Abstract
Integrin-cadherin cross talk is an important aspect of cell function. We explored this signaling using substrates micropatterned with islands of fibronectin surrounded by E-cadherin, capturing the segregation of these signals in normal tissue. While MDCK cells were able to concurrently form adhesive structures with these two proteins, engagement of fibronectin by MCF-7 cells, an adenocarcinoma cell line, inhibited response of these cells to E-cadherin. We further demonstrated that this inhibition is rigidity dependent; on soft elastomer substrates with Young's modulus in the range of tens of kiloPascals, MCF-7 cells were able to engage both integrin and cadherin ligands.
Introduction
The ability of cells to integrate multiple cues of the extracellular environment is central to tissue morphogenesis and a range of diseases. In epithelial tissues, cells coordinate cadherin-based links with adjacent cells and integrin-mediated connections with an underlying extracellular matrix. An important example of cross talk between these pathways is inhibition of cadherin function by integrin engagement (1–3). It has also been established in vitro that integrin signaling is sensitive to the mechanical properties of the underlying substrate, with decreasing rigidity associated with disruption/dissolution of focal adhesion structures (4–6). Combining these ideas poses the intriguing possibility that integrin/cadherin cross talk may be modulated by substrate rigidity. Here, we directly demonstrate this concept by comparing concurrent engagement of fibronectin (FN) and E-cadherin (Ecad) by MCF-7 epithelial cells on substrates of different elastic moduli. To capture the natural spatial separation of these signals, substrates were patterned (Fig. 1) with features of FN surrounded by the extracellular domain of E-cadherin fused with an Fc domain (EcadFc), presenting both components to the basolateral surface of the cell (7,8).
Figure 1.
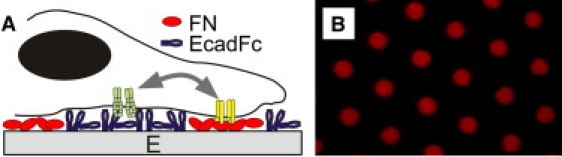
(A) Dual-component surfaces present spatially separated regions of proteins. (B) Surface illustrating 3-μm diameter FN dots, spaced 10 μm center-to-center.
Materials and Methods
Substrate preparation
Glass coverslips were cleaned as previously described (9). Polydimethylsiloxane (PDMS, Sylgard 184, Dow Corning, Midland, MI) was prepared as millimeter-thick layers cast on glass. Rigid PDMS (E = 5 MPa) was prepared using a 1: 10 ratio of curing agent: elastomer base and baked at 85°C overnight, whereas soft PDMS (E = 60 kPa) was prepared at 1:40 and cured at 60°C. Stress-relaxation tests verified the elastic moduli. Control surfaces were prepared by coating with either FN (human plasma, Sigma, St. Louis, MO) at 100 μg/mL for 1 h at 37°C or EcadFc (human E-cad/Fc fusion protein, R&D Systems, Minneapolis, MN) at 20 μg/mL for 1 h at 37°C. Patterned surfaces were prepared by microcontact printing using established methods (9), followed by coating with EcadFc. All surfaces were blocked in 100 μg/mL bovine serum albumin for 1 h at 37°C.
Cell experiments
MCF-7 cells (ATCC, Manassas, VA) were cultured in Dulbecco's Modified Eagle Medium (Gibco, Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum and penicillin/streptomycin. MDCK cells (generously provided by W. James Nelson, Stanford University, Stanford, CA) were cultured in low-glucose Dulbecco's Modified Eagle Medium supplemented with 10% fetal bovine serum and penicillin/streptomycin. For experiments, cells were seeded at 5000 cells/cm2. Cells were fixed (4% paraformaldehyde + 0.5% Triton X-100) and stained using antibodies for the cytosolic domain of endogenous E-cadherin (eEcad; Zymed, San Francisco, CA) and paxillin (Santa Cruz Biotech, Santa Cruz, CA).
Traction force microscopy
PDMS micropillar arrays were prepared and analyzed as previously described (10).
