threshold (P < 0.05). This still yielded voxels above threshold and additionally, a voxel level threshold of P < 0.0001 (uncorrected) for t maps and P < 0.005 (uncorrected) for F maps was applied.
As no region showed a main effect of drug, we performed region-of-interest (ROI) based analyses on areas that showed significant task-related activation in both RWP and RWD conditions. We examined activation magnitude in these ROI for significant correlations between state and drug-driven changes in behavior and the corresponding changes in activation. Only correlations that remained significant at P < 0.05 after the removal of influential outliers (n = 1) was reported (i.e., N = 25).
RESULTS
Behavioral Findings
Encoding
As might be expected, we observed very few non-responses during encoding at rested wakefulness. These increased significantly following sleep deprivation, F1,25 = 17.01, P < 0.001 (Table 1). As observed with the same subjects previously,25 there was neither a main effect of drug, F1,25 = 0.02, P = 0.88, nor an interaction between state and drug, F1,25 = 1.91, P = 0.18.
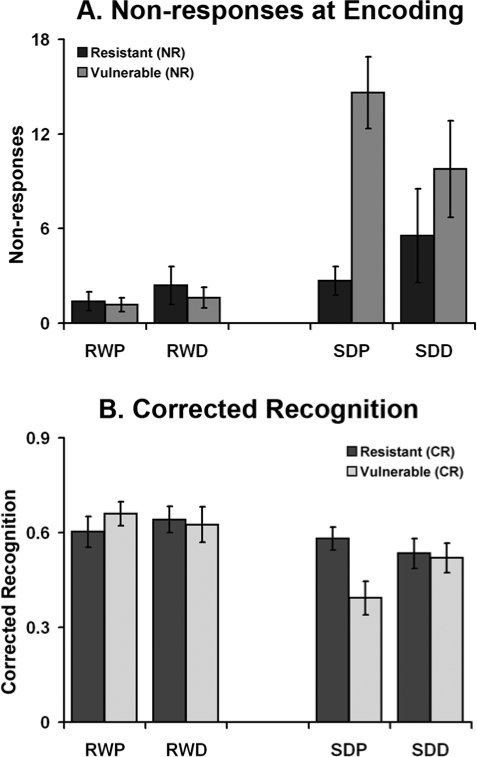
Critically, when vulnerability was included as a between-subjects factor (vulnerability to sleep deprivation here refers to an increase in non-responses), there was a significant interaction between state, drug, and vulnerability, F1,24 = 11.60, P = 0.002 (Figure 2A). This interaction was driven by the number of non-responses following sleep deprivation, F1,24 = 8.73, P = 0.007, but not at rested wakefulness, F1,24 = 0.39, P = 0.54. Paired t-tests showed that donepezil reduced non-responses in sleep-deprived subjects who would otherwise deteriorate if untreated, t12 = 3.79, P = 0.003. This benefit was not present in the more sleep deprivation resistant half of the subjects, t12 = 1.26, P = 0.23 (Figure 2A). Neither subgroup showed treatment effects at rested wakefulness (smaller P = 0.17).
Figure 2.
The extent to which donepezil modulated performance following sleep deprivation was dependent on the extent to which performance declined following sleep deprivation in the untreated condition. Volunteers whose task performance declined following sleep deprivation (Vulnerable) showed drug-related benefit, while those whose performance did not deteriorate following sleep deprivation (Resistant) showed little performance benefit and even marginal decline. No benefit of drug was present at rested wakefulness. RWP: rested wakefulness placebo; RWD: rested wakefulness donepezil; SDP: sleep deprivation placebo; SDD: sleep deprivation donepezil; NR: Non-responses; CR: Corrected Recognition.
Accuracy at encoding was not significantly reduced following sleep deprivation, F1,25 = 1.97, P = 0.17 (Table 1). Hence, when volunteers were not lapsing, they seemed to perform the task accurately. There was no main effect of drug, F1,25 = 0.20, P = 0.66.
Reaction time increased following sleep deprivation, F1,25 = 26.12, P < 0.001 (Supplementary Table 1, available at www.journalsleep.org). There was no significant effect of drug or interaction between state by drug (smaller P = 0.73). No additional effects emerged when vulnerability was added as a between-subjects factor.
Recognition
Sleep deprivation resulted in significant decline in delayed recognition, F1,25 = 31.24, P < 0.001 (Table 2). There was no main effect of drug F1,25 = 0.74, P = 0.40, and no interaction between state and drug, F1,25 = 0.82, P = 0.37.
When vulnerability (defined as the drop in corrected recognition following sleep deprivation) was included as a between-subjects factor, there were significant interactions between state and vulnerability, F1,24 = 10.09, P = 0.004 and between state, drug, and group, F1,24 = 13.10, P = 0.001 (Figure 2B). Separate ANOVA for each state revealed significant interaction between drug and vulnerability in the sleep-deprived condition, F1,24 = 6.16, P = 0.02, but not at rested wakefulness, F1,24 = 2.34, P = 0.14. Post hoc t tests showed that, as was the case for non-responses, sleep deprivation vulnerable subjects showed improved recognition when on donepezil following sleep deprivation, t12 = 3.50, P = 0.004, whereas the sleep deprivation resistant half did not benefit, t12 = 0.78, P = 0.45.
Dissociation of State and Drug Effects of Donepezil
State-related shifts in non-response and corrected recognition scores across subjects in the untreated condition (i.e., RWP-SDP) were not correlated (r = −0.32, P = 0.11). However, donepezil-mediated shifts in the corresponding scores in the sleep-deprived condition (i.e., SDP-SDD) were significantly correlated even after the removal of an influential outlier (r = − 0.46, P < 0.02, for N = 25). These findings suggest that sleep deprivation can have separable effects on lapses in attention (non-responses) and episodic memory (corrected recognition). However, in the context of sleep deprivation, donepezil affects both performance measures to a similar extent in any given individual.
Relationship Between Subjective Sleepiness and Performance
Subjective sleepiness was significantly higher in sleep-deprived subjects at both encoding, F1,25 = 224.60, P < 0.001, and delayed recognition, F1,24 = 142.45, P < 0.001 (Supplementary Table 2, available at www.journalsleep.org) but there were no significant effects of drug. There were no significant correlations between the change in KSS scores and changes in either behavioral measure (non-responses or corrected recognition) across state (i.e., RWP-SDP; smallest P = 0.14). There were also no significant correlations between these variables following donepezil treatment in the sleep deprivation condition (i.e., SDD-SDP; smallest P = 0.15).
