Abstract
A bewildering series of dynamical processes take part in the development of the nervous system. Neuron branching dynamics, the continuous formation and elimination of neural interconnections, are instrumental in constructing distinct neuronal networks, which are the functional building blocks of the nervous system. In this study, we investigate and validate the important regulative role of mechanical tension in determining the final morphology of neuronal networks. To single out the mechanical effect, we cultured relatively large invertebrate neurons on clean quartz surfaces. Applied to these surfaces were isolated anchoring sites consisting of carbon nanotube islands to which the cells and the neurites could mechanically attach. Inspection of branching dynamics and network wiring upon development revealed an innate selection mechanism in which one axon branch wins over another. The apparent mechanism entails the build-up of mechanical tension in developing axons. The tension is maintained by the attachment of the growth cone to the substrate or, alternatively, to the neurites of a target neuron. The induced tension promotes the stabilization of one set of axon branches while causing retraction or elimination of axon collaterals. We suggest that these findings represent a crucial, early step that precedes the formation of synapses and regulates neuronal interconnections. Mechanical tension serves as a signal for survival of the axonal branch and perhaps for the subsequent formation of synapses.
Introduction
The function of the nervous system is strongly linked to its precise connectivity or wiring diagram and the structure of the neuronal networks, which are its underlying building blocks. A bewildering series of dynamical processes take part in the development of the nervous system. Individual neuron growth patterns and the formation of distinct interconnections between neurons are dominant factors that are instrumental in setting the future output of the neural circuit.
One important mechanism that takes part in shaping the connectivity diagram of neuronal systems is axonal branching and arborization. During the intricate process of network organization, neuronal processes go through extensive branching. Axon branching enables a single neuron to connect (form synapses) with multiple targets and is therefore essential for the assembly of complex neural circuits. Establishment of neural connectivity during development involves neurite outgrowth and the pruning of excess or inappropriate axon branches by means of retraction or degeneration (1). New branches are constantly being formed, with many of them being retracted and absorbed.
The process of axonal branching and pruning of inappropriate branches is closely linked to a second important process that takes place during the development of the nervous system. Synaptogenesis, the formation and consolidation of chemical synapses, is instrumental in regulating neuronal interconnections.
Studies have shown that activity-dependent mechanisms play a major role in regulating retraction or elimination of axonal branches (1,2). Similarly, many decades of research have provided insights into the cellular, molecular, and activity-dependent processes that guide synapse formation and stabilization (see articles by Cohen-Cory (3), Li and Sheng (4), Waites et al. (5), Jontes and Phillips (6), and Atkins and Biederer (7)). According to the prevailing view at the time our study was undertaken, an axon targets a dendrite in the initial stage. Interactions at the contact sites of the axonal growth cone and dendritic filopodia culminate in assembly of all the molecular machinery of the active synapse, (e.g., as described by Friedman et al. (8)). It is widely accepted that a critical step in synaptic circuit maturation is the elimination of excess synaptic inputs (9). Extensive efforts focused on elucidating the mechanisms governing synaptic elimination have mostly suggested that the process is activity dependent (10,11). Interestingly, Alsina et al. (12) reported that the majority of axonal branches destined to be eliminated do not express presynaptic markers before retraction. This observation somewhat hampers the strong association between axonal branching mechanisms and synaptogenesis.
Hence, the above schema is incomplete, and additional mechanisms are expected to take an active part in shaping the final structure of neuronal networks. It has been suggested that activity-independent mechanisms (e.g., adhesion and recognition) establish the basic pattern of connectivity that subsequently becomes refined by activity-dependent processes (6). Neural activity may play an important role only at certain specific phases of synapse assembly. Recent studies, for example, suggest that membrane-associated molecules are involved in branching mechanisms (e.g., thalamocortical axons as described by Yamamoto (13)). This finding suggests an instrumental role for cell-surface interactions (mediated by adhesion molecules) in the very early stages of synaptogenesis as well as in the dynamics of axonal arbors (6,7).
An additional activity-independent factor that appears to affect both axonal branching and synaptogenesis is that of mechanical interactions. The question of mechanical tension as a factor influencing axonal growth and differentiation has been the subject of previous studies that demonstrated the important role of mechanical factors (14–16). Although the significance of these effects remains obscure, the wealth of evidence linking mechanical interactions with network branching has prepared the stage for reevaluating the putative regulating role of neurite mechanical tension during the development of nervous systems.
Here, we investigate and validate the importance of the role that mechanical tension has in determining the final morphology of neuronal networks. To this end, we examined the development and branching pattern of cultured invertebrate neurons while they regenerated on clean quartz surfaces to which were attached isolated anchoring sites of carbon nanotube (CNT) islands. With this method, we singled out the effect of mechanical tension by comparing the effect of tension generated when neurites interact with each other versus the effect of tension generated upon interaction of neurites with CNT. We used special cultures of invertebrate neurons with larger cell bodies and neurites that enabled us to inspect the geometry of interactions in greater detail. To explain the results of our observations, we present a model proposing a crucial early step that precedes the formation of synapses and regulates neuronal interconnections. Our hypothesis for the existence of this step is based on the mechanical attachment of neuronal branches to their targets and on the resulting induced tension that serves as a signal for survival of the axonal branch (and perhaps for the subsequent formation of synapses). Hence, we use the findings of previous reports describing the effect of tension on cellular morphology as a basis on which to further the premise that tension has an important role in shaping interneuronal connectivity and, consequently, an overall effect on network morphology and function.
Experimental Procedures
Neuronal cultures
We have previously developed a locust frontal ganglion culture preparation. The procedures for the dissection and dissociation of neurons to prepare the primary cell culture have been previously described in detail (17,18). In brief, the procedures are as follows. After dissection, neurons are dispersed by enzymatic treatment and mechanical dissociation, and plated on prefabricated substrates (see below). Plated neurons are fully differentiated adult neurons that have lost their original dendrites and axon, leaving only the soma. Culture media (L-15; Sigma, St. Louis, MO) are enriched with 5% locust hemolymph, and the cells are maintained in darkness, under controlled temperature and humidity.
