Abstract
Context
Amygdala and ventrolateral prefrontal cortex (vlPFC) dysfunction manifests in adolescents with anxiety disorders when they view negatively valenced stimuli in threatening contexts. Such fear-circuitry dysfunction may also manifest when anticipated social evaluation leads socially anxious adolescents to misperceive peers as threatening.
Objective
To determine whether photographs of negatively evaluated smiling peers viewed during anticipated social evaluation engage the amygdala and vlPFC differentially in adolescents with and without social anxiety.
Design
Case-control study.
Setting
Government clinical research institute.
Participants
Fourteen adolescents with anxiety disorders associated with marked concerns of social evaluation and 14 adolescents without a psychiatric diagnosis matched on sex, age, intelligence quotient, and socioeconomic status.
Main Outcome Measures
Blood oxygenation level–dependent signal measured with event-related functional magnetic resonance imaging. Before and during neuroimaging scans, participants anticipating social evaluation completed peer- and self-appraisals. Event-related analyses were tailored to participants’ ratings of specific peers.
Results
Participants classified 40 pictures of same-age peers as ones with whom they did or did not want to engage in a social interaction. Anxious adolescents showed greater amygdala activation than healthy adolescents when anticipating evaluation from peers previously rated as undesired for an interaction. Psychophysiological interaction connectivity analyses also revealed a significant positive association between amygdala and vlPFC activation in anxious vs healthy adolescents in response to these stimuli.
Conclusions
Anticipating social evaluation from negatively perceived peers modulates amygdala and vlPFC engagement differentially in anxious and healthy adolescents. Amygdala and vlPFC dysfunction manifests in adolescent anxiety disorders in specific contexts of anticipated peer evaluation.
Dramatic changes in the social context of behavior during adolescence are associated with increased incidence of social anxiety.1–4 Information-processing biases about social experiences may contribute to this increase in anxiety. For example, psychological theories posit that social anxiety is associated with excessive fears of judgment and biased appraisals of others as overly critical.5,6 These theories suggest that anticipation of peer evaluation leads anxious adolescents to misperceive peers as overly threatening and uninterested in social interactions with them. Functional neuroimaging provides an opportunity to ground these psychological theories in knowledge of neural function.7
Research in animals and adult humans implicates a circuit connecting the amygdala and ventral prefrontal cortex (vPFC) in social-threat processing.7,8 Animal studies have shown that this fear circuitry is shaped by developmental experiences that may chronically affect social-threat perception.8 For example, studies of nonhuman primates show that the developmental timing of amygdala damage profoundly influences social-threat perception.9,10 These findings raise key questions about the relations between the human amygdala and vPFC function during adolescence when many social-cognitive processes are forming. Activation of this circuit during interactions with peers may occur in adolescent anxiety disorders. Understanding these processes in adolescence is important because this period is associated with an increased focus on peer relationships and increased onset of anxiety disorders, which heightens risk for adult anxiety disorders.2 While few neuroimaging studies pursue this line of research, preliminary work implicates amygdala-vPFC circuitry not only in social anxiety, but also in adolescent anxiety, more broadly.11–14
Heightened amygdala activation in anxiety disorders is thought to generate fear responses to innocuous stimuli misperceived as threatening.15 Indeed, amygdala hyperactivation consistently differentiates adults with and without anxiety about social situations.16–22 However, a unique association between amygdala function and social-threat perception may arise in adolescence. Specifically, amygdala hyperactivation in adult anxiety typically occurs when attention focuses on nonthreatening rather than threatening aspects of negatively valenced social stimuli.20,23 This presumably reflects a lower threshold for threat detection in adult anxiety. In adolescent anxiety, however, amygdala dysfunction manifests specifically when attention focuses on the threatening aspects of social cues.11 This may relate to broader dysfunction in a circuit encompassing vPFC and the amygdala.11,24,25 Focusing attention specifically on the fear of being negatively evaluated may lead some adolescents to misperceive peers as overly critical and to anticipate pejorative peer evaluation.6 Accordingly, extreme concern about social evaluation is expected in the anxious adolescent who confronts peers within a reciprocally evaluative context. Prior imaging work suggests that these anxiety-related concerns relate to amygdala-vPFC function.11
Three issues shaped our current focus on the fear response to social threats in adolescent anxiety. First, although amygdala hyperactivation has been found in response to mild emotional provocations,11,12 studies have yet to assess fear of social evaluation in a salient context for adolescents. This may be a particularly sensitive developmental period for social cognition because the transition into the peer world at large is accompanied by frequent social evaluation. Thus, we developed an ecologically valid paradigm that uses anticipation of peer evaluation with in a simulated internet chatroom to induce feelings of social threat highly relevant to an adolescent’s daily life and to assess the amygdala response. Second, in light of prior work that shows powerful contextual effects on adolescent amygdala and vPFC responsivity,11,14,26 we probed amygdala function using prototypically nonthreatening social cues—smiling faces of novel peers27,28—within the potentially threatening context of peer evaluation. Finally, recent work suggests that between-group differences in functional connectivity with ventrolateral expanses of pre-frontal cortex (vlPFC) parallel differences in amygdala response.11,14,29 However, because only 2 studies using mild threats examined this issue in adolescents,11,14 extension to ecologically valid situations was needed. Therefore, we considered the degree to which amygdala-vlPFC connectivity relates to between-group differences within a real-world social context.
The present study tested 2 hypotheses. First, based on past work in anxious adolescents11,12,14 relative to healthy adolescents, participants with anxiety disorders would exhibit amygdala hyperactivation when anticipating judgment from peers in a context of potential negative evaluation. To elicit anticipation of social evaluation, participants were asked to rate their desire to interact with a series of peers. They were then asked 2 weeks later to rate how they thought these same peers would evaluate them for an interaction. We chose the cognitive task of considering how others judge one’s self to assess self-esteem, a psychological construct related to anxiety.30 Second, as in prior studies of healthy adults and anxious adolescents,11,14,31 we hypothesized that both amygdala hyperactivation and amygdala-vlPFC connectivity would emerge in this context of social concern. We hypothesized that these activations would interact with participants’ own evaluations of peers. We contrasted 2 instances of anxious adolescents’ expectations concerning unfamiliar peers: (1) when peers were rated as undesirable and (2) when they were rated as desirable for anticipated interaction. As part of the paradigm, participants were led to believe that their peer ratings would be revealed to the peers. Hence, appraising how peers deemed undesirable would evaluate them was expected to elicit amygdala hyperactivation and positive amygdala-vlPFC connectivity in the anxious vs nonanxious adolescent. This was expected to reflect neural processes associated with fears of social retaliation when anticipating evaluation from a peer previously rated as undesirable. In addition, because prior data suggest that anxious adolescents exhibit greater amygdala activation11,12,14 and a biased expectation of being negatively evaluated,6,32 both anxiety severity and task ratings related to self-esteem were expected to vary with engagement of amygdala-vlPFC circuitry.15,29
METHODS
PARTICIPANTS
Fourteen adolescents with a current DSM-IV anxiety diagnosis who were not taking medication and 14 healthy adolescents participated. Analyses by t test and χ2 test confirmed that groups did not differ in age, sex, intelligence quotient, or socioeconomic status (Table 1).