Thus increases in subjective sleepiness did not correlate with performance change, regardless of whether a subject was on drug or placebo. Hence, while donepezil may modify cognitive performance, it does not appear to influence subjective feelings of sleepiness.25
Neuroimaging Findings
Areas Activated for High Confidence Hits
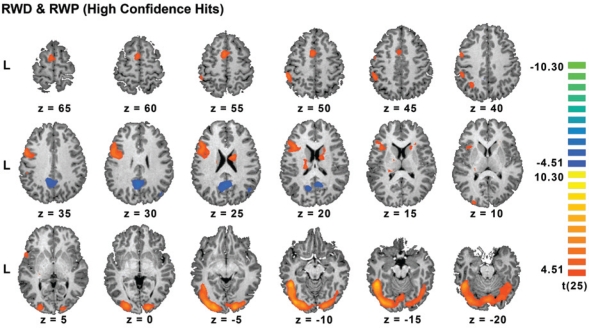
Following a normal night of sleep, high confidence hits in both rested wakefulness conditions elicited activation of the inferior frontal gyrus, pre-supplementary motor area, inferior and superior parietal cortex (all left hemisphere predominant). The thalamus, fusiform gyrus, and ventral occipital cortex were activated bilaterally. Additionally, there was significant deactivation in the posterior cingulate/precuneus bilaterally and in the right temporal parietal junction (Figure 3, Supplementary Table 3, available at www.journalsleep.org). These findings concur with the results of prior fMRI studies on episodic memory.39,41,42,47–49
Figure 3.
Task-related activation associated with high confidence hits following a normal night of sleep (conjunction of RWP (rested wakefulness placebo) and RWD (rested wakefulness donepezil)). All clusters passed a voxel-level threshold of P < 0.0001 (uncorrected).
State-Related Change in Activation
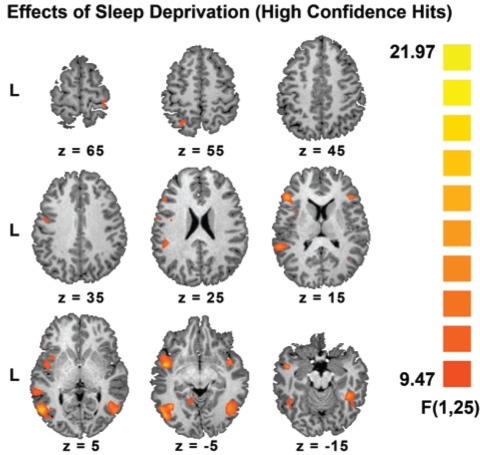
Sleep deprivation attenuated task-related activation within the left inferior frontal gyrus, insula, inferior temporal region, intraparietal sulcus and the fusiform gyri bilaterally (Figures 4 and 5, Supplementary Table 4, available at www.journalsleep.org).
Figure 4.
Regions that showed significant effects of state in a state by drug ANOVA. All clusters passed a voxel-level threshold of P < 0.005 (uncorrected). There were no significant effects of drug.
Figure 5.
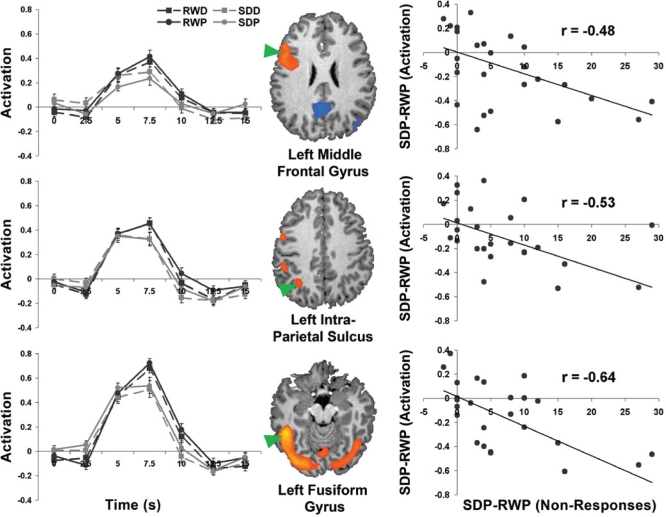
The graphs in the left panel depict encoding-related signal change (± 1 SEM) in brain regions depicted alongside. Task-related activity in these regions was altered during sleep deprivation. Sleep deprivation related change in the number of non-responses (SDP-RWP) at encoding correlated with sleep deprivation related change in activation in the left middle frontal gyrus, the left intraparietal sulcus and the left fusiform gyrus (right panel). Similar correlations were present in 2 ROIs within the inferior frontal gyrus (r = −0.47), the left pre-supplementary motor cortex (r = −0.64), and right fusiform gyrus (r = −0.47). RWP: rested wakefulness placebo; SDP: sleep deprivation placebo.
Relationship Between Sleep Deprivation Related Increase in Non-Responses and Activation in Areas Associated with High Confidence Hits
Previous studies suggest that cognitive decline following sleep deprivation may be contributed by processes that also increase lapses in attention. This led us to examine the relationship between sleep deprivation induced increase (SDP-RWP) in non-response rates and activation magnitude in regions associated with high confidence hits. We found significant correlation between behavioral and imaging metrics in the left middle and inferior frontal gyrus, the intraparietal sulcus, bilateral fusiform gyrus, and the pre-supplementary motor area (Figure 5).
Donepezil Did Not Modulate Brain Activation in Every Sleep-Deprived Subject
In concert with the behavioral findings, ANOVA revealed no main effect of drug on hit-related brain activation. Additionally, the influence of donepezil in each state evaluated by paired comparisons (RWD vs. RWP as well as SDD vs. SDP) also yielded null results. These findings raised 2 possibilities. Either there was no relationship between drug effect and task-related brain activation, or the relationship was masked by inter-individual responses such that an increase in activation in donepezil responders was negated by decreased activation in non-responders.
Donepezil Modulated Activation According to How a Subject Performed When Sleep Deprived
To test this, we evaluated the correlation between activation change associated with the SDD-SDP contrast and corresponding changes in behavior. This analysis was conducted separately for non-responses at encoding and corrected recognition. The analyses were performed on regions known to be involved in successful encoding after a normal night of sleep to ensure that they would be functionally meaningful. Further, in analyzing only successful encoding trials, we reduced the likelihood of including trials where the volunteer was momentarily asleep.
