Abstract
In human mast cells and microvascular endothelial cells, the A2B adenosine receptor controls at least three independent signaling pathways, i.e., Gs-mediated stimulation of adenylate cyclase, Gq-mediated stimulation of phospholipase Cβ, and Gs/Gq-independent upregulation of IL-8. Functional analysis of cells transfected with full-length and truncated receptor constructs revealed that the A2B receptor C-terminus is important for coupling to Gs and Gq proteins. Removal of the entire cytoplasmic portion in the A2B receptor C-terminus rendered it incapable of stimulating adenylate cyclase and phospholipase Cβ. Conversely, removal of the distal 16 amino acids facilitated signal transduction from the receptor to the downstream Gs but not Gq proteins. However, the A2B receptor C-terminus is not essential for upregulation of IL-8. Analysis of chimeric A2A/A2B receptors demonstrated that only chimeras containing the third intracellular loop of the A2B receptor mediated agonist-dependent IL-8 reporter stimulation, suggesting that this domain is important for upregulation of IL-8.
Keywords: Adenosine, Purinergic receptors P1, Interleukin-8, Adenylate cyclase, Type C phospholipases, GTP-binding proteins
Introduction
Adenosine modulates cellular functions by binding to cell surface G-protein-coupled receptors of the P1 purinergic family comprised of A1, A2A, A2B, and A3 subtypes. Adenosine receptors were initially classified according to their effect on adenylate cyclase [1, 2]. A1 and A3 receptors were shown to inhibit adenylate cyclase via coupling to Gi proteins [3]. Both A2 subtypes of adenosine receptors activate adenylate cyclase via Gs protein [4, 5], but only the A2B receptor has been shown to be coupled also to phospholipase Cβ via a GTP-binding protein of the Gq family leading to stimulation of protein kinase C and the release of intracellular calcium [6, 7].
The A2B receptor has emerged recently as an important mediator of pro-inflammatory actions of adenosine. Stimulation of pro-inflammatory cytokines via A2B receptors has been documented in various cell types of different origins. A2B receptors were implicated in stimulation of IL-6 release in peritoneal macrophages [8], airway smooth muscle cells and fibroblasts [9, 10], osteoblasts [11], intestinal epithelial cells [12], pituitary folliculostellate cells [13], astrocytes [14], astrocytoma [15], and astroglioma cells [16]. A2B receptors stimulate IL-8 production in human microvascular endothelial [17], glioblastoma [18], and colon cancer [19] cells. In the human mast cells line HMC-1, A2B receptors regulate production of multiple pro-inflammatory and angiogenic factors including IL-1β, IL-3, IL-4, IL-8, IL-13, and VEGF [6, 20]. The property of A2B receptors to regulate production of diverse cytokines can be attributed to their coupling to multiple signaling pathways within the same cell.
We have previously demonstrated the differential role of Gs-adenylate cyclase and Gq-phospholipase Cβ signaling pathways in A2B receptor-dependent stimulation of IL-4, IL-8, and IL-13 production in mast cells. Regulation of IL-4 production via A2B receptors involves cross-talk between Gs-adenylate cyclase and Gq-phospholipase Cβ signaling pathways [21], whereas regulation of IL-13 is mediated via Gq-phospholipase Cβ signaling independently from stimulation of Gs-adenylate cyclase pathway [8]. In contrast, regulation of IL-8 production is not dependent on coupling of A2B receptors to either Gs or Gq proteins because it was not blocked by inhibitors of Gs-adenylate cyclase and Gq-phospholipase Cβ signaling pathways [21].
Cytoplasmic domains are important determinants of receptor coupling specificity toward different intracellular signaling pathways. In adenosine receptors, cytoplasmic domains are represented by three intracellular loops and a carboxy-terminal tail (C-terminus); the latter varies considerably in length between receptor subtypes ranging from 36 amino acids in the A1 receptor to 120 amino acids in the A2A receptor. Studies in A1 and A2A receptors indicate that structural elements of the C-terminus influence coupling of A1 receptors to Gi proteins, whereas C-terminus contribute little if any to A2A receptor coupling to Gs proteins [22–25]. All but the A2A adenosine receptor subtypes have sites for palmitoylation at their C-termini [3]. The membrane-proximal and membrane-distal segments of the C-terminus divided by the palmitoylated cysteine play a differential role in the regulation of A1 receptor coupling to G-proteins [25]. Like in the A1 receptor, the C-terminus of the A2B receptor is relatively short; it contains approximately 40 amino acids, including a potential cite for palmitoylation at cysteine-311 [3, 26].
In this study, we investigated the role of the C-terminus of the A2B receptor in stimulation of adenylate cyclase, phospholipase Cβ, and interleukin-8 by introducing either partial (16 amino acids) truncation of the C-terminus that leaves the potential site for palmitoylation intact, or by removing the entire cytoplasmic C-terminal extension. Our results show that the C-terminus is important for A2B receptor coupling to Gs-adenylate cyclase and Gq-phospholipase Cβ signaling pathways. Conversely, the 16 amino acid segment most distal to the seventh transmembrane helix restrains signal transduction from the receptor to downstream Gs but not Gq proteins. In contrast, the cytoplasmic C-terminal extension is not essential for A2B receptor-dependent regulation of IL-8 production. Further analysis of chimeric A2A/A2B receptors suggests that the intracellular loop 3 of the A2B receptor may be important for stimulation of IL-8 reporter activity.
Methods
Cell culture and reagents
Chinese hamster ovary cells CHO-K1 and human embryonic kidney cells HEK 293 were obtained from the American Type Culture Collection (Rockville, MD, USA). CHO cells were maintained in Ham’s F12 medium and HEK 293 cells were maintained in Dulbecco's modified Eagle's medium, supplemented with 10% (vol/vol) fetal bovine serum, 2 mM l-glutamine, β-mercaptoethanol, non-essential amino acids, and 1× antibiotic–antimycotic mixture (Sigma-Aldrich, St. Louis, MO, USA). Media for the culture of stably transfected cells were supplemented with 0.4 mg/ml Geneticin (G418) in order to maintain the selection pressure. All cells were kept under humidified atmosphere of air/CO2 (19:1) at 37°C. 5′-N-ethylcarboxamidoadenosine (NECA) and theophylline were purchased from Sigma. [3H]-ZM241385 (4-(2-[7-amino-2-(2-furyl)[1,2,4]triazolo[2,3-a]-[1,3,5]triazin-5-ylamino]ethyl)phenol) was purchased from American Radiolabeled Chemicals (St. Louis, MO, USA).
