Abstract
The vitamin K-dependent carboxylase modifies and renders active vitamin K-dependent proteins involved in hemostasis, cell growth control, and calcium homeostasis. Using a novel mechanism, the carboxylase transduces the free energy of vitamin K hydroquinone (KH2) oxygenation to convert glutamate into a carbanion intermediate, which subsequently attacks CO2, generating the γ-carboxylated glutamate product. How the carboxylase effects this conversion is poorly understood because the active site has not been identified. Dowd and colleagues [Dowd, P., Hershline, R., Ham, S. W. & Naganathan, S. (1995) Science 269, 1684–1691] have proposed that a weak base (cysteine) produces a strong base (oxygenated KH2) capable of generating the carbanion. To define the active site and test this model, we identified the amino acids that participate in these reactions. N-ethyl maleimide inhibited epoxidation and carboxylation, and both activities were equally protected by KH2 preincubation. Amino acid analysis of 14C- N-ethyl maleimide-modified human carboxylase revealed 1.8–2.3 reactive residues and a specific activity of 7 × 108 cpm/hr per mg. Tryptic digestion and liquid chromatography electrospray mass spectrometry identified Cys-99 and Cys-450 as active site residues. Mutation to serine reduced both epoxidation and carboxylation, to 0.2% (Cys-99) or 1% (Cys-450), and increased the Kms for a glutamyl substrate 6- to 8-fold. Retention of some activity indicates a mechanism for enhancing cysteine/serine nucleophilicity, a property shared by many active site thiol enzymes. These studies, which represent a breakthrough in defining the carboxylase active site, suggest a revised model in which the glutamyl substrate indirectly coordinates at least one thiol, forming a catalytic complex that ionizes a thiol to initiate KH2 oxygenation.
The vitamin K-dependent (VKD) carboxylase is an integral membrane enzyme required for the biological activity of proteins involved in hemostasis (prothrombin, factor X, factor VII, factor IX, protein S, protein C, protein Z), calcium homeostasis (bone gla protein and matrix gla protein), cell growth control (gas 6), and possibly signal transduction (PRGP-1 and PRGP-2) (1, 2). Carboxylation is effected via a homologous ≈18-aa sequence in VKD proteins, usually an N-terminal propeptide, which the carboxylase binds with high affinity. Propeptide binding of VKD proteins to the carboxylase leads to the conversion of clusters of glutamyl (Glu) residues to γcarboxylated glutamyl (or Gla) residues, in a region adjacent to the propeptide called the Gla domain. This domain serves as a calcium-dependent membrane-binding module for the attached VKD proteins, for example to effect blood coagulation on cell surfaces. Carboxylation requires a continual supply of the reduced form of the vitamin K cofactor, vitamin K hydroquinone (KH2), and when KH2 is limiting undercarboxylated, inactive VKD proteins are produced. Consequently, understanding the mechanism of carboxylation has important medical ramifications as coumarin-based oral anticoagulant therapies function by limiting cellular KH2, thereby reducing carboxylase activity.
To convert Glus to Glas, the carboxylase uses O2 and KH2 to stereospecifically abstract a γ-hydrogen from the Glus, generating a carbanion intermediate, which then incorporates CO2 via nucleophilic attack. During the reaction, KH2 is oxidized to vitamin K epoxide, and the carboxylase is also an epoxidase. The carboxylase is regulated to prevent uncontrolled epoxidation, as epoxidase activity is low in the absence of the Gla domain substrate (3), but how such regulation is accomplished is not known.
The mechanism by which the carboxylase uses energy from KH2 oxygenation to generate the carbanion intermediate has been the focus of chemical modeling studies, which were based on the observation that thiol-specific reagents inhibit carboxylase activity (4). These studies led to the proposal by Dowd and colleagues (5) of a base-strength amplification mechanism, in which the carboxylase uses a weak base (Cys) to generate a strong base (oxygenated KH2) with sufficient strength to abstract the γ-hydrogen from Glus. Inhibition studies using crude liver microsomes and the thiol modifying reagent N-ethyl maleimide (NEM) indicated the presence of at least two active site Cys residues. Whereas both epoxidase activity and carboxylase activity were inactivated by NEM, preincubation with KH2 partially protected only epoxidase activity (6). Based on these data, Dowd et al. (5) proposed that each Cys residue independently contributed to either KH2 oxygenation or to coordinating CO2 for carbanion attack. Studies with purified carboxylase have verified NEM inactivation of activity (7, 8). However, the residues that effect epoxidation and carboxylation are unknown, and predictions of the base-strength amplification model have not been tested.
The carboxylase has been formidably difficult in attempts to biochemically map functionally relevant amino acids. Because it is a large (95 kDa) integral membrane protein, the amounts of active, pure enzyme that can be obtained are limited, even with the availability of recombinant protein. In addition, the phospholipid and detergent required to purify the carboxylase in active form are incompatible with microsequence technologies. To date, the biochemical mapping has been low in resolution. Cross-linking studies to map the propeptide binding site have identified amino acids 50–225 and 349-≈500, and no explanation has been given for the discrepancy in results (9, 10). A Gla domain-derived peptide was cross-linked to the first third of the carboxylase (11).
