Abstract
Loops connecting the transmembrane (TM) α-helices in membrane proteins are expected to affect the structural organization of the thereby connected helices and the helical bundles as a whole. This effect, which has been largely ignored previously, is studied here by analyzing the x-ray structures of 41 α-helical membrane proteins. First we define the loop flexibility ratio, R, and find that 53% of the loops are stretched, where a stretched loop constrains the distance between the two connected helices. The significance of this constraining effect is supported by experiments carried out with bacteriorhodopsin and rhodopsin, in which cutting or eliminating their (predominately stretched) loops has led to a decrease in protein stability, and for rhodopsin, in most cases, also to the destruction of the structure. We show that for nonstretched loops in the extramembranous regions, the fraction of hydrophobic residues is comparable to that for soluble proteins; furthermore (as is also the case for soluble proteins), the hydrophobic residues in these regions are preferentially buried. This is expected to lead to the compact structural organization of the loops, which is transferred to the TM helices, causing them to assemble. We argue that a soluble protein complexed with a membrane protein similarly promotes compactness; other properties of such complexes are also studied. We calculate complementary attractive interactions between helices, including hydrogen bonds and van der Waals interactions of sequential motifs, such as GXXXG. The relative and combined effects of all these factors on the association of the TM helices are discussed and protein structures with only a few of these factors are analyzed. Our study emphasizes the need for classifying membrane proteins into groups according to structural organization. This classification should be considered when procedures for structural analysis or prediction are developed and applied. Detailed analysis of each structure is provided at http://flan.blm.cs.cmu.edu/memloop/
Introduction
Understanding (and predicting) the structure, dynamics, and function of membrane proteins (in particular α-helical membrane proteins) by experimental and computational methods has been a central goal of extensive research during the last 30 years (1–7). Although considerable effort has been devoted to elucidate the origin of the forces that determine the 3D structural organization of the transmembrane (TM) helices, these forces are still not well understood (8). The focus in previous studies has been on identifying inter- and intrahelical interactions, such as polar-polar attractions (hydrogen bonds), salt bridges, or van der Waals interactions (1–11). The effect of interhelical loops on the structural organization and stability of membrane proteins has been recognized generally (3,12,13), but specific research into this effect has been limited. A recent statistical study based on 56 chains from 27 x-ray structures focused mainly on structure/sequence properties of the water-interface region (14).
In this study, focusing on α-helical membrane proteins, we systematically investigate the contribution of loops to the assembly of TM helices, and compare it to the complementary effect of specific interactions between helices. Our study is based on 41 α-helical membrane protein structures with <30% sequence identity retrieved from the protein data bank of TM proteins (PDB_TM) (15). Detailed analysis of each structure is provided at http://flan.blm.cs.cmu.edu/memloop/. Some of our results (and assumptions) are compared with experimental data available for two membrane proteins, bacteriorhodopsin and bovine rhodopsin.
Materials and Methods
Data sets of membrane protein structures
We retrieved the crystal structures (3.5 Å resolution or better) of the α-helical TM proteins from the Protein Data Bank of TM Proteins (PDB_TM) (February 15 2007 release) (15). Each of these proteins may consist of several TM chains and non-TM (soluble) chains. Using the blastclust program of the standalone BLAST package (16) we selected a set of 41 proteins, where the sequence identity between TM chains of any protein pair is less than 30% (see Table 2). Each protein can contain similar chains. To calculate the frequencies of R values one needs to consider only the nonredundant chains of each protein. Thus, we have compiled a subset of 70 nonredundant TM chains using the 30% sequence identity criterion. Notice that each of the 41 protein structures is in the biological oligomeric state, as retrieved from PDB_TM (15).
Table 2.
Set of 41 proteins
| No. of residues |
f |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Protein name | Topology | Total No. of chains | TM | IN | OUT | TM | IN | OUT | |
| One chain | |||||||||
| 1gzm | Rhodopsin | AO | 1 | 156 | 53 | 119 | 0.75 | 0.28 | 0.50 |
| 1okc | Mitochondrial ADP/ATP carrier | AO | 1 | 164 | 111 | 17 | 0.62 | 0.40 | 0.24 |
| 1pv6 | Lactose permease | AI | 1 | 259 | 97 | 61 | 0.69 | 0.47 | 0.48 |
| 1pw4 | Glycerol-3-phosphate transporter | AO | 1 | 252 | 54 | 128 | 0.71 | 0.59 | 0.45 |
| 1zcd | Sodium/proton antiporter 1 | AI | 1 | 262 | 50 | 64 | 0.72 | 0.42 | 0.45 |
| 2a65 | Neurotrasmitter symporter | AI | 1 | 315 | 87 | 107 | 0.72 | 0.40 | 0.55 |
| 2gfp | Multidrug transporter EmrD | AI | 1 | 252 | 108 | 15 | 0.66 | 0.56 | 0.40 |
| Multiple chains | |||||||||
| 1c3w | Bacteriorhodopsin | AO | 3 | 441 | 93 | 132 | 0.71 | 0.48 | 0.52 |
| 1ots | Clc chloride channel | AI | 2 | 668 | 111 | 106 | 0.62 | 0.41 | 0.60 |
| 1rh5 | Preprotein translocase SecY | AI | 3 | 305 | 156 | 37 | 0.68 | 0.49 | 0.46 |
| 1xfh | Proton glutamate symport protein | AI | 3 | 787 | 193 | 235 | 0.66 | 0.46 | 0.56 |
| 2b2f | Ammonium transporter Amt-1 | AO | 3 | 777 | 183 | 213 | 0.70 | 0.36 | 0.52 |
| 2bhw | Light-harvesting complex II from pea | AO | 3 | 304 | 162 | 203 | 0.65 | 0.33 | 0.44 |
| 2f2b | Aquaporin AqpM | AI | 4 | 716 | 200 | 64 | 0.64 | 0.52 | 0.38 |
| Long beard | |||||||||
| 1iwg | Multidrug efflux transporter AcrB | AI | 3 | 804 | 294 | 1920 | 0.76 | 0.40 | 0.46 |
| 1su4 | Calcium ATPase, E1-2Ca state | AI | 1 | 231 | 682 | 81 | 0.70 | 0.42 | 0.46 |
| Long terminus | |||||||||
| 1ehk | Bacterial cytochrome c oxidase | AI | 3 | 354 | 90 | 299 | 0.75 | 0.47 | 0.58 |
| 1fft | Ubiquinol oxidase | AI | 3 | 419 | 183 | 341 | 0.70 | 0.49 | 0.52 |
| 1kqf | Formate dehydrogenase | BO | 9 (3) | 384 | 258 | 3816 | 0.73 | 0.30 | 0.45 |
| 1p49 | Steryl-sulfatase | AO | 1 | 42 | 8 | 498 | 0.79 | 0.50 | 0.45 |
| 1xl4 | Potassium channel Kirbac3.1 | AI | 4 | 260 | 777 | 95 | 0.70 | 0.43 | 0.47 |
| 1yew | Particulate methane monooxygenase | AO | 9 | 845 | 340 | 1239 | 0.69 | 0.54 | 0.49 |
| 2hyd | Multidrug ABC transporter SAV1866, closed state | AI | 2 | 250 | 776 | 130 | 0.69 | 0.43 | 0.59 |
| Soluble | |||||||||
| 1jb0 | Photosystem I | AI | 9 (3) | 792 | 758 | 693 | 0.66 | 0.43 | 0.53 |
| 1l0v | Fumarate reductase | CI | 4 (4) | 292 | 1748 | 94 | 0.73 | 0.44 | 0.65 |
| 1l7v | ABC transporter BtuCD | AI | 2 (2) | 417 | 576 | 81 | 0.71 | 0.45 | 0.44 |
| 1nek | Succinate dehydrogenase | CI | 2 (2) | 154 | 868 | 46 | 0.71 | 0.44 | 0.57 |
| 1ors | Potassium channel KvAP, sensor domain | CI | 1 (2) | 91 | 27 | 449 | 0.74 | 0.48 | 0.40 |
| 1prc | Photosynthetic reaction center | HO | 3 (1) | 268 | 390 | 528 | 0.68 | 0.47 | 0.51 |
| 1q16 | Respiratory nitrate reductase | CO | 1 (2) | 140 | 1811 | 25 | 0.68 | 0.44 | 0.44 |
| 1q90 | Cytochrome b6f | AI | 16 (2) | 600 | 1076 | 228 | 0.68 | 0.52 | 0.43 |
| 1yq3 | Succinate dehydrogenase | CI | 2 (2) | 144 | 909 | 44 | 0.60 | 0.42 | 0.68 |
| 2bs2 | Fumarate reductase | CI | 2 (4) | 232 | 1928 | 136 | 0.72 | 0.43 | 0.44 |
| Crown | |||||||||
| 1lgh | Light-harvesting complex II from Rhodospirillum molischianum | AI | 16 | 344 | 264 | 184 | 0.81 | 0.42 | 0.70 |
| 1nkz | Light-harvesting complex II from Rhodopseudomonas acidophila | AI | 18 | 414 | 297 | 126 | 0.72 | 0.48 | 0.43 |
| 1r3j | Potassium channel KcsA | CI | 4 | 240 | 72 | 100 | 0.68 | 0.33 | 0.60 |
| 1yce | F-type sodium ATPase | AO | 11 | 416 | 371 | 192 | 0.66 | 0.50 | 0.71 |
| 2ahy | Potassium channel | AI | 4 | 262 | 130 | 22 | 0.61 | 0.54 | 0.18 |
| 2bl2 | V-type sodium ATPase | AO | 10 | 789 | 481 | 290 | 0.67 | 0.54 | 0.48 |
| 2oar | Mechanosensitive channel MscL | AI | 5 | 259 | 280 | 86 | 0.68 | 0.50 | 0.33 |
| 2j58 | Outer membrane lipoprotein Wza | AI | 8 | 204 | 2551 | 5 | 0.48 | 0.45 | 0.00 |
PDB codes and protein names are followed by the Topology column, where superscripts “I” and “O” stand for IN and OUT, respectively; for example, AO means that the N-terminus of chain A is in the OUT layer. Numbers in parentheses indicate the total number of soluble chains attached. The number of residues is given according to the different regions: the central layer (TM), and the outer layers (IN and OUT); the fractions, f, of H residues in these three regions appear in the last three columns. The proteins are divided into different groups according to structural characteristics (see Materials and Methods).