Generation and expression of hemoagglutinin (HA)-tagged full-length, truncated and chimeric versions of the human A2B adenosine receptor
The complementary DNAs (cDNAs) encoding human A2A and A2B adenosine receptors in pRc/CMV expression vector (Invitrogen, Carlsbad, CA, USA) were a generous gift from Andrea Townsend-Nicholson (University College London, UK). The N-terminally HA-tagged full-length human A2B construct was generated in two steps. First, polymerase chain reactions (PCR) were performed with primers carrying HindIII (forward) or KpnI (reverse) sites using the A2B receptor in pRc/CMV as a template. The PCR product was then subcloned into pHM6 expression vector between HindIII and KpnI sites. To generate HA-A2B-316 and HA-A2BΔC-tail constructs, stop codons were introduced at 317S and 294N, respectively, using QuickChange® Site-Direct Mutagenesis Kit (Stratagene, La Jolla, CA, USA) and HA-A2B construct in pHM6 plasmid as a template. The plasmids encoding HA-tagged A2B/A2A chimeric receptors were generated by using HA-A2B construct in pHM6 and A2A construct in pRc/CMV, as templates for a two-step overlap extension PCR. The PCR products were then subcloned into pHM6 expressing vector between HindIII and KpnI sites. All primer sequences used in this study are available upon request. The integrity of each construct was verified by fluorescence DNA sequencing. CHO and HEK 293 cells were transfected using Fugene 6 transfection reagent (Boehringer Mannheim, Germany). Ten micrograms of plasmid DNA was mixed with 500 μl of serum-free medium containing 30 μl of Fugene 6. After 15 min incubation at room temperature, the transfection mixture was added to cells growing on 55 cm2 culture dishes at 70–80% confluency. Stable cell lines expressing HA-tagged versions of the A2B receptor were selected for resistance to G418 (at a concentration of 0.4 mg/ml) and were screened for HA expression.
Measurement of cell surface expression of HA-tagged A2B receptor constructs
A2B adenosine receptor cell surface expression was assessed using an anti-HA antibody as described previously [27] with some modifications. Confluent cell monolayers in 24-well plates were fixed with 0.5% paraformaldehyde in phosphate-buffered saline (PBS) for 5 min at room temperature. Cells were washed three times with PBS, incubated for 45 min with PBS, 1% bovine serum albumin (BSA), and 2% ethylenediamine tetraacetic acid (EDTA) containing 100 μg/ml of whole goat IgG (Jackson ImmunoResearch Laboratories, West Grove, PA, USA), then incubated with a primary anti-HA rabbit antibody (71-5500, Zymed-Invitrogen) or with a negative control antibody (whole rabbit IgG, Jackson ImmunoResearch Laboratories) at a concentration of 2 μg/ml in PBS/BSA/EDTA for 1 h at room temperature. Cells were washed three times with PBS and incubated with a secondary antibody (goat anti-rabbit secondary antibody conjugated with horseradish peroxidase, Jackson ImmunoResearch Laboratories; 1:1,500 dilution in PBS/BSA/EDTA) for 1 h at room temperature. Cells were washed three times with PBS and a colorimetric peroxidase substrate solution (TMB substrate, eBioscience, San Diego, CA, USA) was added. When adequate color change was achieved, 100 μl of sample was added to 100 μl of 1.8 N H2SO4 to terminate the reaction, and the samples were read at 450 nm using a microplate reader. The results were expressed as ΔOD450, with the optic density values for cells incubated with non-specific primary antibody being subtracted from the values obtained with anti-HA antibody.
Preparation of HEK 293 membranes
Monolayers of HEK 293 cells were washed with PBS once and harvested in a buffer containing 10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES; pH 7.4), 10 mM EDTA, and 1:10 dilution of a protease inhibitor cocktail (Roche, Indianapolis, IN, USA). Cells were homogenized by repetitive passing through a syringe needle (21G, ten times) and centrifuged at 20,000×g for 25 min at 4°C. The cell pellets were washed twice with a buffer containing 10 mM HEPES (pH 7.4), 1 mM EDTA, and protease inhibitors and were resuspended in the same buffer supplemented with 10% sucrose. Frozen aliquots were kept at −80°C.
Radioligand binding
A2B adenosine receptor binding was performed using [3H]-ZM214385 as a radioligand. Maximum specific binding (Bmax) was estimated from saturation binding experiments. Binding assays were started by mixing various concentrations of [3H]-ZM214385 with 25 μg membrane proteins in 1 mM EDTA, 10 mM HEPES, pH 7.4 supplemented with 1 U/ml adenosine deaminase. The assays were incubated for 60 min, stopped by filtration using Brandel cell harvester (Gaithersburg, MD, USA), and washed four times with ice-cold 1 mM MgCl2, 10 mM HEPES, pH 7.4. Non-specific binding was determined in the presence of 10 mM theophylline. All binding data were calculated by a non-linear curve-fitting program GraphPad Prism (GraphPad Software, Inc., San Diego, CA, USA).
Measurement of cAMP accumulation
Cells were preincubated for 10 min at 37°C in a buffer containing 150 mM NaCl, 2.7 mM KCl, 0.37 mM NaH2PO4, 1 mM MgSO4, 1 mM CaCl2, 5 g/l d-glucose, 10 mM HEPES–NaOH, pH 7.4, 1 U/ml adenosine deaminase, and the cyclic adenosine monophosphate (cAMP) phosphodiesterase inhibitor papaverine (1 mM). The adenosine receptor agonist NECA was added to cells and incubation was allowed to proceed for 3 min at 37°C. The reaction was stopped by the addition of trichloroacetic acid to a final concentration of 5%. Cell extracts were washed five times with 10 volumes of water-saturated ether. Cyclic AMP concentrations were determined using a cAMP assay kit (GE Healthcare, Little Chalfont, UK).
Measurement of [3H] inositol phosphates formation
Formation of inositol phosphates was determined using the procedure described by Seuwen et al. [28] with modification. Cells were labeled to equilibrium with myo-[3H] inositol (2 μCi/ml, DuPont-NEN, Boston, MA, USA) for 18 h in inositol-free MEM medium. Cells were then washed twice with PBS and preincubated in 150 mM NaCl, 2.7 mM KCl, 0.37 mM NaH2PO4, 1 mM MgSO4, 1 mM CaCl2, 5 g/l d-glucose, 10 mM HEPES–NaOH, pH 7.4, and 1 U/ml adenosine deaminase containing 20 mM LiCl2 for 15 min at 37°C. The adenosine receptor agonist NECA was added to cells, and incubation was allowed to proceed for 30 min at 37°C. Reaction was terminated by replacing the incubation buffer with 200 μl of ice-cold 10 mM formic acid (pH 3). After 30 min, this solution containing the extracted inositol phosphates and inositol was collected and diluted with 800 μl of 5 mM NH3 solution (final pH 8–9). The resulting mixture was then applied to a column containing 0.2 ml anion exchange resin (AG 1-X8, formate form, 200–400 mesh, Bio-Rad Laboratories, Richmond, CA, USA). Free inositol and glycerophosphoinositol were eluted with 1.25 ml of H2O and 1 ml of 40 mM ammonium formate/formic acid, pH 5. Total inositol phosphates were eluted in the single step with 1 ml of 2 M ammonium formate/formic acid, pH 5, and radioactivity was measured by liquid scintillation counting.