Fabrication of CNT islands array
Quartz substrates with CNT islands were prepared using optical lithography and conventional chemical vapor deposition (CVD) CNT growth (Fig. 1). Quartz substrates (1 mm thick; United Silica Products, Franklin, NJ) were first sputtered with a titanium nitride (TiN) layer (100 nm, MRC 8620 sputtering system), which acts as a diffusion barrier in the subsequent CVD process. Samples were prepared by spin-coating with positive photoresist; a standard lithography process was then used to define small islands. Exposed TiN regions were removed by a reactive ion etching process (NE860; Nextral, Grenoble, France), and the photoresist was removed. A second lithography step was conducted to form a photoresist mask for the deposition and lift-off of nickel (Ni) islands directly onto the underlying TiN islands. A thin layer of Ni (5–7 nm) was coated on the sample using an electron beam evaporator (VST Services, Petach-Tiqwa, Israel).
Figure 1.
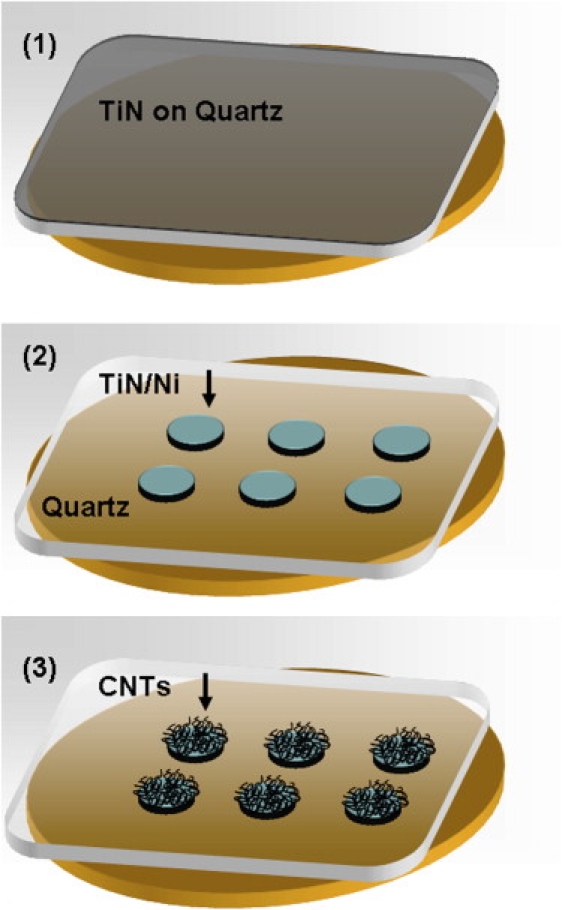
Scheme of CNT microfabrication. (1) The quartz substrate with 100 nm sputtering TiN. (2) The sample after the first standard photolithography process followed by a reactive ion etching process and a second photolithography process to form a 5–7 nm of evaporated Ni catalyst layer. (3) The sample after the CVD process. The thin black lines represent the CNTs. Further details are in the text.
Chemical vapor deposition
After the lithography steps described above, the CVD CNT growth process was performed. This method consisted of an initial purge step in hydrogen after which the temperature was ramped up until it reached the CNT growth-onset temperature. Once the furnace had reached 900°C, a constant volumetric flow of 20 standard cubic centimeter per minute of ethylene gas was added. The duration of this step was 10 min.
Light microscopy and culture monitoring
Cultures were maintained for ≥ 10 days in vitro in a special environmental chamber. The chamber was placed on a microscope stage (TS100; Nikon, Melville, NY), and images were acquired using a charge-coupled device (CCD) camera (Nikon DS-Fi1) with a control unit (Nikon DS-L2) set to take time-lapse images every 5–30 min. The pictures were taken in magnifications of 5×, 10×, 20×, or 40×. Because locust cultures are sensitive to light, a programmed controller unit was used to manage the illumination to minimize their exposure to light. Fixated samples were imaged using a metallurgical light microscope (Meiji Techno, Saitama, Japan) that was equipped with a CCD camera.
Neuronal arborization analysis
Images of extending processes were taken every 10 min. To quantify the length of the processes and the numbers of their branches over time, the following measurements were conducted for each picture taken. The number of branches and the process length were measured between the process' last anchor point, before the growth cone, until the tip of the growth cone or until the process' new anchor point. Lengths were measured and branches were counted using ImageJ software (U.S. National Institutes of Health, Bethesda, MD). Graphs of the normalized process length (divided by the maximum length) versus time and the number of branches versus time were created using these measurements.
Calculation of tension forces
Tension was calculated according to the scheme presented by Bray (14) based on the assumption of equilibrium of forces at neurite branching points. The relative tension in connected segments was estimated by measuring the angles between each two segments in every junction or brunching point. The relative tension was then derived from the following relationship: . Once an initial value was assigned to the first segment (arbitrarily chosen as T0 = 1), the next values could be iteratively calculated. Neurite diameters were measured from light microscope images (the mean value over an appreciable length), and the predicted relation between diameter and tension along a segment was tested (this value should be approximately linear based on Bray's predication and results (14)).