The structures taken from the PDB_TM (15) are already rotated and translated according to a predicted membrane normal, which determines the z axis, where z = 0 is the middle of the bilayer. We consider a (−15,15 Å) slab as the central layer. The topologies of proteins in the membrane bilayer were taken from the Orientation of Membrane Protein Database (17). For a plasma membrane, IN and OUT refer to the intracellular and extracellular parts of the proteins, respectively. All other topology definitions follow Mptopo (18).
Grouping of membrane proteins
For ease of reference, we have given to the groups of proteins descriptive names. Thus, proteins with a single chain are referred to as “one chain”. The “crowns” consist of proteins with multiple helical chains (with or without one loop) arranged together perpendicular to the membrane in a circle forming a central pore. The “long-terminus” proteins have short loops, but at least one of their N- or C-terminals is very long, creating a compact structure on one side of the membrane that interacts with the membrane and with other segments of the protein. Another category, “soluble”, consists of membrane proteins forming a stable complex with a non-TM protein. We refer to two proteins with one or more extremely large loops that form a group as “long beard”. Finally, the “multiple chains” group contains proteins that are assemblies that consist of more than one chain (but do not pertain to the above categories). Notice, however, that even this classification is not comprehensive. For example, most of the proteins that pertain to “soluble”, “crown”, or “long terminus” consist of multiple chains, the outer membrane lipoprotein Wza (2j58) is a crown protein with a long terminus, and formate dehydrogenase (1kqf) interacts with soluble proteins and has a long terminus.
Hydrophobicity scale used
The results reported in this article are based on a hydrophobicity scale, where Ile, Leu, Val, Phe, Trp, Met, Pro, Ala, and Tyr are defined as hydrophobic (H) and the remaining amino acids as polar (19). This set of H residues has been chosen as the preferred set in ref (20) by applying six different hydrophobicity scales to a set of 103 soluble proteins.
TM helices and hydrogen bonds
All helices were assigned initially using the STRIDE software (21), which does not distinguish between TM helices and loop helices, and sometimes might introduce helix breaks in the middle due to helix kinks. We therefore identified the TM helix boundaries automatically using STRIDE assignments together with information from PDB_TM. These assignments were verified further by visual inspection of the structures using the Chimera software (22), where incorrect cases were reassigned manually.
Hydrogen bonds (HBs) were detected using the HBPLUS v3.15 software (23) using default parameters and allowing exchange of the nearly symmetrical side chains of His, Gln, and Asn. All ligands and water molecules were removed from the structures. fTM, the fraction of H residues, is based only on helical segments that lie within the central layer (−15,15 Å), whereas for calculating helix-helix and loop-helix HBs the entire helix is considered even if it goes beyond the central layer. Also, a loop-helix HB is defined only for a loop residue that is at least six residues apart from the helix.
Results and Discussion
An essential factor affecting the structural organization of the loops and the TM helices is the hydrophobic interaction. Therefore, we first discuss general aspects of hydrophobicity related to soluble and membrane proteins.
Hydrophobicity in soluble and membrane proteins
The ability of a globular (soluble) protein to organize its chain in a compact stable structure is expected to depend strongly on the fraction, f, of H residues. For small f (say f < 0.1) of randomly distributed H residues, an H residue could become “wrapped” locally by several polar (P) residues to form a “blob”. This would lead to an effectively shorter random coil chain of blobs connected by flexible segments, which gains further stability from its high entropy. However, when f is large enough, the local coverage of the H residues can no longer be achieved, and the only other thermodynamic way to avoid contact with water is to be buried in the interior of a compact chain structure. If f is too large, the molecule will precipitate and therefore the optimal value observed in real proteins constitutes a balance between these effects and other interactions. Correspondingly, for soluble proteins it has been shown that f calculated around the center of mass is larger than f of the entire protein, and f decreases significantly in concentric spherical layers of increasing radii (20,24).
A membrane protein, on the other hand, resides in a nonhomogenous environment consisting of a central hydrophobic membrane layer (∼30 Å in width) created by the lipid bilayer and flanked from both sides by polar headgroup layers (each of 10–15 Å width), which interact with water. Therefore, a membrane protein can accommodate a larger fraction of H residues than a soluble protein, where the highest fraction, fcentral is in the central hydrophobic layer (defined as a (−15,15 Å) slab along the z axis of the membrane). This indeed is observed in membrane protein structures (see below), and to gain further stability (lower free energy), the polymer chain in this region is typically organized in several TM α-helices, which are assembled by various interactions. In this article, amino acid residues on the outer (inner) side of the membrane (beyond the central (−15,15 Å) slab) are referred to as OUT (IN); see Materials and Methods.
The TM helices might be connected by long loops (which in some cases are extremely long, consisting of 400 residues and more) that can span the headgroup layers and penetrate into the surrounding water, where the contribution of their intramolecular interactions to protein stability must also be taken into account. Thus, if the fraction of H residues in these loops was large, floops ≥ fcentral, one would expect the protein to precipitate, whereas very small floops (i.e., a high fraction of P residues) will cause the loops to separate due to strong interaction with water; this separation might destroy the assembly of TM helices in the central layer. On the other hand, for floops ≈ fsoluble (where fsoluble is the fraction of H residues in soluble proteins), one would expect the loops to organize (like soluble proteins) in a compact structure where the H residues are concentrated more in the interior to avoid contact with water. This compactness by itself would be expected to bring the TM helices together and thus to constitute an important factor in their specific structural organization. The results in Table 1 support part of this picture, where fentire membrane = 0.56 (± 0.01) calculated for our set of 41 α-helical membrane proteins is larger than fsoluble = 0.44 (± 0.006) obtained in Miao et al. (20) for 103 soluble proteins (one-sided t-test, p < 0.001). Also, for the TM helices, fTM = fcentral = 0.69 (± 0.01) is significantly larger than floops = 0.47 (± 0.01) (one-sided t-test, p < 0.001), which is an average of fOUT = 0.48 (± 0.02) and fIN = 0.45 (± 0.01); the latter fractions are indeed close to fsoluble = 0.44. These results are based on a hydrophobicity scale (19) defined in Methods.
Table 1.
Average fractions 〈f〉 of H residues in soluble proteins and in different regions of membrane proteins
| Entire membrane proteins | TM | Loops entire | Loops OUT | Loops IN | Soluble proteins | |
|---|---|---|---|---|---|---|
| 〈f〉 | 0.56 (1) | 0.69 (1) | 0.47 (1) | 0.48 (2) | 0.45 (1) | 0.44 |
Results for soluble proteins are taken from Table 2 of Miao et al. (20). The TM region (central layer) is a (−15,15 Å) slab along the z axis. The set of H residues (19) is defined in Materials and Methods. The statistical errors are (1 SD)/N1/2, where N is 103 and 41 for the soluble and membrane proteins, respectively. The results for the membrane proteins are averages of results appearing in Table 2. The errors of the last digit are denoted by parentheses, e.g., 0.56 (1) = 0.56 ± 0.01. The error for soluble proteins is <0.006.
The names and the PDB codes of the 41 α-helical membrane proteins studied appear in Table 2, which also provides the specific fractions of H residues in the TM region and on both sides of the membrane; these results show that the fluctuations around the average fractions of H residues can be significant (Table 1). In Table 2, we also present the number of residues in the three regions, the topology, and the number of chains in a protein. As is emphasized later, unlike soluble proteins, the loops are not organized (in most cases) in a spherical structure, but appear in various geometrical shapes. Therefore, our analysis requires classifying the membrane proteins into groups according to their structural organization in the IN and OUT loop regions. The six groups are called “one chain”, “multiple chains”, “long beard”, “long terminus”, “soluble”, and “crown”, and they are described in Methods; representative structures of these groups appear in Fig. 1. The results in Table 2 (see also Table 4) are presented according to these groups (see also our web site at http://flan.blm.cs.cmu.edu/memloop/).
Figure 1.
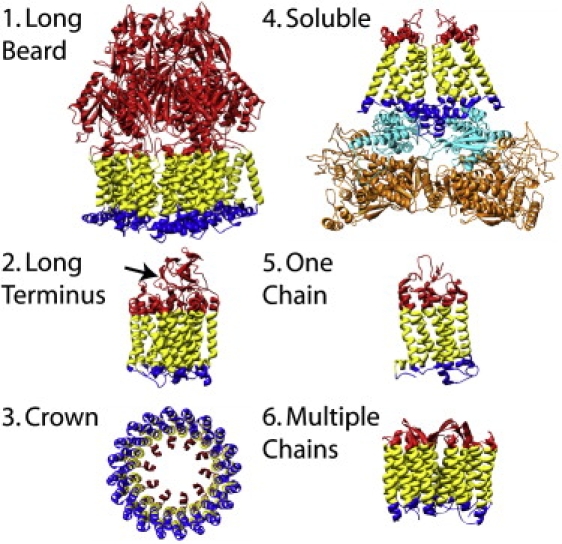
Representative structures of the groups of α-helical TM proteins defined in this article (see Materials and Methods). (1) Multidrug efflux transporter ABC (1iwg). (2) Bacterial cytochrome c oxidase (1ehk); the arrow points to the ∼120-residue-long C-terminus of chain B. (3) V-type sodium ATPase (2bl2). (4) Fumarate reductase (1l0v); chains B and N (cyan) and chains A and M (orange) are the “soluble” part of the protein complex. (5) Rhodopsin (1gzm). (6) Bacteriorhodopsin (1c3w). The membrane protein is colored as follows: yellow, TM; red, OUT; and blue, IN.
Table 4.
