Abstract
Radiation proctitis is an inflammatory process associated with persistent and refractory lower gastrointestinal bleeding. Purinergic signaling regulates hemostasis, inflammation, and angiogenesis. For example, CD39, the vascular ectonucleotidase, blocks platelet activation and is required for angiogenesis. Whether CD39 expression is affected by radiation injury is unknown. The aim of this work was to study CD39 expression patterns after clinical radiation injury to the rectum. We prospectively enrolled eight patients with radiation proctitis and five gender-matched controls. Biopsies were taken from normal-appearing rectal mucosa of controls and from the normal sigmoid and abnormal rectum of patients. Expression patterns of CD39, P2Y2 receptor, CD31, CD61 integrin, and vascular endothelial growth factor receptor 2 were examined by immunostaining; levels of CD39 were further evaluated by Western blots. Chronic inflammatory lesions of radiation proctitis were associated with heightened levels of angiogenesis. Immunohistochemical stains showed increased vascular expression of CD39, as confirmed by Western blots. CD39 was co-localized with vascular endothelial markers CD31 and CD61 integrin, as well as expressed by stromal tissues. Development of neovasculature and associated CD39 expression in radiation proctitis may be associated with the chronic, refractory bleeding observed in this condition.
Keywords: CD39, ENTPD1, Radiation proctitis
Introduction
Ionizing radiation is widely used for the treatment of malignancies but causes varying degrees of damage to the parenchyma, stromal elements, and the blood vessels of affected organs [1]. Radiation damage appears related to vascular effects [2]. Early changes seen during the exposure to radiation include lack of cellular mitotic activity with patchy fibroblastic proliferation [3]. Vascular findings predominate in the late phase of radiation injury that develops within weeks to months after exposure. These later findings are most often seen in the micro-vessels with subsequent capillary rupture and/or thrombosis [1]. In these settings, microscopic examination reveals intimal vascular fibrosis, telangiectasia of capillaries and post-capillary venules, endothelial degeneration, and platelet thrombi formation [3].
Because of the fixed position and anatomical proximity to the radiated target organs such as the prostate and cervix, the rectum is often injured during pelvic radiation [4, 5]. Radiation proctitis presents with mucous rectal discharge, diarrhea, marked tenesmus, and/or pelvic pain [4], with rectal bleeding being the most common symptom [6]. Early transient symptoms of radiation injury to the rectum usually resolve spontaneously within 3 months, while radiation proctitis develops weeks later, is chronic in nature, and exhibits a significant impact on quality of life [7, 8].
Unfortunately, this poorly understood disease has no effective treatments and therapeutic measures are solely supportive [5], The development of radiation proctitis is not entirely dependent on the dose of the radiation delivered, but seems to be related to a complex interaction between multiple factors including the patient’s age, genetic predisposition, and other environmental factors [5].
In the bowel mucosa, endothelial cells damaged by the ionizing radiation undergo apoptosis with early pathological features characterized by swelling and sloughing [9, 10]. Late findings include neovascularization, telangiectasia, and perivascular fibrosis [7, 8]. The replacement of the normal endothelium by a thickened fibrous layer leads to progressive ischemia, with occlusion of the vessels and necrotic changes in the supplied tissue [2, 11]. These reactions are mediated, at least in part, by platelet-derived growth factor, vascular endothelial growth factor (VEGF), and fibroblast growth factor, produced by endothelial cells and/or macrophages [9]. In addition to these findings, Abdollahi et al. have found that radiation upregulates the expression of αvβ3 integrin receptors in endothelial cells. Activation of these receptors is known to have potent pro-angiogenic effects [12].
Multiple in vitro studies have shown the role of extracellular nucleotide-mediated signaling in stimulating endothelial cell mitogenic responses, inflammatory reactions, and angiogenesis via modulation of specific purinergic type 2 P2Y2 and αvβ3 integrin receptors [13]. Roles for extracellular nucleotides, e.g., adenosine diphosphate (ADP) in the development of radiation proctitis, have been also proposed by Wang et al. These suggestions have been based on findings that clopidogrel, an inhibitor of ADP binding to P2Y2 receptors on platelets and endothelium, reduces the inflammatory and vascular changes in radiated intestines of rats [14].
