Abstract
When grown under a variety of stress conditions, cyanobacteria express the isiA gene, which encodes the IsiA pigment-protein complex. Overexpression of the isiA gene under iron-depletion stress conditions leads to the formation of large IsiA aggregates, which display remarkably short fluorescence lifetimes and thus a strong capacity to dissipate energy. In this work we investigate the underlying molecular mechanism responsible for chlorophyll fluorescence quenching. Femtosecond transient absorption spectroscopy allowed us to follow the process of energy dissipation in real time. The light energy harvested by chlorophyll pigments migrated within the system and eventually reaches a quenching site where the energy is transferred to a carotenoid-excited state, which dissipates it by decaying to the ground state. We compare these findings with those obtained for the main light-harvesting complex in green plants (light-harvesting complex II) and artificial light-harvesting antennas, and conclude that all of these systems show the same mechanism of energy dissipation, i.e., one or more carotenoids act as energy dissipators by accepting energy via low-lying singlet-excited S1 states and dissipating it as heat.
Introduction
Cyanobacteria, also known as blue-green algae, are oxygenic photosynthetic organisms found in almost every aquatic habitat, and are among the oldest life forms. Their ubiquitous presence is estimated to be responsible for more than half of the total primary production of organic compounds on Earth. The impressive capacity of cyanobacteria for adaptation stems from a number of regulatory mechanisms that allow them to cope with unfavorable environmental conditions. Of particular importance is their ability to adapt to different iron conditions. In aquatic environments cyanobacteria are exposed to a range of iron concentrations, and in many habitats the iron availability is limiting because of the low solubility of iron ions in water. To cope with this kind of stress, cyanobacteria respond by expressing a number of genes, one of which is the isiA gene encoding the IsiA (or CP43′) protein. This was shown to lead to the formation of photosystem I (PSI)-IsiA supercomplexes (1,2) in which IsiA functions as a light harvester (3,4), and to the accumulation of large IsiA aggregates that are not directly bound to a photosystem (5,6) and show an impressive capacity to dissipate excited-state energy (7,8), in line with the suggestion that IsiA acts as a general photoprotector under stress conditions (9,10). The isiA gene is in fact also expressed under high light (11) and other photooxidative stress conditions (such as heat and salt stress (12,13)), and its deletion leads to a photosensitive phenotype (11). IsiA aggregates are also present in the early stages of iron deprivation when PSI is still abundant in the cell, pointing to a photoprotective role in the early (more physiological) stages of iron deficiency (8). It was shown that IsiA displays a remarkable mobility within the membrane, which suggests that it has a role other than serving as a light-harvesting antenna (14). Furthermore, the dynamics of IsiA transcription shows a pattern that is clearly different from what one would expect for a light-harvesting protein (15). Possible roles of IsiA in photoprotection include the dissipation of energy to prevent overexcitation of photosystem II (PSII) (16) and dissipation of the energy collected by phycobilisomes (17).
Energy dissipation is a ubiquitous and vital process in photosynthesis (18,19). Under conditions of excess light, photoprotective mechanisms are activated that allow the harmless dissipation of excess energy in the form of heat. Evergreens grown under freezing conditions are in a permanently quenched state in which energy is continuously dissipated (20,21). At the other extreme, it has been shown that heat stress can trigger a process of energy dissipation in plants (22), and very dry conditions induce efficient energy dissipation in lichens (23). Most species of cyanobacteria can dissipate energy from phycobilisomes, and this may occur via the recently discovered orange carotenoid protein (24,25).
Energy dissipation processes have been studied in greater detail in green plants, and although no consensus has been reached yet on the underlying molecular mechanism, it is becoming increasingly clear that carotenoids play an essential role in the dissipation mechanism. The importance of carotenoids in nature is evidenced by their ubiquity in living organisms. Carotenoids are active in light harvesting, photoprotection, and structure organization (26,27). Of vital importance for the survival of the photosynthetic organisms is their role in photoprotection (28). Carotenoids quench harmful chlorophyll (Chl) triplet states, which are potential singlet oxygen sensitizers and very effective scavengers of singlet oxygen (27). This capacity relies on the properties of the carotenoid triplet state, whose energy is sufficiently low to effectively quench Chl-triplet states and singlet oxygen.
Another pivotal role of carotenoids is in the quenching of Chl-singlet-excited states under conditions of excess light illumination, a process generally known as nonphotochemical quenching (NPQ) (29,30). The potentially deleterious effect of excess light on a photosynthetic organism is just one example of how too much of a good thing can be extremely harmful. In fact, the absorption of light in excess of that required for maximum CO2 fixation will lead to the formation of a high concentration of Chl-triplet states, mainly within the PSII reaction center. This condition can be very detrimental to the survival of the photosynthetic organism because Chl-triplet states are excellent singlet oxygen sensitizers. Singlet oxygen is an extremely reactive species that can irreversibly damage a photosynthetic organism and eventually lead to its death. To cope with exposure to excess light and the ensuing photoinduced damage, photosynthetic systems have developed a number of strategies that allow the harmless dissipation of excess energy in the form of heat. We recently showed that carotenoids can quench the excited-state energy of a neighboring tetrapyrrole by accepting energy from the latter and dissipating it as heat (31). The same mechanism has been shown to be responsible, at least in part, for NPQ in green plants (32). Alternatively, it has been proposed that carotenoids may quench Chl-excited-state energy via a charge-separation mechanism involving the xanthophyll zeaxanthin (33,34).