Table 1.
Demographic and Clinical Characteristics of Sample and Task Ratings by Group
| Characteristics | Mean (SD) (n=14) |
t26 Statistic | P Value | |
|---|---|---|---|---|
| Patients | Controls | |||
| Age, y | 12.30 (2.76) | 12.58 (2.54) | 0.27 | .79 |
| IQ | 113.36 (14.69) | 117.86 (8.06) | 1.01 | .32 |
| Female sex, No. (%) | 10 (71.4) | 10 (71.4) | χ2=0.0 | 1.00 |
| Parent education levela | 6.27 (0.90) | 6.00 (1.00) | t22=−0.70 | .49 |
| Current DSM-IV diagnosis, No. (%)b | ||||
| GAD | 9 (64.3) | NA | ||
| SP | 8 (57.1) | NA | ||
| SAD | 4 (28.6) | NA | ||
| Pediatric anxiety rating scale scorec | 15.21 (2.69) | NA | ||
| Interest in peersd | 37.57 (19.73) | 40.29 (14.97) | 0.41 | .68 |
| Expected peer evaluationd | 42.14 (12.25) | 53.55 (15.30) | 2.17 | .04 |
| Low-desirability peers | 36.06 (17.42) | 43.79 (21.57) | 1.04 | .31 |
| High-desirability peers | 48.43 (14.27) | 63.44 (15.04) | 2.71 | .01 |
Abbreviations: GAD, generalized anxiety disorder; NA, not applicable; SAD, separation anxiety disorder; SP, social phobia.
Level ranges from 1 (<7 years of education) to 7 (graduate or professional degree).
One patient with SP had comorbid attention-deficit hyperactivity disorder. Five patients with GAD, SP, and/or SAD had comorbid specific phobia.
Items range from 0 (none) to 5 (extreme) anxiety symptoms; total score range is 0 to 25.
Scale ranges from 0 (not interested) to 100 (very interested) for participants’ interest in chatting with peers and participants’ expectation of peers’ interest in them.
Diagnoses were determined using the Kiddie Schedule for Affective Disorders–Present and Lifetime version (K-SADS-PL).33 All patients were recruited when they sought treatment of anxiety about social situations and thus had current, impairing, clinically significant anxiety (Table 1). Patients not diagnosed with current social phobia (SP) (n=6) demonstrated clinically significant fear of social interactions or performance situations on the Pediatric Anxiety Rating Scale34 and/or K-SADS-PL. This selection approach follows Rapee and Heimberg’s model6 that posits that social evaluation concerns exist along a continuum, with SP at the extreme, immediately proximal to other anxiety disorders with high social concerns. We included both patient groups, as Rapee and Heimberg argue that similar cognitive biases manifest in both groups, though to varying degrees. Other inclusion and exclusion criteria were identical to those used in prior studies.11,14 Participants and their parents completed the Screen for Child Anxiety Related Emotional Disorders (SCARED).35 Parent/adolescent SCARED scores were averaged to index anxiety severity.
STUDY PROCEDURES
Study procedures were approved by the National Institute of Mental Health institutional review board. All participants provided written assent; parents or legal guardians provided written informed consent. Participants were informed that they would receive misinformation during testing; all participants were debriefed. No adverse reactions occurred.
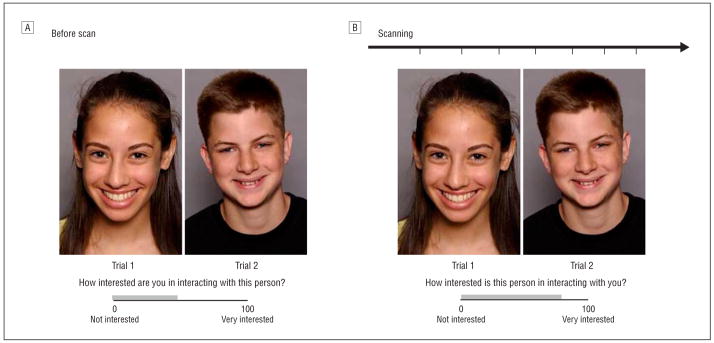
We developed an ecologically valid neuroimaging paradigm, the chat room, to simulate adolescent social interactions in 2 phases. In phase 1 participants were led to believe we were implementing a nationwide investigation of internet-based communication. They were told that after a functional magnetic resonance imaging (fMRI) scan, they would chat online with another teenager. Participants then viewed 40 photographs of peers (20 boys) allegedly participating in the study and rated on a 100-point scale their interest in chatting with each peer (Figure 1A). These ratings were used to sort events during scanning based on participants’ desire to chat with each peer. Participants were also photographed, told that the peers they had rated would similarly evaluate their pictures and view the ratings they had received, and would later chat with a mutually high-interest peer. As part of our hypothesis, knowing that the peers would learn of the participants’ ratings was thought to elicit concern about being evaluated in return. This deceptive approach was intended to increase task sensitivity for engaging symptom-relevant cognitions.
Figure 1.
The chat room paradigm consisted of 2 visits to the laboratory. A, Approximately 2 weeks before the scan, participants rated how interested they were in chatting online with peers based on photographs. A median split divided the ratings into low and high peer-desirability groups. Participants were told that the same peers would learn how they had been rated and rate the participants’ photographs in a similar fashion. B, During the second visit participants were scanned while reviewing previously judged photographs and rated how interested they thought each peer would be in chatting online with them.
In phase 2 approximately 2 weeks later, participants underwent an fMRI scan while reviewing the photographs they had rated previously and were asked to indicate how interested they thought each depicted peer would be in chatting with them (Figure 1B). This cognitive task involves appraisal of expected peer evaluation designed to engage concerns about social evaluation and adolescents’ views of themselves (ie, self-esteem). This appraisal rating was expected to differ in anxious and healthy adolescents, following Rapee and Heimberg’s model.6 Our hypothesis focused on neural activation during appraisal events of anticipated social evaluation by peers sorted by peer-desirability groupings. After the scan, participants answered questions to evaluate their belief in the task and were then debriefed. Only data from successfully deceived participants (80%) were included.