Ectonucleoside triphosphate diphosphohydrolase-1 CD39/ENTPD1 is the dominant endothelial cell ectonucleotidase responsible for phosphohydrolysis of extracellular nucleoside di- (ADP) and tri-phosphates (adenosine triphosphate (ATP) and UTP). The respective nucleosides, e.g., adenosine generated from ATP/ADP by CD39 in tandem with CD73, exhibit inhibitory effect on platelets and exert vasodilatory effects [15].
Control of nucleotide-mediated signaling by CD39 and CD73 is of critical importance for the regulation of inflammatory responses and angiogenesis in response to VEGF and growth factors in several animal models [16]. Expression of this ectoenzyme could well be also pertinent to the heightened inflammation and angiogenesis that impact clinical outcomes following rectal mucosal irradiation [13, 15–18].
In this clinical, prospective study of radiation-induced proctitis, we have investigated the expression of vascular CD39 and P2Y2 receptors together with associated integrins and VEGF receptors as described above.
Methods
We prospectively enrolled eight consecutive patients with radiation-induced proctitis undergoing endoscopic argon laser therapy for persistent rectal bleeding together with five gender-matched asymptomatic controls undergoing colonoscopy for cancer screening (Table 1).
Table 1.
Clinical data and radiation exposures
| Total number | Age | Gender | Radiation dose | Time interval after radiotherapy | |
|---|---|---|---|---|---|
| Controls | 5 | 31–82 (median 57) | 5 males | N/A | N/A |
| Patients | 8 | 61–78 (median 74) | 8 males | 3,000–5,000 cGy | 2–15 years (median 4.5) |
N/A not applicable
After obtaining informed consent from the patients, conscious sedation was administered and colonoscopy was performed using Olympus colonoscopy equipment. Biopsies were taken from endoscopically normal-appearing rectal mucosa (control subjects) as well as abnormal-appearing rectal mucosal areas (patients with proctitis) and normal-appearing adjacent sigmoid mucosa (patients with proctitis). Tissues from the colonic biopsies obtained at endoscopic evaluation were embedded in Optimal Cutting Temperature Compound (OCT), and then frozen in isopentane. The slides were microscopically examined after hematoxylin and eosin (H&E) staining. Patterns of expression of CD39, endothelial cell markers CD31, and CD61/vitronectin receptor (β3 integrin) were determined in the vasculature by immunohistochemistry using monospecific antibodies. This analysis included all areas of the biopsies and a global score was given for CD39 expression, using a semiquantitative four-point scale in a blinded manner: 0 representing the lowest and 3 the highest level of expression. Polyclonal antibodies were used to detect P2Y2R and VEGF receptor 2.
Antibodies and other reagents
The anti-human antibodies against CD31 were manufactured by BD PharmMingen (San Jose, CA, USA)/clone # WM-59. The anti-human antibodies to CD39 were from Ancell (Bayport, MN, USA)/clone# BU61. Anti-hamster/cross-reactive with human CD61 antibodies was from BD PharmMingen/clone # 2C9.G2. Polyclonal antibodies against rat P2Y2 (cross-reactive with human) were produced by Alomone (Jerusalem, Israel)/clone # APR-010. To further validate P2 receptor expression (P2Y2), primary antibodies were blocked with the specific antigen, provided by the manufacturer (Alomone Labs). Anti-human antibodies against vascular endothelial growth factor receptor 2 (VEGFR-2) were manufactured by Upstate Biotechnology (Lake Placid, NY, USA)/clone # JH121. Control antibodies included anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH; Ambion Inc., Woodward, TX, USA).
Western blots
Lysates of tissues were prepared and sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting techniques employed to separate proteins, as described [15–17]. The membranes were probed with CD39 primary antibodies. Appropriate secondary antibodies were used for detection. Equivalent gel loading was confirmed by analyses with GAPDH antibody.