Parameters related to the structural organization of TM helices obtained from 41 proteins
| PDB | No. of stretched loops (NR)∗ |
Hydrophobic (k = IN)† |
Hydrophobic (k = OUT)† |
No. of short-range HB (Nsh)‡ |
NsR = Nsh + NR§ |
No. of long-range HB (Nlo)¶ |
No. of motif (Nmot)‖ |
|
|---|---|---|---|---|---|---|---|---|
| NR/Nloop | Nsh/Nhel | NsRNsR/Nhel | NloNlo/Nhel | Nmot/Nhel | ||||
| One chain | ||||||||
| 1gzm | 5/6 | 0.2 | −0.1 | 3/7 (0.4) | 8 (1.1) | 24 (3.4) | 0.7 | |
| 1okc | 2/5 | 1.0 | 1.4 | 11/6 (1.8) | 13 (2.1) | 30 (5.0) | 1.0 | |
| 1pv6 | 10/11 | 0.1 | 0.5 | 1/12 (0.1) | 11 (0.9) | 34 (2.8) | 0.8 | |
| 1pw4 | 8/11 | 0.1 | 0.4 | 4/12 (0.3) | 12 (1.0) | 13 (1.1) | 1.2 | |
| 1zcd | 7/11 | 0.8 | 0.1 | 3/12 (0.3) | 10 (0.8) | 23 (1.9) | 0.8 | |
| 2a65 | 5/11 | 0.9 | 1.7 | 7/12 (0.6) | 12 (1.0) | 58 (4.8) | 0.6 | |
| 2gfp | 9/10 | 0.0 | 0.0 | 4/12 (0.3) | 13 (1.0) | 8 (0.7) | 0.8 | |
| Multiple chains | ||||||||
| 1c3w | 12/18 | 1.4 | 0.3 | 24/21 (1.1) | 36 (1.7) | 51 (2.4) | 0.9 | |
| 1ots | 10/18 | 1.6 | 1.6 | 9/20 (0.5) | 19 (0.9) | 72 (3.6) | 1.1 | |
| 1rh5 | 3/8 | 1.0 | −0.3 | 6/12 (0.5) | 9 (0.7) | 32 (2.7) | 0.6 | |
| 1xfh | 9/12 | 1.5 | 1.8 | 3/24 (0.1) | 12 (0.5) | 77 (3.2) | 0.5 | |
| 2b2f | 21/30 | 1.3 | 0.7 | 24/33 (0.7) | 45 (1.3) | 177 (5.4) | 0.8 | |
| 2bhw | 0/6 | −0.2 | 0.2 | 0/9 (0.0) | 0 (0.0) | 47 (5.2) | 0.3 | |
| 2f2b | 8/20 | 0.8 | 1.3 | 4/24 (0.2) | 12 (0.5) | 112 (4.7) | 1.3 | |
| Long beard | ||||||||
| 1iwg | 15/33 | 1.2 | 0.8 | 9/36 (0.3) | 24 (0.6) | 45 (1.3) | 0.8 | |
| 1su4 | 1/9 | 1.2 | 1.2 | 7/10 (0.7) | 8 (0.8) | 37 (3.7) | 0.5 | |
| Long terminus | ||||||||
| 1ehk | 6/12 | 0.6 | 0.7 | 20/15 (1.3) | 26 (1.7) | 59 (3.9) | 0.5 | |
| 1fft | 7/16 | 0.8 | 0.5 | 10/19 (0.5) | 17 (0.8) | 47 (2.5) | 0.6 | |
| 1kqf | 0/9 | 1.1 | 0.7 | 16/15 (1.1) | 16 (1.0) | 49 (3.3) | 0.5 | |
| 1p49 | 1/1 | −0.5 | 0.7 | 0/2 (0.0) | 1 (0.5) | 6 (3.0) | 0.0 | |
| 1xl4I | 0/4 | 1.1 | 1.5 | 8/8 (1.0) | 8 (1.0) | 26 (3.3) | 0.5 | |
| 1yew | 12/27 | −0.4 | 1.0 | 34/39 (0.9) | 46 (1.1) | 145 (3.7) | 0.4 | |
| 2hydI | 10/10 | 1.0 | 1.3 | 29/12 (2.4) | 39 (3.2) | 58 (4.8) | 1.7 | |
| Soluble | ||||||||
| 1jb0 | 4/23 | 0.0 | 0.2 | 33/32 (1.0) | 37 (1.1) | 164 (5.1) | 0.7 | |
| 1l0v | 3/8 | 0.5 | 1.6 | 26/12 (2.2) | 29 (2.4) | 4 (0.3) | 0.5 | |
| 1l7v | 11/17 | −0.2 | 0.6 | 4/20 (0.2) | 15 (0.7) | 37 (1.9) | 0.6 | |
| 1nek | 3/4 | −0.1 | 0.7 | 18/6 (3.0) | 21 (3.5) | 1 (0.2) | 0.8 | |
| 1orsO | 2/3 | 0.6 | 2.5 | 3/4 (0.8) | 7 (1.7) | 8 (2.0) | 0.8 | |
| 1prcO | 2/8 | 1.0 | 0.0 | 12/11 (1.1) | 14 (1.2) | 58 (5.3) | 0.8 | |
| 1q16 | 2/4 | 0.5 | −0.5 | 1/5 (0.2) | 3 (0.6) | 17 (3.4) | 1.0 | |
| 1q90 | 2/10 | 0.4 | 0.4 | 20/26 (0.8) | 22 (0.8) | 88 (3.4) | 0.4 | |
| 1yq3 | 4/4 | 1.3 | −0.3 | 20/6 (3.3) | 24 (4.0) | 6 (1.0) | 0.2 | |
| 2bs2 | 2/8 | 0.8 | 0.9 | 29/10 (2.9) | 31 (3.1) | 18 (1.8) | 0.4 | |
| Crown | ||||||||
| 1lgh | 0/0 | 1.9 | −0.2 | 0/16 (0.0) | 0 (0.0) | 48 (3.0) | 0.5 | |
| 1nkz | 0/0 | 0.0 | −2.3 | 0/18 (0.0) | 0 (0.0) | 27 (1.5) | 0.0 | |
| 1r3j | 0/4 | 2.0 | 1.1 | 4/8 (0.5) | 4 (0.5) | 24 (3.0) | 1.5 | |
| 1yce | 11/11 | 1.0 | 2.2 | 61/22 (2.8) | 72 (3.2) | 60 (2.7) | 3.5 | |
| 2ahy | 0/4 | 0.4 | 1.3 | 16/8 (2.0) | 16 (2.0) | 28 (3.5) | 1.0 | |
| 2bl2 | 29/30 | 2.1 | 1.3 | 103/40 (2.6) | 132 (3.3) | 123 (3.1) | 2.2 | |
| 2j58 | 0/0 | 0.9 | −3.0 | 0/8 (0.0) | 0 (0.0) | 0 (0.0) | 0.0 | |
| 2oar | 0/5 | 1.6 | 0.2 | 29/10 (2.9) | 29 (2.9) | 9 (0.9) | 0.0 | |
NR/Nloop = (number of stretched loops with R ≤ 2)/(total number of loops).
Hydrophobicity parameters (Eq. 2) for k = IN (column 3) and k = OUT (column 4). In cases where is found to be significant at the p = 0.05 significance level (see text), the value is given in bold print. If the number of residues in IN or OUT is <30, the result is italicized.
Nsh/Nhel = (number of short-range hydrogen bonds (HB))/(number of TM helices) expressed as a simple fraction and (in parentheses) as a decimal number.
NsR = Nsh + NR is the total number of short-range factors; NsR/Nhel appears as a simple fraction with (in parentheses) a decimal number.
Nlo= number of long-range HBs; Nlo/Nhel appears (in parentheses) as a decimal number.
Nmot= number of motifs. Nmot/Nhel appears as a decimal number.
Before elaborating further on the effect of hydrophobicity, we will discuss the expected contribution of stretched loops to the structural organization of the TM helices.
Stretched loops
For our discussion, it is convenient to assume that α-helices are generated first, then inserted into the membrane, where their assembly occurs in a later stage due to attracting forces; clearly, without these forces the helices would not assemble but would move freely in the inner membrane (subject to constraints imposed by stretched loops) to maximize the protein's entropy. Loops that are stretched in the native structure impose geometrical constraints on the corresponding helices to remain close to each other.
More specifically, a stretched loop is identified by its flexibility ratio, R = l/d, where d is the distance between the α-carbons of the first and last residues of the loop in the x-ray structure, and l is the length of the loop segment if it were removed from the protein and maximally stretched, i.e., arranged in its extended conformation (25). l (in Å) is calculated using the expressions a(n/2 − 1) + b and a(n − 1)/2 for even and odd n (number of residues), respectively; a = 6.046 and b = 3.46 Å are parameters taken from Flory's book (26), where the geometrical constraints of a peptide backbone in the extended conformation are described. Thus, for a fully stretched loop, R should be 1, but has been found in calculations to be slightly different, even smaller than 1, due to a nonperfect fit between the above parameters and x-ray structures. The larger is R, the higher is the expected conformational freedom of the loop's backbone (ignoring geometrical restrictions imposed by neighboring residues).
Notice, however, that bulky side chains can decrease significantly the flexibility of a loop, even for R > 1, inducing thereby restrictions on the mutual movements of the related helices. To illustrate this effect, imagine an extreme case of an OUT side loop with close ends, i.e., with a distance, d, equal to the length of a stretched backbone of two or three residues; the loop includes two consecutive bulky side chains, such as Arg. Now, assume that the Arg side chains point toward the central layer but are not allowed to penetrate it. Under these circumstances, the Arg residues must be flanked by two or three residues on either side, which will enable the loop (of five to seven residues) to bulge out, leaving space for the Arg side chains; this would lead to a nonflexible loop, although R ranges from 3 to 4. Although such effects of the side chains on loop rigidity are expected to decrease as n increases, they should be considered (together with the inaccuracy in the above Flory's parameters), and we thus adopt R ≤ 2 as a reasonable criterion for a stretched loop.