Non-Responses
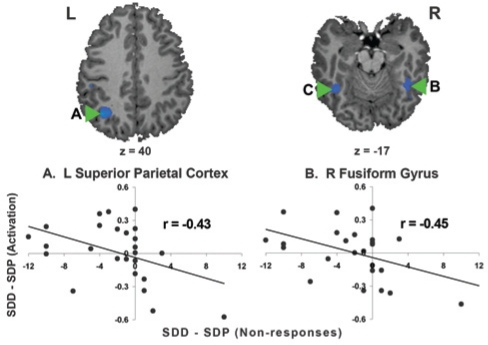
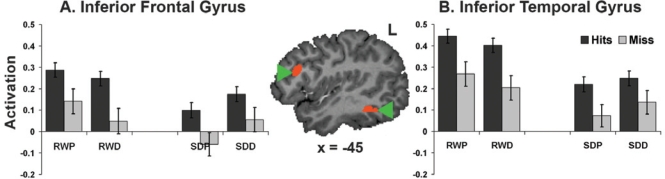
Following the removal of a significant outlier (i.e., N = 25), we found significant correlations between donepezil-induced changes (SDD-SDP) in non-responses and donepezil-related alterations in activation within the left superior parietal cortex (Figure 6A), right fusiform gyrus (Figure 6B), left fusiform gyrus and left ventral occipital cortex.
Figure 6.
Declines in non-responses with donepezil following sleep deprivation (SDD–SDP) correlated (P < 0.05) with activation increases in (A) the left superior parietal cortex (r = −0.43), (B) right fusiform gyrus (r = −0.45), (C) left fusiform gyrus (r = −0.43) and left ventral occipital cortex (r = −0.44). SDD: sleep deprivation donepezil; SDP: sleep deprivation placebo. Note that N = 25 following the exclusion of an outlier.
Corrected Recognition
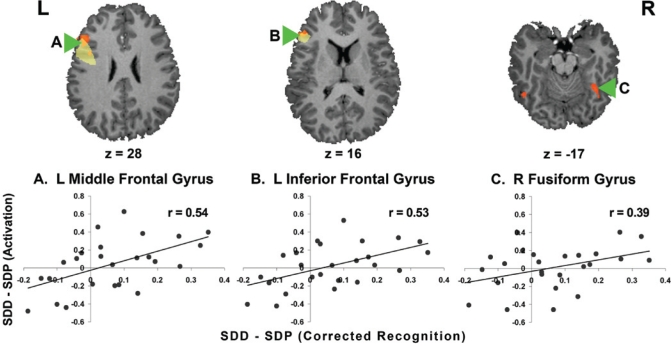
Corresponding significant associations between drug-related improvements in corrected recognition were found in the left middle and inferior frontal gyri (Figure 7A and B). These regions overlap with regions that show a subsequent memory effect in a fixed effects analysis (Supplementary Figure 2, available at www.journalsleep.org). Activation of the right fusiform (Figure 7C) also correlated with donepezil-mediated changes in corrected recognition. There was a marginal, nonsignificant correlation with the left superior parietal ROI mentioned above (r = 0.37, P = 0.07).
Figure 7.
Donepezil-related modulation in corrected recognition correlated (P < 0.05) with activation in (A) the left middle prefrontal cortex (r = 0.54), (B) the left inferior frontal gyrus (r = 0.53), and (C) the right fusiform gyrus (r = 0.39). The highlighted frontal regions overlapped (orange) with those showing a subsequent memory effect (yellow; Supplementary Figure 2 available at www.journalsleep.org). SDD: sleep deprivation donepezil; SDP: sleep deprivation placebo. Note that N = 25 following the exclusion of an outlier.
Absence of Effects in RW
Similar analyses were conducted for possible donepezil-related alterations in activation at RW (RWD-RWP). None of the aforesaid regions showed significant behavior/activation correlations of the type described for the sleep-deprived state.
DISCUSSION
There were three main findings in this present study. The first was that donepezil attenuated decline in verbal episodic memory in accordance with an individual's vulnerability to cognitive impairment following sleep deprivation. Donepezil did not alter episodic memory following a night of habitual sleep. Secondly, treatment-related changes in behavior correlated with corresponding alterations in encoding-related activity within task-relevant regions, suggesting that fMRI can be a functionally relevant imaging probe that tracks drug-related changes in activation. Thirdly, donepezil-related improvement of cognitive performance in the context of sleep deprivation may be attributed to both reduced lapses of attention as well as improved encoding but not to reduced subjective sleepiness.
Vulnerability to Sleep Deprivation Influences the Behavioral Effects of Donepezil
Donepezil improved performance in persons who declined in performance when sleep deprived but not in those who were relatively unaffected by this manipulation. Vulnerable individuals might benefit from exogenous cholinergic augmentation because of the effects of reduced cholinergic transmission arising from sustained wakefulness (Supplementary Figure 1, middle panel, available at www.journalsleep.org). In contrast, persons with relatively preserved behavior despite sleep loss may generate elevated (“compensatory”) task-related activation when sleep deprived50,51 that may relate to an endogenous elevation of cholinergic transmission. As such additional exogenous augmentation would result in little benefit or even slight behavioral decline27,28 (Figure 6; Supplementary Figure 1, bottom panel, available at www.journalsleep.org).
Concern may be raised regarding the absence of donepezil effects in rested wakefulness. However, prior studies on healthy, non-sleep-deprived adults have reported both negative23 and positive22 effects on episodic memory. The latter study argued that it takes at least three weeks of continuous administration for donepezil to elicit behavioral benefit and this could contribute to the null effect in rested wakefulness observed here.
It is noteworthy that while enhancing cholinergic transmission is the primary mode of action of donepezil, cholinesterase inhibitors also influence noradrenergic and dopaminergic transmission13 that are relevant in maintaining wakefulness as well as performance in sleep-deprived persons.26,52 Neurotransmission involving these other substances is altered following sleep deprivation and could explain the aforementioned state differences in donepezil effect in some individuals (see ref. 52 in relation to dopamine). Further, the various cholinergic agonists and antagonists used in functional imaging studies to date affect these transmitters to differing degrees.13 As such, while there is considerable evidence concerning how cholinergic augmentation can benefit attention and memory,53–55 some caution is in order in attributing the observed effects in the current studies solely to the cholinergic system.
Functionally Relevant Relationships Between fMRI Signal and the Effects of State and Drug Highlight Its Value as a Functional Probe
The within-subject design of this study controlled for confounds that could arise from inter-individual variation in response to sleep deprivation, donepezil or both these manipulations. Additionally, imaging the same volunteers as they performed tasks from different cognitive domains allowed us to differentiate task-independent and task-dependent effects on brain activation.
