Abstract
This study examines the action of agonists and antagonists of P2 receptors on mouse mesenteric artery contractions and the possible involvement of these signaling pathways in myogenic tone (MT) evoked by elevated intraluminal pressure. Both ATP and its non-hydrolyzed analog α,β-ATP triggered transient contractions that were sharply decreased in the presence of NF023, a potent antagonist of P2X1 receptors. In contrast, UTP and UDP elicited sustained contractions which were suppressed by MRS2567, a selective antagonist of P2Y6 receptors. Inhibition of Na+, K+, 2Cl− cotransport (NKCC) with bumetanide led to attenuation of contractions in UTP- but not ATP-treated arteries. Both UTP-induced contractions and MT were suppressed by MRS2567 and bumetanide but were insensitive to NF023. These data implicate a P2Y6-mediated, NKCC-dependent mechanism in MT of mesenteric arteries. The action of heightened intraluminal pressure on UTP release from mesenteric arteries and its role in the triggering of P2Y6-mediated signaling should be examined further.
Keywords: Mesenteric arteries; Myogenic tone; P2Y receptors; Na+, K+, 2Cl− cotransport
Discovered more than 100 years ago, myogenic tone (MT) is the intrinsic ability of small arteries to constrict in response to increases in intraluminal pressure in the absence of blood flow [1]. Later on, MT was documented in nearly all arterial vessels with diameters below 150 μm [2]. Because of its key role in peripheral resistance as well as in the maintenance of constant capillary blood flow and hydrostatic pressure [3], the involvement of abnormal MT responses in the pathogenesis of cardiovascular diseases and protection against hypertension-induced organ damage is widely disputed [4–7].
Despite the obvious physiological and pathophysiological significance of MT, upstream mechanisms that translate pressure changes to vascular smooth muscle cell (VSMC) contractions are poorly understood [3, 8–10]. There are gaps in both our knowledge of the molecular make-up of sensory elements and the sensed signal(s) triggered by elevated intraluminal pressure. In accordance with the most widely discussed hypothesis, pressure-sensing is provided by conformational transitions of ion channels and other potential stretch-activated plasma-membrane-bound proteins and/or the cytoskeleton network [3, 11]. However, the molecular origin of pressure-sensitive protein transducers remains unknown. Our recent estimation of plasma membrane reserves indicates that in most physiological situations, tension of the plasma-membrane-unconstrained lipid bilayer is unlikely to rise to the level required for the activation of mechanosensitive proteins [12]. The disruption of actin-microfilaments by cytochalasin D blocked pressure-induced contractions but did not prevent VSMC depolarization, an obligatory step in MT development. It was also shown that mice lacking key elements of the VSMC cytoskeleton network, such as vimetin and desmin, did not exhibit altered MT (for more details, see [11]).
Searches for potential mechanosensitive systems revealed that very modest shear stress, extracellular fluid movement, and cell volume modulation led to the release of ATP, UTP, and other nucleotides from almost all cell types studied so far, including VSMC and endothelial cells [13–15]. It was also documented that activation of ligand-gated P2X receptor cation channels and G protein-coupled P2Y receptors with released nucleotides contributed to the autocrine regulation of blood vessel tone and remodeling [16, 17]. To the best of our knowledge, the role of purinoceptors in blood pressure-sensing and MT development has not yet been explored.
Ubiquitously expressed Na+, K+, 2Cl− cotransporter NKCC1 belongs to the superfamily of Cl−-coupled carriers. Under baseline conditions ([Na+]o>>[Na+]i and [Cl-]o2>>[Cl−]i2), it provides inwardly directed net electroneutral symport of Na+, K+, and Cl- and maintenance of [Cl−]i above values predicted by the Nernst equilibrium potential [18]. Importantly, unlike the dominant contribution of K+ permeability (PK) to resting membrane potential (Em) in skeletal and cardiac muscles, the PK/PCl ratio in VSMC varies from 0.8 to 0.5 [19]. These data suggest that high-ceiling diuretics suppress MT of the renal afferent arterioles via NKCC inhibition, attenuation of [Cl−]i and VSMC hyperpolarization. Indeed, both furosemide and bumetanide decrease [Cl−]i [20–22], hyperpolarize VSMC [21], suppress the activation of L-type Ca2+ channels [22] and reduce the baseline tone of vascular and non-vascular smooth muscles [23–25] as well as contractions triggered by diverse stimuli, including [K+]o-induced depolarization [22], phenylephrine [22, 26–28], histamine [29], angiotensin II [30], thromboxane A2 [31, 32], and oxytocin [33, 34]. Recently, it was demonstrated that high-ceiling diuretics, such as bumetanide and furosemide that are known to be potent NKCC inhibitors, almost completely abolished the MT of renal afferent arterioles in perfused hydronephrotic rat kidneys in vitro [32]. We designed this study to examine the role of P2 receptors and NKCC in MT regulation in mouse mesenteric arteries.