In this study we determined the mechanism responsible for the very pronounced Chl fluorescence quenching observed in the cyanobacterium Synechocystis PCC 6803 grown under iron-depleted growth conditions. Our results suggest that the same dissipation mechanism functions in cyanobacterial IsiA, green plant light-harvesting complex II (LHCII), and model compounds, and that photosynthetic organisms have adopted a general and universal mechanism of energy dissipation whereby carotenoids act as energy sinks by accepting energy via low-lying singlet-excited S1 states and dissipating it as heat.
Materials and Methods
Sample preparation
IsiA aggregates were isolated and purified as described previously (7) from a psaFJ-null mutant of cyanobacterium Synechocystis PCC 6803 cells harvested ∼30 days after growth in a liquid BG11 medium from which iron was omitted. The PsaFJ− mutant was used because of its high content of PSI-free IsiA aggregates under iron-depletion stress conditions. After disruption of the cells, the thylakoid membranes (0.1 mg Chl a mL−1) were solubilized with 0.2% (w/v) n-dodecyl-β,D-maltoside (β-DM) and centrifuged at 11,000 × g for 3 min. The supernatant was subjected to a Mono Q HR 5/5 column (Pharmacia; Roosendaal, The Netherlands) for ion exchange chromatography (IEC). The running buffer consisted of 20 mM Bis-Tris (pH 6.5), 10 mM MgCl2, 20 mM NaCl, 15 mM MgSO4, 1.5% taurine, and 0.03% β-DM. A gradient with MgSO4 up to 500 mM was applied. The IsiA aggregates eluted at an MgSO4 concentration of ∼250 mM. The IEC was monitored with an online diode array detector (Shimadzu SPD-M10Avp).
Time-resolved measurements
Femtosecond pulses were obtained from a titanium/sapphire oscillator-regenerative amplifier (coherent MIRA seed and RegA). The repetition rate was 40 kHz and the initial pulse was ∼60 fs at 800 nm. The beam was split into two beams, one of which (the probe beam) was focused on a CaF2 plate to generate a white light continuum. The other beam was used to pump an optical parametric amplifier (OPA) to obtain the pump beam (∼100 fs) with an excitation wavelength of 682 nm. The data shown in Fig. S2 of the Supporting Material were collected with a kHz system (Coherent Legend-USP) with excitation at 678 nm. The energy per pulse was ∼10 nJ for both experiments and the excitation density was ∼0.7 × 1014 photons · pulse−1 · cm−2. The polarization between the pump and probe beams was set at the magic angle. The measurements were done at room temperature. The data were analyzed with the use of global analysis techniques (35). Since the evolution associated difference spectra (EADS) represent in general a mixture of molecular states, a target analysis, using a specific kinetic scheme, was also applied to the data. In this way, the species associated difference spectra (SADS)—spectra corresponding to pure molecular species—were obtained. The instrument response function was fitted to a Gaussian of ∼120 fs (full width at half-maximum).
Results
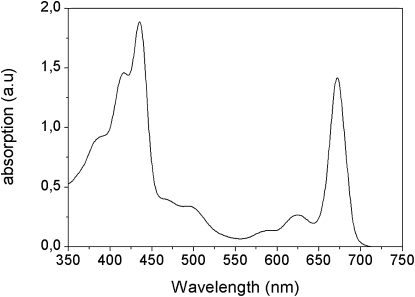
Fig. 1 shows the absorption spectrum of the IsiA aggregates. According to previous results (3), each monomer of IsiA contains ∼16 Chl a, which give rise to the strong absorption in the Qy and Soret regions around 675 nm and 420 nm, respectively. Furthermore, IsiA contains one zeaxanthin, one echinenone, and two β-carotene molecules (7), all of which absorb in the 400–540 nm region.
Figure 1.
Absorption spectrum of IsiA aggregates at room temperature.
Fig. 2 shows selected kinetic traces in different regions of the visible spectrum obtained upon selective excitation of Chl a at 682 nm. The figure shows traces at 679 (A), 495 (B), and 552 nm (C) along with the fit obtained from a global analysis of the data. Four components are needed to fit the data: a 200 fs component to account for ultrafast relaxation and equilibration, 3.9 and 34 ps components that can be explained by both fluorescence quenching and singlet-singlet annihilation, and a small component that does not decay on the timescale of the experiment (1 ns). It must be noted that the lifetimes of the components are shorter than those reported by Ihalainen et al. (7), mainly because of singlet-singlet annihilation and possible small differences in the sample preparation. The trace at 679 nm shows the decay of the Chl Qy bleach. If we compare the decay of this trace with the decay of the 552 nm trace after the initial ultrafast (200 fs) relaxation, we observe that the latter clearly shows a smaller relative decay in the first ∼10 ps. Such a difference would not be expected if the full spectral evolution were determined only by contributions from the decay of excited Chl. Thus, the observed difference between the two kinetics suggests that at 552 nm a species different from excited Chl partly compensates for the decay of Chl excited-state absorption (ESA) by providing a positive contribution to the trace. The opposite trend is seen at 495 nm, where after the initial ultrafast evolution the trace shows a larger relative decay in the first ∼10 ps compared to the 679 nm trace, suggesting that the second species provides a negative contribution (a bleach) to the signal in this region. Both differences at 552 and 495 nm are small and can be better seen in Fig. 4, where the Chl decay (red curve) is not sufficient to fit the two traces (vide infra). In the intervening wavelengths, from 495 nm to 552 nm, the evolution changes progressively from being faster than the Chl decay to being slower (not shown).