Luciferase reporter assay
NECA-dependent effects of adenosine receptor constructs on IL-8 reporter activity were compared after their transient expression in CHO cells. IL-8 promoter-driven luciferase reporter -133-luc, a firefly luciferase reporter plasmid, comprising 5′ flanking −133 to +44 base pairs of the human IL-8 gene, was a generous gift from Dr. Naofumi Mukaida (Kanazawa University, Ishikawa, Japan). A control constitutively active Renilla luciferase plasmid pRL-TK was purchased from Promega (Madison, WI, USA). CHO cells growing in 12-well plates at 70% confluency were co-transfected with cDNA encoding adenosine receptor constructs and luciferase reporters at a ratio of 1:1 using Fugene 6 transfection reagent (Boehringer Mannheim). A ratio of 5:1 was used for experimental firefly luciferase reporter/control Renilla luciferase reporter combination. Twenty-four hours after transfections, the reactions were started in parallel by the addition of 10 μM NECA or its vehicle (0.1% DMSO). Six hours later, cells were lysed and reporter activities were analyzed using a Dual-Luciferase Reporter Assay System (Promega). Chemiluminescence resulted from luciferase activity was measured with a TD 20/20 luminometer (Turner Designs, Sunnyvale, CA, USA). Firefly luciferase reporter activities were normalized against Renilla luciferase activities from the co-expressed pRL-TK to correct for potential fluctuations in cDNA transfection. NECA-dependent stimulation of IL-8 reporter activity mediated by adenosine receptor constructs was determined as an increase in luciferase activity in the presence of NECA over vehicle control (set as 1).
Statistical analysis
Data were analyzed using the GraphPad Prism 4.0 software (GraphPad Software Inc., San Diego, CA, USA) and presented as mean ± SEM. Multiple comparisons between different groups were performed using one-way analysis of variance followed by Bonferroni’s post-tests. Comparisons between several groups and a control group were performed using one-way analysis of variance followed by Dunnett’s post-tests. Comparisons between two groups were performed using two-tailed unpaired t tests.
Results
Expression of full-length and truncated versions of A2B adenosine receptors in CHO cells
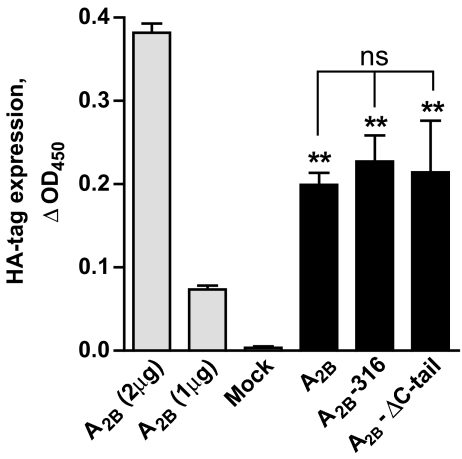
Because CHO cells do not express endogenous adenosine receptors, they represent a convenient cell model to study coupling of transfected adenosine receptors to intracellular signaling [29]. To test a role of the C-terminus in the A2B receptor signaling, CHO cells were stably transfected with plasmids driving the expression of HA-tagged human full-length and truncated A2B receptors, which included two versions, A2B-316 and A2B-ΔC-tail (comprising amino acids 1–316 and 1–293 of the A2B receptor sequence, respectively; Fig. 1). Stable cell clones were obtained by selection with G418. We could not accurately measure receptor density by radioligand binding using [3H]-ZM214385 in agreement with previous reports showing that stable transfection of A2B receptors in CHO cells often results in low levels of expression [29–31]. Instead, we estimated the relative cell surface expression levels of full-length and truncated A2B receptors with an anti-HA antibody, taking advantage of the HA-epitope tag contained in the NH2 terminus of the receptor constructs. This analysis indicated that the cell surface expression of all recombinant receptors was similar (Fig. 2).
Fig. 1.
Schematic representation of receptors designed to study cytoplasmic carboxy-terminal tail of the A2B receptor. HA-tagged human full-length (A2B) and truncated versions (A2B-316, A2B-ΔC-tail) of the A2B receptor were constructed. HA-A2B-293 represents NH2-terminally HA-tagged portion of the A2B molecule that includes amino acids 1–293. Cytoplasmic portions of receptor constructs are presented in single-letter amino acid code. Numbers indicate amino acid positions counted from the receptor NH2-terminus
Fig. 2.
Cell surface expression of HA-tagged receptor constructs in stably transfected CHO cells. Transient transfection of CHO cells (gray bars) with varying doses of cDNA encoding the HA-tagged A2B receptors was assessed by cell surface HA-tag expression as described under “Methods”. A2B (2 μg), A2B (1 μg), and mock denote CHO cells transfected with 2, 1, and 0 μg of HA-tagged A2B cDNA. Cell surface expression of full-length (A2B) and truncated versions (A2B-316, A2B-ΔC-tail) of the A2B receptor was analyzed in stably transfected CHO cells (black bars). Data are mean ± SEM, n = 3. Asterisks indicate significant difference from mock transfection control (**p < 0.01, one-way analysis of variance with Dunnett’s post-test); ns indicates no significant difference in HA-tag cell surface expression between receptor constructs in stably transfected CHO cells (p > 0.05, one-way analysis of variance)
Receptor-mediated stimulation of cAMP accumulation in CHO cells
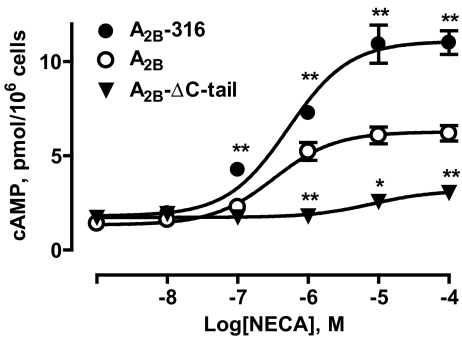
We next analyzed the coupling of full-length and truncated A2B receptors to Gs-adenylate cyclase signaling pathway in CHO cells. As seen in Fig. 3, stimulation of full-length A2B receptors with the adenosine receptor agonist NECA increased cAMP levels in CHO cells in a concentration-dependent manner with an estimated EC50 value of 328 nM (182–590 nM, 95% confidence interval). NECA (100 μM) produced a 5-fold increase in cAMP levels, from 1.26 ± 0.06 to 6.19 ± 0.41 pmol/106 cells. Complete removal of the C-terminus (amino acids 294–332) resulted in a significant loss of the receptor coupling to Gs-adenylate cyclase signaling pathway; stimulation of A2B-ΔC-tail truncated version of the A2B receptor with NECA (100 μM) only slightly raised cAMP levels. Because concentration-dependent stimulation of cAMP with NECA did not reach a plateau, we could not accurately estimate an EC50 value but agonist potency at the truncated A2B-ΔC-tail receptor was obviously much lower than that at the full-length A2B receptor. In contrast, partial truncation of the C-terminus (amino acids 317–332) had little effect on agonist potency (EC50 of 520 nM; 300–898 nM, 95% confidence interval) but significantly increased the maximal cAMP response to NECA (100 μM), compared to that mediated via full-length A2B receptors (11.01 ± 0.63 versus 6.19 ± 0.41 pmol/106 cells, p < 0.01 by one-way analysis of variance with Dunnett’s post-test, n = 3).