Results
Substrate rigidity was modified by using PDMS (Dow Corning) as the cell culture material. Changing the mix of elastomer curing agent:base yielded rigid and soft substrates with Young's modulus (E) of 5 MPa and 60 kPa, respectively. Borosilicate glass was included as a widely-used, rigid cell culture material. Patterned substrates were prepared by microcontact printing islands of FN, followed by coating the substrate with EcadFc. Each pattern contained a hexagonal array of FN dots of 2–11 μm diameter, spaced 10–30 μm center-to-center. Control surfaces containing FN and EcadFc alone were prepared by coating substrates with these proteins. This approach provided identical per-area EcadFc concentrations across all uniformly-coated control surfaces and the regions of the patterned substrates not containing FN, as verified by quantitative fluorescence microscopy of labeled EcadFc. In patterned FN regions, EcadFc was reduced by ∼60%, compared to the neighboring, FN-free areas. Concentrations of FN on patterned surfaces were identical across materials and on coated PDMS. The concentration of FN on coated glass was 3× higher than on PDMS.
We first demonstrate integrin-cadherin cross talk on glass. On FN-coated controls, all cells formed clusters of paxillin along the cell periphery (Fig. 2) indicative of focal adhesions (FAs); these formations were typically observed by 1 h of incubation. Cells on EcadFc-coated surfaces formed elongated cadherin structures (identified by staining for endogenous E-cadherin, i.e., eEcad) similar to the “cadherin adhesions” (CAs) described by Gavard et al. (11), a terminology we adopt here. These structures were observed in 30–40% of cells and took >4 h to form. On FN/EcadFc patterned glass, cells established FAs on the FN features, but >95% of cells exhibited no CA structures. Surprisingly, this inhibition was independent of the size and spacing of FN features over the entire range of diameters and spacing we examined. As such, the arrangement of 3 μm-diameter FN dots spaced 10 μm center-to-center was adopted for the rest of this report. We also compared the per-area staining intensity of eEcad along the basolateral surface, normalized to the EcadFc-coated control. Projected cell area on glass substrates was similar across surfaces (area = 1.24E3 ± 4.7E2, 1.32E3 ± 6.5E2, and 1.34E3 ± 5.1E2 μm2 on FN-coated, EcadFc-coated, and FN/EcadFc-patterned surfaces, respectively, mean ± SD, n = 19 on each surface over three independent experiments, α = 0.05). Basolateral eEcad on the FN/EcadFc-patterned surfaces was similar to that on FN-coated controls, and threefold lower than on EcadFc controls (1 ± 0.23, 0.39 ± 0.16, 0.36 ± 0.19, n = 45 cells/surface for EcadFc-coated, FN-coated, and patterned surfaces). Integrin-cadherin cross talk is not universal across epithelial cells. MDCK cells concurrently formed FAs and CAs on the FN/EcadFc-patterned glass substrates (Fig. 2), suggesting a difference between normal and tumorigenic cells. Given this specificity in response, the rest of this report will focus on MCF-7 cells.
Figure 2.
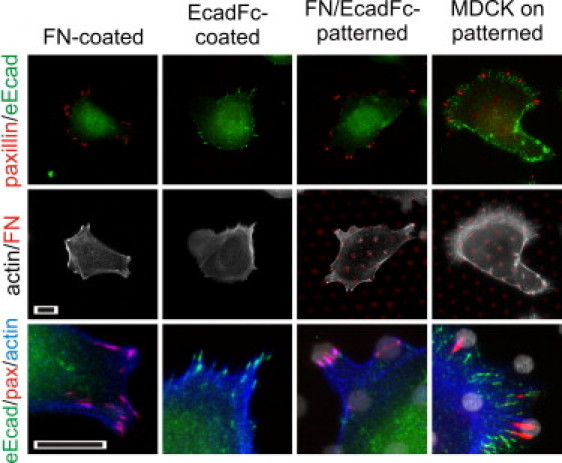
Integrin-cadherin cross talk on rigid, glass substrates. The first three columns illustrate MCF-7 cell interaction with the indicated surfaces, 5 h after seeding. Scale bars indicate 10 μm.