Scanning electron microscopy
For scanning electron microscopy observation, samples were fixated, dried, and then coated with 6 nm of chrome (K575X coating unit; Emitech, Ashford, UK), as described previously (19). In brief, 4- to 9-day-old cultures were fixated for 30 min (37°C) in 2.5% glutaraldehyde (49629; Fluka, Neu-Ulm, Germany) in phosphate-buffered saline ((PBS) 79382; Fluka). The fixed samples were then rinsed for 5 min in increasing concentrations of ethanol (25%, 50%, and 75%), and the sample were kept covered with each of the ethanol solutions. The next step consisted of 10 min rinses with 96% and 100% ethanol solutions. Finally, the dehydrated samples were critical point-dried using a critical-point dryer (Balzers Union, Balzers, Liechtenstein). The samples were examined using a high-resolution scanning electron microscope (JSM-6700; Jeol, Peabody, MA).
Immunohistochemistry
Four-day-old cultures were fixed in 4% paraformaldehyde in PBS for 20 min at room temperature (RT) and then washed twice with PBS for 10 min. Next, the cultures were incubated for 30 min at RT in permeabilization solution (5% goat serum, 1 mg/mL bovine serum albumin, 0.3% Triton in PBS) and again washed twice with PBS. Immunostaining was used to test for the presence of synaptic structures. The primary antibody was a monoclonal mouse antibody raised against the Drosophila synaptic vesicle-associated protein synapsin (SYNORF1; 3C11; kindly provided by E. Buchner, University of Würzburg, Würzburg, Germany). Cultures were incubated overnight at 4°C with the primary antibodies diluted in blocking solution (5% goat serum, 1 mg/mL bovine serum BSA in PBS). The secondary antibody was Cy3-conjugated goat anti-mouse (1:200; Jackson ImmunoResearch, West Grove, PA) and applied for 2 h at RT. To visualize all the neuronal processes, the cultures were costained with primary anti-horseradish peroxidase serum (1:10000; Sigma) overnight at RT and then incubated with a secondary antibody (Cy2-conjugated goat anti-rabbit; 1:200; Jackson ImmunoResearch) for 4 h at RT. Cultures were washed with PBS and mounted in fluorescent mounting medium (Dako, Glostrup, Denmark).
Double-labeled preparations were analyzed with a confocal laser scanning microscope (LSM 510; Carl Zeiss, Jena, Germany). For imaging Cy3 fluorescence, a helium-neon laser with an excitation wavelength of 543 nm and a detection range of 560–700 nm was used; for Cy2 fluorescence, a helium-neon laser with an excitation wavelength of 488 nm and a detection range of 505–530 nm was used. Images were further processed with the LSM 5 Image Browser (Carl Zeiss).
Results
Fig. 1 Our first goal in this study was to identify and establish a preparation in which mechanical tension is readily apparent and can be easily detected and analyzed. Neurons cultured on CNT islands provide such a unique system (19,20). By using (the relatively large) locust neurons, we could significantly reduce the cell density and so monitor the development of the network and the important role of the mechanical forces in greater detail.
Fig. 2 shows a network of locust neurons cultured on a patterned quartz substrate (5 days in culture). The network obtained is marked by two main features. First, the cell bodies are distributed evenly across the surface, and no major clustering is apparent (compare this image to the results described in one of our previous studies (15)). This effect stems from the ability of the CNT islands to anchor the cell bodies (Fig. 2 B). Indeed, most cell bodies are near a CNT island. The strong affinity of the cells to the CNT surface is further demonstrated in the high-resolution scanning electron image (also described by Sorkin et al. (20)). A second important feature is the straightness of the interconnected processes. This apparent tautness provides a clear indication of the existence of tension forces along the neuronal processes.
Figure 2.
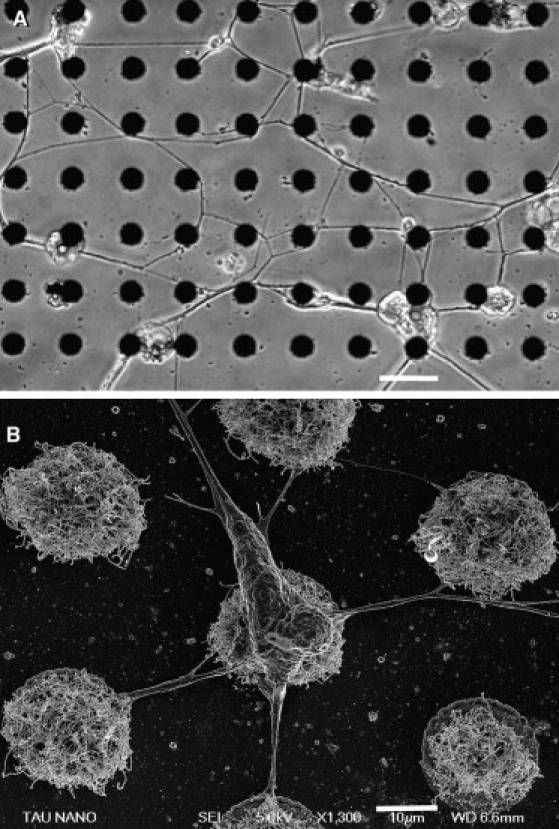
Culture preparation. (A) A circuit of locust neurons cultured and stabilized using 20 μm CNT islands. The cells position themselves according to the spatial distribution of the islands. The topography of the substrata also dictates the neuronal interconnections. Scale bar, 50 μm. (B) A high-resolution scanning electron microscope image of a single locust neuron adhering to an array of CNT islands; also shown are the taut neurites connecting it to neighboring islands. Scale bar, 10 μm.
The nature of the mechanical attachment of neuronal processes to the CNT islands allowing the generation of tension along the neurites is well demonstrated in Fig. 3. This figure shows two examples of the extensive branching of terminal neuronal processes and their entanglement within the CNT islands. As tension along the neurite increased, the forces exerted were such that a “handful” of CNT were broken or torn from the island.
Figure 3.