The chat room task used a rapid event-related design presented in one 7-minute run. Each event was 7.6 to 9.6 seconds, consisting of stimulus face and question presentation (3–5 seconds), which was then superimposed with a rating response screen (4.6 seconds). These 2 subevents were pooled and modeled as a single event. Because the same rating was always performed on every picture, the subject likely begins assessing the picture before actually rating it; thus, from a psychological perspective, they are not distinguishable events. Task stimuli were 40 digital head shots of actors aged 11 to 17 years of varied ethnicities36 posing happy expressions under the direction of an acting coach. Fixation crosses were displayed (4 seconds) randomly throughout the task to serve as a baseline. The interstimulus interval was 1 second.
fMRI DATA ACQUISITION AND PREPROCESSING
Scanning occurred in a Signa 3T magnet (General Electric, Waukesha, Wisconsin). Stimuli were viewed with a head coil–mounted mirror. Participants rated stimuli using a handheld 2-button response box (Research Services Branch, National Institute of Mental Health, Bethesda, Maryland).
For functional image acquisition, each brain volume contained 29 contiguous 3.3-mm axial slices acquired parallel to the anterior commissure–posterior commissure line using a single shot gradient echo with T2* weighting (time to repetition, 2300 milliseconds; echo time, 23 milliseconds; voxel dimensions, 3.3×3.75×3.75 mm; 64×64 matrix; field of view, 24 cm). A high-resolution anatomical image was acquired using a T1-weighted standardized magnetization-prepared spoiled gradient recalled echo sequence (124 1-mm axial slices; time to repetition, 8100 milliseconds; echo time, 32 milliseconds; flip angle, 15°; number of excitations, 1; 256×256 matrix; band-width, 31.2 kHz; field of view, 24 cm).
DATA ANALYSIS
Behavioral rating data collected before and during the scan were analyzed using SPSS 14.0 (SPSS Inc, Chicago, Illinois). Functional MRI data were analyzed using Analysis of Functional and Neural Images (AFNI) software version 2.56b (National Institute of Health, Bethesda, Maryland).37 Standard preprocessing of echoplanar imaging data included slice-time correction, motion correction, reslicing to a 1-mm isotropic voxel, spatial smoothing (6-mm full-width half-maximum Gaussian kernel), removal of large signal deviations of 2.5 SD or greater from the mean using an AFNI despiking algorithm applied on a vox-elwise basis, a bandpass-filtering algorithm to smooth cyclical fluctuations (>.011 Hz or <.15 Hz), and normalization of blood oxygen level–dependent signal intensity to percentage of signal change using each subject’s voxelwise time series mean as a baseline. Motion correction parameters were included as nuisance covariates along with a covariate for mean intensity and linear drift. In addition, subjects who moved more than2.5 mm were excluded.
AMYGDALA ACTIVATION
The statistical model was a γ-variate basis function convolved with the hemodynamic response function from AFNI. The basis function was set to the onset of each event type. Event types consisted of 2 expected–peer-appraisal events: appraising evaluation from peers the participant had rated as being either (1) low or (2) high in desirability, determined using a median split of each participant’s prescan peer ratings. A general linear model was then used to determine the β value and t statistic for each event type at each voxel. Contrasts of whole-brain blood oxygen level-dependent activation were created per subject per event type, followed by a second group-level random-effects analysis of individual contrast values.
Based on past data11,14 and our a priori hypothesis, group-level analyses focused on the left and right amygdala regions of interest (ROI), defined using Talairach anatomical boundaries.38 Mean contrast values were generated for all amygdala voxels and analyzed with t tests. We tested between-group differences in the low- vs high-desirability contrast during expected–peer-appraisal ratings. Statistical significance was based on both height intensity and spatial extent in ROIs, using AFNI AlphaSim to correct for multiple comparisons within the ROIs based on 1000 Monte Carlo simulations for the ROIs. With this algorithm, significant voxels had to exceed a P value of less than .005 whole-brain uncorrected with a 92-voxel cluster size (92 μL), corresponding to an ROI-corrected P value of less than .05.
To separate the contrast and correlate imaging with non-imaging data, secondary analyses were conducted by converting each participant’s data to the percentage of signal change using their voxelwise time series mean as a baseline. The AFNI 3dmaskave program was used to compute and extract per participants’ average activation of all voxels within a functionally defined ROI mask of the low- vs high-desirability contrast.39 Threshold parameters for the mask were based on results from the primary ROI analyses, using t=2.78, P<.005, and cluster size=92 μL. Mean activation values within each ROI cluster were extracted to separate the results using SPSS. Two repeated-measures analysis of variance models included group (patient, control) as a between-subjects factor and peer desirability (low, high) as a within-subjects factor; average left and right amygdala activation during appraisal of expected peer evaluation were the dependent variables. The group×peer desirability interaction on the amygdala response was of primary interest. Secondary regression analyses in AFNI also evaluated the influence of between-group task performance differences on between-group amygdala activation differences. Finally, analyses examined correlations between amygdala activation, self-esteem ratings, and anxiety severity.
FUNCTIONAL CONNECTIVITY
We conducted a psychophysiological interaction analysis to examine connectivity between the amygdala and vlPFC during the low- vs high-desirability contrast. Established procedures were adapted for use with AFNI.14,40,41 Blood oxygen level-dependent signal was deconvolved using an assumed form of the hemodynamic response function before creating the interaction term.41 Each participant’s echoplanar imaging time series was converted to Talairach space. The first eigenvariate time series was extracted from each of 2 seed voxels from the peak t value for the left and right amygdala where between-group differences emerged on the low- vs high-desirability contrast. To examine activation specifically related to the events of interest, we entered the low and high peer-desirability events as covariates. The correlation coefficient of the interaction term between the amygdala seed and the low-vs-high contrast was converted using z score transformation to reduce skew and normalize sampling distribution. The t tests compared groups on coactivation between each amygdala seed and other brain regions. Results of this analysis showed event-related changes in the interaction of the right amygdala seed and left vlPFC (Brodmann area 47). A spatial clustering procedure determined statistical significance with a P value of less than .005 height threshold and spatial extent correction (n=216 voxels) based on 1000 Monte Carlo simulations using the entire echoplanar imaging map, corresponding to a whole-brain corrected P value of less than .01. Secondary analyses separated initial findings. Coactivation correlation values were extracted from the vlPFC region that survived statistically and graphed for presentation purposes. We also examined the correlation of amygdala-vlPFC connectivity with self-esteem ratings and anxiety severity.
RESULTS
BEHAVIORAL RESPONSES
Patients and controls reported similar levels of interest in peers (ie, peer desirability) based on prescan ratings, but data collected during scanning revealed the hypothesized between-group differences (Table 1). Relative to controls, patients expected peers to rate them as less desirable, reflecting expected between-group differences in self-esteem. There was also a positive significant correlation between peer desirability ratings and self-esteem ratings (ie, expected peer evaluation) (Table 2).
Table 2.