Methods
Mice Experiments were performed on 2- to 4-month-old male C57B1/6 mice obtained from Charles River Canada (Montreal, PQ) and the International Biotechnological Research Centre (Moscow, Russia). Before experimentation, the mice were subjected to a lethal dose of CO2 in accordance with the protocol approved by the respective institutional animal care and use committees.
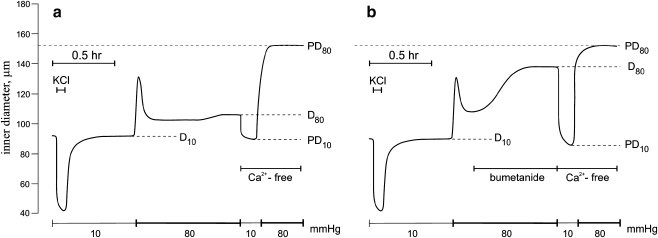
Mesenteric artery contractions The animal intestines were removed surgically with their mesentery beds attached and placed in ice-cold, physiologically balanced salt solution (PSS): 135 mM NaCl, 5 mM KCl, 1.5 mM CaCl2, 1.2 mM MgCl2, 5 mM glucose, 20 mM HEPES; pH 7.4 was adjusted at 37°C with Tris. Dissected segments of the second- and third-order arteries, 1–2 mm in length, were isolated from the intestinal tract and fat tissues by dissection microscopy (Motic, Richmond, BC). They were transferred to a 15-ml arteriographic chamber (Living System Instrumentation, Inc., Burlington, VT), cannulated at both ends with micropipettes and mounted in a Motic microscope supplied with a video-monitored perfusion system. The signal derived from this system was processed by video dimension analyzer, for continuous measurement of intraluminal diameter. MT was triggered by elevation of ultraluminal pressure from 10 to 80 mmHg. We noted that it was sharply decreased in mesenteric arteries with intraluminar diameter below 120 μm and increased in the presence of bicarbonate. Considering this, we used vessels with intraluminar diameters in the range from 70 to 110 μm and PSS containing 25 mM NaHCO3 and 120 mM NaCl; pH was adjusted to 7.4 with HEPES. Passive diameter at 80 mmHg (PD80) was measured after 10-min incubation of arteries in Ca2+-free PSS containing 2 mM EGTA, and MT was quantified as 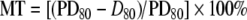 , where D80 was the baseline intraluminar diameter at the same pressure (Fig. 1). Note that MT was absent at 10 mmHg (PD10∼D10), showing that it was triggered by heightened intraluminal pressure. [K+]o-induced contractions were evoked by equimolar substitution of NaCl with 80 mM KCl.
, where D80 was the baseline intraluminar diameter at the same pressure (Fig. 1). Note that MT was absent at 10 mmHg (PD10∼D10), showing that it was triggered by heightened intraluminal pressure. [K+]o-induced contractions were evoked by equimolar substitution of NaCl with 80 mM KCl.
Fig. 1.
Typical recording showing the effect of intraluminal pressure and Ca2+-free medium on the internal diameter of mouse resistance mesenteric arteries in the absence (a) and presence (b) of 100 μM bumetanide
Chemicals Bumetanide, ATP, UTP, UDP, α,β-methylene-ATP (α,β-ATP), pyridoxal-5′-phosphate-6-azo(benzene-2′,4′-disulfonic acid) tetrasodium salt (PPADS), suramin, nicardipine, apyrase—Sigma (St. Louis, MO); 2′-deoxy-N6-methyl adenosine-3′,5′-biphosphate (MRS2567}—Tocris Chemicals, Ellisville, MO; 8,8′-carbonylbis(imino-3,1-phenylene)-bis-1,3,5-naphthalenetrisulfonic acid sodium salt (NF023)—Calbiochem, La Jolla, CA; salts, d-glucose and buffers—Sigma and Anachemia (Montreal, PQ).