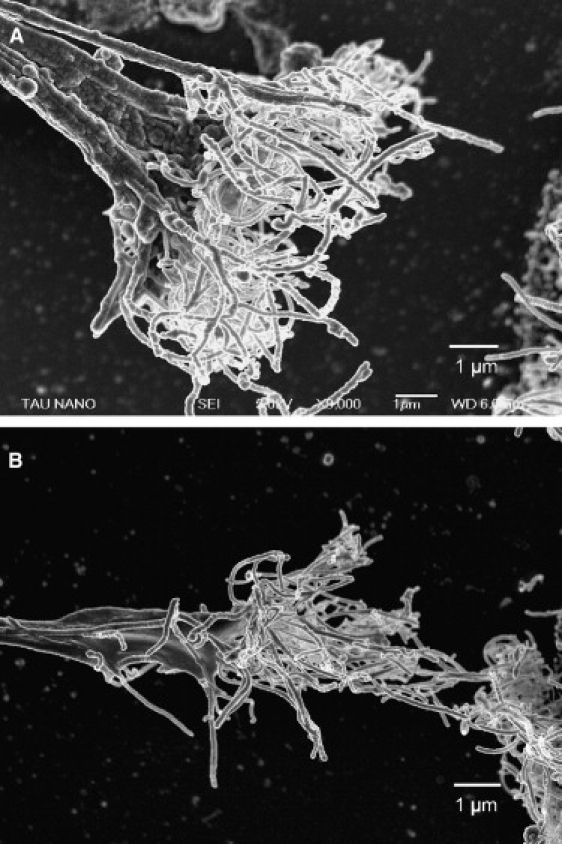
Neuron attachment to the CNT. High-resolution scanning electron microscope images of the point of attachment between the terminals of neuronal processes and the CNT islands. Two examples (A and B) of the force of attachment resulting in breaking or tearing of a “handful” of tubes (shown as whitish mesh) from the CNT island, the edge of which can be seen on the right (the neurite is “arriving” from the left).
Because the CNT islands are produced on transparent quartz surfaces, the morphological development of the network—from the initial stage of isolated cells to that of interconnected circuits—could be effectively monitored using optical (inverted) microscopy. The neurons were initially deposited randomly from solution; within several hours, the cells had self-organized; as mentioned above, they were found deposited almost exclusively in very close proximity to CNT islands. During a span of several days, the cells began regenerating by extending long axonal and dendritic processes, which later consolidated and stretched into a relatively compact interwired system (Fig. 2 A). The temporal evolution of this process will be the main focus of the next sections.
By following the results and predictions described by Bray (14), we could further establish the presence of tension forces in our preparations. Using equilibrium of vectors to estimate the tension of single neurite segments, we obtained the predicted, very high correlation between the calculated tension and the measured diameter of the neurite (Fig. 4).
Figure 4.
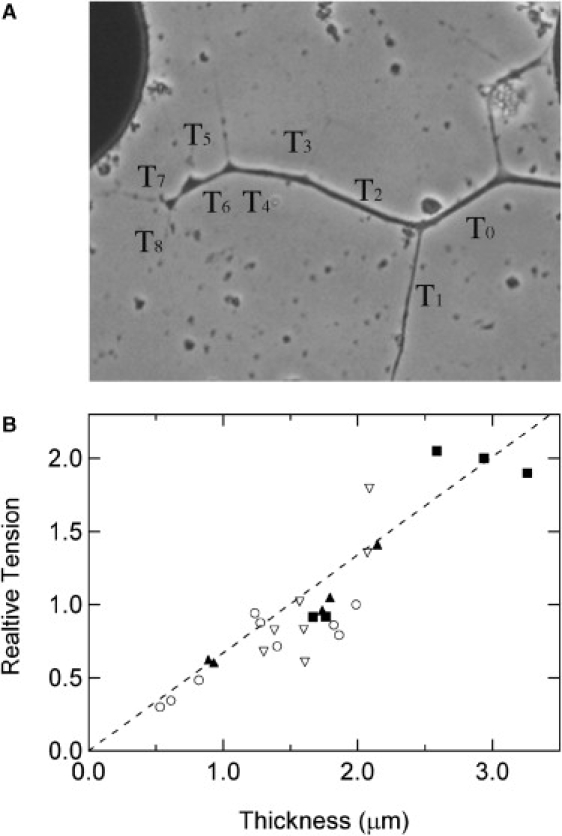
Correlation between tension and diameter of neurite segments. (A) An example of a branched axon with the series of relative tension values. Tension is approximated by measuring the angles between each angles between each two segments in every junction or brunching point. An arbitrary value is assigned T0 = 1, and the relative tension is then derived from the following relationship: (as described by Bray (14))). (B) Four examples of scaled and overlaid relative tension values versus neurite segment thickness values. The scaling was carried out by multiplying all relative tension values for each of the four experiments by a constant number, so that the relative tension of the scaled data is the same for similar diameter values. The dashed line is a linear fit derived from the accumulative scaled data.
We next turned our attention to the dynamics of the neuronal growth process. Fig. 5 A depicts images of the same axonal process and its branches at four different time points. The growth pattern is marked by extensive growth cone arborization and neurite branching; after this stage, the structures are clearly reduced and simplified. These results are in general agreement with our previous reports of culture development (without CNT) in which we observed an initial increase and then a pronounced decrease in the number of processes originating from the cell body as well as a lower average number of neurite segments per cell in later stages of network development (17,21). The same method of growth was observed in all monitored cases, both in different neurons and in different preparations. Data from several such examples are summarized in the graph presented in Fig. 5 B. In Fig. 5 C, the averaged number of branches and the averaged total length of the tree (section) are plotted to establish the consistency of this behavior.
Figure 5.
