Abstract
ATP acts on cellular membranes by interacting with P2X (ionotropic) and P2Y (metabotropic) receptors. Seven homomeric P2X receptors (P2X1–P2X7) and seven heteromeric receptors (P2X1/2, P2X1/4, P2X1/5, P2X2/3, P2X2/6, P2X4/6, P2X4/7) have been described. ATP treatment of Leydig cells leads to an increase in [Ca2+]i and testosterone secretion, supporting the hypothesis that Ca2+ signaling through purinergic receptors contributes to the process of testosterone secretion in these cells. Mouse Leydig cells have P2X receptors with a pharmacological and biophysical profile resembling P2X2. In this work, we describe the presence of several P2X receptor subunits in mouse Leydig cells. Western blot experiments showed the presence of P2X2, P2X4, P2X6, and P2X7 subunits. These results were confirmed by immunofluorescence. Functional results support the hypothesis that heteromeric receptors are present in these cells since 0.5 μM ivermectin induced an increase (131.2 ± 5.9%) and 3 μM ivermectin a decrease (64.2 ± 4.8%) in the whole-cell currents evoked by ATP. These results indicate the presence of functional P2X4 subunits. P2X7 receptors were also present, but they were non-functional under the present conditions because dye uptake experiments with Lucifer yellow and ethidium bromide were negative. We conclude that a heteromeric channel, possibly P2X2/4/6, is present in Leydig cells, but with an electrophysiological and pharmacological phenotype characteristic of the P2X2 subunit.
Keywords: P2X receptors, ATP, Leydig cells, Western blot, Immunofluorescence, Electrophysiology
Introduction
Several studies have shown that extracellular ATP acts as a modulator of function in different endocrine cell types, including Sertoli cells [16], spermatogonia [32], granulosa-luteal cells [47], and adrenocortical fasciculate cells [39]. In Leydig cells of rats and mice, ATP leads to an increase in intracellular calcium concentration ([Ca2+]i) and subsequent testosterone secretion [17, 40]. This effect is due to a calcium modulation of the functioning of the steroidogenic acute regulatory protein (StAR), which represents a limiting step of the steroidogenic process [34, 35]. Although production and secretion of testosterone are controlled mainly by the luteinizing hormone (LH) [37], the above observation supports the hypothesis that ATP is a positive modulator of the process of testosterone production and secretion in Leydig cells.
ATP acts on cellular membranes by interacting with purinergic P2X (ligand-gated ion channels) and P2Y (G protein-coupled) receptors. Seven P2X receptor subunits (P2X1–7) were cloned and characterized in vertebrates, and each of them can form two type of assemblies, homomeric, P2X1 [50], P2X2 [3], P2X3 [8], P2X4 [2], P2X5 [18], P2X7 [46], and P2X6 [23], or heterotrimeric P2X2/3 [31], P2X1/5 [48], P2X4/6 [30], P2X2/6 [27], P2X1/2 [5], P2X1/4 [38], and P2X4/7 [19]. Each subunit is composed by two transmembrane regions (TM1 and TM2) forming the channel pore, a large extracellular domain, which contains the ATP binding site and both N and C termini located intracellularly [14, 48, 22].
Each purinergic receptor assembly presents a particular functional profile [31, 27]. The pharmacological and biophysical properties of homomeric P2X receptors, characterized in heterologous expression systems, allowed their classification into three distinct groups: (1) α,β-methylene-ATP (αβmeATP) sensitive and rapidly desensitizing (P2X1 and P2X3); (2) αβmeATP insensitive and slowly desensitizing (P2X2, P2X4, P2X5); and (3) αβmeATP insensitive, slowly desensitizing and pore formation after long-time exposure to the agonist (P2X7) [15, 33]. On the other side, P2X6 receptors form homotrimeric assemblies only after an extensive glycosylation process. When expressed, they show αβmeATP sensitivity and are slowly desensitizing [23].
Heteromeric assemblies, in most cases, show properties more similar to a given P2X subunit present in the functional receptor, but not exactly equal to the homomeric receptor. Moreover, P2X subunits may have several isoforms, as a result of alternative splicing, which can form heteromeric channels with the same P2X subunits. Each new receptor thus formed has distinct properties when compared with homomeric assemblies [49, 9]. Until now splice variants have been described for P2X1, P2X2, P2X4, P2X5, and P2X7 purinergic receptor subunits [20, 9, 12, 13, 7].
Functional purinergic receptors are present in Leydig cells, as demonstrated through electrophysiological and pharmacological assays, suggesting the presence of a phenotype typical of P2X2 receptors [41]. In fact, the presence of purinergic receptors in Leydig cells was also proposed by Foresta et al. [17] and Perez-Armendariz et al. [40] based on measurements of [Ca2+]i, and also by the finding of P2X4 and P2X7 mRNAs [29]. Despite all these studies and the clear implication of purinergic receptors in Leydig cell function, there remains an open question as to the identity of the purinergic receptor subunits present in these cells.
In this paper, we investigated this issue by analyzing both molecular and electrophysiological properties of the receptors. Western blot and immunofluorescence results indicate the presence of P2X2, P2X4, P2X6, and P2X7 subunits, with distinct spatial distribution. The electrophysiological phenotype of the currents evoked by ATP and the effect of ivermectin on them indicate the presence of heteromers consisting of P2X2/4/6, resembling the properties of homomeric P2X2 subtype. From a physiological point of view the P2X receptors present in Leydig cells may serve as modulators of the production and secretion of testosterone, by allowing calcium influx into the cell.
Materials and methods
All protocols used in this study were approved by the Institutional Ethical Committee on Animal Experimentation of the School of Medicine of Ribeirão Preto—University of São Paulo (#030/2005).
Leydig cells
Freshly isolated Leydig cells were obtained from 50-day-old Swiss mice (∼45 g in weight), after cervical dislocation. The testes were rapidly removed, cleared of their tunica, and placed in Hank’s Balanced Salt Solution (HBSS) (Table 1). Cells were isolated following a basic protocol described by Kawa [24] and Carnio and Varanda [6] as follows: each decapsulated testis was carefully infused several times with HBSS with the aid of a syringe and a 25 × 7-gauge needle. Because Leydig cells are loosely placed in the interstitium between the seminiferous tubules, they were washed away and enter into suspension. For the electrophysiological experiments, they were seeded onto glass coverslips (3 mm × 3 mm) and transferred to an experimental chamber continuously perfused with external solution. Leydig cells readily adhere to glass and contaminant cells, mainly erythrocytes, are washed away by the solution flow. Under the microscope, they are easily recognized by their size, round shape, and presence of lipid droplets in the cytoplasm.