Spearman Correlations Between Task Ratings, Anxiety Severity, Amygdala Activation, and Amygdala–vlPFC Connectivity Across Participantsa
| Interest in Peersb | Expected Peer Evaluationc | Total SCARED scored | Amygdala Activatione |
Right Amygdala-vlPFCe | ||
|---|---|---|---|---|---|---|
| Left | Right | |||||
| Interest in peers | 1.0 | |||||
| Expected peer evaluation | .56f | 1.0 | ||||
| Total SCARED score | −.13 | −.38g | 1.0 | |||
| Left amygdala activation | .12 | −.11 | .34 | 1.0 | ||
| Right amygdala activation | .07 | −.17 | .42g | .69f | 1.0 | |
| Right amygdala-vlPFC | −.09 | −.39g | .60f | .30 | .41g | 1.0 |
Abbreviations: SCARED, screen for child anxiety related emotional disorders; vlPFC, ventrolateral prefrontal cortex.
n=28.
Scale ranges from 0 (not interested) to 100 (very interested) for participants’ interest in peers.
Scale ranges from 0 (not interested) to 100 (very interested) for participants’ expectations of peers’ interest in them.
Items range from 0 (not true) to 2 (very and/or often true) anxiety symptoms; total score range is 0 to 82.
Activation while viewing undesirable vs desirable peers.
P<.01.
P<.05.
As expected, both groups reported higher interest in chatting with same-sex than opposite-sex peers (peer desirability×peer sex interaction, F1,26=51.47, P <.001). A repeated-measure analysis of variance yielded no between-group differences in the proportion of same vs opposite-sex peers nominated to low- vs high-desirability conditions.
AMYGDALA ACTIVATION
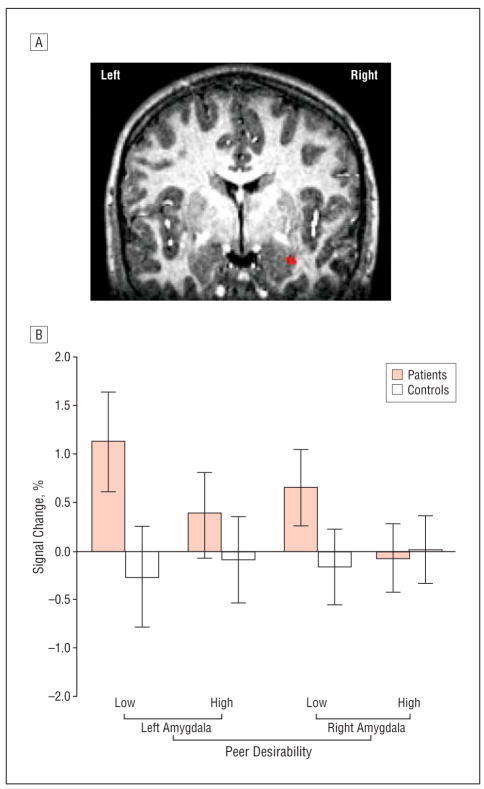
Our a priori hypothesis that the low– vs high–peer-desirability contrast would elicit more amygdala activation in patients vs controls was confirmed by significant group×peer desirability interactions. Patients showed significantly greater bilateral amygdala activation while appraising potential peer evaluation; this effect occurred specifically while viewing undesirable peers. After correcting for multiple comparisons in the ROIs, the maximum intensity value in the left amygdala was t26=3.62 (x=−23, y=3, z=−20) and for the right amygdala was t26=3.53 (x =27, y =−3, z =−21). Figure 2A presents the topography of the maximum intensity t value in the right amygdala.
Figure 2.
A, A significant group difference in right amygdala activity is illustrated on high-resolution images from a representative subject. Bilateral amygdala activation was greater in patients vs controls when appraising expected peer evaluation by low- vs high-desirability peers (P<.005). After correcting for multiple comparisons in the amygdala regions (P<.05), the maximum intensity value for the cluster encompassing the left amygdala was t=3.62 (x=−23, y=3, z=−20) and for the right amygdala was t=3.53 (x=27, y=−3, z=−21). B, Group × peer desirability interaction effects on the event-elicited percentage of signal change in the left (F1,26=13.26; P =.001) and right amygdala (F1,26=12.91; P =.001). Data were collected at functional magnetic resonance imaging scan during appraisal of expected peer evaluation, converted to percentage of signal change using voxelwise time series mean as a baseline, and averaged within each functionally defined amygdala region of interest.
The group×peer desirability interactions were separated through post hoc analyses of the extracted percentage of signal change for each event relative to a null-event baseline. Repeated-measures analyses of variance revealed group×peer desirability interactions (left amygdala F1,26=13.26, P =.001; right amygdala F1,26=12.91, P=.001) consistent with AFNI analyses (Figure 2B). Amygdala activation was greatest among patients specifically when appraising predicted evaluation from undesirable vs desirable peers (P<.001). By contrast, controls showed no amygdala engagement. A significant positive correlation also emerged between anxiety severity ratings and right amygdala activation in the whole sample (Table 2).
We examined differences between participants with SP (n=8), other anxiety disorders plus elevated social concerns on the Pediatric Anxiety Rating Scale (n=6), and controls (n=14). As expected, a significant between-group difference was found in left (F2,25=7.16; P =.003) and right (F2,25=5.93; P =.008) amygdala activation while appraising evaluation from low- vs high-desirability peers. Patients with both SP and other anxiety disorders had significantly greater amygdala activation than controls, but did not differ from each other. Patients with SP had the highest mean (SD) percentage of change in amygdala activation (left amygdala, 0.88[.91]; right amygdala, 0.76[.94]) followed by patients with other anxiety disorders (left amygdala, 0.58 [.54]; right amygdala, 0.68[.83]) and controls (left amygdala, −0.21[.59]; right amygdala, −0.15[.35]).
The AFNI 3dRegAna program was used to conduct a regression analysis of the effects of group- and peer-desirability level on amygdala activation with self-esteem ratings (ie, expected peer evaluation) included as a covariate. These analyses showed that the group differences in bilateral amygdala activation remained significant even after the differences in self-esteem ratings were factored out (left amygdala t26=3.72, P <.005; right amygdala t26=2.53, P <.01).
We entered the study with specific a priori hypotheses concerning greater amygdala activation in patients than in healthy adolescents. Nevertheless, we considered other areas where patients might exhibit greater activation based on peer desirability using the Monte Carlo–based ROI-corrected threshold (Table 3). At this threshold, greater activation in patients vs controls also emerged within the cerebellum. Greater activation in controls vs patients emerged in the left anterior cingulate and the left middle frontal gyrus, encompassing Brodmann area 46.
Table 3.