We found brain regions where donepezil modulated behavior and brain activation in a consistent manner across visual short-term memory, visual perceptual control,25 and episodic memory tasks. These were the intraparietal sulcus that mediates attentional control and bilateral extrastriate regions involved in visual sensory processing. The effect of donepezil on attention (measured by the non-response rate) was reproduced across different tasks (correlation with non-response rate between the present experiment and previously reported tasks were: r = 0.81, P < 0.001 for the short-term memory task and r = 0.58, P = 0.002 for the visual perceptual control task).
In the current study, we also found significant correlation between left inferior prefrontal activation at encoding, and recognition memory that was not previously observed with the visual short-term memory and perceptual control tasks. This represents a task-dependent correlation between brain activation and behavior that may relate specifically to episodic memory.
Our findings support the notion that neurotransmitter modulation can alter brain activation and behavior in a task-specific manner,14,16,31,56 even though neurotransmitter systems mediating wakefulness and arousal, such as the cholinergic system, have very widespread representation throughout the cerebral cortex. Further, the neuroanatomically and psychologically plausible correlations between drug-dependent change in activation and behavior strengthen the case for fMRI serving as a dependable probe in psychopharmacological studies.27,57
Donepezil May Benefit Memory in Sleep-Deprived Persons Through Improvements in Attention and Memory Encoding
The locus at which donepezil altered brain activation in the sleep-deprived state was dependent on whether trials relating to non-responses or recognition were analyzed, yielding clues concerning the underlying mechanism of drug benefit. Activation-behavior correlation for non-responses during encoding was found in the intraparietal sulcus, which mediates attentional control, and bilateral visual extrastriate regions involved in visual sensory processing.
Attention during encoding strongly influences later memory performance58 and numerous functional imaging studies attest to the importance of the parietal cortex in mediating the control of multiple aspects of visual attention.59–61 The downstream effects of attention manifest in the form of greater activation (as well as increased neuronal firing) in the ventral visual cortex in response to attended stimuli59,62,63 and reduced neuronal firing64 in response to ignored stimuli. As such, the effect of donepezil on the aforesaid regions could be to counteract sleep deprivation mediated diminution of activation in the same regions (Figures 4, 5). The current findings together with those relating to the visual short term memory and visual perceptual control tasks25 support the hypothesis that attention and sensory processing are task-independent mechanisms by which cholinergic augmentation could improve cognitive performance.65,66
On the other hand, the finding of activation-behavior correlation in the left lateral prefrontal region that coincidentally showed subsequent memory effects (Figure 7 and Supplementary Figure 2, available at www.journalsleep.org), suggests that donepezil may elicit a task-dependent benefit on episodic memory. However, this surmise should be interpreted cautiously as current opinion is divided as to whether acetylcholine has direct effects on memory22,67 or if these are mediated via indirect effects on attention24 or some combination of attention and memory.68,69
Significance of Reduced Encoding-Related Activation During Sleep Deprivation
Although the left inferior prefrontal signal associated with word encoding was lower following sleep deprivation relative to rested wakefulness, the subsequent memory effect on activation was present in both states (Supplementary Figure 2, available at www.journalsleep.org). Had we not excluded invalid encoding trials, it might be argued that reduced activation could simply have been a consequence of the subject falling asleep. However, this was not the case. A general reduction in blood flow is also unlikely as we have previously shown that the visual cortex responds to alternating check patterns normally under sleep deprivation conditions similar to the current experiment.70
To account for the reduced “successful encoding signal” following sleep deprivation, we speculate that the reduced activation represents some processing elements going “off-line” under the condition of sleep deprivation in a manner that, while not affecting tested memory performance, may compromise encoding so as to affect long-term consolidation. Some effects on memory consolidation may take longer than the experimental period (up to months) to reveal.71,72 A useful metaphor arises from the concept of “safety factor” in neuromuscular transmission.73 Transmission remains effective under various physiological conditions because the amount of transmitter released per nerve impulse is greater than that required to minimally trigger a muscle action potential. Along this line of reasoning, we posit that some of the “redundant” activation observed in association with task performance after a normal night of sleep could serve to ensure robust encoding into long-term memory.
Conclusion
Donepezil reduced decline in recognition performance in individuals vulnerable to the effects of sleep deprivation by improving both attention and enhancing encoding. Additionally our findings demonstrate the utility of combined fMRI–behavior evaluation in psychopharmacological studies.
DISCLOSURE STATEMENT
This work was funded by the Neuroscience Centre of Excellence of Drug Discovery, GlaxoSmithKline (Contract Number 007091) and the Defense Science and Technology Agency, Singapore (POD0713897). The authors indicated that, except for the above research funding, and income received from their primary employer, no other financial support or compensation has been received from any individual or corporate entity over the past 24 months for research or professional services and that there are no personal financial holdings that could be perceived as constituting a potential conflict of interest.
ACKNOWLEDGMENTS
Enhui Yong helped with data collection. We thank Vincenzo Libri, Robert Lai and Martin Pan from GSK for their helpful comments and discussions.
Institution at which work performed: Cognitive Neuroscience Lab, Duke-NUS Graduate Medical School Singapore
Appendices
Supplementary Figure 1.
A schematic illustrating how inter-individual differences in cholinergic transmission might affect behavioral responses to donepezil during rested wakefulness and following sleep deprivation. When young, healthy participants are well rested (top panel), cholinergic neurotransmission is likely to be within the optimal range and further modulation may not elicit further benefit (top panel). Following sustained wakefulness, cholinergic transmission may decline together with cognitive performance (red arrow, red open circle). As such, individuals who are vulnerable to cognitive decline when sleep deprived (middle panel) may benefit from exogenous cholinergic augmentation (middle panel, orange circle). In contrast, there are resistant individuals who show either no decline or minimal cognitive decline after sleep deprivation (bottom panel). Tolerance to the effects of sleep deprivation may be accompanied by elevated task-related brain activation. When sleep deprived, these individuals may manifest endogenous phasic elevation of cholinergic transmission (bottom panel, red solid circle). As such, further exogenous elevation of cholinergic transmission may result in no significant cognitive benefit or even a slight decline in behavioral performance. RWP: rested wakefulness placebo; RWD: rested wakefulness donepezil; SDP: sleep deprivation placebo; SDD: sleep deprivation donepezil.
Supplementary Figure 2.