Fig. 3.
Agonist-dependent cAMP accumulation in CHO cells stably expressing full-length (A2B) and truncated versions (A2B-316, A2B-ΔC-tail) of the A2B adenosine receptor. Data are results from three experiments presented as mean ± SEM. Asterisks indicate significant difference from full-length A2B receptors for each NECA concentration (*p < 0.05, **p < 0.01, one-way analysis of variance with Dunnett’s post-test)
Receptor-mediated stimulation of cAMP accumulation in HEK 293 cells
To ascertain that the enhanced Gs-adenylate cyclase signaling via the A2B-316 truncated version of the A2B receptor is not limited to CHO cells, we transiently transfected HEK 293 cells with expression vectors encoding full-length and truncated A2B receptors. HEK 293 cells transfected with an empty pHM6 vector were used as a negative control. In agreement with the previously reported presence of endogenous A2B receptors in HEK 293 cells [32, 33], 10 μM NECA raised cAMP levels in mock-transfected cells from 0.58 ± 0.03 to 1.59 ± 0.04 pmol/106 cells (p < 0.001 by one-way analysis of variance with Bonferroni’s multiple comparison post-test, n = 4; Table 1). Overexpression of full-length A2B receptors or truncated A2B-316 receptors in HEK 293 increased basal cAMP accumulation by approximately 2.5-fold compared to control. In contrast, expression of A2B-ΔC-tail truncated version of the A2B receptor had no significant effect on basal cAMP levels (Table 1). These data suggest that both full-length A2B and A2B-316, but not A2B-ΔC-tail receptors, interacted with Gs proteins in HEK 293 cells even in the absence of an agonist.
Table 1.
Cyclic AMP accumulation (pmol/106 cells) in HEK 293 cells transiently expressing full-length (A2B) and truncated versions (A2B-316, A2B-ΔC-tail) of the A2B adenosine receptor
| Controla | A2B | A2B-316 | A2B-ΔC-tail | |
|---|---|---|---|---|
| Basalb | 0.58 + 0.03c | 1.42 ± 0.08 (p < 0.01)d | 1.38 ± 0.22 (p < 0.01) | 0.53 ± 0.02 (p > 0.05) |
| Forskolin | 2.32 ± 0.12 | 9.72 ± 0.34 (p < 0.01) | 10.92 ± 0.72 (p < 0.01) | 2.22 ± 0.23 (p > 0.05) |
| NECA | 1.59 ± 0.04 | 2.35 ± 0.05 (p < 0.05) | 5.31 ± 0.37 (p < 0.01) | 1.73 ± 0.05 (p > 0.05) |
aMock-transfected HEK 293 cells were used as a control
bCyclic AMP accumulation was measured in the absence (basal), or in the presence of 1 μM forskolin or 10 μM NECA
cData are results from four experiments presented as mean ± SEM
dp values in parentheses are results of one-way analysis of variance within each group (basal, forskolin, NECA) with Dunnett's post-tests versus corresponding controls
Constitutive activity of full-length A2B receptors in HEK 293 has been previously demonstrated using the inverse agonist MRE 2029F20 [31]. Because the increased levels of free Gsα subunits are known to enhance adenylate cyclase responses to forskolin, this agent can be used to compare constitutive activity of Gs-protein-coupled receptors [34]. Indeed, forskolin magnified the constitutive activity of full-length A2B and truncated A2B-316 but not A2B-ΔC-tail receptors resulting in cAMP levels of 9.73 ± 0.34, 10.92 ± 0.72, and 2.22 ± 0.23 pmol/106 cells, respectively, versus cAMP levels of 2.32 ± 0.12 pmol/106 cells in control HEK 293 cells (Table 1).
Although transfection of CHO cells with A2B-316 and full-length A2B receptors resulted in comparable basal and forskolin-stimulated cAMP accumulation, agonist-induced activity of truncated A2B-316 receptors was significantly greater than that of full-length A2B receptors (5.31 ± 0.37 and 2.35 ± 0.05 pmol/106 cells, respectively; p < 0.001 by one-way analysis of variance with Bonferroni’s multiple comparison post-test). In contrast, there was no significant difference in NECA-induced cAMP levels between cells expressing truncated A2B-ΔC-tail receptors and control HEK 293 cells (1.73 ± 0.05 versus 1.6 ± 0.04 pmol/106 cells; Table 1).
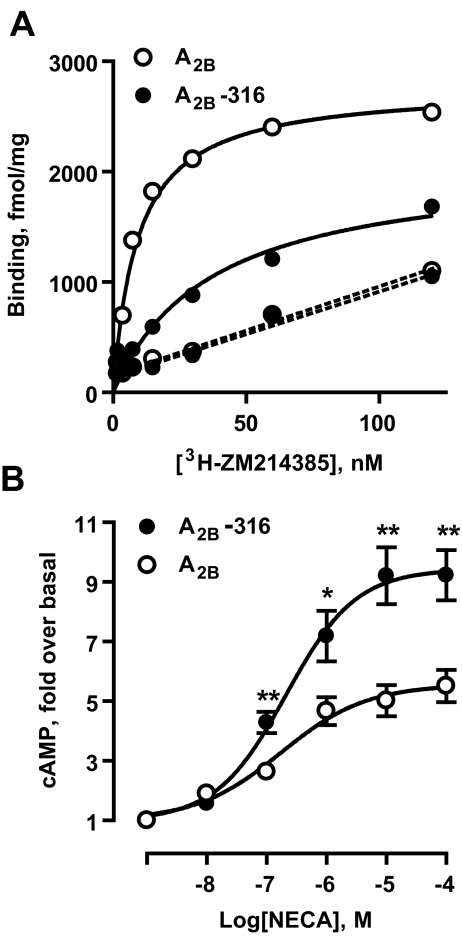
To investigate further the enhancement of Gs-adenylate cyclase signaling by the A2B-316 truncated version of the A2B receptor, we selected two HEK 293 clones stably expressing recombinant A2B-316 and full-length A2B receptors. Based on specific [3H]-ZM214385 binding to membranes obtained from both clones (Fig. 4a), we estimated Bmax values of 2,774 and 2,092 fmol/mg protein for full-length and A2B-316 truncated version of the A2B receptor, respectively. Even though the A2B-316 receptor density was lower, stimulation of these receptors resulted in significantly greater cAMP accumulation, compared to full-length A2B receptors (Fig. 4b). Taken together, our results show that partial truncation of the C-terminus (amino acids 317–332) of the A2B receptor results in enhanced Gs-adenylate cyclase signaling, whereas complete removal of the C-terminus drastically reduces receptor coupling to this pathway.