Activation Areas in Patients vs Controls for the Low vs High Peer-Desirability Contrast While Appraising Expected Peer Evaluation
| Region | xa | ya | za | t26a | BA | Volume, μL |
|---|---|---|---|---|---|---|
| Left anterior cingulate | −15 | 30 | −8 | −3.53 | 32 | 358 |
| Left amygdala | −23 | 3 | −20 | 3.62 | NA | 174 |
| Right amygdala | 27 | −3 | −21 | 3.53 | NA | 164 |
| Left cerebellum | −5 | −70 | −29 | 3.66 | NA | 153 |
| Left middle frontal gyrus | −41 | 43 | 5 | −3.38 | 46 | 153 |
Abbreviation: BA, Brodmann area.
Negative t values indicate greater activation in controls vs patients; positive t values indicate greater activation in patients vs controls. Activations survived a small-volume correction at P less than .01 with a voxel threshold of 92 μL. Talairach coordinates are reported.
FUNCTIONAL CONNECTIVITY
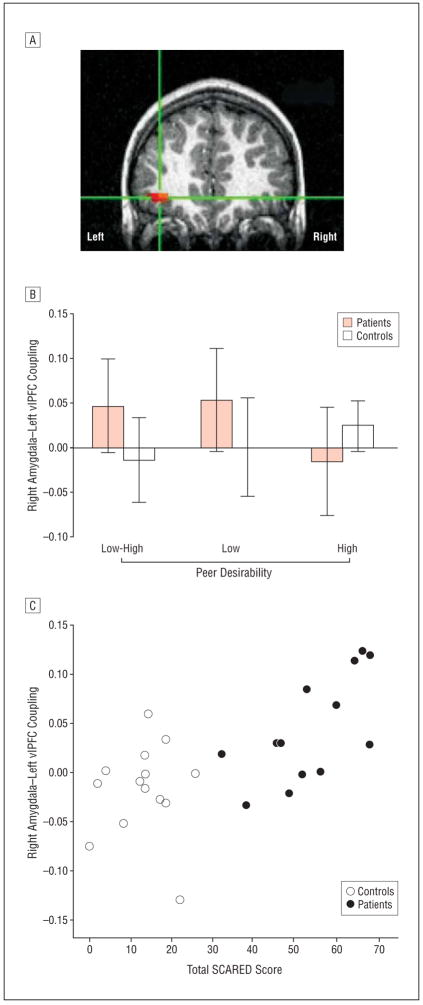
Table 4 summarizes all group differences that surpassed the whole brain–corrected statistical threshold for connectivity with the left and right amygdala. Between-group comparisons showed that, relative to controls, patients demonstrated a positive correlation between the right amygdala seed and left vlPFC-encompassing Brodmann area 47 (Figure 3A) while appraising low- vs high-desirability peers. This difference was driven by positive connectivity emerging only in patients during appraisal of low-desirability peers (Figure 3B). Anxiety severity and self-esteem ratings correlated significantly with amygdala-vlPFC coactivation such that lower self-esteem (Table 2) and higher anxiety severity (Table 2; Figure 3C) were related to positive connectivity.
Table 4.
Group Differences in Activation From the Psychophysiological Connectivity Analysis of the Right and Left Amygdala Seed Voxels for the Low vs High Peer-Desirability Contrasta
| Correlation Direction | x | y | z | t26 | Region | BA | k |
|---|---|---|---|---|---|---|---|
| Connectivity with right amygdala | |||||||
| P+>C − | −32 | 44 | −24 | 3.79 | Left middle frontal gyrus | 47 | 12 |
| C+>P − | 11 | −14 | −16 | −3.32 | Right parahippocampal gyrus | 34/28 | 18 |
| C+>P − | 5 | −38 | −25 | −4.39 | Right cerebellum | NA | 37 |
| C+>P − | −23 | −77 | 33 | −3.62 | Left precuneus | 19 | 46 |
| Connectivity with left amygdala | |||||||
| C+>P − | −14 | −65 | 18 | −3.03 | Left precuneus | 31 | 9 |
| C+>P − | −41 | −11 | −4 | −2.97 | Left insula | 13 | 12 |
| C+>P − | −29 | −62 | 3 | −3.65 | Left middle occipital gyrus | 19 | 13 |
| C+>P − | 17 | 62 | 24 | −3.71 | Right superior frontal gyrus | 10 | 20 |
| P+>C − | 47 | 2 | −25 | 3.48 | Right middle temporal gyrus | 21 | 20 |
| C+>P − | −29 | 38 | −4 | −3.26 | Left parahippocampal gyrus | 36/37 | 22 |
Abbreviations: BA, Brodmann area; C, controls; k, number of surviving voxels; NA, not applicable; P, patients; +, positive correlation; −, negative correlation.
Each line in the table represents 1 voxel within the specific neural region. All activations are corrected for multiple comparisons at P less than .01 and spatial extent of 216 μL. Talairach coordinates are reported.
Figure 3.
Significant group differences in functional connectivity between the right amygdala and left ventrolateral prefrontal cortex (vlPFC) during task performance. Correlation data were averaged within each region that survived a spatial clustering procedure using a corrected P value of less than .05 and extracted to illustrate coactivation patterns within each group. A, Cross-hairs centered on activation indicating significant group difference in coactivation between the right amygdala seed (x=27, y=−3, z=−21) and left vlPFC (x=−32, y=44, z=−5) (t26=3.79; P <.01; activation survives P<.001). B, Extracted correlation data indicated that patients had significantly greater positive connectivity than controls between activation in the amygdala and vlPFC, specifically during the low peer-desirability events. C, Association between amygdala-vlPFC connectivity and total Screen for Child Anxiety Related Emotional Disorders (SCARED) score; Spearman r=0.60, P=.001, n=27 (patient Spearman r=0.62, P=.03, n=13; control Spearman r=0.17, P=.57, n=14).
COMMENT
This is the first study to map neural processing engaged during real-world social interactions in either anxious or healthy adolescents. Two main fMRI findings emerged. First, anxious adolescents showed greater amygdala activation than did healthy adolescents when viewing photographs of peers rated as less desirable relative to those deemed more desirable for an anticipated social interaction. Second, these differences were paralleled by differing patterns of coactivation in the amygdala-vlPFC circuitry. These findings emerged against a backdrop of behavioral findings. Compared with healthy adolescents, anxious adolescents perceived unfamiliar peers as less likely to want to chat with them, consistent with studies demonstrating that anxious youth view themselves as socially unaccepted.30 Thus, the current task replicates and adds to previous work demonstrating a cognitive bias about social evaluation in adolescent anxiety by linking this observation to measures of neural system function. Indeed, social stimuli typically considered non-threatening (ie, smiling peers) elicited robust amygdala responses only in patients, especially when viewing peers whom they had rated negatively.