Integrin-cadherin cross talk was then examined as a function of rigidity. MCF-7 cell response on the rigid PDMS substrates was similar to that on glass. Cells established FAs and CAs on FN- and EcadFc-coated controls, respectively (data not shown), and established only FAs on the FN/EcadFc-patterned surfaces, directed to the peripheral FN features (Fig. 3 A). Cell spreading on each surface was similar between glass and rigid PDMS (area = 9.8E2 ± 4.4E2, 1.24E3 ± 5.5E2, 1.01E3 ± 2.6E2 μm2 on FN-coated, EcadFc-coated, and FN/EcadFC-patterned, rigid elastomer surfaces, n = 19 cells/surface, α = 0.05). In contrast, cells on FN/EcadFc-patterned, soft PDMS substrates were able to concurrently form FAs and CAs (Fig. 3 A). Formation of CAs was observed in 30% of cells on the FN/EcadFc-patterned soft PDMS surfaces, similar to that on EcadFc-coated surfaces (Fig. 3 A). This effect did not correlate with changes typically associated with rigidity-dependent integrin function. First, cell spreading was not decreased on soft PDMS, as the projected cell area was similar to the corresponding glass and rigid elastomer surfaces (area = 1.00E3 ± 3.6E2, 1.29E3 ± 4.2E2, and 1.36E3 ± 6.6E2 μm2 on FN-coated, EcadFc-coated, and FN/EcadFc-patterned surfaces, n = 19, α = 0.05). Second, clusters of paxillin on the FN-coated and FN/EcadFc-patterned surfaces were well defined. However, FAs on the FN-coated, soft PDMS surface were often larger than those observed on the rigid surfaces (Fig. 3 A).
Figure 3.
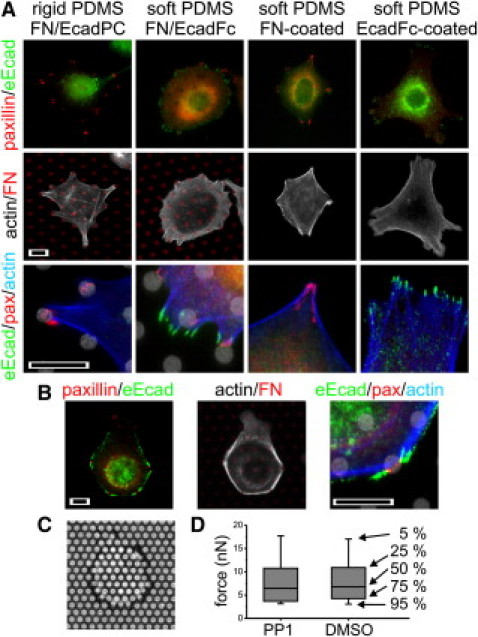
Integrin-cadherin cross talk is dependent on (A) rigidity and (B) SFK activity. Scale bars = 10 μm. (C) MCF-7 cells on an array of pillars, spaced at 3 μm pitch. (D) PP1 does not modulate forces applied by cells. These box plots summarize deflections of all pillars above background from a representative experiment. The rank percentiles of each element of the plots are indicated.
To identify a specific mechanism of this integrin-cadherin cross talk, we examined Src family kinase (SFK) proteins (2). On FN/EcadFc-patterned glass substrates, application of the SFK-inhibitor PP1 (10 μM from a stock solution in dimethyl sulfoxide) over the duration of cell interaction abrogated inhibition of CA formation (Fig. 3 B) and indeed promoted formation of dense CA structures. SFKs have an additional role in cytoskeletal remodeling, and it is possible that CA formation may result from changes in integrin signaling rather than inhibition of integrin-cadherin cross talk itself. However, MCF-7 cells formed well-defined FAs in the presence of PP1 (Fig. 3 B). We also compared traction forces exerted by MCF-7 cells using arrays of microscale pillars (10,12) coated with fibronectin. These arrays consist of 1-μm diameter PDMS pillars separated 3-μm center-to-center and of 6-μm height (providing a spring constant of 6.0 nN/μm). MCF-7 cells applied forces to individual posts on the order of several nN, localized to the periphery of individual cells (Fig. 3 C). The magnitudes of these forces were similar in the presence of PP1 or dimethyl sulfoxide alone (Fig. 3 D, Kruskal-Wallis test, α = 0.05) suggesting that SFKs in this case do not modulate extracellular matrix traction forces, and the effect of PP1 is directly on integrin-cadherin cross talk. However, we note that a higher percentage of cells on EcadFc-coated or FN/EcadFc-patterned glass (70% in both cases) established CAs in the presence of PP1, so the possibility that this effect is a result of heightened cadherin function cannot be ruled out.