To identify the carboxylase active site and biochemically test the base-strength amplification model, we undertook a study to identify NEM-modified carboxylase residues and to assess the importance of these residues to activity. Here, we report that Cys-99 and Cys-450 are the active-site Cys residues. Each is required for both carboxylase and epoxidase activities, necessitating a reconsideration of how base strength amplification occurs. Kinetic analyses indicate that at least one thiol coordinates the glutamyl substrate in a complex that is a prerequisite for KH2 oxygenation.
Materials and Methods
Supplemental Material.
The details for the following experiments are provided as supplemental data, which are published on the PNAS website, www.pnas.org, see Methods: purification of the human r-carboxylase, HPLC and mass spectrometry conditions for analyzing tryptic peptides, construction, expression, and Western analysis of mutants, and carboxylase and epoxidase activity assays.
14C-NEM Modification of the Carboxylase.
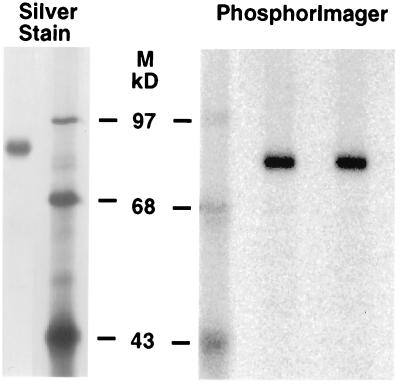
Optimal conditions for NEM inactivation of the carboxylase were determined (Fig. 4, which is published as supplemental data). To modify the carboxylase with 14C-NEM, 900 μl of carboxylase (30 μg) was combined immediately after purification with 100 μl of 1 M BES (pH 6.9) and 100 μl 14C-NEM (NEN, 33 mCi/mmol, 1.1 mM final concentration). An identical reaction using cold NEM (Sigma) was analyzed in parallel. Both reactions were incubated for 30 min at room temperature. Aliquots of the starting material and the cold NEM mixture then were assayed to determine the extent of inactivation, which was >92% in all cases. The remaining NEM was quenched by the addition of DTT (to 10 mM). Aliquots then were subjected to SDS/PAGE and analyzed by silver staining to show that the carboxylase preparation was homogenous (Fig. 1), and by PhosphorImager, where quantitation of the lane showed that >90% of the 14C-NEM comigrated with the carboxylase band.
Figure 1.
Purification and 14C-NEM modification of carboxylase. Carboxylase purified in the absence of reducing agents was assayed for enzyme activity (supplemental data, Methods) and modified with 14C-NEM (Materials and Methods). Duplicate aliquots were loaded on the PhosphorImager gel for quantitation of radioactivity. 14C-BSA standards were processed in parallel (not shown) to quantitate the amount of 14C-NEM incorporation (907 cpm in the band) into a known amount of carboxylase activity (1.5 × 10-4 μmol/min). The preparation shown here also was analyzed by amino acid analysis.
Carboxyamidomethylation and Tryptic Digestion of Modified Carboxylase.
After the addition of DTT, 14C-NEM-modified carboxylase (above) was adjusted to 400 mM Tris⋅HCl, pH 7.4, and urea (Pierce) and CHAPS (3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate, Sigma) then were added to final concentrations of 8 M and 1%, respectively. After 1 h at room temperature, the reaction was brought to 50 mM iodoacetamide (IAN) (Sigma) and incubated for an additional 1 h at room temperature. This experiment also was performed at pH 8, giving identical results to those obtained at pH 7.4.
The sample was concentrated to 0.1 μg/μl by using an Amicon Microcon-30, and then precipitated overnight at 4°C in 30% trichloroacetic acid (TCA). We found that TCA gave the best recovery, and that it was compatible with subsequent mass spectral analysis (i.e., no reactivity with amino acid residues was detected). After centrifugation at 16,000 × g for 30 min at 4°C, the pellet was resuspended in 100 μl H2O and extracted seven times with 1 ml of water-saturated ether to remove 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS) and phosphatidyl choline. The sample then was precipitated in 67% acetone at −20°C overnight and respun, and the pellet was resuspended in 20 μl of 5 M urea, 50% acetonitrile. After 1 h at room temperature, 100 μl of 0.1 M ammonium bicarbonate, pH 8, was added. The sample (300 pmol for 100% recovery) then was digested at 37°C with tosyl-l-phenylalanine chloromethyl ketone-modified trypsin (Promega), added in four equal parts (18 pmol per addition) at 10-h intervals. The high ratio of trypsin to carboxylase was critical, as lower ratios resulted in partial tryptic fragments. Overdigestion was not observed. The trypsin was inhibited by the addition of 720 pmol of 7-amino-1-chloro-3-tosylamido-2-heptanone (Sigma) and incubation for 1 h at 37°C. The carboxylase peptides then were deglycosylated by the addition of 1,500 units (0.83 μg) of PN Glycanase F (New England Biolabs) and overnight incubation at 37°C. Samples were stored at −80°C until analysis by liquid chromatography electrospray mass spectrometry (LC-ESMS) (see supplemental data, Methods).
Amino Acid Analysis of 14C-NEM-Modified Carboxylase.