Figure 2.
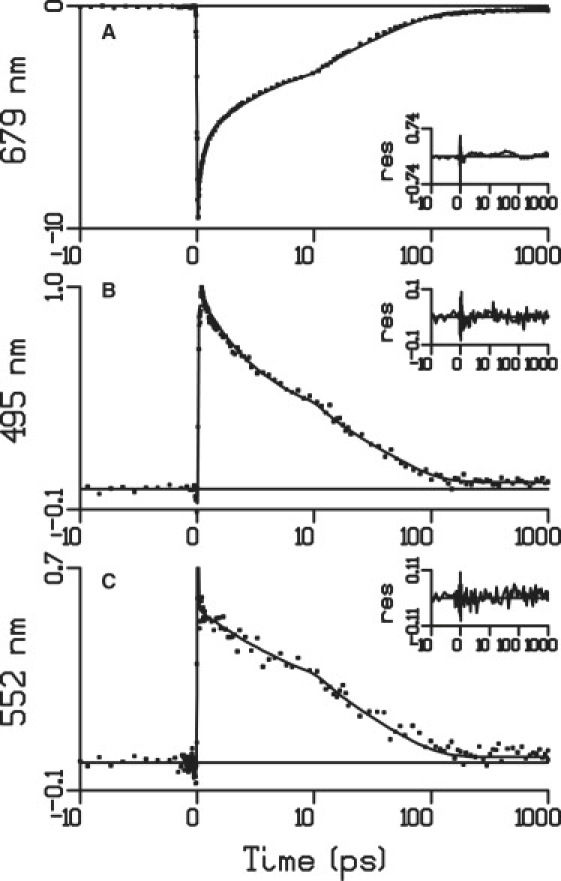
Kinetic traces in different regions of the spectrum along with the fit obtained from a global analysis with a sequential model. The amplitudes are in mOD; the inset shows the residuals.
Figure 4.
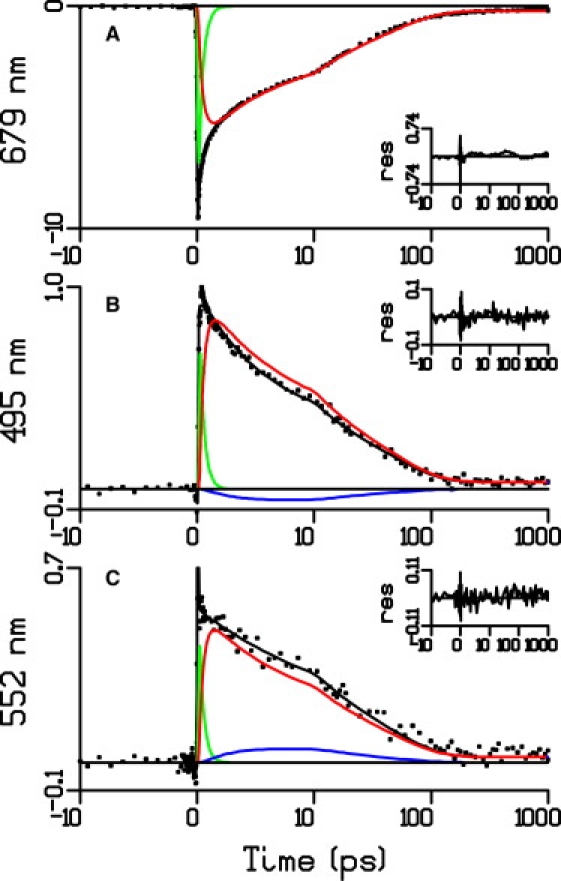
Kinetic traces along with the fit obtained from the target analysis and the contributions of the various compartments. The green line represents the contribution of Chl 1; the red line represents the contribution of Chl 2, Chl 3, and Chl 4; and the blue line represents the contribution from the quencher. The amplitudes are in mOD.
Interestingly, the 495 nm region is the absorption region of the carotenoid S0→S2 transition, whereas the 552 nm region corresponds to the S1→Sn transition region. Thus, the nonhomogeneous decay of the traces suggests that concomitantly with the decay of the Chl-excited state, a carotenoid ground state is depopulated while at the same time a carotenoid-excited state is populated, which would provide a quenching channel for Chl-excited-state energy. It must be noted that the same phenomenon has been detected in artificial caroteno-phthalocyanine dyads (31) and in the quenched state of LHCII of plants (32). In both systems, energy transfer from the excited Chl or tetrapyrrole to a carotenoid with low S1 energy was established to be the mechanism of fluorescence quenching.