Results
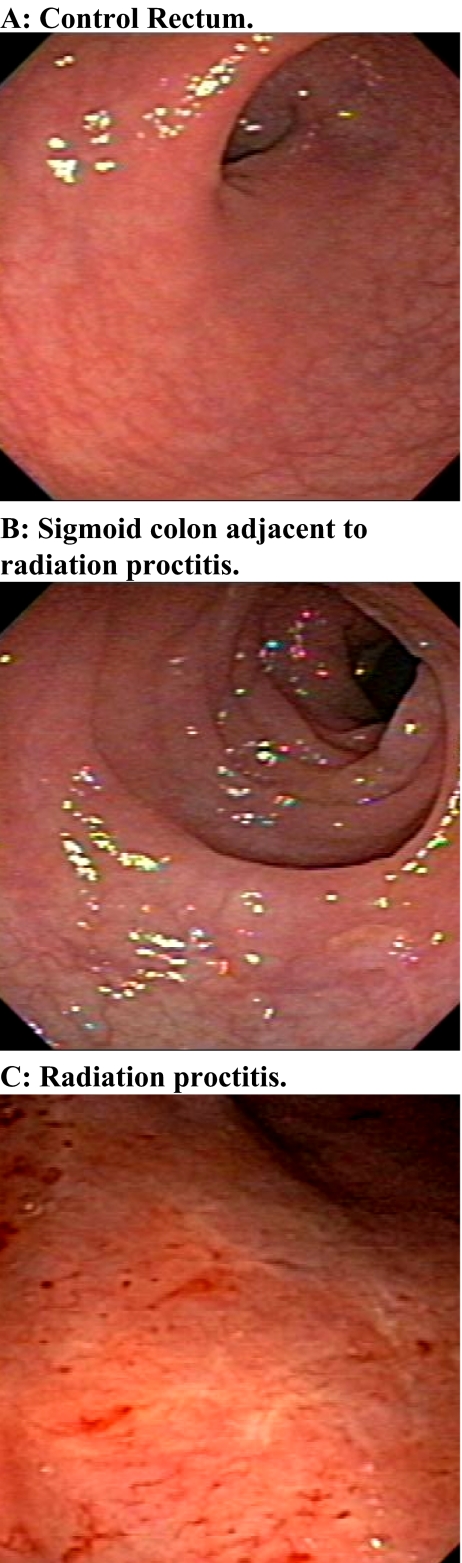
Endoscopically, the control patients all had normal-appearing rectosigmoid and colonic mucosa. Rectal mucosa of the eight patients with radiation proctitis was pale and friable and demonstrated scattered areas of vascular telangiectasia while the non-irradiated sigmoid colon had normal features (Fig. 1).
Fig. 1.
Control and pathological rectosigmoid colonoscopic views. a Normal rectal mucosal image from control patient. b Normal-appearing sigmoid colonic mucosal surfaces from patient with adjacent radiation proctitis. c Radiation proctitis showing telangiectasia and active bleeding sites
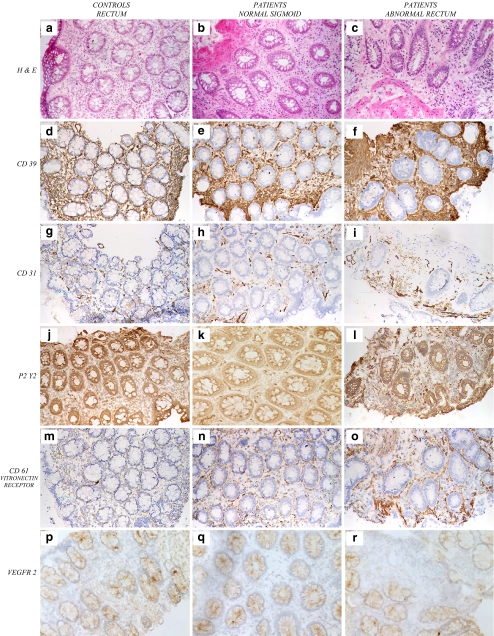
Histological examination showed normal mucosa and submucosa in the rectal biopsies from the control subjects and sigmoid biopsies from patients with radiation proctitis. In contrast, rectal biopsies from patients with proctitis after radiation showed substantial areas of scarring and fibroblastic proliferation in the submucosae with increased numbers of new vessels. Monocyte–macrophages and other mononuclear cells were present within the fibrotic lesions in involved tissues (Fig. 2a–c).
Fig. 2.