Carboxylase (900 μl, 30 μg) was assayed for activity and modified with 14C-NEM as described above. After the 14C-NEM incubation, cold NEM (27 mM final concentration) was added and incubated for an additional 30 min at room temperature. The NEM then was quenched by the addition of DTT to 100 mM. SDS/PAGE and PhosphorImager analysis showed that >90% of the radioactivity comigrated with the carboxylase band (Fig. 1). The 14C-NEM-modified carboxylase was purified over Q-Sepharose to remove the factor X propeptide used to isolate the carboxylase. Carboxylase was diluted into 50 mM Tris⋅HCl (pH 7.4), 50 mM NaCl, 5 mM DTT, 0.25% phosphatidyl choline IIIE (Sigma), 0.25% 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS), adsorbed to Q-Sepharose (100 μl, Amersham Pharmacia), and the resin was washed with 5 ml of the same buffer, all at 4°C. Carboxylase was eluted in 0.5 ml of the same buffer containing 0.5 M NaCl, concentrated in a Microcon-30 to 100 μl and transferred to a glass hydrolysis vial. The carboxylase was precipitated twice in 67% acetone to remove salt. The pellet then was resuspended in 50 μl H2O and extracted seven times with 400 μl H2O-saturated ether. The carboxylase was reprecipitated in 67% acetone, and the pellet was subjected to vapor phase hydrolysis at 150°C for 1 h. After resuspension in 0.25 μg/μl EDTA (20 μl), aliquots were quantitated for radioactivity by scintillation counting. To quantitate the amount of carboxylase, phenylthiocarbamyl amino acid analysis was performed by using an Applied Biosystems model 420H/130/920 automated analysis system (12).
Results
NEM Inhibition of Carboxylation and Epoxidation and Protection by Vitamin K.
Because previous studies indicted that the NEM-modified amino acids were Cys residues (4, 6), the isolation of fully active enzyme was necessary to avoid potential mixed disulfide artifacts. Carboxylase therefore was purified from a cell line coexpressing human r-carboxylase and human r-factor IX, as we had previously found that carboxylase activity in the factor IX-carboxylase complex was very stable (13). The complex was adsorbed to an α-factor IX antibody column, washed, and then eluted with a propeptide to give an active, homogenous preparation of enzyme (Fig. 1). Western analysis capable of detecting <0.1% factor IX contamination showed none was present (data not shown). The purification was performed in the absence of reducing agents to avoid potential disulfide exchange. To test the stability of carboxylase activity, parallel purifications were carried out that differed only in the presence or absence of DTT (see supplemental data, Methods), and no difference in activity between the two preparations was observed. In all subsequent experiments, carboxylase was purified in the absence of DTT, and NEM modification was performed immediately after propeptide elution.
Treatment of the carboxylase with NEM inhibited both carboxylase and epoxidase activities (Table 1, Fig. 4, and supplemental data, Methods), in agreement with earlier results (6, 8). NEM inhibition of both activities was time- and dose-dependent (Fig. 4 and supplemental data, Methods). All NEM incubations were performed at pH 6.9, where thiol reactivity with NEM is orders of magnitude higher than for other amino acids, and so inactivation was most likely at Cys residues (14).
Table 1.
Both carboxylase and epoxidase activities are equally protected by vitamin K
| Reaction conditions
|
Activity, %
|
|||
|---|---|---|---|---|
| KH2 | NEM | Carboxylase | Epoxidase | |
| A | − | − | 100.0 | 100.0 |
| − | + | 4.0 | 8.5 | |
| B | + | − | 100.0 | 100.0 |
| + | + | 62.4 | 63.8 | |
Purified carboxylase (7 nM, 150 μl) was preincubated with KH2 (350 μM, part B) or the equivalent amount of vehicle (ethanol, final concentration of 0.6%, part A) for 10 min, and samples then were incubated for 30 min in NEM (2.5 mM) or the equivalent amount of vehicle (ethanol, final concentration of 1%). DTT (6 mM) was added and 10 min later the samples were assayed for activity for 60 min. Epoxidation during the 10-min preincubation of carboxylase with KH2 also was measured to subtract activity occurring before the addition of NEM. All incubations were performed at room temperature.
Preincubation of the carboxylase with KH2 before the addition of NEM afforded substantial and equal protection of both carboxylase and epoxidase activities, to 62–64% (Table 1). The KH2 preincubation was performed in the absence of a carboxylatable substrate to prevent vitamin K turnover and consequent exposure of the vitamin K protected site to NEM. Recent studies suggest that the carboxylatable substrate may affect KH2 binding (15, 16), and its absence may explain why only 62–64% protection of activity was observed at the concentration of KH2 used (350 μM). Higher concentrations also were attempted but led to inhibition of activity, as reported by others (17).
Identification of 14C-NEM-Modified Cys.
Carboxylase was treated with 14C-NEM and a parallel sample was treated with cold NEM, using identical reaction conditions to assure that NEM inactivation was reproducible among individual experiments. Samples assayed before and after modification showed that the extent of inactivation was at least 92% in each experiment. To quantify and identify Cys residues modified in NEM inactivation of the carboxylase, 14C-NEM-modified carboxylase was analyzed: (i) by SDS/PAGE and PhosphorImager to determine 14C incorporation into protein, (ii) by amino acid analysis to determine mol 14C-NEM incorporated per mol carboxylase, and (iii) by LC-ESMS on tryptic digested sample to identify 14C-NEM containing peptides.