The subsequent memory effect (high confidence hits > high confidence misses) was present in the left lateral prefrontal cortex (middle and inferior frontal gyri) and left middle temporal gyrus for all four conditions. The low number of miss trials during rested wakefulness (approximately half the subjects had fewer than 10 % misses), precluded a random effects analysis and a fixed-effects analysis was performed. The left frontal regions showing this effect overlapped substantially with those described earlier in relation to corrected recognition (Figure 7).
Supplementary Table 1.
Mean Response Time in ms (and standard deviation) at Encoding and Recognition Sorted by State, Drug and Recognition Success
| Encoding |
Delayed Recognition |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| All | Targets |
All | Targets |
Lures |
|||||||
| Hhits | Lhits | Miss | Hhits | Lhits | Miss | HFA | LFA | Crej | |||
| Placebo | |||||||||||
| RW | 955 (221) | 955 (219) | 939 (240) | 933 (275) | 918 (171) | 809 (111) | 1220 (358) | 992 (244) | 884 (234) | 1210 (300) | 970 (182) |
| SD | 1058 (170) | 1059 (179) | 1007 (188) | 1144 (355) | 951 (185) | 864 (113) | 1159 (305) | 1040 (286) | 935 (247) | 1151 (287) | 1008 (227) |
| Donepezil | |||||||||||
| RW | 943 (187) | 945 (203) | 909 (219) | 926 (206) | 902 (131) | 808 (93) | 1132 (272) | 1020 (235) | 990 (312) | 1126 (248) | 941 (137) |
| SD | 1056 (253) | 1056 (278) | 1033 (212) | 1053 (304) | 932 (106) | 860 (100) | 1159 (223) | 991 (242) | 942 (145) | 1146 (210) | 967 (135) |
For delayed recognition, only trials responded to during encoding were considered valid target trials. All reaction time responses below 100 ms were considered invalid trials and were not analysed.
RW: Rested Wakefulness; SD: Sleep Deprivation; P: Placebo, D: Donepezil; HHits: High Confidence Hits; Lhits: Low Confidence Hits; Crej: Correct Rejections; HFA: High Confidence False Alarms; LFA: Low Confidence False Alarms. N = 26.
Supplementary Table 2.
Mean Subjective Sleepiness (and Standard Deviation) Measured on the Karolinska Sleepiness Scale (KSS) as a Function of State (RW: Rested Wakefulness; SD: Sleep Deprivation) and Drug (Placebo; Donepezil)
| Placebo |
Donepezil |
|||
|---|---|---|---|---|
| RW | SD | RW | SD | |
| Encoding | 4.38 (1.48) | 7.87 (0.91) | 4.38 (1.49) | 7.55 (1.11) |
| Recognition | 4.96 (1.61) | 8.50 (0.86) | 4.50 (1.77) | 8.15 (1.29) |
The KSS scores during encoding were derived by averaging the 3 scores provided during each test session. N = 26.
Supplementary Table 3.
Brain Regions Showing Significant Encoding-Related Activity in Both Rested Wakefulness Scanning Sessions (RWD and RWP)
| BA | Hemisphere | Talairach Coordinates |
t(25) | |||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| All Encoding Trials | ||||||
| Activation | ||||||
| Middle Frontal Gyrus | 9 | L | −48 | 26 | 28 | 5.52 |
| Inferior Frontal Gyrus | 44 | L | −48 | 2 | 31 | 6.88 |
| Inferior Frontal Gyrus | 45 | L | −45 | 29 | 15 | 5.47 |
| Pre Supplementary Motor Area | 6 | L | −3 | 5 | 55 | 6.13 |
| Superior Parietal Cortex | 7 | L | −30 | −55 | 40 | 5.50 |
| Inferior Parietal Cortex | 40 | L | −51 | −37 | 49 | 6.40 |
| Fusiform Gyrus | 37 | R | 42 | −46 | −20 | 5.69 |
| Fusiform Gyrus | 37 | L | −48 | −49 | −14 | 9.23 |
| Ventral Occipital Cortex | 18/19 | R | 15 | −91 | −5 | 7.64 |
| Ventral Occipital Cortex | 18/19 | L | −36 | −79 | −14 | 8.62 |
| Caudate Nucleus | R | 12 | −1 | 25 | 6.56 | |
| Caudate Nucleus | L | −18 | −19 | 19 | 5.77 | |
| Deactivation | ||||||
| Posterior Cingulate/Precuneus | 23/30 | R | 9 | −52 | 19 | −6.29 |
| Posterior Cingulate/Precuneus | 23/30 | L | −12 | −58 | 22 | −5.89 |
| Hit Trials Only | ||||||
| Activation | ||||||
| Middle Frontal Gyrus | 9 | L | −48 | 26 | 28 | 6.73 |
| Inferior Frontal Gyrus | 44 | L | −48 | 11 | 28 | 6.56 |
| Inferior Frontal Gyrus | 45 | L | −43 | 26 | 15 | 5.20 |
| Pre-Supplementary Motor Area | 6 | L | −3 | 5 | 55 | 6.12 |
| Superior Parietal Cortex | 7 | L | −30 | −55 | 40 | 5.29 |
| Inferior Parietal Cortex | 40 | L | −51 | −37 | 49 | 6.25 |
| Fusiform Gyrus | 37 | R | 42 | −61 | −14 | 6.04 |
| Fusiform Gyrus | 37 | L | −45 | −46 | −14 | 9.79 |
| Ventral Occipital Cortex | 18/19 | R | 18 | −91 | −5 | 7.89 |
| Ventral Occipital Cortex | 18/19 | L | −21 | −91 | −5 | 8.02 |
| Caudate Nucleus | R | 12 | −1 | 25 | 6.29 | |
| Caudate Nucleus | L | −18 | −19 | 19 | 6.00 | |
| Deactivation | ||||||
| Temporal Parietal Junction | 39 | R | 39 | −76 | 28 | −5.32 |
| Posterior Cingulate/Precuneus | 23/30 | R | 9 | −52 | 22 | −6.11 |
| Posterior Cingulate/Precuneus | 23/30 | L | −14 | −61 | 22 | −5.80 |
All clusters passed a voxel-level threshold of p = 0.0001 (uncorrected).
Supplementary Table 4.