The R values obtained with our set of 41 proteins are shown in Table 3. Because some membrane proteins contain multiple copies of the same chain, and some contain multiple different chains, we created a subset of single chains from the 41 proteins, which resulted in 70 nonredundant chains. The frequency of loops with R ≤ 2 (obtained from the 70 chains) is 0.53 (0.12 ≤ 1; 1 < 0.41 ≤ 2), showing that stretched loops are very common in our dataset. Notice that loops with 1 ≤ R ≤ 2 are not necessarily short due to possible large d values. On average they consist of n = 7.3 residues where the longest loop in the set is of n = 24 (Table 3).
Table 3.
Frequency of R values in 70 nonredundant TM chains
| R | 0–1 | 1–2 | 2–3 | 3–4 | 4–5 | 5–10 | 10–500 |
|---|---|---|---|---|---|---|---|
| Frequency | 0.12 | 0.41 | 0.20 | 0.07 | 0.06 | 0.10 | 0.04 |
| 〈n〉 | 2.9 | 7.3 | 15.8 | 22.3 | 21.3 | 32.5 | 154.9 |
| nmax | 6 | 24 | 44 | 45 | 39 | 64 | 433 |
Frequency of loops with a flexibility ratio, R, based on 236 interhelical loops. 〈n〉 and nmax are the average and maximum number, respectively, of loop residues for each R bin.
In Table 4 (second column), we present for each protein the ratio NR/Nloop, where NR is the number of stretched loops (on both sides) and Nloop is the total number of loops in the entire protein complex. It should be noted that the three crown proteins, 1lgh, 1nkz, and 2j58, consist of individual chains with a single TM helix and do not have loops at all; their non-TM segments are composed exclusively of termini. For some proteins, all loops are stretched (1yce, 1p49, 2hyd, and 1yq3), whereas for others no stretched loops exist (2ahy, 2oar, 1kqf, and 2bhw); for the rest of the proteins, NR/Nloop takes intermediate values between these extreme cases. The fraction NR/Nloop is a meaningful parameter that can be compared among proteins (see below).
Although stretched loops can help to keep the helices together, they only operate on successive TM helices along the chain (we call interactions between successive TM helices “short-range” and interactions between nonsuccessive helices, or between helices and loops, “long-range”). Thus, even with a relatively large fraction of stretched loops (53%), the conformational freedom of the helices might still be considerable, and long-range interactions (such as the hydrophobic interaction discussed in detail below) are imperative for their assembly.
Hydrophobicity in the loop regions
Verifying the hydrophobic effect on the (long) loops is not straightforward, because these loops are not organized (in most cases) in a spherical structure but appear in various geometrical shapes. Therefore, to test the hydrophobic effect we carry out the following analysis. First, we use a simplified model where a residue is represented by its α-carbon, and we treat separately the IN and OUT regions of the protein, which are denoted by k = IN or k = OUT; these parts include segments of the protein chain that are beyond the central layer of 30 Å (±15Å). Second, for each residue i, we calculate mi—the number of neighbor residues (α-carbons) within a sphere of radius r (excluding nearest neighbors along the chain), which leads to the averages
| (1) |
where nH(k) (nP(k)) is the total number of H (P) residues in the IN or OUT parts and () is the average number of neighbors of the H (P) residues. Obviously, surface residues have fewer neighbors than interior residues, and since the P (H) residues are expected to appear more on the surface (interior), a positive difference, , would reflect the hydrophobic effect occurring for k = IN or OUT,
| (2) |
To determine the value of the radius, r, we rely on the fact that the entire residue is adequately modeled by its α-carbons, and on Miyazawa and Jernigan (27), who found that r = 7.5 Å represents most optimally the first-neighbors shell of a residue. To estimate the sensitivity of this parameter we carry out calculations also for r = 6, 6.5, 7, 7.5, and 8 Å. Notice that with our procedure, any residue of the central layer will be counted as a neighbor if it is located within a radius, r, of a residue belonging to the IN (OUT) region. This might lead to a too-large due to the relatively high population of H residues in and close to the central layer of the membrane. To check this effect, we calculated two sets of results, where the OUT and IN regions start at ±15 and ±20 Å from the membrane center. We found that the bias is weak, where only 8 out of 82 results of set 2 differ qualitatively from set 1 (data not shown).
In principle, >0 constitutes a verification of hydrophobicity: the larger is the stronger is the effect. However, values ranging from 0.1 to 0.3, for example, are expected to lie within the statistical uncertainty (which increases for the smaller proteins) and thus are too small to reflect a significant measure. On the other hand, = 1 means that on average, each H residue has one neighbor more than a P residue for k = IN or OUT of a studied protein; therefore, ≥ 1 seems to serve as a significant (physical) measure of hydrophobicity. Furthermore, values that are slightly smaller than 1 (e.g., 0.7–1) might also provide meaningful measures. Therefore, it would be of interest to determine a minimum value of , min, where for ≥ min, the hydrophobic interaction can be considered effective.
To determine the statistically significant values, and in particular min, we carried out a randomization test. For each protein side, k (IN or OUT, starting at ±15 Å), the H (P) residues were distributed randomly over the structure (keeping nH(k) (nP(k)) of Eq. 1 constant), where the corresponding (Eq. 2) was calculated for r = 7.5 Å. This experiment was carried out 104 times for each side of the membrane, leading to a distribution of the values around zero. Our null hypothesis is that the value of is as large as what would be expected if the H residues were distributed by chance in the studied part of the protein structure. This hypothesis is rejected if the actual values of are >95% of the 104 randomized results for (one-sided test, p = 0.05).
The actual results for (r = 7.5 Å) for the two membrane sides appear in Table 4 (columns 3 and 4), where a value is bold-faced when it is statistically significant at the p = 0.05 level. The thresholds r = 6.5, 7, and 8 Å yield similar results (see below). It would be difficult for short loops to arrange themselves according to the hydrophobic forces; therefore, we have not analyzed cases where the number of residues in an outer part (excluding the soluble chains) is <30 (still, their values appear in italics in Table 4). Among the 41 proteins, there are two and seven such cases for the IN and OUT sides, respectively (altogether eight proteins), where both sides of 1ors have short loops (<30 residues). Table 4 shows that the statistical criterion is satisfied by 24 of the 39 proteins for k = IN and 19 of 35 proteins for k = OUT. For 15 proteins, the criterion is satisfied for both sides, i.e., hydrophobicity is predicted to affect the structural organization of 28 of the 41 proteins studied (70% excluding 1ors, where both sides have <30 residues). Interestingly, six of the eight crown proteins (which have few or no interhelical loops) belong to this group; here, in most cases, the hydrophobicity is contributed by the N- or C-terminus.
The number of proteins satisfying the statistical criterion is not very sensitive to the significance level used, as can be learned from Table 5, where results for significance-level probabilities ranging from 0.01 to 0.07 for r = 7 and 7.5 Å are presented. Such sets of results are expected (and found) to increase slightly as r increases from 6.5 to 8 Å, where the average results obtained for r = 7 and 8 Å are very close to those obtained for r = 7.5 (data not shown). In these calculations the loops of all of the protein's chains are considered. In the calculations of the “soluble” group of proteins, the soluble chains are excluded, and for the “long-terminus” group, both the loops and the long terminus are taken into account.
Table 5.
Number of proteins satisfying the statistical criterion of hydrophobicity for different significance level probabilities, p
| p |
r = 7 Å∗ |
r = 7.5 Å∗ |
||
|---|---|---|---|---|
| IN | OUT | IN | OUT | |
| 0.01 | 19 | 16 | 21 | 15 |
| 0.02 | 21 | 17 | 23 | 15 |
| 0.04 | 23 | 21 | 24 | 18 |
| 0.05 | 23 | 21 | 24 | 19 |
| 0.06 | 24 | 21 | 24 | 19 |
| 0.07 | 24 | 21 | 24 | 20 |
r is the radius defining the sphere of nearest-neighbor residues (Cα) around each residue. The results for p = 0.05 are in bold print.
For the “long-terminus” group, when the OUT (IN) side is dominated by an extremely long N- or C-terminus, this terminus is typically compact due to hydrophobicity. Thus, for the two proteins of this category, 1xl4 and 2hyd, the long terminus is located on the IN side and it is found that the hydrophobic effect is satisfied (denoted in Table 4 by a superscript “I” above their names) and for them, IN > 0.7 and the results are in bold print. For the other proteins, the long terminus is located in the OUT side, and for all of these, OUT is given in bold print, meaning that hydrophobicity affects all of the long termini (and the corresponding loops)—in accord with their observed compact structures. Also, Table 4 reveals that (excluding the italicized results) only in two cases are the bold-faced values <0.7 ( = 0.5 and 0.4), and two results satisfying ≥ 0.7 (0.8, 0.7) are not in bold print. Thus, = 0.7 constitutes a suitable value for min discussed earlier, i.e., hydrophobicity is significant for ≥ min = 0.7.
In summary, our results demonstrate that for most of the 41 proteins the H residues in the extramembranous regions show a clear preference to avoid the surface, i.e., the contact with water; this is expected to lead to the loops' collapse and to the assembly of TM helices.
Interactions between membrane proteins and soluble proteins
A complex of a membrane protein with one or more soluble proteins can affect the association of the TM helices. To illustrate this effect, we return to the basic assumption that α-helices are generated first, inserted into the membrane, and (without the effect of attracting forces) move freely in the inner membrane (subject to constraints imposed by stretched loops); in this scenario, the protein will exhibit an open structure of low density in the central, IN, and OUT layers. A soluble protein, on the other hand, is typically compact and, due to its globular shape, would have a relatively small interacting interface with the loops of a membrane protein. To enhance this interaction, the density of loops in the (small) interface should increase where this aggregation is expected to be an additional factor contributing to the assembly of the TM helices. The discussion below supports this theory, where evidence is provided that the interfaces are small, the density of both proteins in an interface is similar, and most of the complexes are “held” predominately by the hydrophobic interaction.