SDS/PAGE and PhosphorImager Analysis.
When 14C-NEM-modified carboxylase was analyzed by SDS/PAGE and PhosphorImager, a single 14C-containing band was detected (Fig. 1). Quantitation showed that >90% of the 14C in the lane was incorporated into the carboxylase band, showing that the 14C-NEM-modified amino acids were in the carboxylase and not in contaminating proteins.
Amino Acid Analysis.
14C-NEM-modified carboxylase was acid-hydrolyzed, and fractions of the hydrolysate were quantitated for radioactivity by scintillation counting and for protein by amino acid analysis. Analysis of three different preparations revealed 1.8, 2.1, and 2.3 mol 14C-NEM per mol carboxylase. These results indicate that of the 10 possible Cys residues in the human carboxylase, two are modified by 14C-NEM with concomitant inactivation of both carboxylase and epoxidase activities.
These experiments also established a more accurate specific activity for the carboxylase. Protein quantitation in previous determinations all have been estimates based on the staining of gel-electrophoresed samples. In the present study, an accurate specific activity was obtained by determining the amount of 14C-NEM incorporation into a known amount of carboxylase activity (907 cpm of 14C-NEM incorporated into 1.5 × 10-4 international units of carboxylase activity, Fig. 1) and the amount of 14C-NEM incorporation into a known amount of carboxylase protein (1,300 cpm of 14C-NEM in 10 pmol protein as quantitated by amino acid analysis). The value obtained, 0.2 μmol/min per mg, is approximately 3-fold lower than previously reported (13, 18). This value, in conventional carboxylase units, corresponds to 7 × 108 cpm of 14CO2 incorporated into substrate/hr per mg.
LC-ESMS.
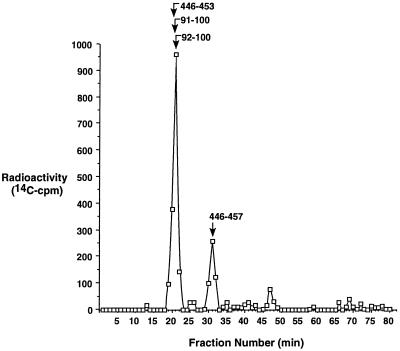
To identify the 14C-labeled Cys, 14C-NEM-modified carboxylase was denatured, reductively carboxyamidomethylated, trichloroacetic acid-precipitated, and then digested with trypsin. The tryptic peptides were treated with PN Glycanase F to remove Asn-linked carbohydrates, and the products were analyzed by LC-ESMS. A postcolumn splitter was used that allowed half of the sample to be collected and counted to detect 14C peaks, with the remainder of the sample being analyzed by mass spectrometry. Of the injected radioactivity, 97% was recovered. Three peaks eluting at 20–21 min, 31 min, and 47 min were observed (Fig. 2), which corresponded to 72%, 21%, and 6% of the radioactivity, respectively. A small amount of radioactivity totaling 1% also was observed over several fractions at the end of the gradient (Fig. 2), where only large (>10 kDa) peptide fragments were detected by mass spectrometry.
Figure 2.
Identification of 14C-NEM-labeled tryptic peptides of the human carboxylase. A tryptic digest of 14C-NEM-modified carboxylase was subjected to LC-ESMS, and postcolumn fractions were split with half of the sample being analyzed by mass spectrometry and aliquots (12% of the total digest) of the remainder analyzed by scintillation counting. The 14C-NEM-labeled peptides identified by mass spectrometry in the radioactive peaks are indicated and described in Table 2.
The chromatogram was scanned for masses predicted for tryptic fragments of the carboxylase, and peptides bearing NEM-modified Cys-99 and Cys-450 were identified (Table 2). In both cases, two different peptides were observed, one corresponding to the complete tryptic digest and the other to a dipeptide. The masses were consistent only with the NEM-modified form and not with any other tryptic peptide predicted for the carboxylase. Three of these peptides eluted at 20–21 min, which corresponded to the major peak (72%) of radioactivity (Fig. 2). The fourth peptide was detected at 31 min where 21% of the radioactivity was observed. Thus, the Cys-99- and Cys-450-containing NEM-modified peptides accounted for 93% of the input radioactivity. This percentage suggests that these two Cys are the only 14C-modified amino acids, consistent with the amino acid determination of 1.8–2.3 mol 14C-NEM per mol carboxylase. The only other radioactive peak, a small one (6%) eluting at 47 min, could not be accounted for, as the amount of material was below the level required for identification by mass spectrometry.
Table 2.