Histology and immunohistology of control and pathological rectosigmoid biopsies. The H&E staining of rectal mucosa from control patients (a) and the sigmoid mucosal biopsies from patients (b) showed normal structures. In contrast, significant fibrosis, neovascularization, and telangiectasia were evident in the rectal mucosa of patients with radiation proctitis (c). Immunohistochemical studies revealed that CD39 expression was confined to the endothelium of arterioles and venules and to low numbers of monocytes in the control biopsies and the endoscopically normal sigmoid (d, e). However, the biopsies of radiation proctitis exhibited increased numbers of new vessels that expressed high levels of CD39, in addition to high levels of CD39 staining within highly vascular myofibroblastic tissues. (f). A global score was given for CD39 expression levels, using a semiquantitative four-point scale: 0 represented the lowest and 3 the highest level of expression. Quantification of all pathological rectal biopsies from patients (n = 8) revealed a cumulative vascular score for staining of CD39 of between 2+ and 3+, whereas control biopsies (n = 5) had vascular scores of 1 to 2+. Stromal scores were all comparably 3+ for the rectal proctitis biopsies and 0–1+ for the control samples. Immunohistochemical studies for CD31 (PECAM) and P2Y2 together with the CD61 component of the vitronectin receptor show high levels of expression in the rectal mucosa affected by radiation proctitis with marked vascular proliferation and angiogenesis (g–o). The expression of VEGFR-2 was decreased in the diseased tissues of the radiation proctitis mucosa, in contrast to the normal and control biopsies (p–r)
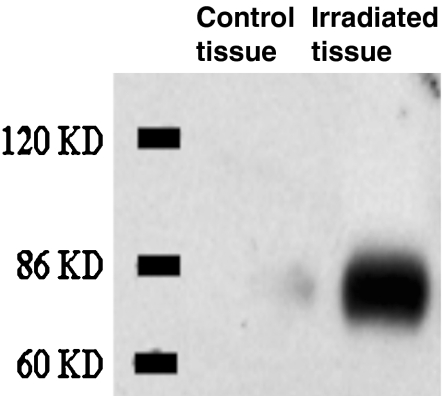
Immunohistological examination using anti-CD39 antibodies showed that the expression of CD39 was confined to the endothelium of arterioles and venules and to low numbers of mononuclear cells in the tissue of control subjects and in the unaffected rectal and sigmoidal biopsies of patients with proctitis. In the affected rectal mucosa showing radiation proctitis, the vessels expressed high levels of CD39, as did the adjacent proliferative stromal elements (Fig. 2d–f). Western blotting of tissue lysates of rectal biopsies obtained from patients with radiation proctitis revealed heightened levels of expression of CD39 (Fig. 3), in concordance with the immunopathological assessments [15, 16].
Fig. 3.
Representative Western blot analysis for CD39: Tissue biopsies from non-irradiated and irradiated rectosigmoid mucosae were processed for SDS-PAGE and immunoblotting with anti-CD39. High-level expression of CD39 was observed in the biopsies from patients with radiation proctitis
Vessels adjacent to the fibroblastic reactions in radiation proctitis displayed increased angiogenic responses indicated by the development of “mother vessel” formation that stained for high levels of CD39 (Fig. 2f). In concordance with these observations, expression of CD31, P2Y2, αvβ3 integrin (vitronectin receptor), and CD31 were all markedly increased in involved tissues in patients with proctitis (Fig. 2g–o). In contrast, VEGFR-2 expression appeared substantially decreased in the mucosal biopsies from radiation proctitis when compared to the endoscopically and histologically normal mucosae from rectum and sigmoid control biopsies (Fig. 2p–r).
Discussion
The etiology of radiation proctitis is poorly understood [19]. Acute symptoms presenting early after irradiation are believed to be largely inflammatory in nature and generally resolve by around 3 months post-exposure. Radiation proctitis is thought to manifest several months later [5]. Radiation sequentially triggers several molecular events leading to functional injury with altered cellular and intercellular signaling resulting in fibrosis and angiogenesis [10]. These more subacute reactions appear to be sustained for unclear reasons in select individuals for months and even years after exposure to radiation.
New vessel growth is modulated by monocyte/macrophages secreting angiogenic factors and metalloproteases that facilitate endothelial cell migration [13, 16, 17]. CD39 generates nucleoside analogs that have mitogenic effects on endothelial cells. The role of CD39 in modulating cellular migration and angiogenesis via P2Y-type receptors has been established by contrasting vascularization responses of control mice and CD39 null mice in response to Matrigel plug and tumor implantation [16, 17].
Eltzschig et al. have shown that CD39 and CD73 are readily induced by hypoxia, thereby resulting in increased metabolism of ATP to adenosine and amplified endothelial responses to adenosine generated at sites of hypoxia [20]. Such conditions of oxygen lack are highly likely to occur in the setting of chronic inflammation, as in radiation proctitis or with ischemia. Indeed, key studies have indicated that such activation of CD39-dependent nucleotide phosphohydrolysis may play a tissue-protective role such as in periods of renal ischemia/hypoxia [21].