Results
Mesenteric artery contractions evoked by P2 agonists
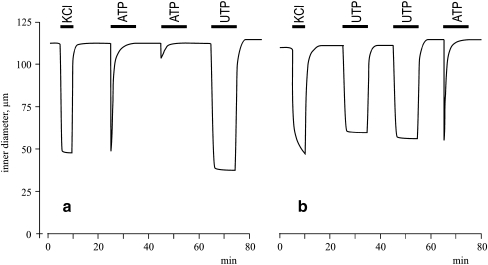
Figure 2A demonstrates that the addition of 100 μM ATP led to transient mesenteric artery contractions that were completely abolished in 3–5 min. We also noted that repeated stimulation with ATP in 10 to 30 min of washing in PSS had a negligible effect compared to the first exposure. Unlike ATP, 100 μM UTP elicited sustained contractions (Fig. 2B) which did not significantly change during 40-min treatment (data not shown). Significantly, both sustained contractions evoked by UTP and transient contractions induced by ATP were preserved in UTP-pretreated vessels (Fig. 2).
Fig. 2.
Typical recording charting the effects of 80 mM KCl, 100 μM ATP and 100 μM UTP on the internal diameter of mouse resistance mesenteric arteries
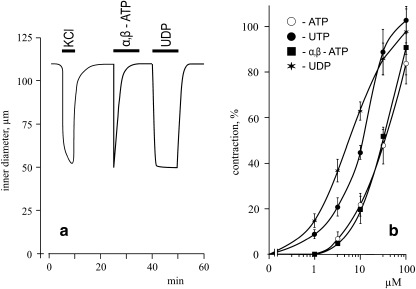
Addition of the non-hydrolyzed ATP analog α,β-ATP and UDP caused transient and sustained contractions, respectively (Fig. 3A). ATP- and α,β-ATP triggered half-maximal mesenteric artery contractions were somewhat similar (∼30 μM), whereas UDP was more effective than UTP (EC50 ∼5 and 10 μM, respectively; Fig. 3B).
Fig. 3.
a Typical recording illustrating the effects of 80 mM KCl, 100 μM α,β-ATP, and 100 μM UDP on the internal diameter of mouse resistance mesenteric arteries. b Dose-dependent actions of ATP, UTP, UDP, and α,β-ATP on mesenteric artery contractions. Maximal values of contractions evoked by 80 mM KCl were taken as 100%. Means ± S.E. of three independent experiments are shown
Effect of P2 antagonists
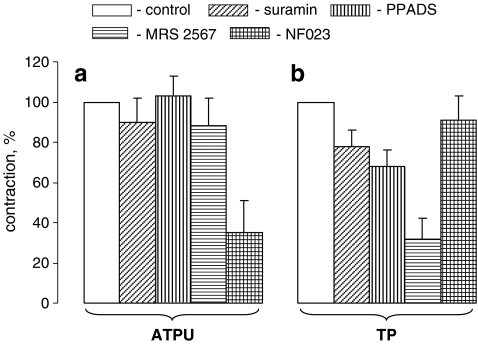
It is well-documented that suramin modestly suppresses several subtypes of P2X and P2Y receptors [14]. We observed that this compound decreased UTP-induced contractions by 22% (p < 0.05), but did not significantly change contractions evoked by ATP (Fig. 4). Contractions triggered by UTP were also suppressed by PPADS, a modest antagonist of P2X2, P2X3, P2X5, P2Y2, and P2Y6 receptors [14, 35], and by MRS2567, a selective antagonist of P2Y6 receptors [35]. ATP-induced contractions were not affected by the above-listed compounds, but were sharply reduced in the presence of NF023, a potent antagonist of cloned P2X1 and P2X3 receptors [14].
Fig. 4.
Effect of purinoceptor antagonists on mesenteric artery contractions evoked by ATP (a) and UTP (b); 100 μM suramin, 100 μM PPADS, 10 μM MRS2567 and 10 μΜ NF023 were added 10 min before the treatment of mesenteric arteries with 100 μM ATP or UTP. Maximal values of contractions measured in the absence of purinergic antagonists were taken as 100%. Means ± S.E. of three independent experiments are reported. *p < 0.02
Effect of bumetanide
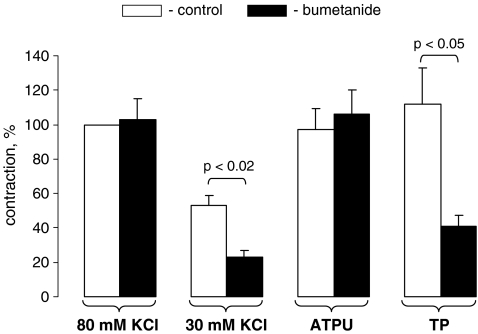
Figure 5 shows that NKCC inhibition with bumetanide led to twofold attenuation of mesenteric artery contractions caused by modest depolarization ([K+]o = 30 mM), but did not affect contractions in vessels subjected to full-scale depolarization ([K+]o = 80 mM). These results are consistent with data obtained in a study on contractions of depolarized endothelium-denuded strips from rat aortae [22]. We did not observe any inhibitory action of bumetanide on contractions triggered by ATP, whereas contractions of UTP-treated arteries were decreased by 60% (Fig. 5).