Identification of Cys residues modified by NEM or IAN
| Cys residue | Peptide amino acids | Modified thiol | Retention time, min | Mass
|
|
|---|---|---|---|---|---|
| Predicted | Observed | ||||
| Cys-99 | 92-100 | NEM | 21 | 1178.3 | 1178.8 |
| 92-100 | IAN | 23 | 1110.3 | 1110.6 | |
| 91-100 | NEM | 21 | 1306.5 | 1306.4 | |
| 91-100 | IAN | 22 | 1238.4 | 1238.7 | |
| Cys-134 | 101-136 | IAN | 64 | 4265.3 | 4265.3 |
| Cys-139 | 137-154 | IAN | ND | 2289.8 | — |
| Cys-288 | 277-317 | IAN | ND | 4842.7 | — |
| Cys-311 | 277-317 | IAN | ND | 4842.7 | — |
| Cys-323 | 318-326 | IAN | 24 | 1150.4 | 1150.6 |
| Cys-343 | 335-346 | IAN | 21 | 1306.5 | 1306.4 |
| Cys-450 | 446-453 | NEM | 20 | 1066.2 | 1066.4 |
| 446-457 | NEM | 31 | 1517.8 | 1516.6 | |
| Cys-598 | 589-622 | IAN | 47 | 3991.5 | 3990.9 |
| Cys-700 | 695-704 | IAN | 32 | 1227.5 | 1228.0 |
Peptides from a chromatogram (Fig. 2) of tryptic-digested modified carboxylase were detected by LC-ESMS (supplemental Methods). No free thiol-containing peptides were detected. Predicted and observed masses are for the actual peptide masses, derived from their positive ion masses. M+H ions were observed for Cys-99, Cys-323, Cys-343, Cys-450, and Cys-700. M+2H ions were observed for Cys-99, Cys-323, Cys-343, Cys-450, Cys-598, and Cys-700. M+3H ions were observed for Cys-134 and Cys-598. M+4H ions were observed for Cys-134. ND is not detected.
Analysis of eight different preparations of tryptic digested NEM-modified carboxylase identified masses consistent with the NEM-modified Cys-99 and Cys-450 peptides (Table 2 and data not shown). In half of these experiments, the Cys-99 peptide was detected only as an NEM-modified peptide, whereas in the remaining experiments both carboxyamidomethylated and NEM-modified peptides were observed. The experiment presented in Table 2 showed a mixture, and amino acid analysis of this sample gave a ratio of 1.8 mol 14C-NEM per mol carboxylase. The reason for the variation in NEM modification is not known but may reflect subtle differences in enzyme conformation or alkylation reaction conditions.
Of the eight remaining Cys residues, five could be identified by LC-ESMS. All of these amino acids, Cys-134, Cys-323, Cys-343, Cys-598, and Cys-700 were modified only by IAN. Three of the peptides (containing Cys-343, Cys-598, and Cys-700) eluted at or near the positions where radioactivity was observed (Table 2, Fig. 2). However, in eight different analyses only carboxyamidomethylated peptides were detected, and NEM-modified forms of these peptides were never observed.
Two peptides, one containing Cys-139 and the other Cys-288 and Cys-311, were not observed in these studies. These peptides should have been detectable: in preliminary studies using carboxyamidomethylated, non-NEM treated tryptic-digested carboxylase, the two peptides eluted before the Cys-134-containing peptide, which was detected in the studies shown here (Table 2 and data not shown). Both peptides reside within a hydrophobic region of the carboxylase (Fig. 3), and their lack of detection is likely because of poor tryptic digestion in this region. Because 93% of the 14C-NEM could be accounted for by incorporation into Cys-99 and Cys-450 (Fig. 2, Table 2), it is highly unlikely that any of these three Cys were modified by NEM. Nonetheless, because of their lack of detection and to determine whether any of these three Cys affected carboxylase function, they were mutated and analyzed in parallel with the two NEM-modified Cys residues.
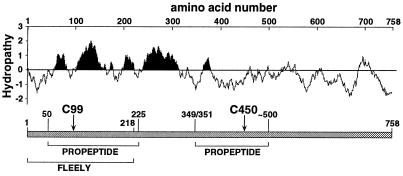
Figure 3.
Functional mapping of the carboxylase. A linear representation of the human carboxylase is shown, along with the two active site Cys residues identified in these studies, and regions that cross link to FLEELY or propeptide, identified in previous biochemical mapping studies (9–11). A hydropathy plot also is shown. Analysis was performed at bioinformatics.weizmann.ac.il/hydroph/plot_hydroph.html, using the Kyte-Doolittle x-1 method of calculating hydrophilicty over a window length of 25 to predict membrane spanning regions. The black shading highlights very hydrophobic regions of the carboxylase.
Mutational Analysis of Cys-99 and Cys-450.
Cys-99, -139, -288, -311, and -450 were individually changed to Ser, and the mutants were expressed by using baculovirus in SF21 cells (Fig. 5, which is published as supplemental material) (19), which do not have endogenous carboxylase activity (20). To control for variability in the amount of carboxylase protein expressed in individual infections, a quantitative Western analysis was performed (see supplemental data, Methods) and then used to normalize the activities of wild-type and mutant carboxylases.
Carboxylase and epoxidase activities were assayed in microsomes from mock or virus-infected cells (Table 3). Substitution of Cys-99 to Ser (C99S) or Cys-450 to Ser (C450S) reduced activity to 0.2% (C99S) or 1% (C450S) that of wild-type carboxylase. This reduction in activity was not a general consequence of a Cys to Ser mutation as the Cys-139 to Ser, Cys-288 to Ser, and Cys-311 to Ser mutants all had wild-type carboxylase and epoxidase activities (Table 3).