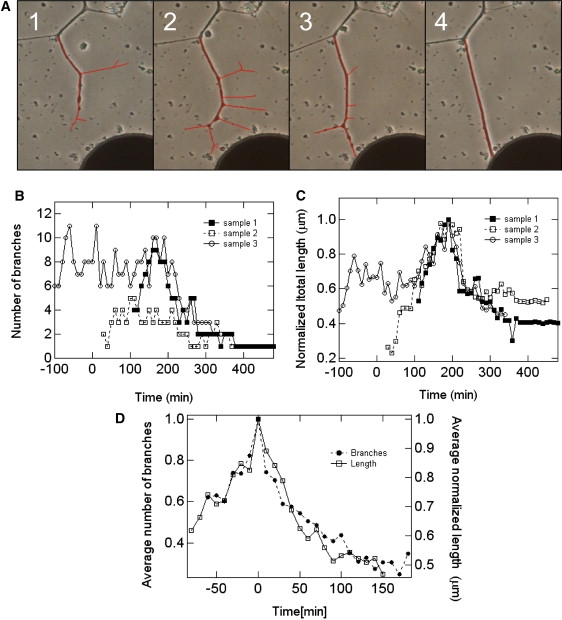
Time-lapse images (see Experimental Procedures) and analysis of process development and neurite branch pruning. (A) An extensive growth cone and neuronal arborization (1 and 2), followed by branch retraction and absorption (3 and 4). The region analyzed is traced in red. The reduction in number and total length of branches accompanies the attachment of one of the branches to a CNT island, the establishment of the connection, and the formation of tension along the axonal arbors. The course of neurite arborization described by following the change in number of secondary branches (B) or the total length of the branches (C). The independent observations were normalized by dividing the data by the maximum value. The images shown in A correspond to the data in sample 1. (D) Average curves calculated for the data shown in B (solid symbols) and C (open symbols). Data in B through D were shifted in time to align the maximum (number of branches or total length).
Close investigation of the data described above revealed that the destiny of a particular branch (branch survival or retraction) was closely tied to whether that branch was attached to a CNT island (Fig. 6; see Movie S1 in the Supporting Material). Moreover, as soon as such a connection to a CNT island was established, tension was generated along the neurites, as evidenced in the clear straightening of the neuronal segments (Fig. 6). Based on these results, we propose the following putative mechanism: as the tension in the connected branch increases, other branches (only loosely connected to the surface) lose their grips on the surface and subsequently retract, leaving only the very taut, single branch still connected (Figs. 2, 5, and 6). This phenomenon is clearly observed in the experiments described in this study, probably because of the weak adhesion of the substrate (outside of the CNT islands).
Figure 6.
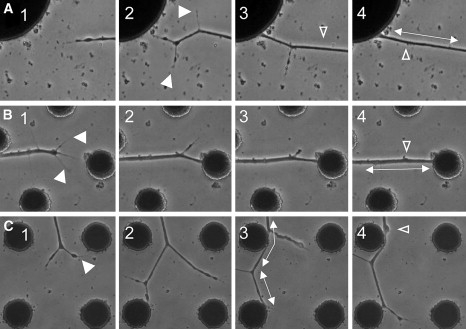
Three examples demonstrating the formation of tension along neurites after attachment to a CNT island (time-lapse images). The generation of tension is accompanied by the retraction and absorption of unconnected neurite branches. Solid arrowheads show newly formed branches. Open arrowheads mark the respective spots of retracted branches as tension is generated (double-headed arrows).
To establish whether this putative mechanism is characteristic only of neuron-CNT interactions or whether it constitutes an important, regulative step in the development and stabilization of the neuronal network structure, we explored several related incidents in which branches were stabilized by connecting to other neuronal processes rather than to the CNT islands. To make this determination, we followed a number of growth cones in different developing networks while they connected to other neurites. As can be seen in Fig. 7, the same steps identified in the neurite–CNT island interactions could be detected during neuron–neuron connections. A growing neurite approaching other neuronal processes branches and rebranches (Fig. 7 A). Connection of one of the second order branches with the perpendicular process is accompanied by increased tension and followed by retraction of the sister branch. Further attachment with the two neuronal processes is also accompanied by retraction of the unconnected first order branch, leaving only the strongly attached branch to be part of the wiring diagram of the developing network. The generation of tension is evident in the straightening of the neuronal segments (Fig. 7 B).
Figure 7.
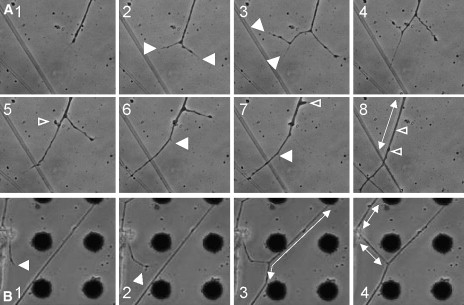
A process similar to the one shown in Fig. 4 is demonstrated in two examples of neurite–neurite interactions. As the connection is established, tension is generated (evidenced in the straightening of the segments), and unconnected branches are retracted and absorbed.
We then considered whether we could relate the generation of tension and establishment of interconnections versus branch pruning directly to synaptogenesis. A first step toward answering this question (although somewhat circumstantial rather then conclusive) was achieved by way of immunohistochemical monitoring of synapse formation during the development of the network on the CNT-applied substrate. As suggested by recent work on cultured insect neurons (22,23), cultured neurons form presynaptic specializations independent of postsynaptic structures (even in isolated neurons). As shown in Fig. 8, localized expressions of presynaptic proteins occurred at neurite terminals after establishment of the connection to the CNT (after pruning of unconnected branches).
Figure 8.