Fig. 5.
Effect of bumetanide on mesenteric artery contractions evoked by KCl, ATP, and UTP. Bumetanide (100 μM) was added 30 min before the treatment of mesenteric arteries with KCl (80 or 30 mM), ATP (100 μM) or UTP (100 μM). Maximal values of contractions triggered by 80 mM KCl and measured in the absence of bumetanide were taken as 100%. Means ± S.E. of three independent experiments are presented
Myogenic tone
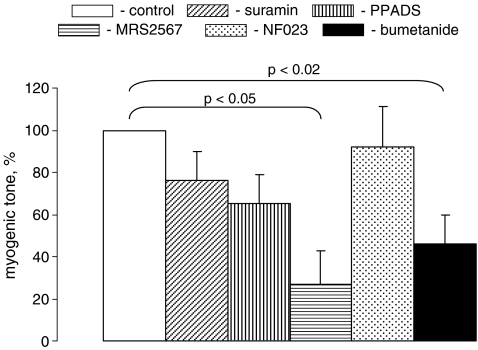
MT was diminished by ∼3.5-fold in mesenteric arteries pretreated for 40 min with MRS2567 (p < 0.02; Fig. 6). It was also suppressed by suramin and PPADS, but these differences were not statistically significant. Bumetanide time-dependently increased mesenteric artery intraluminal diameter pressurized at 80 mmHg (Fig. 1B) which resulted in a twofold attenuation of MT (Fig. 6).
Fig. 6.
Effects of purinoceptor antagonists and bumetanide on MT in mesenteric arteries treated with 100 μM suramin, 100 μM PPADS, 10 μM MRS2567, 10 μΜ NF023 or 100 μM bumetanide 40 min before their transfer in Ca2+-free PSS, as illustrated in Fig. 1. In the absence of the tested compounds, MT varied in the range from 29 to 41% of passive diameter, and these values were taken as 100%. Means ± S.E. of four independent experiments are shown
Discussion
Seven P2X receptor subunits (P2X1–7) and seven P2Y receptors (P2Y1, P2Y2, P2Y4, P2Y6, P2Y11, P2Y12, and P2Y13) have been cloned from mammalian cDNA libraries [14]. Among them, P2X1, P2X2, P2X4, P2X5, P2Y2, P2Y4, and P2Y6 mRNA species and proteins interacting with anti-P2X1, anti-P2Y2, and anti-P2Y6 antibodies have been detected in rodent small arteries [36–40]. Our results show that transient and sustained constrictions of mouse mesenteric arteries evoked by ATP and UTP, respectively, are mainly mediated by P2X1 and P2Y6 receptors. Indeed, the transient kinetics of ATP-induced contractions followed by prolonged inactivation of this signaling pathway (Fig. 2) are consistent with rapid decay of ATP-induced cation current detected in cells transfected with P2X1 and P2X3 but not other P2X isoforms [41, 42]. Significantly, ATP-induced contractions were sharply suppressed by NF023, a compound that inhibited ion currents mediated by cloned P2X1 and P2X3 with IC50 of 0.2 and 8 μM, respectively, but did not affect the activity of the other P2 receptors [14]. The major role of P2X1 receptors in ATP-induced contractions is consistent with the absence of an inhibitory action of PPADS, a potent antagonist of P2X2, P2X3, and P2X5 receptors [14, 35], and with the lack of contractions in ATP-treated mesenteric arteries from P2X1−/− mice [39].
A key function of P2Y6 receptors in sustained constrictions triggered by UTP was supported by several observations. First, sustained constrictions were absent in arteries treated with ATP and α,β-ATP, potent agonists of all cloned P2X and P2Y receptors with the exception of the P2Y6 isoform [14, 40]. Second, the apparent affinity of sustained contractile responses was higher for UDP compared to UTP (Fig. 3B), which is consistent with the rank-order potency for P2Y6 rather than for other UTP- and UDP-sensitive receptors, such as P2Y2 and P2Y4 [40, 43]. Third, UTP-induced constrictions were sharply suppressed in the presence of 10 μM MRS2567 (Fig. 4). At this concentration, the compound almost completely depressed the signaling evoked by P2Y6 without any effect on the activity of cloned P2Y2 and P2Y4 receptors [35].