Target analysis of the time-resolved data
The different evolution of the traces in different regions of the spectrum strongly suggests the involvement of a carotenoid-excited state in the quenching process. To further test this hypothesis, we applied a target analysis to the time-resolved data (35). In a target analysis, the various photophysical and photochemical processes that take place after excitation of the system are disentangled and quantified with the use of a physical (kinetic) model. Contrary to what is obtained from a global analysis, the spectra obtained from the target analysis are expected to represent the pure molecular species involved in the various processes. Our goal was to extract the spectrum of the putative quenching state.
The upper panel of Fig. 3 shows the kinetic scheme (which is basically identical to the scheme used by Ruban et al. (32)). The model consists of five compartments: after excitation, Chl 1 is populated and relaxes within 1 ps. The relaxed Chl consists of three populations: Chl 2 and Chl 3, which decay via the quenching state Q (rate constant kQ) and singlet-singlet annihilation (rate constants k4 and k5), and a small unquenched fraction Chl 4 (3.5%), which does not decay on the timescale of the experiment (1 ns). The four Chl compartments are needed to account for the multiexponential decay of the Chl-excited state because of the multiexponential character of singlet-singlet annihilation (36) and the possible heterogeneity of Chl populations. The quenching state decays to the ground state with rate constant k6.
Figure 3.
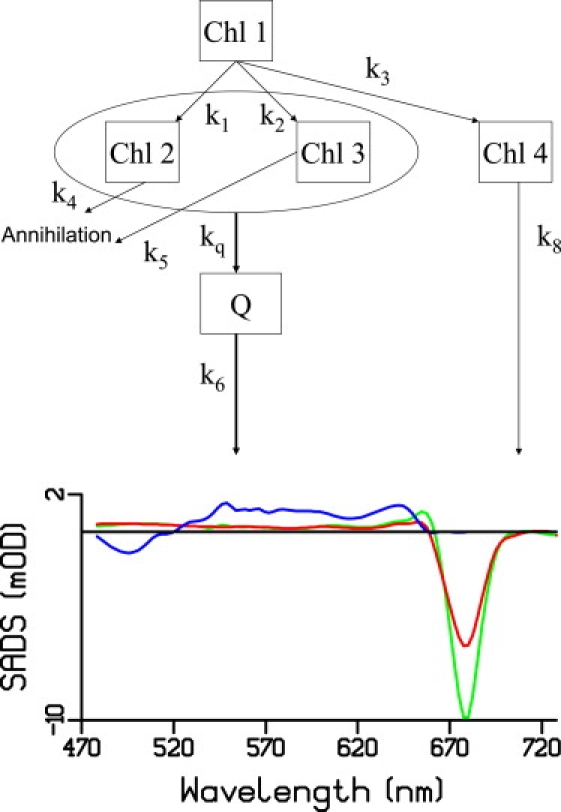
(Upper) Kinetic model. (Lower) SADS obtained from the target analysis. (Green) Chl 1; (red) Chl 2, Chl 3, and Chl 4 (blue) Q.
The spectra (SADS) obtained from the target analysis are shown in Fig. 3 (lower panel) and the rate constants are shown in Table 1. The green spectrum (Chl 1) corresponds to unrelaxed Chls; it displays Chl Qy bleach in the 680 nm region, a peak at ∼665 nm, and a region of flat ESA in the remaining spectral region. The lifetime of the Chl 1 compartment is 200 fs; therefore, its spectral shape suffers from interference with coherent artifacts and cross-phase modulation phenomena. The spectra corresponding to Chls 2, 3, and 4 (relaxed Chls) are shown in red and are assumed to be identical; they show a Chl Qy bleach in the 680 nm region, a less pronounced shoulder around 668 nm, and flat ESA. The spectrum of the quenching state (in blue) shows negative amplitude below ∼522 nm and positive signal from 522 up to 665 nm (above this wavelength the spectrum was set to zero because the amplitude of the quenching state was too small to reliably estimate its spectral shape); its shape resembles that of a carotenoid S1 state (27). In analogy with the quenching mechanism in the dyads (31) and LHCII (32), it must be emphasized that within the framework of the kinetic model the quenching state is populated according to inverted kinetics, i.e., it is slowly populated and quickly depopulated, and therefore attains a very low transient population, which gives rise to uncertainty in the estimate of its spectral shape. Based on the results from the target analysis, we estimate that the quenching via the carotenoid S1 state accounts for 20% of the total decay.
Table 1.
Reciprocal of rate constants (ps−1) obtained from the target analysis (f stands for fixed parameter)
| k1 | k2 | k3 | k4 | k5 | k6 | kQ | k8 |
|---|---|---|---|---|---|---|---|
| 0.76 | 0.87 | 10.86 | 6.3 | 43.5 | 4(f) | 100(f) | inf |
Reproducibility of the results
To test the reproducibility of the results, we repeated the experiment with a kHz system with broadband detection. The SADS obtained are shown in Fig. S1 and the rate constants are displayed in Table 1; the SADS are very similar to those shown in Fig. 3. Fig. S2 shows the results of the same analysis without setting any spectral constraint on the Q spectrum. This results in an erratic behavior of the Q spectrum in the Chl Qy region. The small differences in the spectral shape of the quenching state obtained from the 40 kHz and kHz systems are due to the intrinsically low concentration of the quenching state.
Role of electron transfer in the quenching process
Another mechanism that has been proposed to be at least partly responsible for NPQ in plants is based on the formation of a Chl-zeaxanthin charge-transfer state (33,34). In this case, a carotenoid cation is formed during the quenching process, followed by charge recombination.