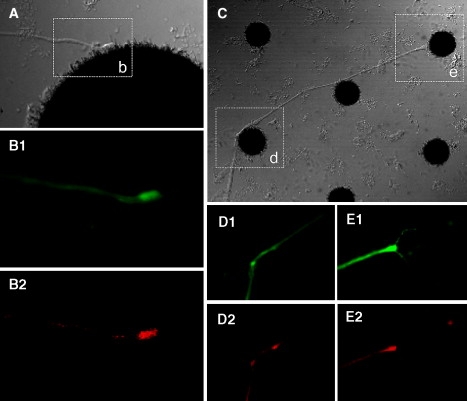
Neuron-CNT interactions and synaptogenesis. (A) The point of attachment of a neurite terminal to a CNT island. The area outlined and labeled as b is enlarged in B. Neuron-specific, anti-horseradish peroxidase immunostaining is shown with green in B1; antisynapsin staining is red in B2. (C) Two additional examples demonstrating established connections, evidenced by the straight segments (induced by tension). The areas outlined and marked as d and e are enlarged in D and E. The immunostaining is the same as that described and used for B.
Discussion
The data presented in this article demonstrate a simple selection mechanism in which one axon branch wins over others. The apparent mechanism entails the build-up of mechanical tension in developing axons. The tension is maintained through the attachment of the growth cone to the substrate or, alternatively, to the neurites of a target neuron. The induced tension promotes the stabilization of one set of axon branches while causing retraction or elimination of axon collaterals. Establishment of the connection of the growth cone may serve as a signal for the early stages of synaptogenesis.
Two primary models have been proposed as taking part in controlling and regulating the pattern of neuronal connectivity in the developing nervous system. The first model relies on molecular codes in the form of adhesion and cell surface molecules that guide the formation of specific neuronal interconnections (6,24). This model promotes cell–cell recognition as the major factor preceding the synaptogenesis. The second class of models suggests dependence on the formation of active synapses (and correlated activity) as a factor that stabilizes the “correct” synaptic connectivity. Several studies have investigated some of the mechanisms whereby electrical activity shapes the growth and branching of axon arbors, and thus the formation of neural circuits during development (25,26). Of course, the two general schemes may not be mutually exclusive and may both fit a sequence of events whereby the early ones are dependent on surface molecules or “molecular addresses” and the latter, predominantly activity-dependent ones, lead to the final refinement of a distinct neuronal network wiring diagram. The picture, however, remains far from complete. Although axon retraction and degeneration are considered instrumental in the formation of the distinct neuronal interconnections and specific neural circuitry (1,27), much work remains before the underlying mechanisms can be well understood.
In contrast to the ample work dedicated to molecular and activity-dependent regulation of axonal growth and branching, far less attention has been focused on physical or mechanical factors, primarily tension. Heidemann and Buxbaum (16) have reviewed studies indicating that mechanical tension is a regulator and stimulator of axonal elongation and retraction. Evidence from these studies includes direct measurements of the tension generated along a growing axon, but they do not ascribe a role to the mechanical forces in network developmental. Bray (14,15) reported a role for tension in promoting branching. In accordance with this finding, further work (e.g., the study by Lamoureux et al. (28)) suggested that tension along a developing neurite must send some kind of signal to regulate the fate of axonal branches and neuronal and network morphology. We have previously reported on the role of tension in controlling some aspects of neural network development (neurite branching angles (29)). The results of this study provide direct evidence for the important role that mechanical tension plays in the control and regulation of the topology of developing networks via control of the retraction and elimination of axonal branches. We present a model (Fig. 9) that expands the role of tension to include the regulation of neuronal interconnections and, subsequently, of the neural circuit wiring diagram.
Figure 9.
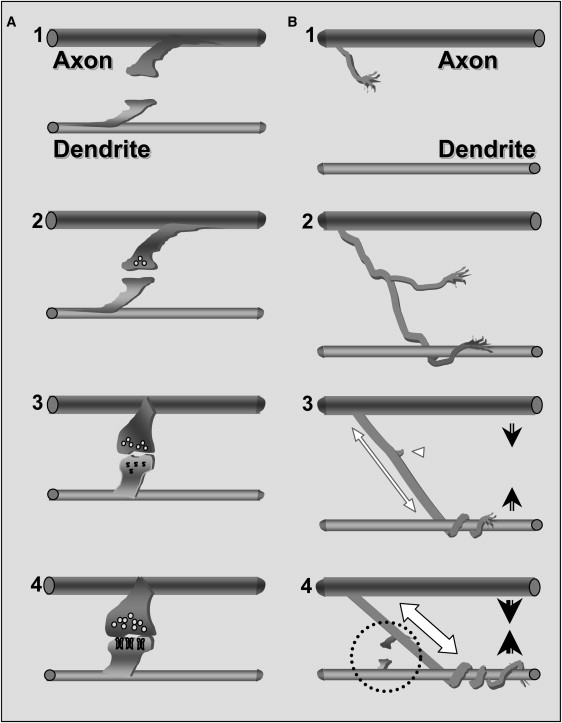
Novel scheme of synaptogenesis. (A) The accepted stages of interneuronal connection and synapse formation (described by Cohen-Cory (3)): (1) Two-way filopodia connections; (2) an unspecialized yet functional connection is formed; (3) synaptic vesicles accumulate and postsynaptic differentiation is triggered; and (4) functional maturation of both the pre- and the postsynaptic sides. (B) The suggested preceding stages: (1) An axonal branch growth cone is approaching a dendrite; (2) entanglement of the axonal branch along the dendrite; (3) tension is formed along the axonal branch (white, double-headed arrow) as the axodendritic connection is established. The axon and dendrite are then pulled closer toward each other (arrows). The unattached branches are retracted (white arrowhead). (4) The mechanical attachment is secured as tension is increased (white, double-headed arrow). The axon and dendrite are pulled yet closer (arrows). Two-way filopodia connections are formed (dashed circle). The subsequent stages are the same as those described in A. Similar to the original model, failure at any of the stages will result in elimination of the connection.