Discussion and Conclusion
Our results provide a new insight into integrin-cadherin balance, namely that cross talk between the underlying mechanisms can be modulated by substrate rigidity. We focus on relatively early cell responses, but recognize that MCF-7 cells are able to form both integrin and cadherin interactions over longer timescales (days) in vitro. Over such times, cells have the opportunity to remodel their environment, and mount more-complex responses, and integration of these short- and long-term processes remains a topic of much investigation. Changes in the rigidity/stiffness of both cells and tissues are associated with a range of physiological processes. An emerging picture is that cells change their mechanical properties in response to their environment, with large impacts on internal cell signaling (6,13); intriguingly, epithelial cell invasiveness has been correlated with cell stiffness (14). We have directly demonstrated, to our knowledge, a new consequence of this rigidity on cross talk between two important signaling pathways. These results have implications in disease progression and processes such as epithelial-mesenchymal transformation.
Acknowledgments
The authors gratefully thank Benoît Ladoux (Université Paris) and Yunfei Cai (Columbia University) for fabrication of the micropillar arrays.
This work was funded by the National Institutes of Health through their Roadmap for Medical Research (grant No. PN2 EY016586).
References and Footnotes
- 1.Chen X., Gumbiner B.M. Crosstalk between different adhesion molecules. Curr. Opin. Cell Biol. 2006;18:572–578. doi: 10.1016/j.ceb.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 2.Wang Y., Jin G., Miao H., Li J.Y.S., Usami S. Integrins regulate VE-cadherin and catenins: dependence of this regulation on Src, but not on Ras. Proc. Natl. Acad. Sci. USA. 2006;103:1774–1779. doi: 10.1073/pnas.0510774103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Rooij J., Kerstens A., Danuser G., Schwartz M.A., Waterman-Storer C.M. Integrin-dependent actomyosin contraction regulates epithelial cell scattering. J. Cell Biol. 2005;171:153–164. doi: 10.1083/jcb.200506152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choquet D., Felsenfeld D.P., Sheetz M.P. Extracellular matrix rigidity causes strengthening of integrin-cytoskeleton linkages. Cell. 1997;88:39–48. doi: 10.1016/s0092-8674(00)81856-5. [DOI] [PubMed] [Google Scholar]
- 5.Pelham R.J., Jr., Wang Y.-l. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc. Natl. Acad. Sci. USA. 1997;94:13661–13665. doi: 10.1073/pnas.94.25.13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Discher D.E., Janmey P., Wang Y.-l. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310:1139–1143. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- 7.Perez T.D., Nelson W.J., Boxer S.G., Kam L. E-cadherin tethered to micropatterned supported lipid bilayers as a model for cell adhesion. Langmuir. 2005;21:11963–11968. doi: 10.1021/la052264a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hunt S.J., Nelson W.J. Fabrication of a dual substrate display to test roles of cell adhesion proteins in vesicle targeting to plasma membrane domains. FEBS Lett. 2007;581:4539–4543. doi: 10.1016/j.febslet.2007.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kam L., Boxer S.G. Cell adhesion to protein-micropatterned-supported lipid bilayer membranes. J. Biomed. Mater. Res. 2001;55:487–495. doi: 10.1002/1097-4636(20010615)55:4<487::aid-jbm1041>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 10.du Roure O., Saez A., Buguin A., Austin R.H., Chavrier P. Force mapping in epithelial cell migration. Proc. Natl. Acad. Sci. USA. 2005;102:2390–2395. doi: 10.1073/pnas.0408482102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gavard J., Lambert M., Grosheva I., Marthiens V., Irinopoulou T. Lamellipodium extension and cadherin adhesion: two cell responses to cadherin activation relying on distinct signaling pathways. J. Cell Sci. 2004;117:257–270. doi: 10.1242/jcs.00857. [DOI] [PubMed] [Google Scholar]
- 12.Tan J.L., Tien J., Pirone D.M., Gray D.S., Bhadriraju K. Cells lying on a bed of microneedles: an approach to isolate mechanical force. Proc. Natl. Acad. Sci. USA. 2003;100:1484–1489. doi: 10.1073/pnas.0235407100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang N., Tolic-Norrelykke I.M., Chen J., Mijailovich S.M., Butler J.P. Cell prestress. I. Stiffness and prestress are closely associated in adherent contractile cells. Am. J. Physiol. Cell Physiol. 2002;282:C606–C616. doi: 10.1152/ajpcell.00269.2001. [DOI] [PubMed] [Google Scholar]
- 14.Guck J., Schinkinger S., Lincoln B., Wottawah F., Ebert S. Optical deformability as an inherent cell marker for testing malignant transformation and metastatic competence. Biophys. J. 2005;88:3689–3698. doi: 10.1529/biophysj.104.045476. [DOI] [PMC free article] [PubMed] [Google Scholar]