Our results indicate that sustained constrictions caused by P2Y6 G protein-coupled receptors rather than transient activation of P2X1 receptor-mediated, inwardly directed Ca2+ currents are involved in the long-term maintenance of slowly-developing MT in mesenteric arteries. Indeed, both UTP-induced contractions and MT were depressed by the NKCC inhibitor bumetanide and the P2Y6 antagonist MRS2567 and were insensitive to the P2X1 antagonist NF023 (Figs. 4B, 5, and 6). In contrast, constrictions of ATP-treated vessels were sharply suppressed by NF023 but were resistant to bumetanide (Fig. 5). The distinct action of bumetanide on ATP- and UTP-induced contractions is consistent with different mechanisms of action of these nucleotides on excitation-constriction coupling. Indeed, in rat mesenteric arteries, UTP triggered modest and sustained depolarization from −60 to −50 mV [44] that was probably caused by the activation of Ca2+-sensitive Cl− channels and could be abolished by the addition of bumetanide that decreases [Cl−]i and approaches Nernst Cl− equilibrium potential to resting Em [20–22]. Unlike UTP, ATP affected VSMC contractions via transient activation of Ca2+ influx through P2X ion channels and independently of Em modulation [44]. Finally, the slow kinetic of MT development in mesenteric arteries is consistent with the presence of P2Y6 receptors in endothelial cells triggering NO-mediated vasorelaxation [45] and suppressing the rapid phase of vasoconstriction evoked by interaction of UTP/UDP with VSMC P2Y6 receptors.
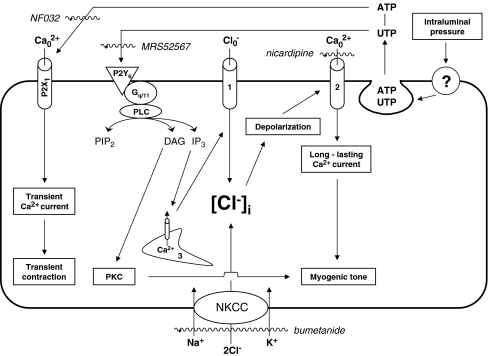
The data considered above are consistent with the signaling mechanism presented schematically in Figure 7. In accordance with the proposed model, intraluminal pressure acting through unidentified sensors leads to the release of intracellular nucleotides, such as ATP and UTP. Extracellular ATP triggers inwardly directed Ca2+ currents via activation of non-selective cation channels assembled from P2X1 subunits. These channels are, however, rapidly inactivated, which results in transient artery contractions rather than long-lasting MT. Unlike ATP, UTP activates P2Y6 Gq/G11-coupled receptors that, in turn, evoke Ca2+ release from the endoplasmic reticulum and activation of protein kinase C (PKC). Ca2+ release from the endoplasmic reticulum elicits activation of Cl− channels, plasma membrane depolarization and activation of long-lasting L-type Ca2+ channels, whose involvement in MT is well-documented in an overwhelming number of vessels studied so far [3]. Significantly, NKCC inhibition partially blocked the depolarizing action of Cl− channel activation via attenuation of [Cl-]i, as demonstrated in bumetanide- and furosemide-treated smooth muscle cells of different origins [20–22], and resulted in the MT suppression documented here (Fig. 6). Side-by-side with the [Ca2+]i signaling precipitated by P2Y6 receptors, MT may involve PKC activation that, in turn, leads to heightened [Ca2+]i sensitivity of the contractile machinery via myosin light chain kinase phosphorylation [3].
Fig. 7.