We suggest that the process of tension-induced selection of some axonal arbors over others is instrumental in shaping the morphology of the neurons and the network. It is related to molecular recognition and adhesion, although independent of them. Tension may be viewed as a selection mechanism during formation of the connectivity between two neurons (Fig. 9). This mechanism could actually limit the number of connection points (and later synapses) between the two cells at a given site (i.e., the first and/or strongest attachment point has a tendency to persist whereas other attachment points are eliminated). Our results suggest that stabilization of the attachment of a neurite terminal, whether to the processes of a neighboring cell or even to an abiotic anchor (e.g., the CNT island), is sufficient to initiate the process of synaptogenesis. This mechanical tension–dependent step precedes the known scheme of activity-dependent stabilization of synaptic specificity (Fig. 9).
The important role of tension by no means diminishes the importance of chemical cues. The advancing growth cone is undoubtedly subjected to a rich chemical environment in the developing nervous system, an environment composed of relatively long-range signals (attractant and repellent molecules) and short-range or contact signals (cell surface molecules). However, our results show that the process of retraction and degeneration of neurite branches can also be seen during the interactions with nonbiological elements (CNT islands). The stabilization of a single branch while its collaterals are being retracted and ultimately absorbed seems to be a direct consequence of both a strong connection between the branch and a target and the mechanical tension generated in all the branches resulting in the retraction of the nonconnected ones.
In a previous report (30), we demonstrated that tension by itself is not sufficient to explain the distribution of branching angles observed in a culture preparation of locust neurons. We concluded that there were attachment points additional to the cell body and growth cone that added an extra force to the equilibrium at a bifurcation point. In this study, the unique nature of the substrate (pristine quartz substrate with isolated CNT anchoring points) is such that the attachment points are mainly at the neurite ends. This material enabled us to use the model suggested by Bray (14) to asses the tension forces as well as to pinpoint or clearly isolate their effect.
How is tension generated? This important question has been frequently addressed in previous reports and is beyond the scope of our study. The accepted mechanism for the generation of tension along the neuronal process is perceived to be dependent on rearrangement of the cellular cytoskeleton (31–33). Rearrangement of actin filaments and microtubules is commonly attributed to complex molecular cues in the nervous system environment (e.g., the study by Challacombe et al. (33)). Given the results of our study, we suggest that the generated tension itself also serves as a signal that induces a different kind of cytoskeleton rearrangement leading to growth cone retraction and axonal branch elimination. Hence, our study supports the findings of Heidemann and Buxbaum (16), who suggested that mechanical tension plays a role in axonal development analogous to that of a second messenger (i.e., a signal molecule).
Finally, Van Essen (34) states that the macroscopic mechanical properties of the brain in vivo are consistent with neurite properties observed in vitro. He also suggests that many structural features of the mammalian central nervous system can be explained by mechanisms that involve mechanical tension along neuronal processes. Our results offer experimental evidence to support this view.
Acknowledgments
This work was supported in part by grant 1138/04 from the Israeli Science Foundation (ISF) to Y.H.
Supporting Material
References
- 1.Luo L., O'Leary D.D. Axon retraction and degeneration in development and disease. Annu. Rev. Neurosci. 2005;28:127–156. doi: 10.1146/annurev.neuro.28.061604.135632. [DOI] [PubMed] [Google Scholar]
- 2.Hua J.Y., Smear M.C., Baier H., Smith S.J. Regulation of axon growth in vivo by activity-based competition. Nature. 2005;434:1022–1026. doi: 10.1038/nature03409. [DOI] [PubMed] [Google Scholar]
- 3.Cohen-Cory S. The developing synapse: construction and modulation of synaptic structures and circuits. Science. 2002;298:770–776. doi: 10.1126/science.1075510. [DOI] [PubMed] [Google Scholar]
- 4.Li Z., Sheng M. Some assembly required: the development of neuronal synapses. Nat. Rev. Mol. Cell Biol. 2003;4:833–841. doi: 10.1038/nrm1242. [DOI] [PubMed] [Google Scholar]
- 5.Waites C.L., Craig A.M., Garner C.C. Mechanisms of vertebrate synaptogenesis. Annu. Rev. Neurosci. 2005;28:251–274. doi: 10.1146/annurev.neuro.27.070203.144336. [DOI] [PubMed] [Google Scholar]
- 6.Jontes J.D., Phillips G.R. Selective stabilization and synaptic specificity: a new cell-biological model. Trends Neurosci. 2006;29:186–191. doi: 10.1016/j.tins.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 7.Akins M.R., Biederer T. Cell-cell interactions in synaptogenesis. Curr. Opin. Neurobiol. 2006;16:83–89. doi: 10.1016/j.conb.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 8.Friedman H.V., Bresler T., Garner C.C., Ziv N.E. Assembly of new individual excitatory synapses: time course and temporal order of synaptic molecule recruitment. Neuron. 2000;27:57–69. doi: 10.1016/s0896-6273(00)00009-x. [DOI] [PubMed] [Google Scholar]