Since carotenoid cations do not absorb in the S1→Sn ESA region (37), the clear difference in the 552 nm kinetics as compared to the Chl Qy decay strongly suggests that a carotenoid-excited state is populated concomitantly with the decay of the Chl-excited state. The results from the target analysis (Fig. 3, Fig. S1, and Fig. S2) allowed us to identify the quenching state as a carotenoid S1 state. To investigate the involvement of a carotenoid cation in the quenching process in IsiA aggregates, we investigated the near-infrared region (880–960 nm) where carotenoid cations are expected to absorb (38). The spectra obtained from a global analysis and selected kinetic traces are shown in Fig. S3. Based on the results from the target analysis, we can estimate the maximum amplitude of a hypothetical carotenoid cation if the quenching occurred via a charge-separated state; we estimate a maximum amplitude of 0.8 mOD. The signal/noise ratio in our experiment would then allow us to detect a signal that is on the order of ∼10% of the maximum estimated amplitude. The near-infrared signal thus reflects the decay of Chl ESA and shows no evidence of the formation of a charge-separated state during the quenching process in IsiA aggregates.
Discussion
The process of nonphotochemical quenching and, more generally, of energy dissipation in photosynthetic organisms has been the subject of extensive investigation (30,39). In particular, the underlying molecular mechanism(s) and the role of carotenoids are still very controversial issues. Two mechanisms by which carotenoids quench Chl-excited states were recently proposed. We have shown (31) that carotenoids are capable of quenching the excited state of a neighboring tetrapyrrole by accepting energy via a low-lying singlet state, and that the process is very sensitive to the conjugation length of the carotenoid. With a difference of one conjugated double bond, the S1 state can be converted from an energy donor into an energy acceptor (40), as originally proposed for the molecular gear-shift mechanism (41). We recently demonstrated that the same mechanism constitutes the quenching mechanism in LHCII aggregates from green plants, where lutein, a 10 double-bond carotenoid, acts as an energy sink by accepting energy from Chl-excited states and harmlessly dissipating it as heat (32). A second mechanism is based on charge separation: the quenching site in this case would be constituted by a Chl-zeaxanthin dimer that, once excited, would lead to the formation of a charge-separated state, Chl−Zea+, which in turn would dissipate energy by charge recombination (33). This second mechanism has been proposed to be active within the minor light-harvesting antenna of PSII, but not in LHCII (34,42).
The results presented here show that the same carotenoid signature detected in artificial caroteno-phthalocyanine dyads, and quenched LHCII is present in quenched IsiA. Thus, our results reaffirm a model for energy dissipation in which the energy is transferred from the excited Chl to a low-lying carotenoid dark state, which in turn dissipates it to heat. The model is schematically shown in Fig. 5. The energy harvested by Chl molecules migrates within the system (upper panel) until it reaches a quenching site where it is dissipated (lower panel).
Figure 5.
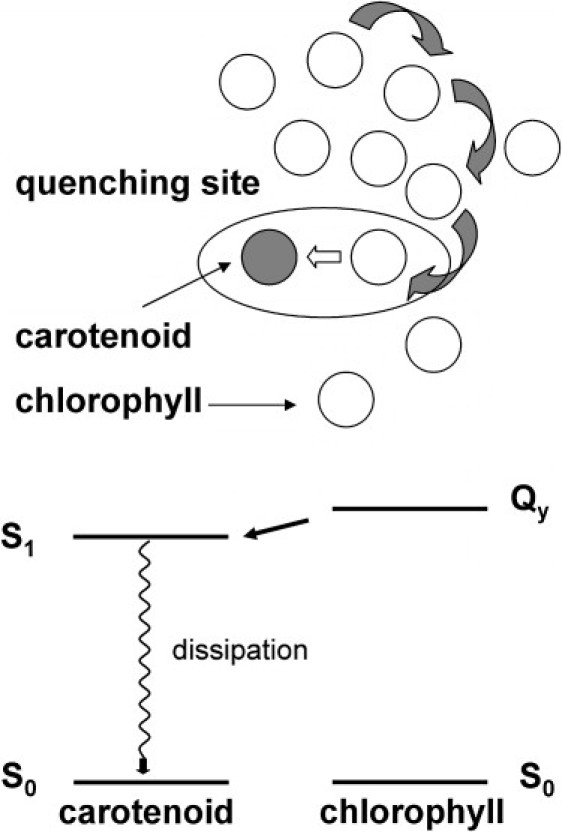
Proposed quenching model. (Upper) Energy is transferred among the Chl pool and eventually reaches a quenching site where it is dissipated as heat. (Lower) The physical mechanism of the quenching: the excited Chl transfers energy to a carotenoid S1 state, which dissipates it by decaying to the ground state on a timescale of a few picoseconds.
The results from the target analysis allowed us to quantify the real quenching via the carotenoid S1 state. We estimate that this accounts for ∼20% of the total quenching. This raises a question as to whether other quenching mechanisms may be active in IsiA aggregates. To this end, it is interesting to note that the average lifetime of the Chl-excited state in IsiA aggregates in the annihilation-free regime was found to be on the order of 100–150 ps (7), similar to the value of kQ in the target kinetic model. This suggests that the heavy loss in the system is due to annihilation processes and not to hypothetical, unknown quenching mechanisms.