Table 1.
Bath (HBSS) and pipette solutions (mM)
| NaCl | KCl | CaCl2 | MgCl2 | HEPES | d-Glucose | EGTA | TEA | NaHCO3 | pHa | |
|---|---|---|---|---|---|---|---|---|---|---|
| HBSS | 140 | 4.6 | 1.6 | 1.13 | 10 | 10 | 5 | 7.4 | ||
| Pipetteb | 140 | 2 | 10 | 11 | 2 | 7.2 |
apH adjusted with NaOH (bath) or KOH (pipette)
bOsmolality = 308 mosm/kg H2O (Mark 3 Osmometer—Fiske Associates, Norwood, Massachusetts, USA)
Osmotic shock
This procedure was used when a cleaner preparation and a small number of Leydig cells were needed, as in the immunofluorescence experiments. Since most of the contaminating cells are red cells, which are easily disrupted in hypotonic solution, we decided to treat the cell suspension, obtained as described above, with a hyposmotic shock. To this end, the cell suspension was initially centrifuged at 800×g for 3 min and resuspended in a known volume of deionized water for 20 s. After that, a given volume of 10× concentrated HBSS was added to bring osmolality back to control conditions. Cells were centrifuged again at 900×g for 5 min, resuspended and seeded on coverslips. The efficiency of this process was confirmed by cytochemical detection of the enzyme 3β-HSD as described elsewhere [28].
Western blots
The expression of purinergic receptors P2X1–7 in Leydig cells was detected by Western blot analysis. Since a large number of high purity Leydig cells are needed for these experiments, we used 20 testes in each preparation. After decapsulation, they were treated for 10 min with 0.4 mg/ml collagenase (Type II; Worthington Biochemical Corporation, Lakewood, NJ, USA) and the tubules and cells dispersed by several cycles of suction and ejection with the aid of a Pasteur pipette. Afterwards, Leydig cells were purified from the suspension by centrifugation in a discontinuous Percoll gradient, as described by Schumacher et al. [44]. After purification, they were suspended in radioimmunoprecipitation assay (RIPA) buffer (750 mM NaCl, 250 mM Tris pH 7.5, 5% NP-40, 2.5% sodium deoxycholate, 0.5% SDS, 0.5% Triton X-100) containing a cocktail of protease and phosphatase inhibitors (Sigma Fast Protease Inhibitor), diluted 1:100, respectively. After that, cells were sonicated on an ice bath for 30 s, centrifuged at 10,000×g for 5 min at 4°C and cell lysates collected from the supernatant fractions. Protein concentration was determined using a Bradford Assay Kit (Bio-Rad Laboratories, Hercules, CA, USA). Each sample of 100 μg of extracted protein was separated by SDS-PAGE 12%. Proteins were then transferred to nitrocellulose membranes (GE Healthcare Bio-Sciences Corp., Piscataway, NJ, USA) by Bio-Rad Trans-Blot SD semidry transfer cell (Bio-Rad Laboratories Inc., Richmond, CA, USA). Membranes were blocked with 5% BSA in TBST (100 mM Tris–HCl, pH 7.5, 100 mM NaCl, 0.1% Tween 20) for 1 h, and then incubated for 1 h with anti-P2X1–7 (1:400) polyclonal antibodies (Alomone Labs Ltd., Jerusalem, Israel) diluted in 5% BSA in TBST. After washing, membranes were incubated for 1 h with goat anti-rabbit IGg conjugated to horseradish peroxidase, diluted in TBST. Finally, the immune complexes were developed with an enhanced chemiluminescence method (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and the membranes were then immediately exposed to autoradiographic film (Hyperfilm, Amersham Bioscience, UK).
Immunofluorescence
Leydig cells, isolated by mechanical dispersion and cleaned by the osmotic shock described above, were seeded on coverslips (13 mm diameter) pre-treated with 2% Biobond (Biobond-Tissue Adhesive, Electron Microscopy Sciences, Hatfield, PA, USA), fixed in 4% formaldehyde in 0.01 M PBS (tablets; Sigma Chemical Co., St. Louis, MO, USA) for 15 min and blocked with 0.01 M PBS, 0.5% Triton X-100, 1% BSA and 5% goat serum for 60 min. The coverslips were incubated 120 min with the same primary antibodies anti-P2X1–7 (1:200; rabbit) and anti-calreticulin (Chemicon International, Temecula, California, USA; 1:200; chicken) diluted with 0.01 M PBS, 0.5% Triton X-100, 1% BSA. Then, the cells were incubated with secondary antibodies Alexa Fluor 488 (goat, anti-rabbit) and 594 (goat, anti-chicken; Molecular Probes Inc., Eugene, USA) for 60 min, both in a dilution of 1:800. All incubations were done at room temperature and cells were washed three times in PBS for 5 min before each incubation. Finally, cells were washed with deionized water and mounted onto glass slides with Prolong (Invitrogen Corporation, Carlsbad, CA, USA). Immunofluorescence analysis was made with the aid of a confocal microscope (TCS SP5, Leica, Bensheim, Germany). The images were analyzed using the software Image J (W. S. Rasband/National Institutes of Health, Bethesda, MD, USA).
Dye uptake
Leydig cells were isolated by mechanical dispersion, purified by osmotic shock and seeded on Petri dishes. ATP application is known to cause permeabilization in cells that express P2X7 purinergic receptors. To confirm that P2X7 receptors are functional in Leydig cells, uptake of the fluorescent dyes Lucifer yellow (5 mM; Sigma Chemical Co., St. Louis, MO, USA) and ethidium bromide (10 μM; USB Corporation, Cleveland, OH, USA) was tested by incubating cells in the absence or presence of 5 mM ATP diluted in HBSS. Following a 10-min incubation at room temperature, cells were visualized in the confocal microscope. Cells incubated with Lucifer yellow were washed twice with HBSS before visualization.