- 9.Sanes J.R., Lichtman J.W. Development of the vertebrate neuromuscular junction. Annu. Rev. Neurosci. 1999;22:389–442. doi: 10.1146/annurev.neuro.22.1.389. [DOI] [PubMed] [Google Scholar]
- 10.Goodman C.S., Shatz C.J. Developmental mechanisms that generate precise patterns of neuronal connectivity. Cell. 1993;72(Suppl.):77–98. doi: 10.1016/s0092-8674(05)80030-3. [DOI] [PubMed] [Google Scholar]
- 11.Ruthazer E.S., Akerman C.J., Cline H.T. Control of axon branch dynamics by correlated activity in vivo. Science. 2003;301:66–70. doi: 10.1126/science.1082545. [DOI] [PubMed] [Google Scholar]
- 12.Alsina B., Vu T., Cohen-Cory S. Visualizing synapse formation in arborizing optic axons in vivo: dynamics and modulation by BDNF. Nat. Neurosci. 2001;4:1093–1101. doi: 10.1038/nn735. [DOI] [PubMed] [Google Scholar]
- 13.Yamamoto N. Cellular and molecular basis for the formation of lamina-specific thalamocortical projections. Neurosci. Res. 2002;42:167–173. doi: 10.1016/s0168-0102(01)00324-8. [DOI] [PubMed] [Google Scholar]
- 14.Bray D. Mechanical tension produced by nerve cells in tissue culture. J. Cell Sci. 1979;37:391–410. doi: 10.1242/jcs.37.1.391. [DOI] [PubMed] [Google Scholar]
- 15.Bray D. Axonal growth in response to experimentally applied mechanical tension. Dev. Biol. 1984;102:379–389. doi: 10.1016/0012-1606(84)90202-1. [DOI] [PubMed] [Google Scholar]
- 16.Heidemann S.R., Buxbaum R.E. Mechanical tension as a regulator of axonal development. Neurotoxicology. 1994;15:95–107. [PubMed] [Google Scholar]
- 17.Shefi O., Ben-Jacob E., Ayali A. Growth morphology of two-dimensional insect neural networks. Neurocomputing. 2002;44:635–643. [Google Scholar]
- 18.Shefi O., Golding I., Segev R., Ben-Jacob E., Ayali A. Morphological characterization of in vitro neuronal network. Phys. Rev. E. 2002;66:021905. doi: 10.1103/PhysRevE.66.021905. [DOI] [PubMed] [Google Scholar]
- 19.Sorkin R., Gabay T., Blinder P., Baranes D., Ben-Jacob E. Compact self-wiring in cultured neural networks. J. Neural Eng. 2006;3:95–101. doi: 10.1088/1741-2560/3/2/003. [DOI] [PubMed] [Google Scholar]
- 20.Sorkin R., Greenbaum A., David-Pur M., Anava S., Ayali A., Ben-Jacob E., Hanein Y. Process entanglement as a neuronal anchorage mechanism. Nanotechnology. 2009;20:015101. doi: 10.1088/0957-4484/20/1/015101. [DOI] [PubMed] [Google Scholar]
- 21.Ayali A., Shefi O., Ben-Jacob E. Self-organization of two-dimensional insect neural networks. In: Experimental ChaosBoccaletti S., Gluckmam B.J., Kurths J., Pecora L.M., Spano M.L., editors. American Institute of Physics; Melville, NY: 2002. pp. 465–475. [Google Scholar]
- 22.Küppers-Munther B., Letzkus J.J., Lüer K., Technau G., Schmidt H. A new culturing strategy optimises Drosophila primary cell cultures for structural and functional analyses. Dev. Biol. 2004;269:459–478. doi: 10.1016/j.ydbio.2004.01.038. [DOI] [PubMed] [Google Scholar]
- 23.Oh H.W., Campusano J.M., Hilgenberg L.G., Sun X., Smith M.A. Ultrastructural analysis of chemical synapses and gap junctions between Drosophila brain neurons in culture. Dev. Neurobiol. 2008;68:281–294. doi: 10.1002/dneu.20575. [DOI] [PubMed] [Google Scholar]
- 24.Washbourne P., Dityatev A., Scheiffele P., Biederer T., Weiner J.A. Cell adhesion molecules in synapse formation. J. Neurosci. 2004;24:9244–9249. doi: 10.1523/JNEUROSCI.3339-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Katz L.C., Shatz C.J. Synaptic activity and the construction of cortical circuits. Science. 1996;274:1133–1138. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- 26.Zhang L.I., Poo M.M. Electrical activity and development of neural circuits. Nat. Neurosci. 2001;4:1207–1214. doi: 10.1038/nn753. [DOI] [PubMed] [Google Scholar]
- 27.Low L.K., Cheng H.J. Axon pruning: an essential step underlying the developmental plasticity of neuronal connections. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2006;361:1531–1544. doi: 10.1098/rstb.2006.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lamoureux P., Ruthel G., Buxbaum R.E., Heidemann S.R. Mechanical tension can specify axonal fate in hippocampal neurons. J. Cell. Biol. 2002;159:499–508. doi: 10.1083/jcb.200207174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shefi O., Golobovitz S., Ben Jacob E., Ayali A. A two-phase growth strategy in cultured neuronal networks as reflected by the distribution of neurite branching angles. J. Neurobiol. 2005;62:361–368. doi: 10.1002/neu.20108. [DOI] [PubMed] [Google Scholar]
- 30.Shefi O., Harela A., Chklovskii D.B., Ben-Jacobb E., Ayali A. Biophysical constraints on neuronal branching. Neurocomputing. 2004;58-60:487–495. [Google Scholar]
- 31.Lin C.H., Thompson C.A., Forscher P. Cytoskeletal reorganization underlying growth cone motility. Curr Opin Neurobiol. 1994;4:640–647. doi: 10.1016/0959-4388(94)90004-3. [DOI] [PubMed] [Google Scholar]
- 32.Mitchison T.J., Cramer L.P. Actin-based cell motility and cell locomotion. Cell. 1996;84:371–379. doi: 10.1016/s0092-8674(00)81281-7. [DOI] [PubMed] [Google Scholar]
- 33.Challacombe J.F., Snow D.M., Letourneau P.C. Actin filament bundles are required for microtubule reorientation during growth cone turning to avoid an inhibitory guidance cue. J. Cell Sci. 1996;109:2031–2040. doi: 10.1242/jcs.109.8.2031. [DOI] [PubMed] [Google Scholar]
- 34.Van Essen D.C. A tension-based theory of morphogenesis and compact wiring in the central nervous system. Nature. 1997;385:313–318. doi: 10.1038/385313a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