Each monomeric IsiA contains on average two molecules of β-carotene, one molecule of zeaxanthin, and one of echinenone (7). Based on our study of model compounds (31), all three types of carotenoids could potentially quench Chl-excited-state energy because they have an effective conjugation length higher than 10 (31). The isosbestic point of the spectrum of the quencher (Fig. 3 and Fig. S1) is above 520 nm, pointing to echinenone as the quencher (43) because β-carotene and zeaxanthin S1 spectra are expected to have an isosbestic point more to the blue (44–47). Furthermore, the ESA region of the quencher appears to be very broad (Fig. 3 and Fig. S1), which suggests that the quenching carotenoid may possess an intramolecular charge-transfer character in its excited state, and again points to echinenone (43). Even if the unequivocal assignment of the quenching carotenoid requires further investigation (experiments on a number of mutants are under way to this end), we propose echinenone as the likely candidate. Interestingly, the same carotenoid was recently proposed to be the quencher in the orange carotenoid protein-mediated energy dissipation mechanism in cyanobacteria (25).
Taken together, our results point to a universal mechanism of energy dissipation in photosynthesis whereby carotenoid(s) of proper conjugation length act as energy sinks when fast and effective energy dissipation is required.
Acknowledgments
We thank Jos Thieme for technical support. R.B was supported by the Netherlands Organization for Scientific Research through the Earth and Life Sciences Council (NWO-ALW) and a Rubicon grant. J.T.M.K. was supported by the NWO-ALW through a VIDI fellowship.
Supporting Material
References
- 1.Bibby T.S., Nield J., Barber J. Iron deficiency induces the formation of an antenna ring around trimeric photosystem I in cyanobacteria. Nature. 2001;412:743–745. doi: 10.1038/35089098. [DOI] [PubMed] [Google Scholar]
- 2.Boekema E.J., Hifney A., Yakushevska A.E., Piotrowski M., Keegstra W. A giant chlorophyll-protein complex induced by iron deficiency in cyanobacteria. Nature. 2001;412:745–748. doi: 10.1038/35089104. [DOI] [PubMed] [Google Scholar]
- 3.Andrizhiyevskaya E.G., Schwabe T.M.E., Germano M., D'Haene S., Kruip J. Spectroscopic properties of PSI-IsiA supercomplexes from the cyanobacterium Synechococcus PCC 7942. Biochim. Biophys. Acta. 2002;1556:265–272. doi: 10.1016/s0005-2728(02)00371-7. [DOI] [PubMed] [Google Scholar]
- 4.Melkozernov A.N., Barber J., Blankenship R.E. Light harvesting in photosystem I supercomplexes. Biochemistry. 2006;45:331–345. doi: 10.1021/bi051932o. [DOI] [PubMed] [Google Scholar]
- 5.Yeremenko N., Kouril R., Ihalainen J.A., D'Haene S., van Oosterwijk N. Supramolecular organization and dual function of the IsiA chlorophyll-binding protein in cyanobacteria. Biochemistry. 2004;43:10308–10313. doi: 10.1021/bi048772l. [DOI] [PubMed] [Google Scholar]
- 6.Kouril R., Arteni A.A., Lax J., Yeremenko N., D'Haene S. Structure and functional role of supercomplexes of IsiA and photosystem I in cyanobacterial photosynthesis. FEBS Lett. 2005;579:3253–3257. doi: 10.1016/j.febslet.2005.03.051. [DOI] [PubMed] [Google Scholar]
- 7.Ihalainen J.A., D'Haene S., Yeremenko N., van Roon H., Arteni A.A. Aggregates of the chlorophyll-binding protein IsiA (CP43′) dissipate energy in cyanobacteria. Biochemistry. 2005;44:10846–10853. doi: 10.1021/bi0510680. [DOI] [PubMed] [Google Scholar]
- 8.van der Weij-de Wit C.D., Ihalainen J.A., van de Vijver E., D'Haene S., Matthijs H.C.P. Fluorescence quenching of IsiA in early stage of iron deficiency and at cryogenic temperatures. Biochim. Biophys. Acta. 2007;1767:1393–1400. doi: 10.1016/j.bbabio.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 9.Sandstrom S., Park Y.I., Oquist G., Gustafsson P. CP43′, the isiA gene product, functions as an excitation energy dissipator in the cyanobacterium Synechococcus sp PCC 7942. Photochem. Photobiol. 2001;74:431–437. doi: 10.1562/0031-8655(2001)074<0431:ctigpf>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 10.Sandstrom S., Ivanov A.G., Park Y.I., Oquist G., Gustafsson P. Iron stress responses in the cyanobacterium Synechococcus sp PCC7942. Physiol. Plant. 2002;116:255–263. doi: 10.1034/j.1399-3054.2002.1160216.x. [DOI] [PubMed] [Google Scholar]