First, we defined a set of common interfaces consisting of α-carbon pairs of both proteins with a distance <r, where r = 5, 5.5, 6, 6.5, 7, 7.5, and 8 Å. For each interface, we calculated the corresponding fractions of H residues, finterface-soluble and finterface-loops (see Table 6S in the Supporting Material). In principle, an interface defined by a small r is the most adequate, since it is expected to consist mainly of the surface residues most likely to participate in protein-protein interactions. However, small r might be inadequate when the two proteins are distant from each other, and their interface would contain a small number of residues. Therefore, for each pair of a membrane and a soluble protein, we define an “optimal” r—the lowest value of r for which each protein contributes to the interface at least ∼20 H residues; this relatively large number is needed to avoid strong statistical fluctuations in the above fractions for a small change in the number of H residues.
Eleven membrane/soluble protein pairs are studied, including 1kqf, which appears in the long-terminus group. However, 1nek and 1ors and their soluble counterparts do not satisfy the ∼20 H condition, even for r = 8 Å (the corresponding numbers of H residues are 9,19 and 4,5); therefore, results for these proteins are not provided in Table 6 (but appear in Table 6S), meaning that only nine protein pairs are analyzed. Table 6 shows that the optimal r varies within the entire range of 5–8 Å, and the results for each pair of proteins (which are given for the optimal r) are not changed significantly for a relatively wide range of r values.
Table 6.
Fractions (f) of H residues in various regions of membrane protein-soluble protein complexes
| PDB | r (Å) Δr (Å) | fentire-loops | fouter-shell-loops | finterface-loops | fentire-soluble | fouter-shell-soluble | finterface-soluble |
|---|---|---|---|---|---|---|---|
| 1jb0I | 6.5 6.5–8.0 | 200/471 (0.42) | 26/98 (0.27) | 19/53 (0.36) | 125/287 (0.44) | 31/80 (0.39) | 22/48 (0.46) |
| 1kqfO | 5.0 | 387/873 (0.44) | 21/75 (0.28) | 18/36 (0.50) | 1317/2943 (0.45) | 42/114 (0.37) | 27/45 (0.60) |
| 1l0vI | 8.0 7.5–8.0 | 23/52 (0.44) | 11/28 (0.39) | 14/32 (0.44) | 363/819 (0.44) | 27/81 (0.33) | 21/42 (0.50) |
| 1l7vI | 8.0 5.5–8.0 | 56/128 (0.44) | 23/49 (0.47) | 18/36 (0.50) | 204/448 (0.46) | 20/60 (0.33) | 26/51 (0.51) |
| 1prcO | 6.0 5.0–8.0 | 113/196 (0.58) | 22/42 (0.52) | 24/45 (0.53) | 155/332 (0.47) | 34/78 (0.44) | 22/39 (0.56) |
| 1q16I | 8.0 5.0–8.0 | 24/60 (0.40) | 11/31 (0.35) | 18/37 (0.49) | 773/1751 (0.44) | 24/80 (0.30) | 21/57 (0.37) |
| 1q90I | 8.0 6.5–8.0 | 223/412 (0.54) | 19/45 (0.42) | 16/24 (0.67) | 57/126 (0.45) | 27/64 (0.42) | 11/18 (0.61) |
| 1yq3I | 7.5 6.5–8.0 | 22/53 (0.42) | 12/33 (0.36) | 15/26 (0.58) | 364/856 (0.43) | 32/89 (0.36) | 19/40 (0.48) |
| 2bs2I | 7.0 7.0–8.0 | 52/140 (0.37) | 20/62 (0.32) | 20/50 (0.40) | 778/1778 (0.44) | 20/66 (0.30) | 20/44 (0.45) |
PDB number is the code of the membrane protein-soluble protein complex; subscripts “I” and “O” denote the membrane side (IN or OUT, respectively) where the soluble chain is located; 1jb0 and 1q16 are italicized to emphasize that for these proteins our conditions 1–3 for a protein-protein hydrophobic interaction were not satisfied (see text). r (Å) is the optimal distance defining an interface, i.e., the distance for which the number of H residues of each protein in the interface is ∼20; Δr(Å) is the range of distances for which conditions 1–3 are satisfied. fentire-loops and fentire-soluble are the fractions of H residues in the entire loops' region and the entire soluble protein, respectively; thus, fentire-soluble = 125/287 = (0.44) means that the number of H residues is 125 and the total number of residues of the soluble protein is 287. fouter-shell-loops and fouter-shell-soluble are the fractions of H residues in an external spherical shell around the center of mass of the loops' residues and the soluble protein residues, respectively; the radius of the outer shell is determined by requiring that the shell contain ∼20 H residues. finterface-loops and finterface-soluble are the fractions of H residues of the loops and soluble protein in the interface, respectively; for example, finterface-loops = (number of H residues of the loops in the interface)/(total number of loops' residues in the interface). If the positive difference fentire − fouter-shell (for the membrane or soluble protein) satisfies a statistical significance test, the value of fouter-shell in the table is in bold print, and if the positive difference finterface − fouter-shell (for the membrane or soluble protein) satisfies this test, the value of finterface in the table is in bold print; for details about the test, see text.
For our analysis, we also calculate fentire-soluble and fentire-loops—the fractions of H residues in the entire soluble protein and in the entire loops region (of the membrane protein), respectively. Finally, we define fouter-shell-soluble and fouter-shell-loops—the fractions of H residues in the outer spherical shells of the soluble protein and in the loops region of the membrane protein. fouter-shell-soluble is obtained by defining two concentric spherical shells around the center of mass of the protein. The radius R of the inner shell is adjusted (for each protein) such that the number of H residues (α-carbons) in the outer shell (R,∞) is ∼20. In the same way we calculate the center of mass of the residues of the loops' region of the membrane protein; however, to eliminate the effect of the predominantly hydrophobic residues in the (nonsurface) interior, a slab of residues that is parallel and close to the central layer (TM part) is ignored (see Fig. 2).
Figure 2.
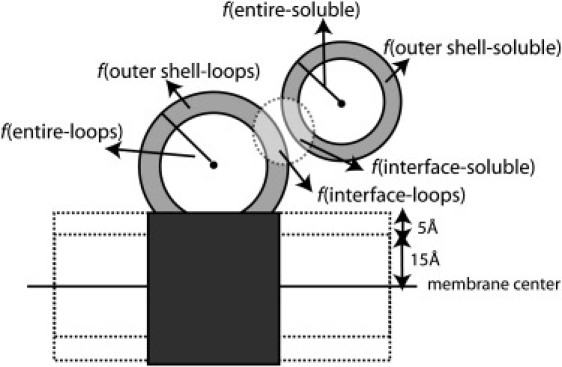
Illustration of the different regions of a membrane protein-soluble protein complex. The loops are defined beyond the central layer (±15 Å). The interface and the outer spherical shells of the two proteins are shown. Residues in the 5 Å slab are not considered in the calculation of fouter-shell-loops.
Notice that for most proteins, the soluble part consists of a complex of two or three soluble chains lumped together; in this case fentire-soluble, fouter-shell-soluble, and finterface-soluble are calculated with respect to the soluble complex. Also, for 1l0v and 1q90, the complex consists of two symmetric (or close-to-symmetric) parts, where the (symmetric) soluble parts are positioned relatively far from each other. In these cases, the calculations are based on one part only (where the results obtained from the second part are identical). Therefore, the occupancy (in Table 6) of the outer shells and interfaces of these proteins is relatively low.
In accord with our finding in the previous section, one would have the following expectations:
-
1.
fentire > fouter shell, i.e., the outer (spherical) shell of both proteins is less hydrophobic than the entire protein (entire loops' region for the membrane protein).
-
2.
For an H-H complex, finterface > fouter shell, for both proteins, the interface will be more hydrophobic than the outer shell.
-
3.
For an H-H complex, finterface should be relatively large, and we require that finterface-soluble ≥ fentire-soluble and finterface-loops ≥ fentire-loops.
Criterion 3 is the most important for defining an H-H complex.
Table 6 reveals that conditions 1–3 above are satisfied for seven (of the nine) proteins analyzed—1kqf, 1l0v, 1l7v, 1prc, 1q90, 1yq3, and 2bs2—which suggests that the related complexes are held together by the hydrophobic interaction. Notice, however, that for 1yq3, fouter-shell-loops is based on only 12 H residues, and for 1prc finterface-loops = 0.53 is slightly smaller than fentire-loops = 0.58. However, 0.53 is a relatively large fraction that exceeds the average fractions of H residues, 0.45 (± 0.01) and 0.48 (± 0.02) in the IN and OUT parts, respectively, of membrane proteins (see Table 1). For two complexes, the value of finterface of one member is slightly smaller than f(entire): for 1jb0, finterface-loops = 0.36 < fentire-loops = 0.42, and for 1q16 finterface-soluble = 0.37 is smaller than fentire-soluble = 0.44. However, in these cases, the complex might still be held by the hydrophobic interaction, where its validation requires a more detailed analysis, which also considers side-chain conformations. Thus, conditions 1–3 are satisfied in 89% of the cases (besides for 1jb0 (loops) and 1q16 (soluble)), suggesting that the complexes are held predominately by the hydrophobic interaction.
Our expectation that the interfaces would be relatively small also materialized. Indeed, Table 6S reveals that the number of residues contributed by the membrane proteins does not exceed 95 and 18 for r = 8 and 5 Å, respectively, and the corresponding numbers for the soluble proteins are 90 and 20 (this should be compared to the considerably larger number of residues in the entire soluble proteins and the loops regions; see Table 6S). (Only for 1kqf, which also possesses a long C-terminus, are the numbers larger: 204 and 36, and 237 and 45, respectively.) Moreover, each interface consists of a comparable number of residues from both proteins, supporting our assumption that the soluble protein contributes to the collapse of the loops. Notice, however, that this long-range effect is expected to be of a second order compared to the hydrophobic effect measured by the criterion; we shall return to this subject in a later section.