CD39 has significant thromboregulatory roles that involve multiple cell types interacting together with soluble phase elements to maintain adequate fluidity of the blood. This is crucial in normal circumstances and also impacts the development and localization of thrombi in the case of vascular injury with loss of CD39 activity [13, 15, 16]. In this study, we show high levels of CD39 expression in patients with radiation proctitis. We also show comparable changes in the increased expression of P2Y2 and the vitronectin receptors that co-localize on endothelial cells. The activation of these receptors is thought to modulate the process of endothelial cell adhesion, proliferation, and angiogenesis [22]. Extracellular nucleotides have been shown to further modulate the function of integrins via G protein-coupled receptor (GPCR) P2Y-mediated signaling. Functional levels of P2 receptor expression and activation by nucleotides are in turn regulated by endothelial cell expression of CD39 [13, 17, 22–24].
Curiously, we noted distinct downregulation of VEGFR-2 in these chronically inflamed tissues biopsied months to years after radiation exposure, possibly in keeping with these late stages of irradiation injury [25]. Radiation proctitis was, however, associated with high-level vascular expression of three key components of extracellular nucleotide-mediated cellular responses (P2Y2, vitronectin receptor and CD39). CD39 expression is essential for angiogenesis [15] and high-level expression may be functionally associated with the development of the hypervascularity, as seen in radiation proctitis. In addition, anti-hemostatic actions of CD39 upon platelet ADP stimulation may contribute to rectal bleeding. The vasodilatory effect of derived adenosine may further contribute to the development of telangiectasia and refractory hemorrhage.
The expression and biochemical activity of CD39 can be modulated by various modalities, including oxygen tension [15, 20, 21]. Further animal studies with experimental radiation injury are planned that will use mutant mice null for Cd39 or transgenic for CD39 to determine the impact of altered expression at sites of radiation injury. These studies have implications in the understanding and potential treatment of rectal bleeding and disordered angiogenesis associated with radiation proctitis in the clinic.
Acknowledgments
This work has been supported by the National Institutes of Health grants HL076540 and HL63972. We thank Drs. Douglas K. Pleskow, J. Thomas Lamont, Richard Farell, Harold Greenberg, and Harry Anastopoulos from the Department of Medicine, Beth Israel Deaconess Medical Center for their help in patient referrals and clinical support and Ms. Hema Mahase for secretarial help.
References
- 1.Fajardo LF (2005) The pathology of ionizing radiation as defined by morphologic patterns. Acta Oncol 44(1):13–22 [DOI] [PubMed]
- 2.Sindelar WF, Kinsella TJ (2003) Normal tissue tolerance to intraoperative radiotherapy. Surg Oncol Clin N Am 12(4):925–942 [DOI] [PubMed]
- 3.Haboubi NY, Schofield PF, Rowland PL (1988) The light and electron microscopic features of early and late phase radiation-induced proctitis. Am J Gastroenterol 83(10):1140–1144 [PubMed]
- 4.Cotti G, Seid V, Araujo S, Souza AH Jr, Kiss DR, Habr-Gama A (2003) Conservative therapies for hemorrhagic radiation proctitis: a review. Rev Hosp Clin Fac Med Univ Sao Paulo 58(5):284–292 [DOI] [PubMed]
- 5.Andreyev J (2005) Gastrointestinal complications of pelvic radiotherapy: are they of any importance? Gut 54(8):1051–1054 [DOI] [PMC free article] [PubMed]
- 6.Johnston MJ, Robertson GM, Frizelle FA (2003) Management of late complications of pelvic radiation in the rectum and anus: a review. Dis Colon Rectum 46(2):247–259 [DOI] [PubMed]
- 7.Hayne D, Vaizey CJ, Boulos PB (2001) Anorectal injury following pelvic radiotherapy. Br J Surg 88(8):1037–1048 [DOI] [PubMed]