Brain Regions that Exhibited Significant Effects of State in a State (Rested Wakefulness, Sleep Deprivation) by Drug (Donepezil, Placebo) by Time (7.5 s, 10 s) ANOVA
| BA | Hemisphere | Talairach Coordinates |
F(1,25) | |||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| All Encoding Trials | ||||||
| Inferior Frontal Gyrus | 45 | L | −45 | 26 | 13 | 22.38 |
| Inferior Frontal Gyrus | 44 | L | −57 | 2 | 25 | 13.13 |
| Insula | L | −36 | 8 | 7 | 16.52 | |
| Temporo-Occipital Junction | 37 | L | −54 | −61 | 1 | 21.11 |
| Hit Trials Only | ||||||
| Inferior Frontal Gyrus | 45 | L | −45 | 29 | 16 | 18.00 |
| Inferior Frontal Gyrus | 45 | R | 39 | 29 | 16 | 14.16 |
| Inferior Frontal Gyrus | 6/44 | L | −39 | −4 | 28 | 13.13 |
| Insula | L | 42 | 2 | −5 | 14.68 | |
| Insula | R | −45 | −1 | −5 | 20.43 | |
| Postcentral Gyrus | 1/2/5 | R | 36 | −34 | 61 | 17.80 |
| Intraparietal Sulcus | 7 | L | −24 | −58 | 52 | 12.78 |
| Middle Temporal Gyrus | 22/39 | R | 42 | −55 | 10 | 17.85 |
| Inferior Temporal Gyrus | 37 | L | −54 | −61 | 1 | 20.97 |
| Inferior Temporal Gyrus | 37 | R | 42 | −61 | −8 | 15.05 |
| Fusiform Gyrus | 37 | R | 39 | −43 | −14 | 18.75 |
No regions showed a significant effect of drug. All clusters passed a voxel-level threshold of p = 0.005 (uncorrected).
REFERENCES
- 1.Born J, Rasch B, Gais S. Sleep to remember. Neuroscientist. 2006;12:410–24. doi: 10.1177/1073858406292647. [DOI] [PubMed] [Google Scholar]
- 2.Maquet P. The role of sleep in learning and memory. Science. 2001;294:1048–52. doi: 10.1126/science.1062856. [DOI] [PubMed] [Google Scholar]
- 3.Walker MP, Stickgold R. Sleep, memory, and plasticity. Annu Rev Psychol. 2006;57:139–66. doi: 10.1146/annurev.psych.56.091103.070307. [DOI] [PubMed] [Google Scholar]
- 4.Chee MW, Chuah LY. Functional neuroimaging insights into how sleep and sleep deprivation affect memory and cognition. Curr Opin Neurol. 2008;21:417–23. doi: 10.1097/WCO.0b013e3283052cf7. [DOI] [PubMed] [Google Scholar]
- 5.Bonnet MH, Balkin TJ, Dinges DF, Roehrs T, Rogers NL, Wesensten NJ. The use of stimulants to modify performance during sleep loss: a review by the sleep deprivation and Stimulant Task Force of the American Academy of Sleep Medicine. Sleep. 2005;28:1163–87. doi: 10.1093/sleep/28.9.1163. [DOI] [PubMed] [Google Scholar]
- 6.Porrino LJ, Daunais JB, Rogers GA, Hampson RE, Deadwyler SA. Facilitation of task performance and removal of the effects of sleep deprivation by an ampakine (CX717) in nonhuman primates. PLoS Biol. 2005;3:e299. doi: 10.1371/journal.pbio.0030299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wesensten NJ, Reichardt RM, Balkin TJ. Ampakine (CX717) effects on performance and alertness during simulated night shift work. Aviat Space Environ Med. 2007;78:937–43. doi: 10.3357/asem.2055.2007. [DOI] [PubMed] [Google Scholar]
- 8.Boutrel B, Koob GF. What keeps us awake: the neuropharmacology of stimulants and wakefulness-promoting medications. Sleep. 2004;27:1181–94. doi: 10.1093/sleep/27.6.1181. [DOI] [PubMed] [Google Scholar]
- 9.Sarter M, Gehring WJ, Kozak R. More attention must be paid: the neurobiology of attentional effort. Brain Res Rev. 2006;51:145–60. doi: 10.1016/j.brainresrev.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 10.Murphy PC, Sillito AM. Cholinergic enhancement of direction selectivity in the visual cortex of the cat. Neuroscience. 1991;40:13–20. doi: 10.1016/0306-4522(91)90170-s. [DOI] [PubMed] [Google Scholar]
- 11.Sato H, Hata Y, Masui H, Tsumoto T. A functional role of cholinergic innervation to neurons in the cat visual cortex. J Neurophysiol. 1987;58:765–80. doi: 10.1152/jn.1987.58.4.765. [DOI] [PubMed] [Google Scholar]
- 12.Hasselmo ME. The role of acetylcholine in learning and memory. Curr Opin Neurobiol. 2006;16:710–5. doi: 10.1016/j.conb.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giacobini E. Cholinesterase inhibitors do more than inhibit cholinesterase. In: Becker R, Giacobini E, editors. Alzheimer Disease: From molecular biology to therapy. Boston Birkhauser; 1996. pp. 187–204. [Google Scholar]
- 14.Thiel CM. Cholinergic modulation of learning and memory in the human brain as detected with functional neuroimaging. Neurobiol Learn Mem. 2003;80:234–44. doi: 10.1016/s1074-7427(03)00076-5. [DOI] [PubMed] [Google Scholar]
- 15.Bentley P, Driver J, Dolan RJ. Cholinesterase inhibition modulates visual and attentional brain responses in Alzheimer's disease and health. Brain. 2008;131:409–24. doi: 10.1093/brain/awm299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bentley P, Husain M, Dolan RJ. Effects of cholinergic enhancement on visual stimulation, spatial attention, and spatial working memory. Neuron. 2004;41:969–82. doi: 10.1016/s0896-6273(04)00145-x. [DOI] [PubMed] [Google Scholar]
- 17.Furey ML, Pietrini P, Haxby JV. Cholinergic enhancement and increased selectivity of perceptual processing during working memory. Science. 2000;290:2315–9. doi: 10.1126/science.290.5500.2315. [DOI] [PubMed] [Google Scholar]
- 18.Furey ML, Pietrini P, Haxby JV, et al. Cholinergic stimulation alters performance and task-specific regional cerebral blood flow during working memory. Proc Natl Acad Sci U S A. 1997;94:6512–6. doi: 10.1073/pnas.94.12.6512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sperling R, Greve D, Dale A, et al. Functional MRI detection of pharmacologically induced memory impairment. Proc Natl Acad Sci U S A. 2002;99:455–60. doi: 10.1073/pnas.012467899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lawrence NS, Ross TJ, Stein EA. Cognitive mechanisms of nicotine on visual attention. Neuron. 2002;36:539–48. doi: 10.1016/s0896-6273(02)01004-8. [DOI] [PubMed] [Google Scholar]