The condition fentire-loops > fouter-shell-loops manifests the effect of hydrophobicity on the loops, which thus is expected to correlate with the hydrophobic effect measured by in the previous section. Our results show that only for 1l7v (and only marginally for 1l0v) is the condition fentire-loops > fouter-shell-loops not satisfied, and indeed Table 4 reveals that the IN values for these proteins are small (and are not in bold print), i.e., hydrophobicity is not effective according to the analysis either. For five of the seven remaining proteins, hydrophobicityIN is effective according to both tables (where 1q16 is a marginal case, = 0.5), and only for two proteins out of nine (1jb0 and 1prc) do the results in the two tables disagree. This strong correlation (∼78%) provides a further support for our analysis.
Although conditions 1–3 above are satisfied in 89% of the cases studied, various differences (e.g., fentire − fouter shell) were found sometimes to be relatively small and it is of interest to test their statistical significance further by comparing them to differences obtained from a random distribution of residues. Thus, the H and P residues (α-carbons) of the loops of a membrane protein and its soluble counterpart were randomly distributed 104 times, keeping the number of H and P residues unchanged (i.e., fentire-loops and fentire-soluble are constant). Although for both proteins the averages based on the random distributions satisfy (up to small statistical errors) 〈finterface〉 = 〈fouter shell〉 = fentire, the individual values fluctuate, leading to the corresponding standard deviations σ (or 2σ, denoted for brevity by σ2).
Condition 1, fentire − fouter shell > 0 is satisfied (for the optimal values of r) for all proteins (besides for the membrane protein 1l7v). However, this difference is >σ2(fouter shell) (i.e., it is statistically significant on the σ2 level) only for three of the membrane proteins and five of the soluble proteins, and these (eight) values of fouter shell are in bold print in Table 6. According to condition 2, the interface is expected to be more hydrophobic than the outer shell (finterface − fouter shell > 0), which is satisfied for all cases in Table 6. The statistical test reveals that these differences (for the optimal values of r) are >95% of the randomized differences (p = 0.05 significance level) for four membrane proteins and five soluble proteins; these nine values of finterface are also in bold in Table 6. Condition 3, finterface ≥ fentire, is satisfied for eight of the nine proteins analyzed in Table 6.
In summary, the statistical tests demonstrate that the hydrophobic effect is more pronounced in the soluble proteins, probably because they are subject to fewer geometrical constraints than their membrane counterparts. The fact that conditions 1–3 are largely satisfied (even though conditions 1 and 2 do not always reach the 0.05 significance level) suggests that the protein-protein interaction is predominantly hydrophobic. The interfaces are relatively small, with the densities of both proteins comparable, reflecting the loops' aggregation, which helps the assembly of the TM helices.
Other helix-helix interactions
It is of interest to relate the long-range hydrophobic effect to other interactions that affect the assembly of the TM helices, such as hydrogen bonds and van der Waals interactions.
First, we identified the hydrogen bonds (HBs) and divided them into two categories, “short-range” and “long-range”, where the first group includes HBs between consecutive TM helices (along the chain) and the second group consists of HBs between nonconsecutive TM helices (and also between TM helices and loops). The numbers of HBs (for each protein) are denoted by Nsh (short) and Nlo (long). The short-range HBs (like stretched loops) can potentially bring consecutive helices together, but cannot lead by themselves to the 3D assembly of the TM helices. In Table 4, column 5, we provide Nsh/Nhel, where Nhel is the total number of TM helices for a protein. In parentheses, we present the result of this ratio (decimal number), which makes it possible to compare the results for the number of HBs per helix among different proteins. In column 6, results for NsR = Nsh + NR (and for NsR/Nhel (in decimal numbers)) are presented as a combined measure of the short-range effect. We have also added to NsR the number of short-range disulfide bonds, which exist only for 1ors. In column 7, the numbers of long-range HBs, Nlo (and Nlo/Nhel (in decimal numbers)) are provided, which for 1p49 and 1gzm also include one and two long-range disulfide bonds, respectively.
Next, we identified the sequence motifs, GXXXG, GXXXA, AXXXA, IXXXI, [GAS]XXXGXXXG, GXXXGXXX[GST], GXXGXXX[GST], and SXXXSSXXT, and calculated their number, Nmot, where a long motif is counted as two; these motifs are expected to lead to strong van der Waals attractions between TM helices (28–30). In Table 4, column 8, we provide the ratios Nmot/Nhel as decimal numbers. It should be noted that this set of motifs is not complete, and we have not grouped them according to their short- or long-range interactions, because we have not identified their counterpart segments.
The combined effect of various interactions on the assembly of TM helices
The ratios discussed above can be compared for a specific protein as well as for different proteins. Although the magnitude of each ratio reflects its potential contribution to the organization of the TM helices, the effect of all ratios would provide a more complete picture. Table 4 reveals that for 1yce, 2bl2 (crowns), and 2hyd (long terminus), all of these ratios are relatively high. On the other hand, for 1nkz, 2j58 (crowns), and 1gfp (one chain), very low ratios are observed, and it is intriguing to reveal whether other factors govern the structure of their TM helices.
However, combining these ratios into a single measure would require experimental data (currently unavailable) about their relative effect on the folding and stability of membrane proteins. Still, long-range interactions are expected to be more important than short-range ones, and because the typical energy of HBs and van der Waals sequence motifs lies within a range of ∼4 kcal/mol, their weights (w) should be comparable. However, Nmot also consists of short-range motifs, so one would expect w(Nlo/Nhel) > w(Nmot/Nhel) > w(NsR/Nhel). Although it is difficult to estimate the relative contribution of the hydrophobic interaction, it is expected to be significant, and doubled if on both sides of the membrane. On the other hand, the effect of a soluble protein is small, in particular, when the complex is on the side of the membrane where the loops are affected by hydrophobicity (i.e., the value of is in bold print in Table 4).
It is of interest to examine the cases mentioned above, where only a few interactions are operative. This category includes 1nkz, a (crown) nonamer of dimers consisting of 18 nonconnected helices in total, where hydrophobicity is not effective, and it has been pointed out before that no direct helix-helix interactions exist within the TM helices (31). We observed 27 HBs formed between the intracellular end of a helix and the N-terminus of a neighbor helix, as has already been observed by others (31). For this protein, however, essential “glue” that holds the chains together is provided by three chlorophyll and two caretonoid molecules per dimer that are bound noncovalently to each dimer. Mutation studies have shown that in the absence of caretonoid the protein fails to assemble (32). Moreover, time-resolved fluorescence experiments showed that chlorophyll a is essential in the early steps of the protein's folding (33).
The second protein is Wza (2j58)—a (crown) octamer forming a ring structure surrounding a central cavity used for export. Each monomer has a single TM helix at the C-terminus (345–376), and a bulky OUT “tail”. It appears that the hydrophobicity of the helices plays an important role in the assembly of this protein. First, fTM = 0.48 (Table 2) is significantly smaller than the average fraction, fTM = 0.69 (Table 1)—in accord with the formation of an open cavity. Indeed, the surface of the helices facing the cavity has been found to be highly polar (34), leading to protection of the H residues from water. However, this effect is not taken into account by our five interactions. Thus, the hydrophobic effect ( = 0.9) observed for the tails, constitutes only a partial contribution to the structural organization of the TM helices.
An interesting case is the (long-terminus) protein steryl-sulfatase (1p49). This protein is composed of two TM helices connected by a six-residue stretched loop, where the other end of the helices is linked by a disulfide bridge between Cys170 and Cys242 and a single HB exists between the loop and one of these helices. Furthermore, the helices are tightly packed by bringing together side chains of Ile, Val, Ala, and Phe (35). For this unusual protein with only two TM helices, these interhelical constraints alone would probably be insufficient to lock the helices nearby, which thus is being “helped” by the effect of hydrophobicity on the OUT side of the membrane ( = 0.7 (Table 4)).
Are structural constraints imposed by loops observed experimentally?
One of our assumptions is that stretched loops provide a short-range constraint that helps bring the connected helices together. In this section, we address the question of whether this constraint is observed experimentally. Extensive experimental studies on the effect of loops on the association and stability of TM helices have been carried out for bacteriorhodopsin (BR) and, to a lesser extent, mammalian rhodopsin, both of which are bundles composed of seven TM helices, denoted A–G. They are particularly suitable systems for such investigations, because loops 4 and 5 of their six loops are stretched, respectively; also, hydrophobicity does not play a role in rhodopsin and is effective only slightly in BR, where the main part involved is the C-terminus (IN). In these experimental studies, a loop was cut, eliminated, or replaced by a mutation, where two or more complementary fragments were either coexpressed or separated, denatured, and reconstituted again in vesicles or micelles. Most of these studies have led to the conclusion that with the presence of the retinal ligand the function of the associated (noncovalently bonded) fragments is similar to that of the intact protein, suggesting that the corresponding structures are similar as well; in some studies with BR, the identity of these structures was validated by x-ray crystallography. However, in most cases, the stability of the associated fragments has been found to be lower than that of the wild-type (WT). Furthermore, most complementary fragments of rhodopsin studied were not able to fold correctly, even though the fragments were coexpressed in vivo. These experimental results demonstrate collectively that the geometrical constraints imposed by loops can affect the association and stability of the TM helices. Below we compare in detail the structural factors for BR and rhodopsin in relationship to these experiments.