- 8.Hauer-Jensen M, Wang J, Denham JW (2003) Bowel injury: current and evolving management strategies. Semin Radiat Oncol 13(3):357–371 [DOI] [PubMed]
- 9.Brenn T, Fletcher CD (2006) Postradiation vascular proliferations: an increasing problem. Histopathology 48(1):106–114 [DOI] [PubMed]
- 10.Anscher MS, Vujaskovic Z (2005) Mechanisms and potential targets for prevention and treatment of normal tissue injury after radiation therapy. Semin Oncol 32(2 Suppl 3):S86–S91 [DOI] [PubMed]
- 11.Hasleton PS, Carr N, Schofield PF (1985) Vascular changes in radiation bowel disease. Histopathology 9(5):517–534 [DOI] [PubMed]
- 12.Abdollahi A, Griggs DW, Zieher H, Roth A, Lipson KE, Saffrich R, Grone HJ, Hallahan DE, Reisfeld RA, Debus J, Niethammer AG, Huber PE (2005) Inhibition of alpha(v) beta3 integrin survival signaling enhances antiangiogenic and antitumor effects of radiotherapy. Clin Cancer Res 11(17):6270–6279 [DOI] [PubMed]
- 13.Kittel A, Csapo ZS, Csizmadia E, Jackson SW, Robson SC (2004) Co-localization of P2Y1 receptor and NTPDase1/CD39 within caveolae in human placenta. Eur J Histochem 48(3):253–259 [PubMed]
- 14.Wang J, Albertson CM, Zheng H, Fink LM, Herbert JM, Hauer-Jensen M (2002) Short-term inhibition of ADP induced platelet aggregation by clopidogrel ameliorates radiation induced toxicity in rat small intestine. Thromb Haemost 87(1):122–128 [PubMed]
- 15.Robson SC, Wu Y, Sun X, Knosalla C, Dwyer K, Enjyoji K (2005) Ectonucleotidases of CD39 family modulate vascular inflammation and thrombosis in transplantation. Semin Thromb Hemost 31(2):217–233 [DOI] [PubMed]
- 16.Goepfert C, Sundberg C, Sevigny J, Enjyoji K, Hoshi T, Csizmadia E, Robson S (2001) Disordered cellular migration and angiogenesis in cd39-null mice. Circulation 104(25):3109 [DOI] [PubMed]
- 17.Jackson SW, Hoshi T, Wu Y, Sun X, Enjyoji K, Cszimadia E, Sundberg C, Robson SC (2007) Disordered purinergic signaling inhibits pathological angiogenesis in cd39/Entpd1-null mice. Am J Pathol 171(4):1395–1404 [DOI] [PMC free article] [PubMed]
- 18.Satterwhite CM, Farrelly AM, Bradley ME (1999) Chemotactic, mitogenic, and angiogenic actions of UTP on vascular endothelial cells. Am J Physiol 45:H1091–H1097 [DOI] [PubMed]
- 19.Beckman JA, Thakore A, Kalinowski BH, Harris JR, Creager MA (2001) Radiation therapy impairs endothelium-dependent vasodilation in humans. J Am Coll Cardiol 37(3):761–765 [DOI] [PubMed]
- 20.Eltzschig HK, Ibla JC, Furuta GT, Leonard MO, Jacobson KA, Enjyoji K, Robson SC, Colgan SP (2003) Coordinated adenine nucleotide phosphohydrolysis and nucleoside signaling in posthypoxic endothelium: role of ectonucleotidases and adenosine A2B receptors. J Exp Med 198(5):783–796 [DOI] [PMC free article] [PubMed]
- 21.Grenz A, Zhang H, Hermes M, Eckle T, Klingel K, Huang DY, Müller CE, Robson SC, Osswald H, Eltzschig HK (2007) Contribution of E-NTPDase1 (CD39) to renal protection from ischemia–reperfusion injury. FASEB J 21(11):2863–2873 [DOI] [PubMed]
- 22.Erb L, Liu J, Ockerhausen J, Kong Q, Garrad RC, Griffin KNC, Krugh B, Santiago-Perez LI, Gonzalez FA, Gresham HD et al (2001) An RGD sequence in the P2Y(2) receptor interacts with alpha(v) beta(3) integrins and is required for G(o)-mediated signal transduction. J Cell Biol 153:491–501 [DOI] [PMC free article] [PubMed]
- 23.Carmeliet P (2000) Mechanisms of angiogenesis and arteriogenesis. Nat Med 6(4):389–395 [DOI] [PubMed]
- 24.Hodivala-Dilke KM, Reynolds AR, Reynolds LE (2003) Integrins in angiogenesis: multitalented molecules in a balancing act. Cell Tissue Res 314:131–144 [DOI] [PubMed]
- 25.Zips D, Eicheler W, Geyer P, Hessel F, Dorfler A, Thames HD, Haberey M, Baumann M (2005) Enhanced susceptibility of irradiated tumor vessels to vascular endothelial growth factor receptor tyrosine kinase inhibition. Cancer Res 65(12):5374–5379 [DOI] [PubMed]