Electrophysiological recordings
For electrophysiological experiments, Leydig cells were isolated by simple mechanical dispersion and seeded on glass coverslips (3 mm × 3 mm). Whole-cell voltage-clamp recording was carried out at room temperature (23–25°C) using an Axopatch 200B amplifier (Axon Instruments, Foster City, CA, USA). Membrane potential was held at −50 mV. Recording electrodes (resistance 2–4 MΩ) were filled with pipette solution (Table 1) and had resistance around 3 MΩ. Access resistance (9.9 ± 0.26 MΩ, n = 218) and cell capacitance (22.07 ± 0.51 pF) were electronically corrected using the amplifier settings (mean ± SEM). The bath solution was HBSS. Data were acquired using pCLAMP10 software (Molecular Devices, Sunnyvale, CA, USA). Signals were filtered at 2 kHz, and then digitized at 5 kHz (Digidata 1440A, Molecular Devices, Sunnyvale, CA, USA). Traces were analyzed using Clampfit 10 and/or Origin 7 (Microcal, Northampton, MA, USA). Solutions were delivered by gravity from independent reservoirs using a RSC160 rapid solution changer (Biologic Science Instruments, Claix, France). One barrel was used to apply drug-free solution to enable rapid termination of drug applications. ATP was separately applied for 6 s at 3 min intervals, a washing time sufficient for the responses to be reproducible. Ivermectin was present for 3 min before and during the reapplication of ATP (after one control application). After application of ATP with ivermectin cells were washed for 5 min to remove the drug.
Drugs
Triton X-100 is from Beckman Instruments (Fullerton, CA, USA), ATP, ivermectin, and all other reagents were obtained from Sigma Chemical Co. (St. Louis, MO, USA). Solutions of ATP and other drugs were prepared using deionized water and stored frozen, except for ivermectin, which was dissolved in dimethylsulfoxide (DMSO) to 10 mM. The final concentration of DMSO in the bathing solutions was always <0.01%. All drugs were diluted in the extracellular solution to the desired final concentration.
Data analysis
All whole-cell recordings were normalized to the current evoked by 100 μM ATP in the same cell. Data are expressed as mean ± SEM and statistical significance was tested by the Wilcoxon signed rank test using R (version 2.5.1, 2007-06-27, Copyright (C), The R Foundation for Statistical Computing, ISBN 3-900051-07-0).
Results
Partial purification of Leydig cells by osmotic shock
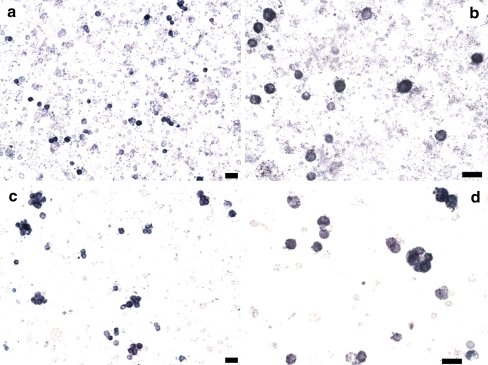
In order to check if the osmotic shock procedure leads to enrichment of the preparation in Leydig cells we performed cytochemical detection of the enzyme 3β-HSD. As can be seen in Fig. 1a and b, the preparation clearly contains Leydig cells (marked in deep blue), but a number of other cells, mainly red blood cells and spermatozoa, are also present. After the osmotic shock (Fig. 1c and d), much of the contaminating cells disappear, making Leydig cells more evident. This protocol was used thereafter for cell isolation in the immunofluorescence experiments, where a small number of cells is required.
Fig. 1.
Cytochemical detection of 3β-HSD on cell suspensions before and after hyposmotic shock. a and b The cell suspension was obtained by mechanical dispersion only and stained for 3β-HSD. c and d Mechanically isolated cells were also subjected to a hyposmotic shock and then stained for 3β-HSD. Leydig cells are marked in intense blue. Scale bar 20 μm
Molecular identification of P2X receptors in mouse Leydig cells
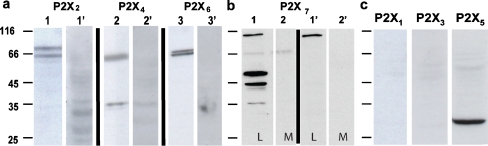
Western Blot experiments were performed in order to detect P2X receptor protein expression in lysates of Leydig cells using subunit specific antibodies against P2X1–7 receptors. As shown in Fig. 2a, bands were marked at a molecular weight around 66 kDa, corresponding to the glycosylated form of the P2X2, P2X4, and P2X6 subunits. As far as P2X7 is concerned, more than one band was marked (Fig. 2b). The band around 75 kDa corresponds to the usual marker for the glycosylated P2X7 receptor. This is supported by the fact that the same band is also disclosed in lysates of macrophages, used as positive control. Nevertheless, smaller molecular weight bands (around 45 and 53 kDa) are also present, probably representing truncated splice variants of P2X7. Alternatively, Leydig P2X7 may have undergone partial degradation. In all cases, the bands are specific for the subunits described above, since pre-absorption of the antibodies with the corresponding cognate peptides knocked down their expression. Figure 2c shows that subunits P2X1, P2X3, and P2X5 are not detected in Leydig cells.
Fig. 2.
Western Blot analysis of purinergic receptors P2X1–7. a Western blot analysis of lysates of mouse Leydig cells performed with specific antibodies against rat P2X2 (lane 1), P2X4 (lane 2), and P2X6 (lane 3) receptors. Positively marked bands are seen at ∼66 kDa corresponding to these subunits. These same bands are absent in the experiments where the antibodies were pre-incubated with their respective cognate peptides (lane 1′, lane 2′, and lane 3′ for P2X2, P2X4, and P2X6, respectively). b P2X7 receptors were recognized in both Leydig cells (lane 1) and macrophages (lane 2). Again, the reaction is specific because pre-incubation of the antibody with the cognate peptide resulted in negative results (lane 1′ and lane 2′, respectively). As expected there are bands marked around 70 kDa, for both Leydig cells and macrophages used as positive control. Besides this, there are three other bands in Leydig cells between 35 and 66 kDa. c Antibodies against P2X1, P2X3, and P2X5 receptors did not show any positively marked band. Numbers in the left side of the blots are molecular weight markers
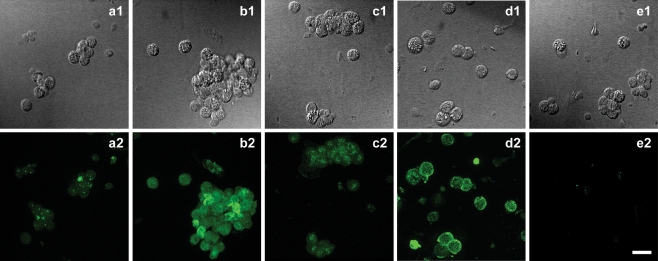
The Western Blot results described above were corroborated by immunofluorescence experiments. Figure 3 shows immunofluorescence labeling due to P2X2, P2X4, P2X6, and P2X7 receptor subunits (a2, b2, c2, and d2, respectively). Figure 3e2 is a negative control where the primary antibody was omitted during the incubation, indicating specificity of the antibodies used. The fluorescence exhibited by P2X1, P2X3, and P2X5 do not differ from the control experiment. These receptors are absent in Leydig cells corroborating the Western Blot results.