First, we discuss fragment complementation studies in BR. The earliest work by the Khorana and Engelman groups (36–41) targeted the BC loop, where the fragment pair AB•CDEFG (with and without the retinal) was studied. (The symbol “•” indicates the position of a cut in the loop connecting two helices, e.g., A•B means that no covalent bond connects helices A and B.) The structure of AB•CDEFG was found to be similar to the WT structure. BR was also divided into three fragments, A•B•CDEFG, targeting the AB loop in addition to the BC loop. The WT structure still formed, but the yield and stability was drastically reduced (40,42). Marti later reconstituted in micelles all possible complementary pairs of BR fragments and confirmed that loops are not essential for the correct association of the TM helices, but that reconstituted fragments display decreased stability (43). In particular, covalent linkages in the BC and DE loops were found to be least important for BR stability (43). BR loops were also subjected to sequence replacements, insertions, and deletions. For example, replacements of the CD, EF, and FG loops with glycine-serine containing sequences were found to affect folding kinetics (44), and the CD and EF replacement mutants were also biochemically the least stable (45). Even more drastic changes in the sequence of each BR loop were introduced by Teufel et al. (46). Only the sequence alteration of the AB and FG loops resulted in a loss of function, most plausibly due to folding defects of the respective proteins (46). In BC and EF loop deletion experiments, again the structures of all associated fragments were similar to that of WT, but their stability was reduced (41).
Similar studies were carried out also for rhodopsin (47–50) and references therein). In the earliest work (e.g., (49,51)), proteolytically cleaved but otherwise intact rhodopsin was studied with absorbance spectroscopy and circular dichroism. The major conclusion from these and similar studies was that in the membrane and upon solubilization with detergent, the rhodopsin structure remains intact. Recent differential scanning calorimetry of the proteolytically cleaved rhodopsin proteins showed that the CD and EF loops are important for rhodopsin stability (52). The role of the loops in folding was also assessed through coexpression experiments (47,50). First, five bovine opsin gene fragments cut in the CD and EF loops were studied (47). The sites were chosen based on the known proteolytic cleavage sites. When two or three of the complementary fragments were coexpressed in mammalian cells and retinal was added, ABC•DEFG, ABCE•FG, and ABC•DE•FG formed pigments with spectral properties similar to those of WT rhodopsin, i.e., the associated fragments kept the structure of the intact protein. In a later article (50), of 10 possible additional pairs of fragment combinations testing AB, DE, EF, and FG loops, only fragments 1–194 and 195–348 (ABCD•EFG) and 1–248 and 249–348 (ABCDE•FG) were stably associated to a noncovalently-linked rhodopsin. Still, the yield of the DE loop cut was only 10%. Thus, the two studies showed that only the CD and EF loops are dispensable for rhodopsin folding and only when cut at certain positions. Furthermore, unlike most experiments with BR, the rhodopsin fragments studied were not removed and reconstituted in micelles or liposomes separately before combining fragments, but were present simultaneously inside the cell during folding. Thus, it cannot be excluded that partially folded intermediates associate and that final folding requires tertiary interactions across fragments.
In summary, although stretched loops only provide a short-range effect, the above experimental studies suggest that this effect on protein structure and stability cannot be ignored. For BR, most of the loops are dispensable as regards folding, but typically contribute to protein stability. This is in marked contrast to rhodopsin, whose folding appears to be severely compromised by the vast majority of cuts in its loop regions, with the exception of the cytoplasmic CD and EF loops, which are nonetheless critical for rhodopsin stability.
Summary
The objective of this article has been twofold: to study the effect of loops (in particular their hydrophobicity) on the assembly of TM helices and to understand this assembly from a global perspective, where the effect of loops is combined with interhelical interactions such as hydrogen bonds and sequence motifs. However, unlike globular proteins, which are largely spherical, the structural organization of membrane proteins varies significantly, in particular in the non-TM regions; therefore, our analysis required dividing the proteins into different groups.
Our working assumption is that α-helices are generated first, then inserted into the membrane, and that without the existence of attracting forces, they would not assemble but would move freely in the inner membrane (subject to constraints imposed by stretched loops) to maximize entropy. Correspondingly, we defined the loop flexibility ratio, R, and showed that a significant fraction—53% of the loops in a set of 41 proteins—are stretched, where a stretched loop constrains the distance between the two connected helices. Experimental data demonstrate that this limited (short-range) effect cannot be ignored. Thus, when the (mostly stretched) loops of BR were cut, eliminated, or replaced by a mutation, BR stability in most cases was decreased (even though the structure was not affected). On the other hand, in related studies of bovine rhodopsin the majority of loops were found to be critical for the formation of the structure.
Nonstretched (long) loops that span the headgroup region and the surrounding water (on each side of the membrane) tend to collapse (like soluble proteins) to protect their H residues from contact with water and this “togetherness” may be transferred to the TM helices, causing them to assemble. To check whether this hydrophobicity effect exists in known membrane protein structures, we calculated the average occupancy, and of residues (in spheres of different radii) around H and P residues, respectively, where a higher average occupancy is expected for the H residues that are located in the interior of a loop region; thus, a positive difference in occupancy, , above some threshold constitutes a significant measure of hydrophobicity (k is IN or OUT). Through a randomization test, we identified values that are significantly larger than would be expected by chance, finding that for 70% of the proteins, at least one side of the membrane is affected significantly by hydrophobicity (at significance level p = 0.05); this statistical test is equivalent (approximately) to a (physical) criterion based on min = 0.7, where for ≥ min, the hydrophobic interaction is significant. This aggregation of loops is a long-range type of interaction, which is expected to affect the assembly of all helices (not only consecutive ones).
We have demonstrated that a complex of a membrane protein and a soluble protein is characterized by a relatively small interface at which the density of both proteins is similar, meaning that the loops undergo aggregation, which would be expected to constrain the TM helices as well. We have also shown that the interaction between these proteins is predominantly hydrophobic. However, this long-range effect is expected to be weaker than the structural organization of loops due to their hydrophobicity, which is expressed by the criterion.
To take into account the combined effect of various interactions, we divide them (as mentioned above) into short- and long-range, where the latter have the stronger influence on the assembly of the TM helices. More specifically, the number of occurrences of each interaction is divided by the number of TM helices, Nhel, where the corresponding ratios for different proteins can be compared. Although we do not assign values to the weights (w), we expect them to satisfy w(Nlo/Nhel) > w(Nmot/Nhel) > w(NsR/Nhel), where Nlo is the number of long-range HBs, Nmot is the number of van der Waals sequence motifs, and NsR is the combined number of stretched loops and short-range HBs. In addition, we expect the weight of hydrophobicity (which is expected to lead to the compactness of the longer loops) to be significant. It is of interest to understand the structure and stability of proteins with extremely low ratios. A close examination of such proteins has shown that additional factors, such as protein-ligand interactions, might be important and should be taken into account. Finally, one hopes that understanding the relative contribution of various interactions will provide better insight into protein structure and might lead to improved algorithms for structure prediction of membrane proteins.
Acknowledgments
This work was supported in part by the Sofya Kovalevskaya Prize of the Humboldt Foundation, Germany/Zukunftsinvestitionsprogramm der Bundesregierung Deutschland, by National Science Foundation grants EIA0225636/EIA0225656 and CAREER CC044917, National Institutes of Health grants NLM108730 and 2-R01 GM066090-4 A2, and by the Pennsylvania Department of Health.
Supporting Material
References
- 1.Curran A.R., Engelman D.M. Sequence motifs, polar interactions and conformational changes in helical membrane proteins. Curr. Opin. Struct. Biol. 2003;13:412–417. doi: 10.1016/s0959-440x(03)00102-7. [DOI] [PubMed] [Google Scholar]
- 2.White S.H., Wimley W.C. Membrane protein folding and stability: physical principles. Annu. Rev. Biophys. Biomol. Struct. 1999;28:319–365. doi: 10.1146/annurev.biophys.28.1.319. [DOI] [PubMed] [Google Scholar]
- 3.Popot J.L., Engelman D.M. Helical membrane protein folding, stability, and evolution. Annu. Rev. Biochem. 2000;69:881–922. doi: 10.1146/annurev.biochem.69.1.881. [DOI] [PubMed] [Google Scholar]
- 4.Senes A., Ubarretxena-Belandia I., Engelman D.M. The Cα-H.O hydrogen bond: a determinant of stability and specificity in transmembrane helix interactions. Proc. Natl. Acad. Sci. USA. 2001;98:9056–9061. doi: 10.1073/pnas.161280798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bowie J.U. Solving the membrane protein folding problem. Nature. 2005;438:581–589. doi: 10.1038/nature04395. [DOI] [PubMed] [Google Scholar]