Table 3.
Mutation of Cys-99 or Cys-450 inhibits both carboxylase and epoxidase activity
| Carboxylase construct | Specific activity
|
|
|---|---|---|
| Carboxylase* | Epoxidase† | |
| Mock-infected | 0.03 | 0.02 |
| Wild type (-K) | 0.03 | — |
| Wild type | 63.30 | 69.30 |
| C99S | 0.13 | 0.11 |
| C139S | 64.40 | 72.00 |
| C288S | 61.40 | 69.90 |
| C311S | 66.60 | 67.80 |
| C450S | 0.80 | 0.90 |
Solubilized microsomes (50 μl, 100 μg total protein) from mock- or r-carboxylase containing baculovirus-infected insect cells (19) were assayed for 30 min for carboxylase and epoxidase activity. Samples were assayed in triplicate and each value was within 10% error. The amount of carboxylase protein expressed in each preparation was determined by quantitative Western analysis, and the expression levels were normalized to the wild-type levels. The wild type (-K) control was a sample assayed in the absence of KH2. The pmol 14CO2 value is based on a determination of the specific activity of 14CO2 as 55 cpm/pmol (supplemental Methods).
pmol 14CO2 incorporated/hr per μg total protein.
pmol vitamin K epoxide generated/hr per μg total protein.
Interestingly, both the C99S and C450S mutants exhibited the same decrease in epoxidase activity as seen for carboxylase activity (Table 3), which was not predicted by the base amplification model (5). To further characterize how the Cys-99 and Cys-450 mutations impaired activity, the effects of varying concentrations of cofactors or substrate on activity were examined (Table 4). C99S and C450S showed a 6- to 8-fold increase in Km for the substrate EEL and 6- to 7-fold decrease in Km for KH2. In all cases, the Vmax for the mutants was substantially less than for wild-type carboxylase.
Table 4.
Kinetic analyses of the active site mutants
| Substrate/cofactor | Carboxylase | Km, μM | Vmax, pmol/min | Vmax/Km, pmol/min per μM × 102 |
|---|---|---|---|---|
| EEL | Wild type | 200 | 545 | 273 |
| C99S | 1,600 | 5 | <1 | |
| C450S | 1,100 | 38 | 3 | |
| KH2 | Wild type | 29 | 493 | 1,700 |
| C99S | 4 | 5 | 125 | |
| C450S | 5 | 40 | 800 | |
| CO2 | Wild type | 900 | 513 | 57 |
| C99S | 1,000 | 5 | <1 | |
| C450S | 1,100 | 38 | 3 |
Lysates were used instead of microsomes because the higher activities obtained for the mutants made these assays possible. A total of 100 μg of lysate was assayed, and the carboxylase levels, determined by quantitative Western analysis, were normalized to the wild-type levels. Each value was determined three times (supplemental Methods), and the error for each experiment was +/− 5%.
Discussion
The carboxylase has been proposed to contain active site Cys residues that participate in a base strength amplification mechanism (5). In this model, one Cys is proposed to act as a weak base, which ionizes KH2 to a strong base able to generate a carbanion intermediate. The second Cys residue is proposed to serve to coordinate CO2 for nucleophilic attack by the carbanion. Our mapping of the active site Cys residues and determination of their importance to carboxylase activity provides a biochemical test of this hypothesis. The thiol-specific inhibitor NEM abolished both carboxylase and epoxidase activities, as reported (6, 8), and amino acid analysis showed that 2 mol 14C-NEM were incorporated per mol of carboxylase. To map the NEM-modified amino acids, 14C-NEM carboxylase was digested with trypsin and analyzed by LC-ESMS (Fig. 2, Table 2), and 93% of the radioactivity was detected in NEM-modified peptides that contained Cys-99 or Cys-450. Mutation of each of these amino acids to Ser reduced activity to 0.2% (C99S) or 1% (C450S) that of the wild-type enzyme, whereas the independent conversion of three other Cys residues to Ser had no effect on activity (Table 3). The combined biochemical identification and mutational analyses show that Cys-99 and Cys-450 comprise part of the active site.
The C99S and C450S mutations each caused an equivalent inhibition of epoxidation and carboxylation, a result that was unexpected from the original base amplification hypothesis. According to this model, the mutation of the Cys affecting KH2 ionization should inhibit epoxidation and consequent carboxylation. However, the mutation of the other Cys residue coordinating CO2 would be predicted to still allow the production of a strong base by the first Cys residue and retention of epoxidase activity (5), a prediction that was not fulfilled by our results. The original hypothesis was based on previous studies in crude liver microsomes showing that KH2 protected epoxidase but not carboxylase activity from NEM inhibition (6). In contrast, our studies with pure carboxylase showed that KH2 protected both activities equally well (Table 1). This discrepancy in results is most likely because of the fact that with the microsomal studies (6) the large number of carboxylase VKD substrates present may have affected those experiments.