Fig. 3.
Immunofluorescence detection of purinergic receptors. Differential interference contrast (DIC) microphotography of Leydig cells (a1, b1, c1, and d1) and respective immunofluorescence labeling of P2X2 (a2), P2X4 (b2), P2X6 (c2), and P2X7 (d2) subunits. e1 is a DIC image of cells and e2 the respective immunofluorescence labeling of control experiment, performed in the absence of primary antibodies (e2). Scale bar 20 μm
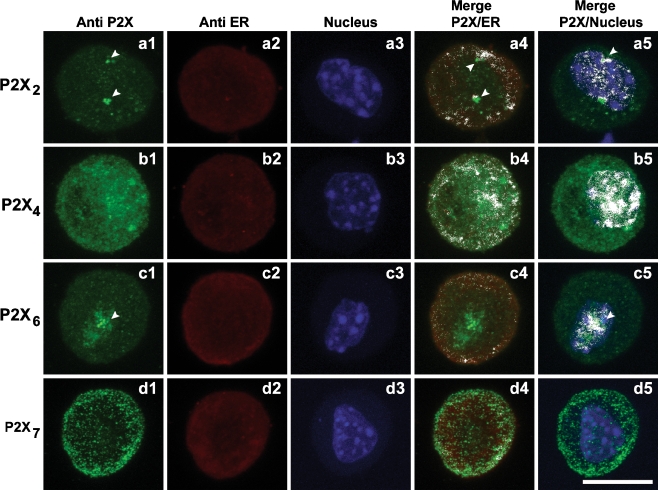
The cellular distribution of P2X2, P2X4, P2X6, and P2X7 subunits is particular for each type of receptor as shown in Fig. 4 (a1, b1, c1, and d1, respectively). Colocalization with the endoplasmic reticulum (ER) was detected by staining calreticulin and with the nucleus by staining with DAPI. The Manders Overlap Coefficient (MOC) was used as an index of colocalization and calculated using Image J. As can be seen in the merged images (Fig. 4a4 and a5), P2X2 subunits colocalize with the ER (MOC for P2X2-ER = 0.699) and with the nucleus (MOC P2X2-NC = 0.660). They also form clusters as indicated by the arrow heads. Figure 4b4 and b5 show the merged images for the P2X4 subunit with respect to the ER (MOC for P2X4-ER = 0.927) and nucleus (MOC for P2X4-CN = 0.750), respectively. The P2X6 receptor subunit shows the most clustered distribution with MOC for P2X6-ER = 0.816 (Fig. 4c4) and MOC for P2X6-CN = 0.778 (Fig. 4c5). P2X7 subunits also colocalize with the ER (MOC for P2X7-ER = 0.818) and less strongly with the nucleus (MOC P2X7-NC = 0.607). Figure 4d4 and d5 show the merged images for the P2X7 subunit with respect to the ER and nucleus. This indicates that most of these subunits colocalizing with the ER are not functional.
Fig. 4.
Immunofluorescence detection of purinergic receptors. Immunofluorescence cellular localization of P2X2 (a1), P2X4 (b1), P2X6 (c1) and P2X7 (d1) subunits. Colocalization of purinergic receptors and endoplasmatic reticulum (ER) by Immunofluorescent staining of ER and respective merge with P2X2 (a2; a4), P2X4 (b2; b4), P2X6 (c2; c4), and P2X7 (d2; d4) subunits. Colocalization of purinergic receptors and cell nucleus (CN) by immunofluorescent staining of CN and respective merge with P2X2 (a3; a5), P2X4 (b3; b5), P2X6 (c3; c5), and P2X7 (d3; d5) subunits. White arrow heads indicate clusters of P2X subunits. P2X2 colocalized with ER and CN and P2X6 with the nucleus. White color on merged images indicates colocalization points. P2X7 is the only subunit that do not colocalize with CN. Scale bar 10 μm
Functional profile of P2X receptors in mouse Leydig cells
To elucidate which receptor subunits are functional in Leydig cells we performed two types of experiments: fluorescent dye uptake for testing the P2X7 receptor and the effect of ivermectin on the ATP-activated currents to look at P2X2, P2X4, and P2X6 receptors.
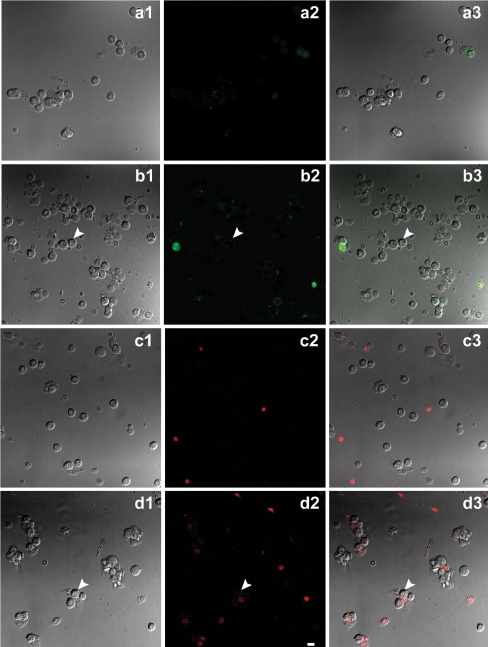
Figure 5 shows that treatment of Leydig cells with 5 mM ATP for 10 min did not induce any uptake of either Lucifer yellow (Fig. 5a1, a2, and a3) or ethidium bromide (Fig. 5b1, b2, and b3) in intact cells, as indicted by the arrows in Fig. 5. There is only a small fluorescent staining in damaged cells. Therefore, although present in Leydig cells, P2X7 receptors are not functional under the present conditions.
Fig. 5.