Possible mechanisms involving P2Y6 receptors and Na+, K+, 2Cl− cotransporter (NKCC) in the maintenance of MT. IP3 inositol 1,4,5-triphosphate, PIP2 triphosphoinositide, PKC protein kinase C, PLC phospholipase C; 1 Ca2+-activated Cl− channels, 2 voltage-gated L-type Ca2+ channels, 3 endoplasmic reticulum, ? unknown pressure-sensing mechanism. Compounds used in the study appear in italics
The model considered above assumes that MT is suppressed by apyrase (ATP diphosphohydralase), providing ATP and UTP hydrolysis to yield di- and monophosphonucleosides [46]. However, we failed to detect any significant action of 2 U/ml apyrase on MT of pressurized mesenteric arteries (data not shown). To explain these negative results, two hypotheses may be proposed. First, the diffusion limit caused by adventitial and endothelial cells protects released ATP and UTP from degradation by apyrase. Second, apyrase-mediated accumulation of diphosphonucleosides is sufficient to activate purinoceptors. Indeed, we demonstrated that UDP is a more potent activator of mesenteric artery contractions than UTP (Fig. 3B). Precise measurements of tri-, di-, and monophosphonucleosides released from pressurized vessels in space-limited compartments should be performed to further evaluate their relative impact on MT maintenance.
Acknowledgments
This work was supported by grants from the Canadian Institutes of Health Research and the Heart and Stroke Foundation of Canada. The editorial assistance of Ovid Da Silva (irtc.inc@hotmail.com) is acknowledged.
References
- 1.Bayliss WM (1902) On the local reactions of the arterial wall to changes of internal pressure. J Physiol 28:220–231 [DOI] [PMC free article] [PubMed]
- 2.Uchida E, Bohr DF (1969) Myogenic tone in isolated perfused vessels. Occurrence among vascular beds and along vascular trees. Circ Res 25:549–555 [DOI] [PubMed]
- 3.Davis MJ, Hill MA (1999) Signaling mechanisms underlying the vascular myogenic response. Physiol Rev 79:387–423 [DOI] [PubMed]
- 4.Zhang J, Lee MY, Cavalli M et al (2005) Sodium pump a2 subunits control myogenic tone and blood pressure in mice. J Physiol 569:243–256. doi:10.1113/jphysiol.2005.091801 [DOI] [PMC free article] [PubMed]
- 5.Murphy TV (2007) I don't need this pressure on; src-family kinases, ERK 1/2 kinase and mechanotransduction in arteries. J Hypertens 25:1791–1793. doi:10.1097/HJH.0b013e3282ef45f9 [DOI] [PubMed]
- 6.Hughes JM, Bund SJ (2002) Arterial myogenic properties of the spontaneously hypertensive rats. Exp Physiol 87:527–534. doi:10.1113/eph8702399 [DOI] [PubMed]
- 7.Loutzenhiser R, Griffin K, Williamson G et al (2006) Renal autoregulation: new perspectives regarding the protective and regulatory roles of the underlying mechanisms. Am J Physiol Regul Integr Comp Physiol 290:R1153–R1167. doi:10.1152/ajpregu.00402.2005 [DOI] [PMC free article] [PubMed]
- 8.Zhang J, Berra-Romani R, Sinnegger-Brauns MJ et al (2007) Role of Cav1.2 L-type Ca2+ channels in vascular tone: effects of nifedipine and Mg2+. Am J Physiol Heart Circ Physiol 292:H415–H425. doi:10.1152/ajpheart.01214.2005 [DOI] [PubMed]
- 9.Lucchesi PA, Sabri A, Belmadani S et al (2004) Involvement of metalloproteinases 2/9 in epidermal growth factor receptor transactivation in pressure-induced myogenic tone in mouse mesenteric resistance arteries. Circulation 110:3587–3593. doi:10.1161/01.CIR.0000148780.36121.47 [DOI] [PubMed]
- 10.Schubert R, Lidington D, Bolz S-S (2008) The emerging role of Ca2+ sensitivity regulation in promoting myogenic vasoconstriction. Cardiovasc Res 77:8–18 [DOI] [PubMed]
- 11.Hill MA, Davis MJ, Meininger GA et al (2006) Arteriolar myogenic signaling mechanisms: implications for local vascular functions. Clin Hemorheol Microcirc 34:67–79 [PubMed]