- 21.Schon K, Atri A, Hasselmo ME, Tricarico MD, LoPresti ML, Stern CE. Scopolamine reduces persistent activity related to long-term encoding in the parahippocampal gyrus during delayed matching in humans. J Neurosci. 2005;25:9112–23. doi: 10.1523/JNEUROSCI.1982-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gron G, Kirstein M, Thielscher A, Riepe MW, Spitzer M. Cholinergic enhancement of episodic memory in healthy young adults. Psychopharmacology. 2005;182:170–9. doi: 10.1007/s00213-005-0043-2. [DOI] [PubMed] [Google Scholar]
- 23.Beglinger LJ, Tangphao-Daniels O, Kareken DA, Zhang L, Mohs R, Siemers ER. Neuropsychological test performance in healthy elderly volunteers before and after donepezil administration: a randomized, controlled study. J Clin Psychopharmacol. 2005;25:159–65. doi: 10.1097/01.jcp.0000155822.51962.b4. [DOI] [PubMed] [Google Scholar]
- 24.Everitt BJ, Robbins TW. Central cholinergic systems and cognition. Annu Rev Psychol. 1997;48:649–84. doi: 10.1146/annurev.psych.48.1.649. [DOI] [PubMed] [Google Scholar]
- 25.Chuah YML, Chee MWL. Cholinergic augmentation modulates visual task performance in sleep-deprived young adults. J Neurosci. 2008;28:11369–77. doi: 10.1523/JNEUROSCI.4045-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bodenmann S, Xu S, Luhmann UF, et al. Pharmacogenetics of modafinil after sleep loss: catechol-O-methyltransferase genotype modulates waking functions but not recovery sleep. Clin Pharmacol Ther. 2009;85:296–304. doi: 10.1038/clpt.2008.222. [DOI] [PubMed] [Google Scholar]
- 27.Honey G, Bullmore E. Human pharmacological MRI. Trends Pharmacol Sci. 2004;25:366–74. doi: 10.1016/j.tips.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 28.Mattay VS, Goldberg TE, Fera F, et al. Catechol O-methyltransferase val158-met genotype and individual variation in the brain response to amphetamine. Proc Natl Acad Sci U S A. 2003;100:6186–91. doi: 10.1073/pnas.0931309100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van Dongen HP, Baynard MD, Maislin G, Dinges DF. Systematic interindividual differences in neurobehavioral impairment from sleep loss: evidence of trait-like differential vulnerability. Sleep. 2004;27:423–33. [PubMed] [Google Scholar]
- 30.Leproult R, Colecchia EF, Berardi AM, Stickgold R, Kosslyn SM, Van Cauter E. Individual differences in subjective and objective alertness during sleep deprivation are stable and unrelated. Am J Physiol Regul Integr Comp Physiol. 2003;284:R280–90. doi: 10.1152/ajpregu.00197.2002. [DOI] [PubMed] [Google Scholar]
- 31.Saykin AJ, Wishart HA, Rabin LA, et al. Cholinergic enhancement of frontal lobe activity in mild cognitive impairment. Brain. 2004;127:1574–83. doi: 10.1093/brain/awh177. [DOI] [PubMed] [Google Scholar]
- 32.Horne JA, Ostberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 1976;4:97–110. [PubMed] [Google Scholar]
- 33.Schmidt C, Collette F, Leclercq Y, et al. Homeostatic sleep pressure and responses to sustained attention in the suprachiasmatic area. Science. 2009;324:516–9. doi: 10.1126/science.1167337. [DOI] [PubMed] [Google Scholar]
- 34.Dinges DF, Pack F, Williams K, et al. Cumulative sleepiness, mood disturbance, and psychomotor vigilance performance decrements during a week of sleep restricted to 4-5 hours per night. Sleep. 1997;20:267–77. [PubMed] [Google Scholar]
- 35.Doran SM, Van Dongen HP, Dinges DF. Sustained attention performance during sleep deprivation: evidence of state instability. Arch Ital Biol. 2001;139:253–67. [PubMed] [Google Scholar]
- 36.Dorrian J, Rogers NL, Dinges DF. Psychomotor vigilance performance: Neurocognitive assay sensitive to sleep loss. In: Kushida CA, editor. Sleep deprivation: Clinical issues, pharmacology and sleep loss effects. New York: Marcel Dekker; 2005. pp. 39–70. [Google Scholar]
- 37.Horne JA, Reyner LA. Sleep related vehicle accidents. BMJ. 1995;310:565–7. doi: 10.1136/bmj.310.6979.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chee MW, Hon NH, Caplan D, Lee HL, Goh J. Frequency of concrete words modulates prefrontal activation during semantic judgments. Neuroimage. 2002;16:259–68. doi: 10.1006/nimg.2002.1061. [DOI] [PubMed] [Google Scholar]
- 39.Chee MW, Westphal C, Goh J, Graham S, Song AW. Word frequency and subsequent memory effects studied using event-related fMRI. Neuroimage. 2003;20:1042–51. doi: 10.1016/S1053-8119(03)00335-5. [DOI] [PubMed] [Google Scholar]
- 40.Chee MW, Venkatraman V, Westphal C, Siong SC. Comparison of block and event-related fMRI designs in evaluating the word-frequency effect. Hum Brain Mapp. 2003;18:186–93. doi: 10.1002/hbm.10092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Otten LJ, Henson RN, Rugg MD. State-related and item-related neural correlates of successful memory encoding. Nat Neurosci. 2002;5:1339–44. doi: 10.1038/nn967. [DOI] [PubMed] [Google Scholar]
- 42.Wagner AD, Schacter DL, Rotte M, et al. Building memories: remembering and forgetting of verbal experiences as predicted by brain activity. Science. 1998;281:1188–91. doi: 10.1126/science.281.5380.1188. [DOI] [PubMed] [Google Scholar]
- 43.Kirchhoff BA, Wagner AD, Maril A, Stern CE. Prefrontal-temporal circuitry for episodic encoding and subsequent memory. J Neurosci. 2000;20:6173–80. doi: 10.1523/JNEUROSCI.20-16-06173.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Snodgrass JG, Corwin J. Pragmatics of measuring recognition memory: Applications to dementia and amnesia. J Exp Psychol Gen. 1988;117:34–50. doi: 10.1037//0096-3445.117.1.34. [DOI] [PubMed] [Google Scholar]
- 45.Donaldson W. Measuring recognition memory. J Exp Psychol Gen. 1992;121:275–7. doi: 10.1037//0096-3445.121.3.275. [DOI] [PubMed] [Google Scholar]