Fluorescent dye uptake by Leydig cells. DIC image of cells (a1, b1), and respective visualization of Lucifer yellow fluorescence in cells before and after incubation with 5 mM ATP for 10 min (a2, b2, respectively), and merge (a3, b3). DIC image (c1, d1) and respective ethidium bromide fluorescence in cells before and after incubation with 5 mM ATP for 10 min (c2, d2, respectively), and merge (c3, d3). Intact cells do not present fluorescent staining for both dyes (white arrows) after incubation with ATP. Scale bar 20 μm
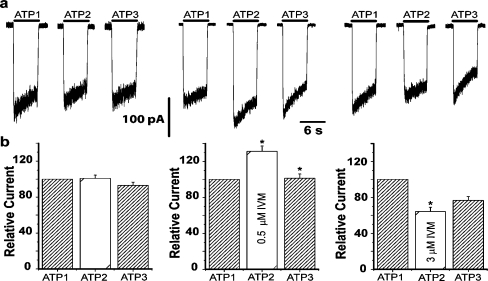
As shown in Fig. 6a, application of 100 μM ATP to the Leydig cells induces an inward current (at −50 mV holding potential) which slowly desensitizes, as already described [41]. Washing the preparation for 3 min restores the response to control level (Fig. 6a and b, left panels). On the other hand, 0.5 μM ivermectin causes an increase (131.2 ± 5.9%; mean ± SE, n = 12, p < 0.05) in the ATP elicited current (Fig. 6a and b, middle panels) while 3 μM ivermectin leads to a significant reduction in the ATP current (64.2 ± 4.8% mean ± SE, n = 19, p < 0.05, Fig. 6a and b, right panels). These results indicate that the phenotype of the currents evoked by ATP can be determined by heteromers containing P2X4 subunits.
Fig. 6.
Effect of ivermectin on ATP-activated currents. a Representative recordings of inward currents evoked by sequential applications of 100 μM ATP for 6 s. The holding potential was −50 mV in all cases. The left panel shows control traces in response to three applications of ATP (ATP1, ATP2, and ATP3), indicating that desensitization of the receptor is removed under these conditions. The middle panel refers to the application of ATP in the presence of 0.5 μM ivermectin (ATP2). There is a clear increase in the current amplitude in relation to control (ATP1) which is reversed upon wash out of IVM. The right panel shows ATP-evoked current before (ATP1) and after incubation with 3 μM IVM. A small decrease in the amplitude of the ATP-evoked current (ATP2) is now observed, which is partially reversed upon wash out of the drug (ATP3). b Bar graphs of the peak current amplitudes evoked by applications of ATP in control conditions (left panel, n = 17 cells), after incubation with 0.5 μM IVM (middle panel, n = 10 cells), and after incubation with 3 μM IVM (right panel, n = 11 cells). Numbers are normalized to the first control condition taken as 100%. Bars represent mean ± SEM (*p < 0.05, Wilcoxon test). Washout interval between second and third applications was 3 and 5 min, respectively
Taken together our results indicate the presence of multiple receptor subtypes in Leydig cells. Our main finding is that the receptor may be formed by a heteromeric assembly of P2X2, P2X4, and P2X6 subunits with an electrophysiological and pharmacological phenotype mainly determined by the P2X2 subunit.
Discussion
Leydig cells are easily isolated using a mechanical dispersion method. However, for immunofluorescence experiments it is easier to localize these cells when the preparation is made free of other cell types. To achieve a preparation with lower contamination, we associated mechanical dispersion with a hyposmotic shock. This new procedure results in a less-contaminated Leydig cell preparation. This conclusion is confirmed by the results shown on Fig. 1, where the cells were recognized by cytochemical detection of the enzyme 3β-HSD.
Purinergic receptors in mouse Leydig cells show fast activation and slow desensitization, mostly resembling the properties of homomeric P2X2 ionotropic receptors [41]. However, the Western Blot results show the presence of bands for four different subtypes of purinergic receptors, P2X2, P2X4, P2X6, and P2X7 (Fig. 2a, lanes 1, 2, and 3; b, lane 1; respectively). We also find three other bands marked by the anti-P2X7 antibody, with lower molecular weights. In this experiment, positive control with macrophage cells, which have endogenous expression of P2X7 homomeric receptors [11], confirms the band corresponding to the P2X7 subunit at approximately 70 kDa. Therefore, Leydig cells endogenously express the classical P2X7 subunits and other P2X7 subunits probably derived from alternative splice. It is known that expression of alternatively spliced subunits does not always result in functional channels, almost certainly because of lacking of protein segments important for their function [12, 7]. Seven isoforms of alternatively spliced human P2X7 purinergic receptors were already described. For mice P2X7 receptors, four isoforms were described, “a”, “b”, “c”, and “d” (NCBI respective numbers: NP 035157, NP 001033934, NP 001033928, and NP 001033976). The larger isoform “a” is 99% similar to mice P2X7 subunit and the other three are not recognized by antibody used for Western Blot experiments.
The immunofluorescence experiments corroborated the Western Blot results, showing staining for anti-P2X2, anti-P2X4, anti-P2X6, and anti-P2X7 antibodies (Fig. 3, a2, b2, c2, and d2, respectively). Each subunit shows a distinct distribution and colocalization with the endoplasmatic reticulum (ER) and the cell Nucleus (CN). Staining of the P2X2 subunit reveals a sparse distribution and appears as agglomerates of fluorescent points colocalized with both ER and CN (Fig. 4a). The P2X6 subunit agglomerates are mainly colocalized with the CN (Fig. 4c). The agglomerated points observed for both P2X2 and P2X6 subunits represent “clusters” of more than three equal purinergic subunits, which do not form functional channels. These clusters stay in the ER and are not incorporated into the plasma membrane [1]. This point is particularly relevant for the P2X6 subunits, which requires an extensive glycosylation process in order to form functional homotrimeric channels [23]. The P2X4 subunit is colocalized with the ER and CN, showing a dense and uniform distribution (Fig. 4b4 and b5). The P2X7 subunit appears as stained points distributed mainly close to the membrane and also colocalized with the ER (Fig. 4d4 and d5). Since all subunits show colocalization with the ER and, in this compartment they are non-functional, we can assume that this may represent a modulatory step for the receptors expression in the plasma membrane.