- 11.Havaux M., Guedeney G., Hagemann M., Yeremenko N., Matthijs H.C.P. The chlorophyll-binding protein IsiA is inducible by high light and protects the cyanobacterium Synechocystis PCC6803 from photooxidative stress. FEBS Lett. 2005;579:2289–2293. doi: 10.1016/j.febslet.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 12.Vinnemeier J., Kunert A., Hagemann M. Transcriptional analysis of the isiAB operon in salt-stressed cells of the cyanobacterium Synechocystis sp. PCC 6803. FEMS Microbiol. Lett. 1998;169:323–330. doi: 10.1111/j.1574-6968.1998.tb13336.x. [DOI] [PubMed] [Google Scholar]
- 13.Geiss U., Vinnemeier J., Schoor A., Hagemann M. The iron-regulated isiA gene of Fischerella muscicola strain PCC 73103 is linked to a likewise regulated gene encoding a Pcb-like chlorophyll-binding protein. FEMS Microbiol. Lett. 2001;197:123–129. doi: 10.1111/j.1574-6968.2001.tb10593.x. [DOI] [PubMed] [Google Scholar]
- 14.Sarcina M., Mullineaux C.W. Mobility of the IsiA chlorophyll-binding protein in cyanobacterial thylakoid membranes. J. Biol. Chem. 2004;279:36514–36518. doi: 10.1074/jbc.M405881200. [DOI] [PubMed] [Google Scholar]
- 15.Singh A.K., Sherman L.A. Iron-independent dynamics of IsiA production during the transition to stationary phase in the cyanobacterium Synechocystis sp PCC 6803. FEMS Microbiol. Lett. 2006;256:159–164. doi: 10.1111/j.1574-6968.2006.00114.x. [DOI] [PubMed] [Google Scholar]
- 16.Wilson A., Boulay C., Wilde A., Kerfeld C.A., Kirilovsky D. Light-induced energy dissipation in iron-starved cyanobacteria: roles of OCP and IsiA proteins. Plant Cell. 2007;19:656–672. doi: 10.1105/tpc.106.045351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Joshua S., Bailey S., Mann N.H., Mullineaux C.W. Involvement of phycobilisome diffusion in energy quenching in cyanobacteria. Plant Physiol. 2005;138:1577–1585. doi: 10.1104/pp.105.061168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Demmig-Adams B., Adams W.W. Photoprotection in an ecological context: the remarkable complexity of thermal energy dissipation. New Phytol. 2006;172:11–21. doi: 10.1111/j.1469-8137.2006.01835.x. [DOI] [PubMed] [Google Scholar]
- 19.Kirilovsky D. Photoprotection in cyanobacteria: the orange carotenoid protein (OCP)-related non-photochemical-quenching mechanism. Photosynth. Res. 2007;93:7–16. doi: 10.1007/s11120-007-9168-y. [DOI] [PubMed] [Google Scholar]
- 20.Gilmore A.M., Ball M.C. Protection and storage of chlorophyll in overwintering evergreens. Proc. Natl. Acad. Sci. USA. 2000;97:11098–11101. doi: 10.1073/pnas.150237697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oquist G., Huner N.P.A. Photosynthesis of overwintering evergreen plants. Annu. Rev. Plant Biol. 2003;54:329–355. doi: 10.1146/annurev.arplant.54.072402.115741. [DOI] [PubMed] [Google Scholar]
- 22.Tang Y.L., Wen X.G., Lu Q.T., Yang Z.P., Cheng Z.K. Heat stress induces an aggregation of the light-harvesting complex of photosystem II in spinach plants. Plant Physiol. 2007;143:629–638. doi: 10.1104/pp.106.090712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Veerman J., Vasil'ev S., Paton G.D., Ramanauskas J., Bruce D. Photoprotection in the lichen Parmelia sulcata: The origins of desiccation-induced fluorescence quenching. Plant Physiol. 2007;145:997–1005. doi: 10.1104/pp.107.106872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilson A., Ajlani G., Verbavatz J.M., Vass I., Kerfeld C.A. A soluble carotenoid protein involved in phycobilisome-related energy dissipation in cyanobacteria. Plant Cell. 2006;18:992–1007. doi: 10.1105/tpc.105.040121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilson A., Punginelli C., Gall A., Bonetti C., Alexandre M. A photoactive carotenoid protein acting as light intensity sensor. Proc. Natl. Acad. Sci. USA. 2008;105:12075–12080. doi: 10.1073/pnas.0804636105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frank H.A., Cogdell R.J. Carotenoids in photosynthesis. Photochem. Photobiol. 1996;63:257–264. doi: 10.1111/j.1751-1097.1996.tb03022.x. [DOI] [PubMed] [Google Scholar]
- 27.Polívka T., Sundström V. Ultrafast dynamics of carotenoid excited states. From solution to natural and artificial systems. Chem. Rev. 2004;104:2021–2071. doi: 10.1021/cr020674n. [DOI] [PubMed] [Google Scholar]
- 28.Griffiths M., Sistrom W.R., Cohenbazire G., Stanier R.Y. Function of carotenoids in photosynthesis. Nature. 1955;176:1211–1214. doi: 10.1038/1761211a0. [DOI] [PubMed] [Google Scholar]
- 29.Demmig-Adams B., Adams W.W. Photoprotection and other responses of plants to high light stress. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1992;43:599–626. [Google Scholar]