Because participants were told that peers would learn of their ratings, having rated certain peers as undesirable was expected to generate particularly heightened levels of concern about evaluation in the anxious participant. However, the amygdala response to the low-desirability peers may reflect other processes as well, eg, avoidance of specific individuals or concern about the possibility of chatting with peers that the participant did not like. Further work is needed to extend the current findings to delineate the precise feature of low-desirability peers that elicits a heightened amygdala response in socially anxious adolescents. However, we documented a strong relationship between participants’ initial ratings of each peer’s desirability and later ratings of expected peer evaluation; this provides some evidence that participants’ initial impressions relate to a lasting aspect of the social-evaluative processes that can be engaged 2 weeks later. Regardless of the precise psychological context that most robustly elicits between-group differences in the adolescent amygdala response, the present findings add to prior work in both adolescents and adults. Specifically, the current and prior findings suggest that psychological interpretations initiate or maintain social information processing biases associated with neural function in anxiety disorders.11,20,22
The present study underscores the powerful role of interactions between attention and stimulus properties in shaping the amygdala response, adding to previous work. Specifically, adult social phobia also involves a pronounced amygdala response to socially threatening faces, but this heightened response occurs when attention is constrained to a nonemotional context.20,23 As in the current work, our previous research in at-risk or anxious adolescents also shows that focusing attention on disorder-relevant cognitions elicits amygdala hyperactivation.11,26 Moreover, the current study adds to these findings by showing that appraisal variations influence amygdala response even for social stimuli that prototypically appear nonthreatening. This finding again illustrates the powerful effect of viewing context as mediated by changes in attention. The lack of amygdala engagement during appraisal of peer evaluation in controls suggests that adolescents without social anxiety do not demonstrate the same cognitive biases and associated neural responses that reflect fear of social evaluation.
Amygdala-response differences were paralleled by differential amygdala-vlPFC coactivation, circuitry previously implicated in attention modulation42 and behavioral flexibility.43 These results complement prior findings in adolescents.11,13,14 As in other research,29 our measures of anxiety severity and task performance both correlated with amygdala-vlPFC connectivity. This centrally implicates perturbed amygdala-vlPFC engagement in core cognitive features of adolescent social anxiety. However, beyond our work on vlPFC engagement in adolescent anxiety, considerable research in adult humans and animal models also implicates ventral-medial PFC (vmPFC) in amygdala regulation, particularly in extinction-related processes.44–46 Accordingly, one might also expect between-group differences in vmPFC-amygdala connectivity in the current study. As noted, our prior work more consistently implicates the vlPFC than the vmPFC in adolescent anxiety. Nevertheless, because neither our prior nor our present work specifically targets extinction and related emotion-regulatory processes most consistently shown to engage vmPFC,11,13,14 the current and prior data do not firmly address questions on the role of vmPFC engagement in adolescent anxiety.
The current and prior findings in adolescent anxiety disorders suggest that distinct amygdala-vlPFC perturbations manifest in distinct psychological contexts. The vlPFC and amygdala are strongly interconnected anatomically,47 and the 2 regions can reliably be engaged by the same class of stimuli.11,25,31 However, available data also show that the vlPFC and amygdala perform different functions. For example, while the amygdala is more closely associated with controlling attention to orchestrate stimulus-reinforcement learning, vlPFC is more closely associated with stimulus-response learning, as manifest on response-reversal and flexibility tasks.43 In the context of the present task, these data suggest that vlPFC activity might reflect positive vlPFC-amygdala coupling arising from the amygdala, which signals the need to avoid an interaction and adjust behavior accordingly. In other work on attention orienting, briefly presented threats served as implicit distracters from task-related goals. In this context, negative amygdala-vlPFC connectivity occurs in healthy adolescents but not in adolescents with generalized anxiety disorder.14 This may reflect the specific effect on orienting behavior and vlPFC response that emerges when threat cues draw attention away from a competing attention-orienting task; in this instance, engagement of the vlPFC in healthy subjects in tandem with associated negative vlPFC-amygdala coupling might facilitate competent task performance by maintaining representations of task-related goals despite the presence of salient emotional distractors. Regardless, evidence of stronger positive amygdala-vlPFC connectivity or stronger vlPFC engagement in patients than controls emerges across many neuroimaging studies.11,13,22,48,49 This is consistent with data implicating amygdala-vlPFC circuitry in decision making and behavioral response where salient emotional cues appear.24
The present study has several limitations. First, the sample size is relatively small. Because results derived from small samples are associated more commonly with type II rather than type I error, the potential for masking true effects increases; however, observation of expected significant findings reduces this possibility. Nonetheless, given limitations in statistical power associated with small samples, greater caution is needed when interpreting negative rather than positive findings. Second, roughly half of the patients did not meet criteria for SP, though all reported significant concerns about social interactions. Limiting probands to those with SP could provide a more homogenous sample, reduce variance, and increase statistical power; again, this limitation is also likely to contribute more to type II than type I errors, further suggesting the need to emphasize positive more than negative findings. However, the sample selection approach did not hinder our ability to detect hypothesized group differences and a secondary analysis suggested that amygdala dysfunction might indeed occur in both SP and other adolescent anxiety disorders involving heightened social concerns. Observation of correlations among anxiety symptom ratings, task performance, and neural engagement also support this possibility. Nevertheless, an important future step will be to conduct a larger, more definitive study of participants with varying levels of social anxiety across the full range of the social anxiety continuum. This would provide vital data on key questions arising from this initial study concerning the degree to which perturbed amygdala and associated amygdala-vlPFC circuitry function in adolescent social anxiety is best viewed as a categorical or continuous construct. Third, the social evaluation task has some limitations. Task sensitivity to group differences may have been reduced because our key event incorporated 2 subcomponents rather than examining each component separately and additional “jitter” time was not interspersed between subcomponents. This limitation may have been offset by the advantages gained in task feasibility and psychological fidelity that was maintained, particularly given confirmation of expected findings. Nonetheless, future studies should attempt to separate neural response to picture presentation and to rating.
Despite these limitations, the present study has several strengths. First, the task paradigm is unique in that it engaged psychological processes central to clinical adolescent anxiety concerning social events. Moreover, our past work used photographs of adults to elicit amygdala hyperactivation to negative-valence faces,11,13,14,26 whereas the present study used positive-valence adolescent faces. Second, the present findings support theoretical models. Anxious adolescents demonstrated neural abnormalities when assessing how peers whom they rated negatively would evaluate them in return. This effect occurred despite the positive accepting cues depicted in the peers. Third, functional connectivity patterns support prior studies on circuitry encompassing the vPFC and the amygdala. Future studies may examine connectivity to add further insight on cognitive modulation of emotion and its role in cognitive-behavioral treatments. These results also inform a more precise model of the brain’s response to complex social interactions, increasing precision in attempts to understand neurophysiological and cognitive mechanisms that underlie adolescent social anxiety.
Acknowledgments
Funding/Support: This research was supported by the NIMH intramural research program and NIMH career development grant K99 MH080076 (Dr Guyer).
Footnotes
Financial Disclosure: None reported.