Fig. 4.
Stable expression of full-length (A2B) and truncated version (A2B-316) of the A2B adenosine receptor in HEK 293. aSolid lines represent saturation isotherm for specific binding of [3H]-ZM241385 to membranes from stably transfected HEK 293 cells. Dashed lines represent non-specific binding. Data are means from duplicate determinations. b NECA-dependent cAMP accumulation. Data are results from six experiments presented as mean ± SEM. Asterisks indicate significant difference from full-length A2B receptors for each NECA concentration (*p < 0.05, **p < 0.01, unpaired two-tailed t test)
Receptor-mediated stimulation of phosphoinositide turnover in HEK 293 cells
In addition to coupling to Gs-adenylate cyclase, A2B receptors can stimulate phospholipase Cβ via coupling to Gq proteins [6, 7, 21]. Coupling of A2B receptors to Gq proteins has been reported in several cell types, but it is not a universal phenomenon [26]. For instance, it is absent in CHO cells expressing recombinant A2B receptors and HEK 293 cells expressing endogenous A2B receptors. We were not able to detect an increase in phosphoinositide turnover in these cells upon stimulation with NECA. However, this signaling pathway can be simulated in HEK 293 cells overexpressing recombinant A2B receptors [7]. Therefore, we transiently transfected HEK 293 cells with plasmids encoding HA-tagged recombinant full-length and truncated A2B receptors and confirmed their expression with an anti-HA antibody (data not shown).
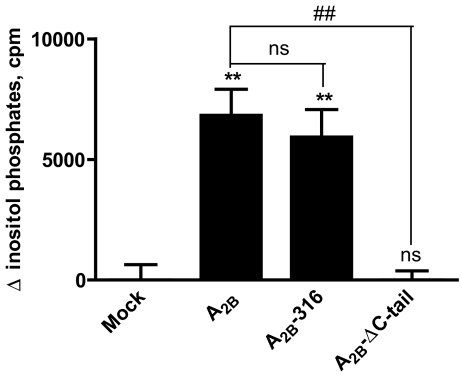
To test the role of the C-terminus in A2B receptor coupling to Gq-phospholipase C signaling pathway, we measured agonist-induced accumulation of inositol phosphates. As shown in Fig. 5, 100 μM NECA induced significant accumulation of inositol phosphates in HEK 293 cells expressing recombinant full-length A2B receptors. Similar to stimulation of cAMP accumulation, complete removal of the A2B receptor C-terminus in A2B-ΔC-tail caused loss of the receptor’s ability to stimulate inositol phosphates accumulation. In contrast to stimulation of cAMP accumulation, however, partial truncation of the A2B receptor C-terminus in A2B-316 did not result in enhancement of inositol phosphates accumulation. These results suggest a differential role of C-terminal segments distal to the seventh transmembrane helix in A2B receptor-dependent stimulation of Gs-adenylate cyclase and Gq-phospholipase Cβ signaling pathways.
Fig. 5.
Agonist-dependent inositol phosphate formation in HEK 293 cells transiently expressing full-length (A2B) and truncated versions (A2B-316, A2B-ΔC-tail) of the A2B adenosine receptor. Accumulation of total inositol phosphates was measured in the absence or in the presence of 100 μM NECA. Mock-transfected HEK 293 cells were used as a control. Basal concentrations of [3H]IPs were 5,000–6,000 cpm/tube. Values are presented as difference between inositol phosphates accumulation in the presence and in the absence of agonist. Data are results from three experiments presented as mean ± SEM. Asterisks indicate significant difference from mock transfection control (**p < 0.01, one-way analysis of variance with Dunnett’s post-test). Pounds indicate significant difference from full-length A2B receptor (##p < 0.01, one-way analysis of variance with Dunnett’s post-test)
Receptor-mediated stimulation of IL-8 reporter in CHO cells
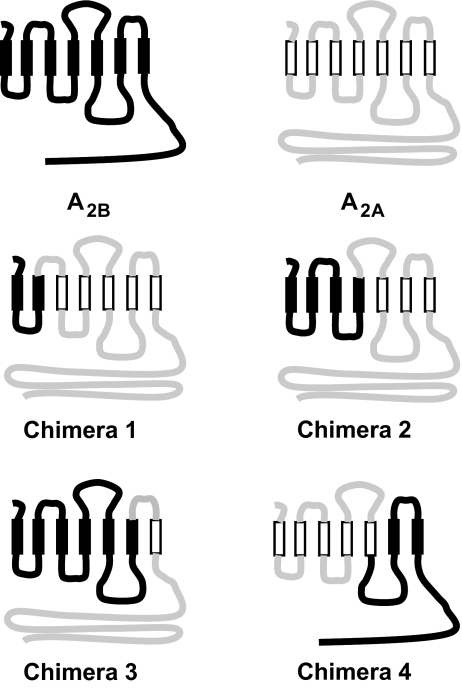
We have previously reported that 100 μM NECA stimulated IL-8 reporter in CHO cells co-transfected with A2B but had no effect in cells co-transfected with A2A receptors [17]. In the current study, we used this reporter system to evaluate the role of A2B receptor C-terminus in the receptor-dependent regulation of IL-8 production. The cell surface expression of all adenosine receptor constructs was verified using an anti-HA antibody (Table 2). NECA (10 μM) had no effect on IL-8 reporter activity (0.95 ± 0.03-fold change) in control CHO cells co-transfected with an empty vector (mock). In contrast, NECA significantly increased IL-8 reporter activity in CHO cells co-transfected with full-length A2B receptors by 1.97 ± 0.16-fold (p < 0.01, Dunnett's post-test versus mock control). Although NECA-dependent stimulation of IL-8 reporter activity in CHO cells co-transfected with A2B-ΔC-tail receptors was moderately reduced compared to cells co-transfected with full-length A2B receptors, NECA significantly stimulated IL-8 reporter activity by 1.56 ± 0.12-fold (p < 0.01, Dunnett's post-test versus mock control). Thus, in spite of the apparent loss of function in the regulation of adenylate cyclase and phospholipase C, the truncated A2B-ΔC-tail receptor retained its ability to stimulate IL-8 reporter. Because complete removal of the C-terminus led only to partial loss of stimulation of IL-8 reporter activity, we evaluated the role of other intracellular domains in the A2B receptor molecule in the regulation of IL-8. For this purpose, we created a series of A2B/A2A chimeric receptors. The N-terminal parts of chimeras 1, 2, and 3 included the first, both the first and the second, and all three intracellular loops of the A2B receptor, respectively, whereas the C-terminal parts of molecules represented A2A receptor sequences corresponding to the remaining domains of the A2B receptor (Fig. 6). Of these constructs, only chimera 3 mediated NECA-induced increase in IL-8 reporter activity, which was comparable to that mediated by the full-length A2B receptor (Table 2). As a complementary approach, we used another chimeric receptor, in which the N-terminal part included two intracellular loops of the A2A receptor and the C-terminal part that included the intracellular domain 3 and the C-terminus of the A2B receptors (chimera 4, Fig. 6). As seen in Table 2, there was no significant difference in the effects of NECA on IL-8 reporter activities between CHO cells expressing this chimera and the full-length A2B receptor. Taken together, our results point to the intracellular loop 3 of the A2B receptor as a potential interface for signaling leading to upregulation of IL-8.