Interestingly, the C99S and C450S mutations both resulted in an apparent decrease in affinity of the carboxylase for the glutamyl substrate (Table 4). One explanation for the decrease is that normally these thiols are part of a complex in which they are indirectly coordinated with the glutamyl substrate. This complex may be required for efficient KH2 oxygenation, which would explain previous results showing that the glutamyl substrate increases epoxidase activity (3). The proposed complex also could provide a mechanism to account for the observation that the Cys to Ser mutants retained some activity (Tables 3 and 4) despite the high pK (>13) of Ser (14). Partial functional substitution suggests that the carboxylase has a mechanism for ionizing Cys/Ser, a possibility that also would reconcile the known pH optima for the wild-type carboxylase (6.9, data not shown) and pK (8.4) of Cys. It is therefore likely that the carboxylase uses a mechanism similar to that of other active site thiol enzymes (e.g., papain, cathepsins, caspases; refs. 21 and 22), where a histidyl (His) residue increases the nucleophilicity of the reactive thiol. The Glu substrate, then, could coordinate a carboxylase thiol through His, and even enhance thiol ionization, analogous to the role of aspartate in the serine protease catalytic triad. The simplest explanation for these data, then, which we propose as a working model for the carboxylase active site, is that the glutamyl substrate is recruited by one or both active site thiols into a complex where it functions as part of the active site to initiate KH2 oxygenation. The revised model is mechanistically attractive because KH2 oxygenation would be regulated, occurring only in the presence of the glutamyl substrate.
The current data cannot identify which of the two thiols actually initiates epoxidation. KH2 ionization by Cys-99 is likely, because its mutation effected the largest decrease in activity (Table 3). However, Cys-450 substitution also showed a significant decrease, and so participation of this residue in KH2 ionization cannot be excluded. Both mutants exhibited an increased Km for the glutamyl substrate, and Glu binding has been shown to increase carboxylase affinity for KH2 (15, 16), raising the possibility that Cys-99 and Cys-450 both are required to coordinate KH2 in a substrate-dependent manner. The two thiols must be in close proximity to KH2 because it protected them from NEM inactivation (Table 1). With both mutants, the increase in Km for the Glu substrate was accompanied by a comparable decrease in Km for KH2 (Table 4), which could be because of increased affinity of the thiols for KH2 in the absence of appropriate Glu substrate binding. Nonetheless, epoxidation was still poor (Table 3) and therefore implicates a role for both Cys-99 and Cys-450 in KH2 oxygenation.
The identification of Cys-99 and Cys-450 as part of the active site provides significant insight into carboxylase topology. Unlike most transmembrane proteins, the carboxylase is an enzyme that uses both a hydrophobic cofactor (vitamin K) and hydrophilic substrates (CO2 and glutamate). Thus, some of the hydrophobic sequences probably comprise part of the active site, as opposed to playing only a structural role as membrane spanning sequences. Cys-99 borders a hydrophobic portion of the carboxylase (Fig. 3), which implicates this region as near the active site. The membrane topology of the carboxylase is unknown: Cys-450 must be in the endoplasmic reticulum (ER) lumen because its neighboring glycosylation sites in this hydrophilic region of the molecule appear to be used (13, 23). Algorithms for predicting the membrane topology (e.g., Fig. 3) are equivocal and place the region containing Cys-99 on either the cytoplasmic or luminal side of the ER membrane. Our identification of Cys-99 and Cys-450 as components of the active site indicate that Cys-99 must be in the ER lumen and that these two regions of the molecule come together to form a multicomponent active site containing hydrophobic and hydrophilic regions of the enzyme.
The identification of active site residues that are separated by a large distance in the primary sequence reconciles previous low-resolution mapping studies. Attempts to identify the carboxylase active site succeeded only in mapping large regions of the molecule: two different groups reported cross-linking of the factor IX propeptide to either amino acids 50–225 (9) or 349-≈500 (10), and a glutamate peptide substrate (NBrFLEELY) was cross-linked to the first 218 aa (11) of the carboxylase (Fig. 3). Although no explanation was previously provided for the conflicting propeptide mapping results, our data indicate that both regions contain amino acids in the active site. Both regions may therefore also contain residues involved in propeptide binding, and differences in the experimental approach used by each group could account for the different propeptide cross-linking results. Likewise, the NBrFLEELY cross-linking experiments indicated binding to the N terminus, whereas our kinetic analysis suggests that the C-terminal Cys-450 is required to coordinate Glu. NBrFLEELY, then, most likely interacts with multiple residues in the carboxylase active site, which originate from both the N and C terminus. Thus, a more meaningful way to consider the carboxylase active site is in terms of its overall conformation rather than the primary sequence source of the residue.
Now that the carboxylase active site has been identified, further molecular probing of this environment can be used to determine how the reactive oxygenated KH2 intermediate is produced and the energy harnessed for the conversion of Glus to Glas. Refined studies will both define this unique mechanism and facilitate the design of new anticoagulants that directly target the carboxylase.