Despite the fact that purinergic receptors present in Leydig cells show functional properties consistent with homomeric P2X2 receptors [41], our present results suggest that P2X2, P2X4, P2X6, and P2X7 subunits can interact to form heteromeric channels. To elucidate the assembly of functional purinergic receptors, we analyzed other functional properties of these receptors. P2X7 homomeric receptors show singular properties in comparison to other purinergic subunits. Pharmacologically, the main agonist for native mice receptors is not ATP (EC50 = 734 μM), like other subunits, but the analog BzATP (EC50 = 90 μM) [10]. Leydig cells show an EC50 for ATP around 44 μM [41], which are not consistent with the presence of homomeric P2X7 receptors. Moreover, the necessity of millimolar concentrations of ATP to stimulate these channels in vivo poses a question about the possible physiological role of these receptors [21]. Another singular property of these receptors is that they have a large pore-forming phenotype specific for P2X7 after long-term stimulation with the agonist, allowing large molecules (>900 Da) to pass [46]. Our results on dye uptake in intact Leydig cells are negative for both Lucifer yellow (457.24 Da; Fig. 5a and b) and ethidium bromide (394.31 Da; Fig. 5c and d). Only damaged cells show some fluorescence for both dyes. These results are consistent with the absence of functional P2X7 receptors in Leydig cells under our experimental conditions.
Ivermectin is known to act only on P2X4 receptors by binding to two distinct sites. The higher-affinity binding site (EC50 = 0.25 µM) is able to increase the maximal ATP-activated currents without changing the ATP concentration–response relationship. The action at the lower affinity binding site (EC50 = 2 µM) results in a slower deactivation rate and enhancement of the ATP affinity [43]. Moreover, ivermectin does not act on P2X2, P2X3, P2X2/3, and P2X7 receptors but does so on heteromeric channels containing the P2X4 subunit [25, 4, 19]. After ATP stimulation of Leydig cells, we observed a 30% non-reversible increased in the peak currents subsequent to the incubation with 0.5 μM ivermectin (Fig. 6a and b, middle panel). However, a reversible decrease of the peak currents, amounting to 35%, was detected by using 3 μM ivermectin (Fig. 6a and b, right panel). The increase in current supports the idea that P2X4 subunit is part of the receptor. Furthermore, the current decrease, which was not expected if only P2X4 subunits were present, indicates that other subunits are involved in the receptor assembly [43]. Conversely, if P2X4/7 heteromeric receptors were present, we should have observed an increase on the peak currents after 3 min of incubation with 3 μM ivermectin, a fact not seen in our results [4, 19]. Heteromeric P2X4/6 receptors show high affinity for αβMeATP (threshold = 10 μM) [25]. Nevertheless, purinergic receptors in mouse Leydig cells do not present purinergic currents after stimulation with 100 μM αβMeATP [41]. Therefore, we can exclude the possibility of functional P2X4/6 heteromeric receptors in Leydig cells.
The presence of homomeric P2X2 receptors can also be detected by observing the response to ATP at different extracellular pHs. P2X2 homomeric receptors show only an increase in peak currents when pH is changed from control (pH 7.4) to a pH of 6.5 [26, 45, 51]. In addition, pH variations to 5.5 caused an increase in P2X2 peak currents but a decrease on P2X2/6 peak currents [27]. In mouse Leydig cells, bringing the pH to 5.5 induced a decrease on peak currents [41], corroborating the hypothesis that functional purinergic receptors are formed by P2X2 and P2X6 subunits. In summary, our results suggest that purinergic receptors in mouse Leydig cells are heteromers of P2X2/4/6 subunits, although showing a dominant functional profile of P2X2 subunits [41]. Similar results were seen on Purkinje neurons of postnatal rats [36]. We also show that P2X7 subunits, probably alternatively spliced variants, are present in mouse Leydig cells but certainly non-functional.
From a physiological perspective, it should be noted that LH leads to testosterone secretion in Leydig cells by increasing the intracellular concentrations of cAMP and calcium. Although the levels of intracellular calcium ions can be controlled in other ways, Foresta et al. [17] and Perez-Armendariz et al. [40] have shown that ATP induces an increase in testosterone production and secretion coupled to an increase in the intracellular calcium concentration. These results support the hypothesis that ATP may play a modulatory role, either autocrine and/or paracrine, on this process. In keeping with this hypothesis, our laboratory has recently shown the presence of volume-activated chloride channels in Leydig cells, which may serve as a pathway for ATP release [42].
Acknowledgments
We thank Mr. José Fernando Aguiar for excellent technical assistance and Ana Leticia G.C. Maragno for help with the Western blots. Confocal microscopy was performed in the “Laboratório de Microscopia Confocal da Faculdade de Medicina de Ribeirão Preto—USP”. This work was supported by grants from Fundação de Amparo a Pesquisa do Estado de São Paulo (FAPESP # 06/50954-7). LSA had a fellowship from CNPq.