Table 2.
Adenosine receptor structure–activity analysis of IL-8 reporter stimulation in CHO cells
| HA-tag expressiona | IL-8 promoter stimulationb | |
|---|---|---|
| Mock | 0.004 ± 0.002 | 0.95 ± 0.03 |
| A2B | 0.021 ± 0.007 | 1.97 ± 0.16 |
| A2B-ΔC-tail | 0.029 ± 0.011 (p > 0.05)c | 1.56 ± 0.12 (p < 0.05) |
| Chimera 1 | 0.032 ± 0.004 (p > 0.05) | 1.08 ± 0.03 (p < 0.01) |
| Chimera 2 | 0.024 ± 0.002 (p > 0.05) | 1.08 ± 0.04 (p < 0.01) |
| Chimera 3 | 0.018 ± 0.003 (p > 0.05) | 1.74 ± 0.14 (p > 0.05) |
| Chimera 4 | 0.036 ± 0.007 (p > 0.05) | 1.94 ± 0.08 (p > 0.05) |
Cells were co-transfected with reporters and vectors encoding full-length (A2B), truncated (A2B-ΔC-tail), or chimeric A2A/A2B (depicted in Fig. 6 as chimeras 1, 2, 3, and 4) receptors, or with empty pHM6 vector (mock). Cell surface HA-tag expression (ΔOD450) and NECA-dependent stimulation of IL-8 promoter (fold) were determined as described under “Methods”
aData are results from three experiments presented as mean ± SEM
bData are results from six experiments presented as mean ± SEM
cp values in parentheses are results of one-way analysis of variance with Dunnett's post-tests versus full-length A2B receptor
Fig. 6.
Schematic representation of the wild type and chimeric A2B and A2A adenosine receptors. The A2B sequence is denoted in black lines and transmembrane domains are presented as closed rectangles. The A2A sequence is denoted in gray lines and transmembrane domains are presented as open rectangles
Discussion
A2B adenosine receptors stimulate production of multiple pro-inflammatory and angiogenic factors through activation of divergent signaling pathways [6, 8, 17, 20, 21]. Initiation of signaling in response to agonist stimulation requires the interaction of receptor cytoplasmic domains with other proteins. In this study, we focused on the role of cytoplasmic C-terminus of the A2B receptor in the regulation of adenylate cyclase and phospholipase Cβ, which depends on receptor interaction with Gs and Gq proteins, respectively. In addition, we evaluated the role of cytoplasmic C-terminus and other intracellular domains of the A2B receptor in the stimulation of IL-8 mediated via a signaling pathway independent from adenylate cyclase and phospholipase Cβ [21].
Our results show that removal of the C-terminal cytoplasmic portion of the A2B receptor rendered it incapable of stimulating adenylate cyclase and phospholipase Cβ. The loss of these functions could not be explained by deficiency in receptor expression or agonist binding. Cell surface expression of the HA-epitope tag contained in the NH2 terminus of the receptor constructs was similar in cells transfected with either full-length or truncated versions of the A2B receptor. Furthermore, the A2B receptor lacking C-terminal cytoplasmic tail responded to agonist stimulation with NECA by increasing IL-8 reporter activity. Thus, our results suggest that the C-terminus is important for A2B receptor interaction with Gs and Gq proteins, but not essential for a signaling pathway leading to stimulation of IL-8 production.
Although complete removal of the cytoplasmic portion of the C-terminus resulted in the loss of A2B receptor coupling to both Gs-adenylate cyclase and Gq-phospholipase Cβ, partial truncation of the C-terminus had a differential effect on these signaling pathways. Deletion of the distal 16 amino acids in the C-terminus enhanced A2B receptor efficacy in the stimulation of adenylate cyclase but not phospholipase Cβ. This enhancement was evident upon both transient and stable transfections and was independent of the cell line used. It is likely that truncation of distal 16 amino acids alters the structure of the remaining portion of the A2B receptor in a way favorable for interaction with Gs proteins over other potential receptor-binding partners. The existence of sequence elements in the C-terminus that restrain signaling to Gs protein has been demonstrated in other receptors, e.g., β-adrenoceptors [35]. Of interest, deletion of the last 18 amino acids in the C-terminus of the A1 adenosine receptor also enhanced receptor-mediated signaling [25]. In contrast, partial truncation of the C-terminus of the A2A adenosine receptor had no effect on the receptor function [24]. In contrast to the 120-amino-acid-long A2A receptor C-tail lacking palmitoylation sites, the cytoplasmic portions of both A1 and A2B receptor C-termini are relatively short (36–40 amino acids) and can be divided into membrane-proximal and membrane-distal segments by the palmitoylated cysteine [3]. In this respect, the A2B receptor structurally resembles more the A1 receptor than the A2A receptor. In this study, we also found that partial truncation of the C-terminus of the A2B adenosine receptor has a differential effect on coupling to intracellular pathways, enhancing stimulation of adenylate cyclase but not of phospholipase Cβ. Thus, our results suggest that the C-terminal segment most distal to the seventh transmembrane helix restrains signal transduction from the A2B receptor to downstream Gs but not Gq proteins.
We have previously shown that stimulation of IL-8 production by A2B receptors was not affected by inhibition of adenylate cyclase or phospholipase C, suggesting that this signaling pathway does not require receptor binding to Gs and Gq proteins [21]. Accordingly, our results show that the A2B receptor can stimulate IL-8 reporter even in the absence of the C-terminus, a cytoplasmic domain critical for the binding to both Gs and Gq proteins. Based on our previous observation that only A2B but not A2A receptors stimulated IL-8 reporter upon co-expression in CHO cells [17], we profiled the functional responses of a series of chimeric A2A/A2B receptors in order to define the cytoplasmic regions of the A2B receptors responsible for IL-8 stimulation. We found that only chimeras containing the third intracellular loop of the A2B receptor considerably stimulated IL-8 reporter activity. This finding suggests that integrity of the third intracellular loop is critical for interaction of the A2B receptor with components of a signaling pathway leading to IL-8 stimulation. The nature of these binding partners is unclear. One mechanism for establishing regulatory protein complexes is via protein–protein interaction with submembrane scaffolding proteins, which are known as PDZ domain proteins. The third intracellular loop of the A2B receptor contains the PDZ consensus (QRTEL) sequence enabling it to interact with the scaffold-based regulatory proteins containing PDZ-1 domain. Indeed, the A2B receptor was shown to associate with ezrin (a cytoskeletal linker protein), protein kinase A, and the PDZ-1 domain protein NHERF-2 to form a multiprotein signaling complex in intestinal epithelial cells [36]. Studies in other G-protein-coupled receptors, including β-adrenoceptors [37] and κ-opioid receptors [38], suggested a critical role of their C-termini in receptor interactions with NHERF. However, association of ezrin–NHERF-2 complexes with the A2B receptor is preserved after removal of entire cytoplasmic portion of the C-terminus (S. Ryzhov, unpublished data). Furthermore, formation of these multiprotein signaling complexes with the A2B receptor was abolished after mutation of the PDZ consensus sequence in the third intracellular loop [36]. It remains to be determined whether this protein–protein interaction domain could be used by A2B receptors to bypass the requirement of G proteins to activate intracellular signaling pathways leading to IL-8 upregulation.