Supplementary Material
Acknowledgments
We thank Kurt W. Runge for help throughout this project and Vivien Yee for critical reading of the manuscript. We also thank Jim Sadowski for providing the vitamin K epoxide used for standardization. This work was funded by National Institutes of Health Grant RO1 HL55666 to K.L.B., American Heart Association Grant 97340 to B.N.P., National Institutes of Health Grant RO1 EY06603 to J.W.C., and National Institutes of Health Grant RO1 HL54329 to K.S.M.
Abbreviations
- VKD
vitamin K-dependent
- KH2
vitamin K hydroquinone
- NEM
N-ethyl-maleimide
- IAN
iodoacetamide
- Gla
gamma-carboxylated glutamic acid
- LC-ESMS
liquid chromatography electrospray mass spectrometry
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Berkner K L. J Nutr. 2000;130:1877–1880. doi: 10.1093/jn/130.8.1877. [DOI] [PubMed] [Google Scholar]
- 2.Furie B, Bouchard B A, Furie B C. Blood. 1999;93:1798–1808. [PubMed] [Google Scholar]
- 3.Sugiura I, Furie B, Walsh C T, Furie B C. Proc Natl Acad Sci USA. 1997;94:9069–9074. doi: 10.1073/pnas.94.17.9069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suttie J W. Annu Rev Biochem. 1985;54:459–477. doi: 10.1146/annurev.bi.54.070185.002331. [DOI] [PubMed] [Google Scholar]
- 5.Dowd P, Hershline R, Ham S W, Naganathan S. Science. 1995;269:1684–1691. doi: 10.1126/science.7569894. [DOI] [PubMed] [Google Scholar]
- 6.Canfield L M. Biochem Biophys Res Commun. 1987;148:184–191. doi: 10.1016/0006-291x(87)91093-x. [DOI] [PubMed] [Google Scholar]
- 7.Morris D P, Soute B A, Vermeer C, Stafford D W. J Biol Chem. 1993;268:8735–8742. [PubMed] [Google Scholar]
- 8.Bouchard B A, Furie B, Furie B C. Biochemistry. 1999;38:9517–9523. doi: 10.1021/bi9907375. [DOI] [PubMed] [Google Scholar]
- 9.Yamada M, Kuliopulos A, Nelson N P, Roth D A, Furie B, Furie B C, Walsh C T. Biochemistry. 1995;34:481–489. doi: 10.1021/bi00002a012. [DOI] [PubMed] [Google Scholar]
- 10.Wu S M, Mutucumarana V P, Geromanos S, Stafford D W. J Biol Chem. 1997;272:11718–11722. doi: 10.1074/jbc.272.18.11718. [DOI] [PubMed] [Google Scholar]
- 11.Kuliopulos A, Nelson N P, Yamada M, Walsh C T, Furie B, Furie B C, Roth D A. J Biol Chem. 1994;269:21364–21370. [PubMed] [Google Scholar]
- 12.Crabb J W, West K A, Dodson W S, Hulmes J D. In: Unit 11.9. Coligan J E, Ploegh H L, Smith J A, Speicher D W, editors. New York: Wiley; 1997. pp. 11.19.11–11.19.42. [Google Scholar]
- 13.Lingenfelter S E, Berkner K L. Biochemistry. 1996;35:8234–8243. doi: 10.1021/bi9523318. [DOI] [PubMed] [Google Scholar]
- 14.Wong S S. Reactive Groups of Proteins and Their Modifying Agents. Boca Raton, FL: CRC; 1993. [Google Scholar]
- 15.Houben R J, Jin D, Stafford D W, Proost P, Ebberink R H, Vermeer C, Soute B A. Biochem J. 1999;341:265–269. [PMC free article] [PubMed] [Google Scholar]
- 16.Soute B A, Ulrich M M, Watson A D, Maddison J E, Ebberink R H, Vermeer C. Thromb Haemostasis. 1992;68:521–525. [PubMed] [Google Scholar]
- 17.Furie B C, Kuliopulos A, Roth D A, Sugiura I, Walsh C T, Furie B. Methods Enzymol. 1997;282:333–346. doi: 10.1016/s0076-6879(97)82118-0. [DOI] [PubMed] [Google Scholar]
- 18.Wu S M, Morris D P, Stafford D W. Proc Natl Acad Sci USA. 1991;88:2236–2240. doi: 10.1073/pnas.88.6.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berkner K L, McNally B A. Methods Enzymol. 1997;282:313–333. doi: 10.1016/s0076-6879(97)82117-9. [DOI] [PubMed] [Google Scholar]
- 20.Roth D A, Rehemtulla A, Kaufman R J, Walsh C T, Furie B, Furie B C. Proc Natl Acad Sci USA. 1993;90:8372–8376. doi: 10.1073/pnas.90.18.8372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Earnshaw W C, Martins L M, Kaufmann S H. Annu Rev Biochem. 1999;68:383–424. doi: 10.1146/annurev.biochem.68.1.383. [DOI] [PubMed] [Google Scholar]
- 22.McGrath M E. Annu Rev Biophys Biomol Struct. 1999;28:181–204. doi: 10.1146/annurev.biophys.28.1.181. [DOI] [PubMed] [Google Scholar]
- 23.Wu S M, Cheung W F, Frazier D, Stafford D W. Science. 1991;254:1634–1636. doi: 10.1126/science.1749935. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.