- 46.Goebel R, Esposito F, Formisano E. Analysis of functional image analysis contest (FIAC) data with brainvoyager QX: From single-subject to cortically aligned group general linear model analysis and self-organizing group independent component analysis. Hum Brain Mapp. 2006;27:392–401. doi: 10.1002/hbm.20249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miller SL, Celone K, DePeau K, et al. Age-related memory impairment associated with loss of parietal deactivation but preserved hippocampal activation. Proc Natl Acad Sci U S A. 2008;105:2181–6. doi: 10.1073/pnas.0706818105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Otten LJ, Rugg MD. When more means less: neural activity related to unsuccessful memory encoding. Curr Biol. 2001;11:1528–30. doi: 10.1016/s0960-9822(01)00454-7. [DOI] [PubMed] [Google Scholar]
- 49.Yoo SS, Hu PT, Gujar N, Jolesz FA, Walker MP. A deficit in the ability to form new human memories without sleep. Nat Neurosci. 2007;10:385–92. doi: 10.1038/nn1851. [DOI] [PubMed] [Google Scholar]
- 50.Chee MW, Choo WC. Functional imaging of working memory after 24 hr of total sleep deprivation. J Neurosci. 2004;24:4560–7. doi: 10.1523/JNEUROSCI.0007-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Drummond SP, Brown GG, Salamat JS, Gillin JC. Increasing task difficulty facilitates the cerebral compensatory response to total sleep deprivation. Sleep. 2004;27:445–51. [PubMed] [Google Scholar]
- 52.Volkow ND, Wang GJ, Telang F, et al. Sleep deprivation decreases binding of [11C]raclopride to dopamine D2/D3 receptors in the human brain. J Neurosci. 2008;28:8454–61. doi: 10.1523/JNEUROSCI.1443-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Robbins TW. Chemistry of the mind: neurochemical modulation of prefrontal cortical function. J Comp Neurol. 2005;493:140–6. doi: 10.1002/cne.20717. [DOI] [PubMed] [Google Scholar]
- 54.Sarter M, Hasselmo ME, Bruno JP, Givens B. Unraveling the attentional functions of cortical cholinergic inputs: interactions between signal-driven and cognitive modulation of signal detection. Brain Res Brain Res Rev. 2005;48:98–111. doi: 10.1016/j.brainresrev.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 55.Sarter M, Parikh V. Choline transporters, cholinergic transmission and cognition. Nat Rev Neurosci. 2005;6:48–56. doi: 10.1038/nrn1588. [DOI] [PubMed] [Google Scholar]
- 56.Mentis MJ, Sunderland T, Lai J, et al. Muscarinic versus nicotinic modulation of a visual task. a pet study using drug probes. Neuropsychopharmacology. 2001;25:555–64. doi: 10.1016/S0893-133X(01)00264-0. [DOI] [PubMed] [Google Scholar]
- 57.Matthews PM, Honey GD, Bullmore ET. Applications of fMRI in translational medicine and clinical practice. Nat Rev Neurosci. 2006;7:732–44. doi: 10.1038/nrn1929. [DOI] [PubMed] [Google Scholar]
- 58.Mack A, Rock I. Inattention blindness. Cambridge: MIT Press; 1998. [Google Scholar]
- 59.Kastner S, Ungerleider LG. Mechanisms of visual attention in the human cortex. Annu Rev Neurosci. 2000;23:315–41. doi: 10.1146/annurev.neuro.23.1.315. [DOI] [PubMed] [Google Scholar]
- 60.Culham JC, Kanwisher NG. Neuroimaging of cognitive functions in human parietal cortex. Curr Opin Neurobiol. 2001;11:157–63. doi: 10.1016/s0959-4388(00)00191-4. [DOI] [PubMed] [Google Scholar]
- 61.Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–15. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- 62.Brefczynski JA, DeYoe EA. A physiological correlate of the ‘spotlight' of visual attention. Nat Neurosci. 1999;2:370–4. doi: 10.1038/7280. [DOI] [PubMed] [Google Scholar]
- 63.Gandhi SP, Heeger DJ, Boynton GM. Spatial attention affects brain activity in human primary visual cortex. Proc Natl Acad Sci U S A. 1999;96:3314–9. doi: 10.1073/pnas.96.6.3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Reynolds JH, Chelazzi L. Attentional modulation of visual processing. Ann Rev Neurosci. 2004;27:611–47. doi: 10.1146/annurev.neuro.26.041002.131039. [DOI] [PubMed] [Google Scholar]
- 65.Rombouts SA, Barkhof F, Van Meel CS, Scheltens P. Alterations in brain activation during cholinergic enhancement with rivastigmine in Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2002;73:665–71. doi: 10.1136/jnnp.73.6.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rosier A, Cornette L, Orban GA. Scopolamine-induced impairment of delayed recognition of abstract visual shapes. Neuropsychobiology. 1998;37:98–103. doi: 10.1159/000026486. [DOI] [PubMed] [Google Scholar]
- 67.Gold PE. Acetylcholine modulation of neural systems involved in learning and memory. Neurobiol Learn Mem. 2003;80:194–210. doi: 10.1016/j.nlm.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 68.Mesulam M. The cholinergic lesion of Alzheimer's disease: pivotal factor or side show? Learn Mem. 2004;11:43–9. doi: 10.1101/lm.69204. [DOI] [PubMed] [Google Scholar]
- 69.Sarter M, Bruno JP, Givens B. Attentional functions of cortical cholinergic inputs: what does it mean for learning and memory? Neurobiol Learn Mem. 2003;80:245–56. doi: 10.1016/s1074-7427(03)00070-4. [DOI] [PubMed] [Google Scholar]
- 70.Chee MW, Tan JC, Zheng H, et al. Lapsing during sleep deprivation is associated with distributed changes in brain activation. J Neurosci. 2008;28:5519–28. doi: 10.1523/JNEUROSCI.0733-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gais S, Albouy G, Boly M, et al. Sleep transforms the cerebral trace of declarative memories. Proc Natl Acad Sci U S A. 2007;104:18778–83. doi: 10.1073/pnas.0705454104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Takashima A, Petersson KM, Rutters F, et al. Declarative memory consolidation in humans: a prospective functional magnetic resonance imaging study. Proc Natl Acad Sci U S A. 2006;103:756–61. doi: 10.1073/pnas.0507774103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wood SJ, Slater CR. Safety factor at the neuromuscular junction. Prog Neurobiol. 2001;64:393–429. doi: 10.1016/s0301-0082(00)00055-1. [DOI] [PubMed] [Google Scholar]