In summary, our study demonstrated that segments of the C-terminus most distal to the seventh transmembrane helix restrain signal transduction from the receptor to downstream Gs but not Gq proteins. The C-terminus is important for A2B receptor coupling to Gs-adenylate cyclase and Gq-phospholipase Cβ signaling pathways, but not essential for upregulation of IL-8. Our data indicate that the third intracellular loop of the A2B receptor may be involved in Gs- and Gq-independent stimulation of IL-8 production. Whereas these results contribute to our understanding of how cytoplasmic domains of the A2B adenosine receptor couple to multiple intracellular signaling pathways, further investigation is needed to elucidate basic mechanisms by which these receptors exert their pro-inflammatory functions in many pathological conditions.
Acknowledgments
This work was supported by the U.S. National Institutes of Health grant R01 HL076306 and by the American Heart Association Southeastern Affiliate Grant-in-Aid 0755221B.
References
- 1.Londos C, Wolff J (1977) Two distinct adenosine-sensitive sites on adenylate cyclase. Proc Natl Acad Sci U S A 74:5482–5486 doi:10.1073/pnas.74.12.5482 [DOI] [PMC free article] [PubMed]
- 2.Londos C, Cooper DMF, Wolff J (1980) Subclasses of external adenosine receptors. Proc Natl Acad Sci U S A 77:2551–2554 doi:10.1073/pnas.77.5.2551 [DOI] [PMC free article] [PubMed]
- 3.Fredholm BB, Ijzerman AP, Jacobson KA, Klotz KN, Linden J (2001) International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol Rev 53:527–552 [PMC free article] [PubMed]
- 4.Bruns RF, Lu GH, Pugsley TA (1987) Adenosine receptor subtypes: binding studies. In: Gerlach E, Becker BF (eds) Topics and perspectives in adenosine research. Springer, Berlin, pp 59–73
- 5.Feoktistov I, Biaggioni I (1993) Characterization of adenosine receptors in human erythroleukemia cells. Further evidence for heterogeneity of adenosine A2 receptors. Mol Pharmacol 43:909–914 [PubMed]
- 6.Feoktistov I, Biaggioni I (1995) Adenosine A2B receptors evoke interleukin-8 secretion in human mast cells. An enprofylline-sensitive mechanism with implications for asthma. J Clin Invest 96:1979–1986 doi:10.1172/JCI118245 [DOI] [PMC free article] [PubMed]
- 7.Linden J, Thai T, Figler H, Jin X, Robeva AS (1999) Characterization of human A2B adenosine receptors: radioligand binding, western blotting, and coupling to Gq in human embryonic kidney 293 cells and HMC-1 mast cells. Mol Pharmacol 56:705–713 [PubMed]
- 8.Ryzhov S, Zaynagetdinov R, Goldstein AE, Novitskiy SV, Dikov MM, Blackburn MR, Biaggioni I, Feoktistov I (2008) Effect of A2B adenosine receptor gene ablation on proinflammatory adenosine signaling in mast cells. J Immunol 180:7212–7220 [DOI] [PMC free article] [PubMed]
- 9.Zhong H, Belardinelli L, Maa T, Feoktistov I, Biaggioni I, Zeng D (2004) A2B adenosine receptors increase cytokine release by bronchial smooth muscle cells. Am J Respir Cell Mol Biol 30:118–125 doi:10.1165/rcmb.2003-0118OC [DOI] [PubMed]
- 10.Zhong H, Belardinelli L, Maa T, Zeng D (2005) Synergy between A2B adenosine receptors and hypoxia in activating human lung fibroblasts. Am J Respir Cell Mol Biol 32:2–8 doi:10.1165/rcmb.2004-0103OC [DOI] [PubMed]
- 11.Evans BA, Elford C, Pexa A, Francis K, Hughes AC, Deussen A, Ham J (2006) Human osteoblast precursors produce extracellular adenosine, which modulates their secretion of IL-6 and osteoprotegerin. J Bone Miner Res 21:228–236 doi:10.1359/JBMR.051021 [DOI] [PubMed]
- 12.Sitaraman SV, Merlin D, Wang L, Wong M, Gewirtz AT, Si-Tahar M, Madara JL (2001) Neutrophil-epithelial crosstalk at the intestinal lumenal surface mediated by reciprocal secretion of adenosine and IL-6. J Clin Invest 107:861–869 doi:10.1172/JCI11783 [DOI] [PMC free article] [PubMed]
- 13.Rees DA, Lewis BM, Lewis MD, Francis K, Scanlon MF, Ham J (2003) Adenosine-induced IL-6 expression in pituitary folliculostellate cells is mediated via A2b adenosine receptors coupled to PKC and p38 MAPK. Br J Pharmacol 140:764–772 doi:10.1038/sj.bjp.0705488 [DOI] [PMC free article] [PubMed]
- 14.Schwaninger M, Neher M, Viegas E, Schneider A, Spranger M (1997) Stimulation of interleukin-6 secretion and gene transcription in primary astrocytes by adenosine. J Neurochem 69:1145–1150 [DOI] [PubMed]
- 15.Fiebich BL, Akundi RS, Biber K, Hamke M, Schmidt C, Butcher RD, van Calker D, Willmroth F (2005) IL-6 expression induced by adenosine A2b receptor stimulation in U373 MG cells depends on p38 mitogen activated kinase and protein kinase C. Neurochem Int 46:501–512 doi:10.1016/j.neuint.2004.11.009 [DOI] [PubMed]
- 16.Fiebich BL, Biber K, Guyfko K, Berger M, Bauer J, van Calker D (1996) Adenosine A2b receptors mediate an increase in interleukin (IL)-6 mRNA and IL-6 protein synthesis in human astroglioma cells. J Neurochem 66:1426–1431 [DOI] [PubMed]
- 17.Feoktistov I, Goldstein AE, Ryzhov S, Zeng D, Belardinelli L, Voyno-Yasenetskaya T, Biaggioni I (2002) Differential expression of adenosine receptors in human endothelial cells: role of A2B receptors in angiogenic factor regulation. Circ Res 90:531–538 doi:10.1161/01.RES.0000012203.21416.14 [DOI] [PubMed]
- 18.Zeng D, Maa T, Wang U, Feoktistov I, Biaggioni I, Belardinelli L (2003) Expression and function of A2B adenosine receptors in the U87MG tumor cells. Drug Dev Res 58:405–411 doi:10.1002/ddr.10212 [DOI]