References
- 1.Aschrafi A, Sadtler S, Niculescu C, Rettinger J, Schmalzing G (2004) Trimeric architecture of homomeric P2X2 and heteromeric P2X1+2 receptor subtypes. J Mol Biol 342:333–343 doi:10.1016/j.jmb.2004.06.092 [DOI] [PubMed]
- 2.Bo X, Zhang Y, Nassar M, Burnstock G, Schoepfer R (1995) A P2X purinoceptor cDNA conferring a novel pharmacological profile. FEBS Lett 375:129–133 doi:10.1016/0014-5793(95)01203-Q [DOI] [PubMed]
- 3.Brake AJ, Wagenbach MJ, Julius D (1994) New structural motif for ligand-gated ion channel defined by ionotropic ATP receptor. Nature 371:519–523 doi:10.1038/371519a0 [DOI] [PubMed]
- 4.Brône B, Moechars D, Marrannes R, Mercken M, Meert T (2007) P2X currents in peritoneal macrophages of wild type and P2X4 −/− mice. Immunol Lett 113:83–89 doi:10.1016/j.imlet.2007.07.015 [DOI] [PubMed]
- 5.Brown SG, Townsend-nicholson A, Jacobson KA, Burnstock G, King BF (2002) Heteromultimeric P2X1/2 receptors show a novel sensitivity to extracellular pH. J Pharmacol Exp Ther 300:673–680 doi:10.1124/jpet.300.2.673 [DOI] [PMC free article] [PubMed]
- 6.Carnio EC, Varanda WA (1995) Calcium-activated potassium channels are involved in the response of mouse Leydig cells to human chorionic gonadotropin. Braz J Med Biol Res 28:813–824 [PubMed]
- 7.Cheewatrakoolpong B, Gilchrest H, Anthes JC, Greenfeder S (2005) Identification and characterization of splice variants of the human P2X7 ATP channel. Biochem Biophys Res Commun 332(1):17–27 doi:10.1016/j.bbrc.2005.04.087 [DOI] [PubMed]
- 8.Chen CC, Akopian AN, Sivilotti L, Colquhoun D, Burnstock G, Wood JN (1995) A P2X purinoceptor expressed by a subset of sensory neurons. Nature 377(6548):428–431 doi:10.1038/377428a0 [DOI] [PubMed]
- 9.Chen CC, Parker MS, Barnes AP, Deininger P, Bobbin RP (2000) Functional expression of three P2X(2) receptor splice variants from guinea pig cochlea. J Neurophysiol 83:1502–1509 [DOI] [PubMed]
- 10.Chessel IP, Michel AD, Humphrey PPA (1998) Effects of antagonists at the human recombinant P2X7 receptor. Br J Pharmacol 124:1314–1320 doi:10.1038/sj.bjp.0701958 [DOI] [PMC free article] [PubMed]
- 11.Coutinho-Silva R, Persechini PM, Bissagio RD, Perfettini JL, Neto AC, Kanellopoulos JM, Motta-ly I, Dautry-varsat A, Ojcius DM (1999) P2Z/P2X7 receptor-dependent apoptosis of dendritic cells. Am J Physiol 276(5 Pt 1):C1139–C1147 [DOI] [PubMed]
- 12.Dhulipala PD, Wang YX, Kotlikoff MI (1998) The human P2X4 receptor gene is alternatively spliced. Gene 207:259–266 doi:10.1016/S0378-1119(97)00647-1 [DOI] [PubMed]
- 13.Duckwitz W, Hausmann R, Aschrafi A, Schmalzing G (2006) P2X5 subunit assembly requires scaffolding by the second transmembrane domain and a conserved aspartate. J Biol Chem 281:39561–39572 doi:10.1074/jbc.M606113200 [DOI] [PubMed]
- 14.Egan TM, Haines WR, Voigt MM (1998) A domain contributing to the ion channel of ATP-gated P2X2 receptors identified by the substituted cysteine accessibility method. J Neurosci 18:2350–2359 [DOI] [PMC free article] [PubMed]
- 15.Evans RJ, Lewis C, Buell G, Valera S, North RA, Surprenant A (1995) Pharmacological characterization of heterologously expressed ATP-gated cation channels (P2X purinoceptors). Mol Pharmacol 48:178–183 [PubMed]
- 16.Filippini A, Riccioli A, de Cesaris P, Paniccia R, Teti A, Stefanini M, Conti M, Ziparo E (1994) Activation of inositol phospholipid turnover and calcium signaling in rat Sertoli cells by P2-purinergic receptors: modulation of follicle-stimulating hormone responses. Endocrinology 134(3):1537–1545 doi:10.1210/en.134.3.1537 [DOI] [PubMed]
- 17.Foresta C, Rossato M, Nogara A, Gottardello F, Bordon P, di Virgilio F (1996) Role of P2-purinergic receptors in rat Leydig cell steroidogenesis. Biochem J 320:499–504 [DOI] [PMC free article] [PubMed]
- 18.Garcia–Guzman M, Soto F, Laube B, Stühmer W (1996) Molecular cloning and functional expression of a novel rat heart P2X purinoceptor. FEBS Lett 388:123–127 doi:10.1016/0014-5793(96)00499-1 [DOI] [PubMed]
- 19.Guo C, Masin M, Qureshi OS, Murrell-Lagnado RD (2007) Evidence for functional P2X4/P2X7 heteromeric receptors. Mol Pharmacol 72:1447–1456 doi:10.1124/mol.107.035980 [DOI] [PubMed]
- 20.Hardy LA, Harvey IJ, Chambers P, Gillespie JI (2000) A putative alternatively spliced variant of the P2X(1) purinoreceptor in human bladder. Exp Physiol 85:461–463 doi:10.1017/S0958067000003572 [DOI] [PubMed]
- 21.Hibell AD, Kidd EJ, Chessell IP, Humphrey PPA, Michel AD (2000) Apparent species differences in the kinetic properties of P2X7 receptors. Br J Pharmacol 130:167–173 doi:10.1038/sj.bjp.0703302 [DOI] [PMC free article] [PubMed]
- 22.Jiang LH, Rassendren F, Spelta V, Surprenant A, North RA (2001) Amino acid residues involved in gating identified in the first membrane-spanning domain of the rat P2X2 receptor. J Biol Chem 276:14902–14908 doi:10.1074/jbc.M011327200 [DOI] [PubMed]
- 23.Jones CA, Vial C, Sellers LA, Humphrey PPA, Evans RJ, Chessell IP (2004) Functional regulation of P2X6 receptors by N-linked glycosylation: identification of a novel αβ-methylene ATP-sensitive phenotype. Mol Pharmacol 65:979–985 doi:10.1124/mol.65.4.979 [DOI] [PubMed]
- 24.Kawa K (1987) Existence of calcium channels and intercellular couplings in the testosterone-secreting cells of the mouse. J Physiol 393:647–666 [DOI] [PMC free article] [PubMed]
- 25.Khakh BS, Bao XR, Labarca C, Lester HA (1999) Neuronal P2X transmitter-gated cation channels change their ion selectivity in seconds. Nat Neurosci 2(4):322–330 doi:10.1038/7233 [DOI] [PubMed]
- 26.King BF, Ziganshina LE, Pintor J, Burnstock G (1996) Full sensitivity of P2X2 purinoceptor to ATP revealed by changing extracellular pH. Br J Pharmacol 117(7):1371–1373 [DOI] [PMC free article] [PubMed]