Additional Contributions: Kenneth A. Towbin, MD, Alan Zametkin, MD, and Jennifer Cameron, PhD, provided medical oversight and Harvey A. Iwamoto, MA, assisted with task development and performed programming. We thank the families who participated.
References
- 1.Nelson EE, Leibenluft E, McClure EB, Pine DS. The social re-orientation of adolescence: a neuroscience perspective on the process and its relation to psychopathology. Psychol Med. 2005;35(2):163–174. doi: 10.1017/s0033291704003915. [DOI] [PubMed] [Google Scholar]
- 2.Pine DS, Cohen P, Gurley D, Brook J, Ma Y. The risk for early-adulthood anxiety and depressive disorders in adolescents with anxiety and depressive disorders. Arch Gen Psychiatry. 1998;55(1):56–64. doi: 10.1001/archpsyc.55.1.56. [DOI] [PubMed] [Google Scholar]
- 3.Costello EJ, Pine DS, Hammen C, March JS, Plotsky PM, Weissman MM, Biederman J, Goldsmith HH, Kaufman J, Lewinsohn PM, Hellander M, Hoagwood K, Koretz DS, Nelson CA, Leckman JF. Development and natural history of mood disorders. Biol Psychiatry. 2002;52(6):529–542. doi: 10.1016/s0006-3223(02)01372-0. [DOI] [PubMed] [Google Scholar]
- 4.Steinberg L, Morris AS. Adolescent development. Annu Rev Psychol. 2001;52:83–110. doi: 10.1146/annurev.psych.52.1.83. [DOI] [PubMed] [Google Scholar]
- 5.Clark DM, Wells A. A cognitive model of social phobia. In: Heimberg RG, Liebowitz MR, Hope DA, Schneier FR, editors. Social Phobia: Diagnosis and Treatment. New York, NY: Guilford Press; 1995. pp. 69–93. [Google Scholar]
- 6.Rapee RM, Heimberg RG. A cognitive-behavioral model of anxiety in social phobia. Behav Res Ther. 1997;35(8):741–756. doi: 10.1016/s0005-7967(97)00022-3. [DOI] [PubMed] [Google Scholar]
- 7.Adolphs R. Is the human amygdala specialized for processing social information? Ann N Y Acad Sci. 2003;985:326–340. doi: 10.1111/j.1749-6632.2003.tb07091.x. [DOI] [PubMed] [Google Scholar]
- 8.Gross C, Hen R. The developmental origins of anxiety. Nat Rev Neurosci. 2004;5(7):545–552. doi: 10.1038/nrn1429. [DOI] [PubMed] [Google Scholar]
- 9.Amaral DG. The amygdala, social behavior, and danger detection. Ann N Y Acad Sci. 2003;1000:337–347. doi: 10.1196/annals.1280.015. [DOI] [PubMed] [Google Scholar]
- 10.Sabatini MJ, Ebert P, Lewis DA, Levitt P, Cameron JL, Mirnics K. Amygdala gene expression correlates of social behavior in monkeys experiencing maternal separation. J Neurosci. 2007;27(12):3295–3304. doi: 10.1523/JNEUROSCI.4765-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McClure EB, Monk CS, Nelson EE, Parrish JM, Adler A, Blair RJ, Fromm S, Charney DS, Leibenluft E, Ernst M, Pine DS. Abnormal attention modulation of fear circuit function in pediatric generalized anxiety disorder. Arch Gen Psychiatry. 2007;64(1):97–106. doi: 10.1001/archpsyc.64.1.97. [DOI] [PubMed] [Google Scholar]
- 12.Thomas KM, Drevets WC, Dahl RE, Ryan ND, Birmaher B, Eccard CH, Axelson D, Whalen PJ, Casey BJ. Amygdala response to fearful faces in anxious and depressed children. Arch Gen Psychiatry. 2001;58(11):1057–1063. doi: 10.1001/archpsyc.58.11.1057. [DOI] [PubMed] [Google Scholar]
- 13.Monk CS, Nelson EE, McClure EB, Mogg K, Bradley BP, Leibenluft E, Blair RJ, Chen G, Charney DS, Ernst M, Pine DS. Ventrolateral prefrontal cortex activation and attentional bias in response to angry faces in adolescents with generalized anxiety disorder. Am J Psychiatry. 2006;163(6):1091–1097. doi: 10.1176/ajp.2006.163.6.1091. [DOI] [PubMed] [Google Scholar]
- 14.Monk CS, Telzer EH, Mogg K, Bradley BP, Mai X, Louro HM, Chen G, McClure-Tone EB, Ernst M, Pine DS. Amygdala and ventrolateral prefrontal cortex activation to masked angry faces in children and adolescents with generalized anxiety disorder. Arch Gen Psychiatry. 2008;65(5):568–576. doi: 10.1001/archpsyc.65.5.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pine DS. Research review: a neuroscience framework for pediatric anxiety disorders. J Child Psychol Psychiatry. 2007;48(7):631–648. doi: 10.1111/j.1469-7610.2007.01751.x. [DOI] [PubMed] [Google Scholar]
- 16.Birbaumer N, Grodd W, Diedrich O, Klose U, Erb M, Lotze M, Schneider F, Weiss U, Flor H. fMRI reveals amygdala activation to human faces in social phobics. Neuroreport. 1998;9(6):1223–1226. doi: 10.1097/00001756-199804200-00048. [DOI] [PubMed] [Google Scholar]
- 17.Lorberbaum JP, Kose S, Johnson MR, Arana GW, Sullivan LK, Hamner MB, Ballenger JC, Lydiard RB, Brodrick PS, Bohning DE, George MS. Neural correlates of speech anticipatory anxiety in generalized social phobia. Neuroreport. 2004;15(18):2701–2705. [PubMed] [Google Scholar]
- 18.Veit R, Flor H, Erb M, Hermann C, Lotze M, Grodd W, Birbaumer N. Brain circuits involved in emotional learning in antisocial behavior and social phobia in humans. Neurosci Lett. 2002;328(3):233–236. doi: 10.1016/s0304-3940(02)00519-0. [DOI] [PubMed] [Google Scholar]
- 19.Stein MB, Goldin PR, Sareen J, Zorrilla LT, Brown GG. Increased amygdala activation to angry and contemptuous faces in generalized social phobia. Arch Gen Psychiatry. 2002;59(11):1027–1034. doi: 10.1001/archpsyc.59.11.1027. [DOI] [PubMed] [Google Scholar]
- 20.Straube T, Kolassa IT, Glauer M, Mentzel HJ, Miltner WH. Effect of task conditions on brain responses to threatening faces in social phobics: an event-related functional magnetic resonance imaging study. Biol Psychiatry. 2004;56(12):921–930. doi: 10.1016/j.biopsych.2004.09.024. [DOI] [PubMed] [Google Scholar]
- 21.Phan KL, Fitzgerald DA, Nathan PJ, Tancer ME. Association between amygdala hyperactivity to harsh faces and severity of social anxiety in generalized social phobia. Biol Psychiatry. 2006;59(5):424–429. doi: 10.1016/j.biopsych.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 22.Tillfors M, Furmark T, Marteinsdottir I, Fischer H, Pissiota A, Långström B, Fredrikson M. Cerebral blood flow in subjects with social phobia during stressful speaking tasks: a PET study. Am J Psychiatry. 2001;158(8):1220–1226. doi: 10.1176/appi.ajp.158.8.1220. [DOI] [PubMed] [Google Scholar]