- 19.Merighi S, Benini A, Mirandola P, Gessi S, Varani K, Simioni C, Leung E, Maclennan S, Baraldi PG, Borea PA (2007) Caffeine inhibits adenosine-induced accumulation of hypoxia-inducible factor-1alpha, vascular endothelial growth factor, and interleukin-8 expression in hypoxic human colon cancer cells. Mol Pharmacol 72:395–406 doi:10.1124/mol.106.032920 [DOI] [PubMed]
- 20.Ryzhov S, Goldstein AE, Matafonov A, Zeng D, Biaggioni I, Feoktistov I (2004) Adenosine-activated mast cells induce IgE synthesis by B lymphocytes: an A2B-mediated process involving Th2 cytokines IL-4 and IL-13 with implications for asthma. J Immunol 172:7726–7733 [DOI] [PubMed]
- 21.Ryzhov S, Goldstein AE, Biaggioni I, Feoktistov I (2006) Cross-talk between Gs- and Gq-coupled pathways in regulation of interleukin-4 by A2B adenosine receptors in human mast cells. Mol Pharmacol 70:727–735 doi:10.1124/mol.106.022780 [DOI] [PubMed]
- 22.Olah ME (1997) Identification of A2a adenosine receptor domain involved in selective coupling to Gs. Analysis of chimeric A1/A2a adenosine receptors. J Biol Chem 272:337–344 [DOI] [PubMed]
- 23.Tucker AL, Jia LG, Holeton D, Taylor AJ, Linden J (2000) Dominance of Gs in doubly Gs/Gi-coupled chimaeric A1/A2A adenosine receptors in HEK-293 cells. Biochem J 352:203–210 doi:10.1042/0264-6021:3520203 [DOI] [PMC free article] [PubMed]
- 24.Klinger M, Kuhn M, Just H, Stefan E, Palmer T, Freissmuth M, Nanoff C (2002) Removal of the carboxy terminus of the A2A-adenosine receptor blunts constitutive activity: differential effect on cAMP accumulation and MAP kinase stimulation. Naunyn Schmiedebergs Arch Pharmacol 366:287–298 doi:10.1007/s00210-002-0617-z [DOI] [PubMed]
- 25.Pankevych H, Korkhov V, Freissmuth M, Nanoff C, Pankevych H, Freissmuth M, Nanoff C (2003) Truncation of the A1 adenosine receptor reveals distinct roles of the membrane-proximal carboxyl terminus in receptor folding and G protein coupling. J Biol Chem 278:30283–30293 doi:10.1074/jbc.M212918200 [DOI] [PubMed]
- 26.Feoktistov I, Biaggioni I (1997) Adenosine A2B receptors. Pharmacol Rev 49:381–402 [PubMed]
- 27.Daunt DA, Hurt C, Hein L, Kallio J, Feng F, Kobilka BK (1997) Subtype-specific intracellular trafficking of alpha2-adrenergic receptors. Mol Pharmacol 51:711–720 [DOI] [PubMed]
- 28.Seuwen K, Lagarde A, Pouyssegur J (1988) Deregulation of hamster fibroblast proliferation by mutated ras oncogenes is not mediated by constitutive activation of phosphoinositide-specific phospholipase C. EMBO J 7:161–168 [DOI] [PMC free article] [PubMed]
- 29.Fredholm BB, Irenius E, Kull B, Schulte G (2001) Comparison of the potency of adenosine as an agonist at human adenosine receptors expressed in Chinese hamster ovary cells. Biochem Pharmacol 61:443–448 doi:10.1016/S0006-2952(00)00570-0 [DOI] [PubMed]
- 30.Matharu AL, Mundell SJ, Benovic JL, Kelly E (2001) Rapid agonist-induced desensitization and internalization of the A2B adenosine receptor is mediated by a serine residue close to the COOH terminus. J Biol Chem 276:30199–30207 doi:10.1074/jbc.M010650200 [DOI] [PubMed]
- 31.Varani K, Gessi S, Merighi S, Vincenzi F, Cattabriga E, Benini A, Klotz KN, Baraldi PG, Tabrizi MA, Lennan SM, Leung E, Borea PA (2005) Pharmacological characterization of novel adenosine ligands in recombinant and native human A2B receptors. Biochem Pharmacol 70:1601–1612 doi:10.1016/j.bcp.2005.08.018 [DOI] [PubMed]
- 32.Cooper J, Hill SJ, Alexander SP (1997) An endogenous A2B adenosine receptor coupled to cyclic AMP generation in human embryonic kidney (HEK 293) cells. Br J Pharmacol 122:546–550 doi:10.1038/sj.bjp.0701401 [DOI] [PMC free article] [PubMed]
- 33.Gao Z, Chen T, Weber MJ, Linden J (1999) A2B adenosine and P2Y2 receptors stimulate mitogen-activated protein kinase in human embryonic kidney-293 cells. cross-talk between cyclic AMP and protein kinase C pathways. J Biol Chem 274:5972–5980 doi:10.1074/jbc.274.9.5972 [DOI] [PubMed]
- 34.Alewijnse AE, Smit MJ, Rodriguez Pena MS, Verzijl D, Timmerman H, Leurs R (1997) Modulation of forskolin-mediated adenylyl cyclase activation by constitutively active GS-coupled receptors. FEBS Lett 419:171–174 doi:10.1016/S0014-5793(97)01440-3 [DOI] [PubMed]
- 35.Parker EM, Ross EM (1991) Truncation of the extended carboxyl-terminal domain increases the expression and regulatory activity of the avian beta-adrenergic receptor. J Biol Chem 266:9987–9996 [PubMed]
- 36.Sitaraman SV, Wang L, Wong M, Bruewer M, Hobert M, Yun CH, Merlin D, Madara JL (2002) The adenosine 2b receptor is recruited to the plasma membrane and associates with E3KARP and ezrin upon agonist stimulation. J Biol Chem 277:33188–33195 doi:10.1074/jbc.M202522200 [DOI] [PubMed]
- 37.Hall RA, Ostedgaard LS, Premont RT, Blitzer JT, Rahman N, Welsh MJ, Lefkowitz RJ (1998) A C-terminal motif found in the beta2-adrenergic receptor, P2Y1 receptor and cystic fibrosis transmembrane conductance regulator determines binding to the Na+/H+ exchanger regulatory factor family of PDZ proteins. Proc Natl Acad Sci U S A 95:8496–8501 doi:10.1073/pnas.95.15.8496 [DOI] [PMC free article] [PubMed]
- 38.Lau AG, Hall RA (2001) Oligomerization of NHERF-1 and NHERF-2 PDZ domains: differential regulation by association with receptor carboxyl-termini and by phosphorylation. Biochemistry 40:8572–8580 doi:10.1021/bi0103516 [DOI] [PubMed]