- 27.King BF, Townsend-Nicholson A, Wildman SS, Thomas T, Spyer KM, Burnstock G (2000) Coexpression of rat P2X2 and P2X6 subunits in Xenopus oocytes. J Neurosci 20:4871–4877 [DOI] [PMC free article] [PubMed]
- 28.Klinefelter GR, Hall PF, Ewing LL (1987) Effect of luteinizing hormone deprivation in situ on steroidogenesis of rat Leydig cells purified by a multistep procedure. Biol Reprod 36:769–783 doi:10.1095/biolreprod36.3.769 [DOI] [PubMed]
- 29.Ko WH, Au CL, Yip CY (2003) Multiple purinergic receptors lead to intracellular calcium increases in cultured rat Sertoli cells. Life Sci 72:1519–1535 doi:10.1016/S0024-3205(02)02410-4 [DOI] [PubMed]
- 30.Le KT, Babinski K, Séguéla P (1998) Central P2X4 and P2X6 channel subunits coassemble into a novel heteromeric ATP receptor. J Neurosci 18:7152–7159 [DOI] [PMC free article] [PubMed]
- 31.Lewis C, Neidhart S, Holy C, North RA, Buell G, Surprenant A (1995) Coexpression of P2X2 and P2X3 receptor subunits can account for ATP-gated currents in sensory neurons. Nature 377:432–435 doi:10.1038/377432a0 [DOI] [PubMed]
- 32.Loir M (1999) Spermatogonia of rainbow trout: III. In vitro study of the proliferative response to extracellular ATP and adenosine. Mol Reprod Dev 53(4):443–450 doi:10.1002/(SICI)1098-2795(199908)53:4<443::AID-MRD10>3.0.CO;2-7 [DOI] [PubMed]
- 33.Mackenzie AB, Surprenant A, North RA (1999) Functional and molecular diversity of purinergic ion channel receptors. Ann N Y Acad Sci 868:716–729 doi:10.1111/j.1749-6632.1999.tb11351.x [DOI] [PubMed]
- 34.Manna PR, Kero J, Tena-Sempere M, Pakarinen P, Stocco DM, Huhtaniemi IT (2001) Assessment of mechanisms of thyroid hormone action in mouse Leydig cells: regulation of the steroidogenic acute regulatory protein, steroidogenesis, and luteinizing hormone receptor function. Endocrinology 142(1):319–331 doi:10.1210/en.142.1.319 [DOI] [PubMed]
- 35.Manna PR, Wang XJ, Stocco DM (2003) Involvement of multiple transcription factors in the regulation of steroidogenic acute regulatory protein gene expression. Steroids 68(14):1125–1134 doi:10.1016/j.steroids.2003.07.009 [DOI] [PubMed]
- 36.Mateo J, Garcia-Lecea M, Miras-Portugal MT, Castro E (1998) Ca2+ signals mediated by P2X-type purinoceptors in cultured cerebellar Purkinje cells. J Neurosci 18:1704–1712 [DOI] [PMC free article] [PubMed]
- 37.Miller WL (1988) Molecular biology of steroid hormone synthesis. Endocr Rev 9:295–318 [DOI] [PubMed]
- 38.Nicke A, Kerschensteiner D, Soto F (2005) Biochemical and functional evidence for heteromeric assembly of P2X1 and P2X4 subunits. J Neurochem 92:925–933 doi:10.1111/j.1471-4159.2004.02939.x [DOI] [PubMed]
- 39.Nishi H, Kato F, Masaki E, Kawamura M (2002) ADP-sensitive purinoceptors induce steroidogenesis via adenylyl cyclase activation in bovine adrenocortical fasciculata cells. Br J Pharmacol 137(2):177–184 doi:10.1038/sj.bjp.0704847 [DOI] [PMC free article] [PubMed]
- 40.Perez-Armendariz EM, Nadal A, Fuentes E, Spray DC (1996) Adenosine 5′-triphosphate (ATP) receptors induce intracellular calcium changes in mouse Leydig cells. Endocrine 4:239–247 doi:10.1007/BF02738690 [DOI] [PubMed]
- 41.Poletto-Chaves LA, Pontelli EP, Varanda WA (2006) P2X receptors in mouse Leydig cells. Am J Physiol Cell Physiol 290:1009–1017 doi:10.1152/ajpcell.00506.2005 [DOI] [PubMed]
- 42.Poletto-Chaves LA, Varanda WA (2008) Volume-activated chloride channels in mice Leydig cells. Pflugers Arch. doi:10.1007/s00424-008-0525-2 [DOI] [PubMed]
- 43.Priel A, Silberberg SD (2004) Mechanism of ivermectin facilitation of human P2X4 receptor channels. J Gen Physiol 123:281–293 doi:10.1085/jgp.200308986 [DOI] [PMC free article] [PubMed]
- 44.Schumacher M, Schafer G, Holstein AF, Hilz H (1978) Rapid isolation of mouse Leydig cells by centrifugation in Percoll density gradients with complete retention of morphological and biochemical integrity. FEBS Lett 91:333–338 doi:10.1016/0014-5793(78)81204-6 [DOI] [PubMed]
- 45.Stoop R, Surprenant A, North RA (1997) Different sensitivities to pH of ATP-induced currents at four cloned P2X receptors. J Neurophysiol 78:1837–1840 [DOI] [PubMed]
- 46.Surprenant A, Rassendren F, Kawashima E, North RA, Buell G (1996) The cytolytic P2Z receptor pf extracellular ATP identified as a P2X receptor (P2X7). Science 272:735–738 doi:10.1126/science.272.5262.735 [DOI] [PubMed]
- 47.Tai CJ, Kang SK, Leung PC (2001) Adenosine triphosphate-evoked cytosolic calcium oscillations in human granulosa-luteal cells: role of protein kinase C. J Clin Endocrinol Metab 86(2):773–777 doi:10.1210/jc.86.2.773 [DOI] [PubMed]
- 48.Torres GE, Egan TM, Voigt MM (1998) Topological analysis of the ATP-gated ionotropic P2X2 receptor subunit. FEBS Lett 425:19–23 doi:10.1016/S0014-5793(98)00179-3 [DOI] [PubMed]
- 49.Townsend-Nicholson A, King BF, Wildman SS, Burnstock G (1999) Molecular cloning, functional characterization and possible cooperativity between the murine P2X4 and P2X4a receptors. Brain Res Mol Brain Res 64:246–254 doi:10.1016/S0169-328X(98)00328-3 [DOI] [PubMed]
- 50.Valera S, Hussy H, Evans RJ, Adami N, North RA, Surprenant A, Buell G (1994) A new class of ligand-gated ion channel defined by P2X receptor of extracellular ATP. Nature 371:516–519 doi:10.1038/371516a0 [DOI] [PubMed]
- 51.Wildman SS, Brown SG, Rahman M, Noel CA, Churchill L, Burnstock G, Unwin RJ, King BF (2002) Sensitization by extracellular Ca2+ of rat P2X5 receptor and its pharmacological properties compared with rat P2X1. Mol Pharmacol 62:957–966 doi:10.1124/mol.62.4.957 [DOI] [PubMed]