- 23.Blair K, Shaywitz J, Smith BW, Rhodes R, Geraci M, Jones M, McCaffrey D, Vythilingam M, Finger E, Mondillo K, Jacobs M, Charney DS, Blair RJ, Drevets WC, Pine DS. Response to emotional expressions in generalized social phobia and generalized anxiety disorder: evidence for separate disorders [published online ahead of print May 15, 2008] Am J Psychiatry. doi: 10.1176/appi.ajp.2008.07071060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baxter MG, Parker A, Lindner CC, Izquierdo AD, Murray EA. Control of response selection by reinforcer value requires interaction of amygdala and orbital pre-frontal cortex. J Neurosci. 2000;20(11):4311–4319. doi: 10.1523/JNEUROSCI.20-11-04311.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hariri AR, Mattay VS, Tessitore A, Fera F, Weinberger DR. Neocortical modulation of the amygdala response to fearful stimuli. Biol Psychiatry. 2003;53(6):494–501. doi: 10.1016/s0006-3223(02)01786-9. [DOI] [PubMed] [Google Scholar]
- 26.Pérez-Edgar K, Roberson-Nay R, Hardin MG, Poeth K, Guyer AE, Nelson EE, McClure EB, Henderson HA, Fox NA, Pine DS, Ernst M. Attention alters neural responses to evocative faces in behaviorally inhibited adolescents. Neuroimage. 2007;35(4):1538–1546. doi: 10.1016/j.neuroimage.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang TT, Menon V, Eliez S, Blasey C, White CD, Reid AJ, Gotlib IH, Reiss AL. Amygdalar activation associated with positive and negative facial expressions. Neuroreport. 2002;13(14):1737–1741. doi: 10.1097/00001756-200210070-00009. [DOI] [PubMed] [Google Scholar]
- 28.Breiter HC, Etcoff NL, Whalen PJ, Kennedy WA, Rauch SL, Buckner RL, Strauss MM, Hyman SE, Rosen BR. Response and habituation of the human amygdala during visual processing of facial expression. Neuron. 1996;17(5):875–887. doi: 10.1016/s0896-6273(00)80219-6. [DOI] [PubMed] [Google Scholar]
- 29.Pezawas L, Meyer-Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, Kolachana BS, Egan MF, Mattay VS, Hariri AR, Weinberger DR. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nat Neurosci. 2005;8(6):828–834. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- 30.La Greca AM, Stone WL. Social Anxiety Scale for Children–Revised: factor structure and concurrent validity. J Clin Child Psychol. 1993;22(1):117–127. [Google Scholar]
- 31.Nomura M, Ohira H, Haneda K, Iidaka T, Sadato N, Okada T, Yonekura Y. Functional association of the amygdala and ventral prefrontal cortex during cognitive evaluation of facial expressions primed by masked angry faces: an event-related fMRI study. Neuroimage. 2004;21(1):352–363. doi: 10.1016/j.neuroimage.2003.09.021. [DOI] [PubMed] [Google Scholar]
- 32.La Greca AM, Lopez N. Social anxiety among adolescents: linkages with peer relations and friendships. J Abnorm Child Psychol. 1998;26(2):83–94. doi: 10.1023/a:1022684520514. [DOI] [PubMed] [Google Scholar]
- 33.Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36(7):980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 34.The Pediatric Anxiety Rating Scale (PARS): development and psychometric properties. J Am Acad Child Adolesc Psychiatry. 2002;41(9):1061–1069. doi: 10.1097/00004583-200209000-00006. [DOI] [PubMed] [Google Scholar]
- 35.Birmaher B, Khetarpal S, Brent D, Cully M, Balach L, Kaufman J, Neer SM. The Screen for Child Anxiety Related Emotional Disorders (SCARED): scale construction and psychometric characteristics. J Am Acad Child Adolesc Psychiatry. 1997;36(4):545–553. doi: 10.1097/00004583-199704000-00018. [DOI] [PubMed] [Google Scholar]
- 36.Nelson EE. Adolescent Facial Expressions Stimuli Database: Section on Development and Affective Neuroscience. Bethesda, MD: National Institute of Mental Health; 2004. [Google Scholar]
- 37.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 38.Talairach J, Tournoux P. Co-planar Stereotaxic Altas of the Human Brain. Stuttgart, Germany: Georg Thieme Verlag; 1988. [Google Scholar]
- 39.Clark A. AFNI and NIfTI Server. NIMH; Bethesda, MD USA: [Accessed February 27, 2008]. Creating Regions of Interest (ROI) in AFNI. Web site. http://afni.nimh.nih.gov/afni/doc/misc/afni_roi/index_html/view?searchterm=3dmaskave. Updated August 11, 2005. [Google Scholar]
- 40.Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6(3):218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- 41.Gitelman DR, Penny WD, Ashburner J, Friston KJ. Modeling regional and psychophysiologic interactions in fMRI: the importance of hemodynamic deconvolution. Neuroimage. 2003;19(1):200–207. doi: 10.1016/s1053-8119(03)00058-2. [DOI] [PubMed] [Google Scholar]
- 42.Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- 43.Budhani S, Marsh AA, Pine DS, Blair RJ. Neural correlates of response reversal: considering acquisition. Neuroimage. 2007;34(4):1754–1765. doi: 10.1016/j.neuroimage.2006.08.060. [DOI] [PubMed] [Google Scholar]
- 44.Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: role of the amygdala and vmPFC. Neuron. 2004;43(6):897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- 45.Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005;48(2):175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 46.Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420(6911):70–74. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- 47.Ongür D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex. 2000;10(3):206–219. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- 48.Bystritsky A, Pontillo D, Powers M, Sabb FW, Craske MG, Bookheimer SY. Functional MRI changes during panic anticipation and imagery exposure. Neuroreport. 2001;12(18):3953–3957. doi: 10.1097/00001756-200112210-00020. [DOI] [PubMed] [Google Scholar]
- 49.Rauch SL, Savage CR, Alpert NM, Fischman AJ, Jenike MA. The functional neuroanatomy of anxiety: a study of three disorders using positron emission tomography and symptom provocation. Biol Psychiatry. 1997;42(6):446–452. doi: 10.1016/S0006-3223(97)00145-5. [DOI] [PubMed] [Google Scholar]