- 12.Groulx N, Boudreault F, Orlov SN et al (2006) Membrane reserves and hypotonic cell swelling. J Membr Biol 214:43–56. doi:10.1007/s00232-006-0080-8 [DOI] [PubMed]
- 13.Tatur S, Groulx N, Orlov SN et al (2007) Ca2+-dependent ATP release from A549 cells involves synergic autocrine stimulation by coreleased uridine nucleotides. J Physiol 584:419–435. doi:10.1113/jphysiol.2007.133314 [DOI] [PMC free article] [PubMed]
- 14.Burnstock G (2007) Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev 87:659–797. doi:10.1152/physrev.00043.2006 [DOI] [PubMed]
- 15.Zhao Y, Migita K, Sato C et al (2007) Endoplasmic reticulum is a key organella in bradykinin-triggered ATP-release from cultured smooth muscle cells. J Pharmacol Sci 105:57–65. doi:10.1254/jphs.FP0070865 [DOI] [PubMed]
- 16.Erlinge D, Burnstock G (2008) P2 receptors in cardiovascular regulation and disease. Purinergic Signal 4:1–20. doi:10.1007/s11302-007-9078-7 [DOI] [PMC free article] [PubMed]
- 17.Burnstock G (2006) Vessel tone and remodeling. Nat Med 12:16–17. doi:10.1038/nm0106-16 [DOI] [PubMed]
- 18.Gamba G (2005) Molecular physiology and pathophysiology of electroneutral cation-chloride cotransporters. Physiol Rev 85:423–493. doi:10.1152/physrev.00011.2004 [DOI] [PubMed]
- 19.Chipperfield AR, Harper AA (2001) Chloride in smooth muscle. Prog Biophys Mol Biol 74:175–221. doi:10.1016/S0079-6107(00)00024-9 [DOI] [PubMed]
- 20.Kreye VA, Bauer PK, Villhauer I (1981) Evidence for furosemide-sensitive active chloride transport in vascular smooth muscle. Eur J Pharmacol 73:91–95. doi:10.1016/0014-2999(81)90150-3 [DOI] [PubMed]
- 21.Davis JPL, Chipperfield AR, Harper AA (1993) Accumulation of intracellular chloride by (Na–K–Cl) cotransport in rat arterial smooth muscle is enhanced in deoxycorticosterone acetate (DOCA)/salt hypertension. J Mol Cell Cardiol 25:233–237. doi:10.1006/jmcc.1993.1029 [DOI] [PubMed]
- 22.Anfinogenova YJ, Baskakov MB, Kovalev IV et al (2004) Cell-volume-dependent vascular smooth muscle contraction: role of Na+, K+, 2Cl- cotransport, intracellular Cl- and L-type Ca2+ channels. Pflugers Arch 449:42–55. doi:10.1007/s00424-004-1316-z [DOI] [PubMed]
- 23.Barthelmebs M, Stephan D, Fontaine C et al (1994) Vascular effects of loop diuretics: an in vivo and in vitro study in the rat. Naunyn Schmiedebergs Arch Pharmacol 349:209–216. doi:10.1007/BF00169839 [DOI] [PubMed]
- 24.Lavallee SL, Iwamoto LM, Claybaugh JR et al (1997) Furosemide-induced airway relaxation in guinea pigs: relation to Na-K-2Cl cotransporet function. Am J Physiol 273:L211–L216 [DOI] [PubMed]
- 25.Tian R, Aalkjaer C, Andreasen F (1990) Mechanisms behind the relaxing effect of furosemide on the isolated rabbit ear artery. Pharmacol Toxicol 67:406–410 [DOI] [PubMed]
- 26.Akar F, Skinner E, Klein JD et al (1999) Vasoconstrictors and nitrovasodilators reciprocally regulate the Na+-K+-2Cl- cotransporter in rat aorta. Am J Physiol 276:C1383–C1390 [DOI] [PubMed]
- 27.Garg P, Martin C, Elms SC et al (2007) Effect of the Na–K–2Cl cotransporter NKCC1 on systematic blood pressure and smooth muscle tone. Am J Physiol Heart Circ Physiol 292:H2100–H2105. doi:10.1152/ajpheart.01402.2006 [DOI] [PMC free article] [PubMed]
- 28.Palacios J, Espinoza F, Munita C et al (2006) Na+–K+–2Cl− cotransporter is implicated in gender differences in the response of the rat aorta to phenylephrine. Br J Pharmacol 148:964–972. doi:10.1038/sj.bjp. 0706818 [DOI] [PMC free article] [PubMed]
- 29.Kovalev IV, Baskakov MB, Medvedev MA et al (2008) Na+, K+, 2Cl− cotransport and chloride permeability of the cell membrane in mezaton and histamine regulation of electrical and contractile activity in smooth muscle cells from the guinea pig ureter. Russ Phys J 93:306–317 [PubMed]