- 30.Horton P., Ruban A.V., Walters R.G. Regulation of light harvesting in green plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1996;47:655–684. doi: 10.1146/annurev.arplant.47.1.655. [DOI] [PubMed] [Google Scholar]
- 31.Berera R., Herrero C., van Stokkum I.H.M., Vengris M., Kodis G. A simple artificial light-harvesting dyad as a model for excess energy dissipation in oxygenic photosynthesis. Proc. Natl. Acad. Sci. USA. 2006;103:5343–5348. doi: 10.1073/pnas.0508530103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ruban A.V., Berera R., Ilioaia C., van Stokkum I.H.M., Kennis J.T.M. Identification of a mechanism of photoprotective energy dissipation in higher plants. Nature. 2007;450:575–579. doi: 10.1038/nature06262. [DOI] [PubMed] [Google Scholar]
- 33.Holt N.E., Zigmantas D., Valkunas L., Li X.P., Niyogi K.K. Carotenoid cation formation and the regulation of photosynthetic light harvesting. Science. 2005;307:433–436. doi: 10.1126/science.1105833. [DOI] [PubMed] [Google Scholar]
- 34.Ahn T.K., Avenson T.J., Ballottari M., Cheng Y.C., Niyogi K.K. Architecture of a charge-transfer state regulating light harvesting in a plant antenna protein. Science. 2008;320:794–797. doi: 10.1126/science.1154800. [DOI] [PubMed] [Google Scholar]
- 35.van Stokkum I.H.M., Larsen D.S., van Grondelle R. Global and target analysis of time-resolved spectra. Biochim. Biophys. Acta. 2004;1657:82–104. doi: 10.1016/j.bbabio.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 36.Valkunas L., van Stokkum I.H.M., Berera R., van Grondelle R. Exciton migration and fluorescence quenching in LHCII aggregates: target analysis using a simple nonlinear annihilation scheme. Chem. Phys. 2009;357:17–20. [Google Scholar]
- 37.Berera R., Moore G.F., van Stokkum I.H.M., Kodis G., Liddell P.A. Charge separation and energy transfer in a caroteno-C-60 dyad: photoinduced electron transfer from the carotenoid excited states. Photochem. Photobiol. Sci. 2006;5:1142–1149. doi: 10.1039/b613971j. [DOI] [PubMed] [Google Scholar]
- 38.Galinato M.G.I., Niedzwiedzki D., Deal C., Birge R.R., Frank H.A. Cation radicals of xanthophylls. Photosynth. Res. 2007;94:67–78. doi: 10.1007/s11120-007-9218-5. [DOI] [PubMed] [Google Scholar]
- 39.Holt N.E., Fleming G.R., Niyogi K.K. Toward an understanding of the mechanism of nonphotochemical quenching in green plants. Biochemistry. 2004;43:8281–8289. doi: 10.1021/bi0494020. [DOI] [PubMed] [Google Scholar]
- 40.Berera R., van Stokkum I.H.M., Kodis G., Keirstead A.E., Pillai S. Energy transfer, excited-state deactivation, and exciplex formation in artificial caroteno-phthalocyanine light-harvesting antennas. J. Phys. Chem. B. 2007;111:6868–6877. doi: 10.1021/jp071010q. [DOI] [PubMed] [Google Scholar]
- 41.Frank H.A., Cua A., Chynwat V., Young A., Gosztola D. Photophysics of the carotenoids associated with the xanthophyll cycle in photosynthesis. Photosynth. Res. 1994;41:389–395. doi: 10.1007/BF02183041. [DOI] [PubMed] [Google Scholar]
- 42.Avenson T.J., Ahn T.K., Zigmantas D., Niyogi K.K., Li Z. Zeaxanthin radical cation formation in minor light-harvesting complexes of higher plant antenna. J. Biol. Chem. 2008;283:3550–3558. doi: 10.1074/jbc.M705645200. [DOI] [PubMed] [Google Scholar]
- 43.Polivka T., Kerfeld C.A., Pascher T., Sundstrom V. Spectroscopic properties of the carotenoid 3′-hydroxyechinenone in the orange carotenoid protein from the cyanobacterium Arthrospira maxima. Biochemistry. 2005;44:3994–4003. doi: 10.1021/bi047473t. [DOI] [PubMed] [Google Scholar]
- 44.Larsen D.S., Papagiannakis E., van Stokkum I.H.M., Vengris M., Kennis J.T.M. Excited state dynamics of [β]-carotene explored with dispersed multi-pulse transient absorption. Chem. Phys. Lett. 2003;381:733–742. [Google Scholar]
- 45.Billsten H.H., Pan J.X., Sinha S., Pascher T., Sundstrom V. Excited-state processes in the carotenoid zeaxanthin after excess energy excitation. J. Phys. Chem. A. 2005;109:6852–6859. doi: 10.1021/jp052227s. [DOI] [PubMed] [Google Scholar]
- 46.Polívka T., Zigmantas D., Sundström V., Formaggio E., Cinque G. Carotenoid S1 state in a recombinant light-harvesting complex of photosystem II. Biochemistry. 2002;41:439–450. doi: 10.1021/bi011589x. [DOI] [PubMed] [Google Scholar]
- 47.de Weerd F.L., Kennis J.T.M., Dekker J.P., van Grondelle R. β-Carotene to chlorophyll singlet energy transfer in the photosystem I core of Synechococcus elongatus proceeds via the β-carotene S2 and S1 states. J. Phys. Chem. B. 2003;107:5995–6002. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.