- 6.Fleishman S.J., Ben-Tal N. Progress in structure prediction of α-helical membrane proteins. Curr. Opin. Struct. Biol. 2006;16:496–504. doi: 10.1016/j.sbi.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 7.Mackenzie K.R. Folding and stability of alpha-helical integral membrane proteins. Chem. Rev. 2006;106:1931–1977. doi: 10.1021/cr0404388. [DOI] [PubMed] [Google Scholar]
- 8.Gratkowski H., Lear J.D., DeGrado W.F. Polar side chains drive the association of model transmembrane peptides. Proc. Natl. Acad. Sci. USA. 2001;98:880–885. doi: 10.1073/pnas.98.3.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adamian L., Liang J. Interhelical hydrogen bonds and spatial motifs in membrane proteins: polar clamps and serine zippers. Proteins. 2002;47:209–218. doi: 10.1002/prot.10071. [DOI] [PubMed] [Google Scholar]
- 10.White S.H. How hydrogen bonds shape membrane protein structure. Adv. Protein Chem. 2005;72:157–172. doi: 10.1016/S0065-3233(05)72006-4. [DOI] [PubMed] [Google Scholar]
- 11.Hildebrand P.W., Gunther S., Goede A., Forrest L., Frommel C. Hydrogen-bonding and packing features of membrane proteins: functional implications. Biophys. J. 2008;94:1945–1953. doi: 10.1529/biophysj.107.110395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liang J., Adamian L., Jackups R., Jr. The membrane-water interface r region of membrane proteins: structural bias and the anti-snorkeling effect. Trends Biochem. Sci. 2005;30:355–357. doi: 10.1016/j.tibs.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 13.Klein-Seetharaman J. Dual role of interactions between membranous and soluble portions of helical membrane receptors for folding and signaling. Trends Pharmacol. Sci. 2005;26:183–189. doi: 10.1016/j.tips.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 14.Granseth E., von Heijne G., Elofsson A. A study of the membrane-water interface region of membrane proteins. J. Mol. Biol. 2005;346:377–385. doi: 10.1016/j.jmb.2004.11.036. [DOI] [PubMed] [Google Scholar]
- 15.Tusnady G.E., Dosztanyi Z., Simon I. Transmembrane proteins in the Protein Data Bank: identification and classification. Bioinformatics. 2004;20:2964–2972. doi: 10.1093/bioinformatics/bth340. [DOI] [PubMed] [Google Scholar]
- 16.Altschul S.F., Madden T.L., Schaffer A.A., Zhang J., Zhang Z. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lomize M.A., Lomize A.L., Pogozheva I.D., Mosberg H.I. OPM: orientations of proteins in membranes database. Bioinformatics. 2006;22:623–625. doi: 10.1093/bioinformatics/btk023. [DOI] [PubMed] [Google Scholar]
- 18.Jayasinghe S., Hristova K., White S.H. MPtopo: a database of membrane protein topology. Protein Sci. 2001;10:455–458. doi: 10.1110/ps.43501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schwartz R., Istrail S., King J. Frequencies of amino acid strings in globular protein sequences indicate suppression of blocks of consecutive hydrophobic residues. Protein Sci. 2001;10:1023–1031. doi: 10.1110/ps.33201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miao J., Klein-Seetharaman J., Meirovitch H. The optimal fraction of hydrophobic residues required to ensure protein collapse. J. Mol. Biol. 2004;344:797–811. doi: 10.1016/j.jmb.2004.09.061. [DOI] [PubMed] [Google Scholar]
- 21.Heinig M., Frishman D. STRIDE: a web server for secondary structure assignment from known atomic coordinates of proteins. Nucleic Acids Res. 2004;32:W500–W502. doi: 10.1093/nar/gkh429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pettersen E.F., Goddard T.D., Huang C.C., Couch G.S., Greenblatt D.M. UCSF Chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 23.McDonald I.K., Thornton J.M. Satisfying hydrogen bonding potential in proteins. J. Mol. Biol. 1994;238:777–793. doi: 10.1006/jmbi.1994.1334. [DOI] [PubMed] [Google Scholar]
- 24.Meirovitch H.S., Scheraga H.A. Empirical studies of hydrophobicity. 2. Distribution of the hydrophobic, hydrophilic, neutral, and ambivalent amino acids in the interior and exterior layers of native proteins. Macromolecules. 1980;13:1406–1414. [Google Scholar]
- 25.Das B., Meirovitch H. Optimization of solvation models for predicting the structure of surface loops in proteins. Proteins. 2001;43:303–314. doi: 10.1002/prot.1041. [DOI] [PubMed] [Google Scholar]
- 26.Flory P. Hanser; New York: 1998. Statistical Mechanics of Chain Molecules. [Google Scholar]
- 27.Miyazawa S., Jernigan R.L. Estimation of effective inter-residue contact energies from protein crystal structures: quasi-chemical approximation. Macromolecules. 1985;18:534–552. [Google Scholar]
- 28.Dawson J.P., Weinger J.S., Engelman D.M. Motifs of serine and threonine can drive association of transmembrane helices. J. Mol. Biol. 2002;316:799–805. doi: 10.1006/jmbi.2001.5353. [DOI] [PubMed] [Google Scholar]
- 29.Kleiger G., Grothe R., Mallick P., Eisenberg D. GXXXG and AXXXA: common α-helical interaction motifs in proteins, particularly in extremophiles. Biochemistry. 2002;41:5990–5997. doi: 10.1021/bi0200763. [DOI] [PubMed] [Google Scholar]
- 30.Kim S., Jeon T.J., Oberai A., Yang D., Schmidt J.J. Transmembrane glycine zippers: physiological and pathological roles in membrane proteins. Proc. Natl. Acad. Sci. USA. 2005;102:14278–14283. doi: 10.1073/pnas.0501234102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Law C.J., Roszak A.W., Southall J., Gardiner A.T., Isaacs N.W. The structure and function of bacterial light-harvesting complexes. Mol. Membr. Biol. 2004;21:183–191. doi: 10.1080/09687680410001697224. [DOI] [PubMed] [Google Scholar]
- 32.Zurdo J., Fernandez-Cabrera C., Ramirez J.M. A structural role of the carotenoid in the light-harvesting II protein of Rhodobacter capsulatus. Biochem. J. 1993;290:531–537. doi: 10.1042/bj2900531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Horn R., Paulsen H. Early steps in the assembly of light-harvesting chlorophyll a/b complex: time-resolved fluorescence measurements. J. Biol. Chem. 2004;279:44400–44406. doi: 10.1074/jbc.M407188200. [DOI] [PubMed] [Google Scholar]
- 34.Dong C., Beis K., Nesper J., Brunkan-Lamontagne A.L., Clarke B.R. Wza the translocon for E. coli capsular polysaccharides defines a new class of membrane protein. Nature. 2006;444:226–229. doi: 10.1038/nature05267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hernandez-Guzman F.G., Higashiyama T., Pangborn W., Osawa Y., Ghosh D. Structure of human estrone sulfatase suggests functional roles of membrane association. J. Biol. Chem. 2003;278:22989–22997. doi: 10.1074/jbc.M211497200. [DOI] [PubMed] [Google Scholar]
- 36.Huang K.S., Bayley H., Liao M.J., London E., Khorana H.G. Refolding of an integral membrane protein. Denaturation, renaturation, and reconstitution of intact bacteriorhodopsin and two proteolytic fragments. J. Biol. Chem. 1981;256:3802–3809. [PubMed] [Google Scholar]
- 37.Liao M.J., London E., Khorana H.G. Regeneration of the native bacteriorhodopsin structure from two chymotryptic fragments. J. Biol. Chem. 1983;258:9949–9955. [PubMed] [Google Scholar]
- 38.Popot J.L., Gerchman S.E., Engelman D.M. Refolding of bacteriorhodopsin in lipid bilayers. A thermodynamically controlled two-stage process. J. Mol. Biol. 1987;198:655–676. doi: 10.1016/0022-2836(87)90208-7. [DOI] [PubMed] [Google Scholar]
- 39.Kahn T.W., Engelman D.M. Bacteriorhodopsin can be refolded from two independently stable transmembrane helices and the complementary five-helix fragment. Biochemistry. 1992;31:6144–6151. doi: 10.1021/bi00141a027. [DOI] [PubMed] [Google Scholar]
- 40.Kahn T.W., Sturtevant J.M., Engelman D.M. Thermodynamic measurements of the contributions of helix-connecting loops and of retinal to the stability of bacteriorhodopsin. Biochemistry. 1992;31:8829–8839. doi: 10.1021/bi00152a020. [DOI] [PubMed] [Google Scholar]
- 41.Gilles-Gonzalez M.A., Engelman D.M., Khorana H.G. Structure-function studies of bacteriorhodopsin XV. Effects of deletions in loops B-C and E-F on bacteriorhodopsin chromophore and structure. J. Biol. Chem. 1991;266:8545–8550. [PubMed] [Google Scholar]
- 42.Liao M.J., Huang K.S., Khorana H.G. Regeneration of native bacteriorhodopsin structure from fragments. J. Biol. Chem. 1984;259:4200–4204. [PubMed] [Google Scholar]
- 43.Marti T. Refolding of bacteriorhodopsin from expressed polypeptide fragments. J. Biol. Chem. 1998;273:9312–9322. doi: 10.1074/jbc.273.15.9312. [DOI] [PubMed] [Google Scholar]
- 44.Allen S.J., Kim J.M., Khorana H.G., Lu H., Booth P.J. Structure and function in bacteriorhodopsin: the effect of the interhelical loops on the protein folding kinetics. J. Mol. Biol. 2001;308:423–435. doi: 10.1006/jmbi.2001.4604. [DOI] [PubMed] [Google Scholar]
- 45.Kim J.M., Booth P.J., Allen S.J., Khorana H.G. Structure and function in bacteriorhodopsin: the role of the interhelical loops in the folding and stability of bacteriorhodopsin. J. Mol. Biol. 2001;308:409–422. doi: 10.1006/jmbi.2001.4603. [DOI] [PubMed] [Google Scholar]
- 46.Teufel M., Pompejus M., Humbel B., Friedrich K., Fritz H.J. Properties of bacteriorhodopsin derivatives constructed by insertion of an exogenous epitope into extra-membrane loops. EMBO J. 1993;12:3399–3408. doi: 10.1002/j.1460-2075.1993.tb06014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ridge K.D., Lee S.S., Yao L.L. In vivo assembly of rhodopsin from expressed polypeptide fragments. Proc. Natl. Acad. Sci. USA. 1995;92:3204–3208. doi: 10.1073/pnas.92.8.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Litman B.J. Rhodopsin: its molecular substructure and phospholipid interactions. Photochem. Photobiol. 1979;29:671–677. doi: 10.1111/j.1751-1097.1979.tb07747.x. [DOI] [PubMed] [Google Scholar]
- 49.Albert A.D., Litman B.J. Independent structural domains in the membrane protein bovine rhodopsin. Biochemistry. 1978;17:3893–3900. doi: 10.1021/bi00612a001. [DOI] [PubMed] [Google Scholar]
- 50.Ridge K.D., Lee S.S., Abdulaev N.G. Examining rhodopsin folding and assembly through expression of polypeptide fragments. J. Biol. Chem. 1996;271:7860–7867. doi: 10.1074/jbc.271.13.7860. [DOI] [PubMed] [Google Scholar]
- 51.Pober J.S., Stryer L. Letter to the editor: Light dissociates enzymatically-cleaved rhodopsin into two different fragments. J. Mol. Biol. 1975;95:477–481. doi: 10.1016/0022-2836(75)90204-1. [DOI] [PubMed] [Google Scholar]
- 52.Landin J.S., Katragadda M., Albert A.D. Thermal destabilization of rhodopsin and opsin by proteolytic cleavage in bovine rod outer segment disk membranes. Biochemistry. 2001;40:11176–11183. doi: 10.1021/bi0100539. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