- 30.Stanke F, Devillier P, Breant D et al (1998) Furosemide inhibits angiotensin II-induced contraction on human vascular smooth muscle. Br J Clin Pharmacol 46:571–575. doi:10.1046/j.1365-2125.1998.00820.x [DOI] [PMC free article] [PubMed]
- 31.Stanke-Labesque F, Craciwski JL, Bedouch P et al (2000) Furosemide inhibits thrombaxane A2-induced contraction in isolated human internal artery and saphenous vein. J Cardiovasc Pharmacol 35:531–537. doi:10.1097/00005344-200004000-00003 [DOI] [PubMed]
- 32.Wang X, Breaks J, Loutzenhiser K et al (2007) Effects of inhibition of the Na+/K+/2Cl− cotransporter on myogenic and angiotensin II responses of the rat afferent arteriole. Am J Physiol Renal Physiol 292:F999–F1006. doi:10.1152/ajprenal.00343.2006 [DOI] [PubMed]
- 33.Mozhayeva MG, Bagrov YY (1995) The inhibitory effects of furosemide on Ca2+ influx pathways associated with oxytocin-induced contractions of rat myometrium. Gen Physiol Biophys 14:427–436 [PubMed]
- 34.Mozhayeva MG, Bagrov YY, Ostretsova IB et al (1994) The effect of furosemide on oxytocin-induced contractions of the rat myometrium. Exp Physiol 79:661–667 [DOI] [PubMed]
- 35.von Kügelgen I (2006) Pharmacological profiles of cloned mammalian P2Y-receptor subtypes. Pharmacol Ther 110:415–4322. doi:10.1016/j.pharmthera.2005.08.014 [DOI] [PubMed]
- 36.Nori SL, Fumagalli L, Bo X et al (1998) Coexpression of mRNAs for P2X1, P2X2 and P2X4 receptors in rat vascular smooth muscle: an in situ hybridization and RT-PCR study. J Vasc Res 35:179–185. doi:10.1159/000025582 [DOI] [PubMed]
- 37.Phillips JK, McLean AL, Hill CE (1998) Receptors involved in nerve-mediated vasoconstriction in small arteries of the rat mesentery. Br J Pharmacol 124:1403–1412. doi:10.1038/sj.bjp.0701976 [DOI] [PMC free article] [PubMed]
- 38.Volchanova L, Arvidsson U, Riedl M et al (1996) Differential distribution of two ATP-gated ion channels (P2X receptors) determined by immunohistochemistry. Proc Natl Acad Sci USA 93:7617–7622 [DOI] [PMC free article] [PubMed]
- 39.Vial C, Evans RJ (2002) P2X1 receptor-deficient mice establish the native P2X receptor and P2Y6-like receptor arteries. Mol Pharmacol 62:1438–1445. doi:10.1124/mol.62.6.1438 [DOI] [PubMed]
- 40.Rayment SJ, Latif ML, Ralevic V et al (2007) Evidence for the expression of multiple uracil nucleotide-stimulated P2 receptors coupled to smooth muscle contraction in porcine isolated arteries. Br J Pharmacol 150:604–612. doi:10.1038/sj.bjp. 0707120 [DOI] [PMC free article] [PubMed]
- 41.Roberts JA, Vial C, Digby HR et al (2006) Molecular properties of P2X receptors. Pfluger Arch 452:486–500. doi:10.1007/s00424-006-0073-6 [DOI] [PubMed]
- 42.Egan TM, Samways DSK, Li Z (2006) Biohysics of P2X receptors. Pflugers Arch 452:501–512. doi:10.1007/s00424-006-0078-1 [DOI] [PubMed]
- 43.Cipleu CD, Palant CE, Sanders KM et al (2002) Separation of two Cl− currents in cultured human and murine mesangial cells: biphysical and pharmacological characteristics of ICl.vol. and ICl. Ca. J.Vasc Res 39:426–436 [DOI] [PubMed]
- 44.Juul B, Plesner L, Aalkjaer C (1992) Effects of ATP and UTP on [Ca2+]i, membrane potential and force in isolated rat small arteries. J Vasc Res 29:385–395. doi:10.1159/000158955 [DOI] [PubMed]
- 45.Bar I, Guns PJ, Metallo J et al (2008) Knockout mice reveal a role for P2Y6 receptor in macrophages, endothelial cells, and vascular smooth muscle cells. Mol Pharmacol 74:777–784. doi:10.1124/mol.108.046904 [DOI] [PubMed]
- 46.Shibata K, Morita Y, Abe S et al (1999) Apyrase from pea stems: isolation, purification, characterization and identification of a NTPase from the cytoskeleton fraction of pea stem tissue. Plant Physiol Biochem 37:881–888. doi:10.1016/S0981-9428(99)00102-3 [DOI]