Abstract
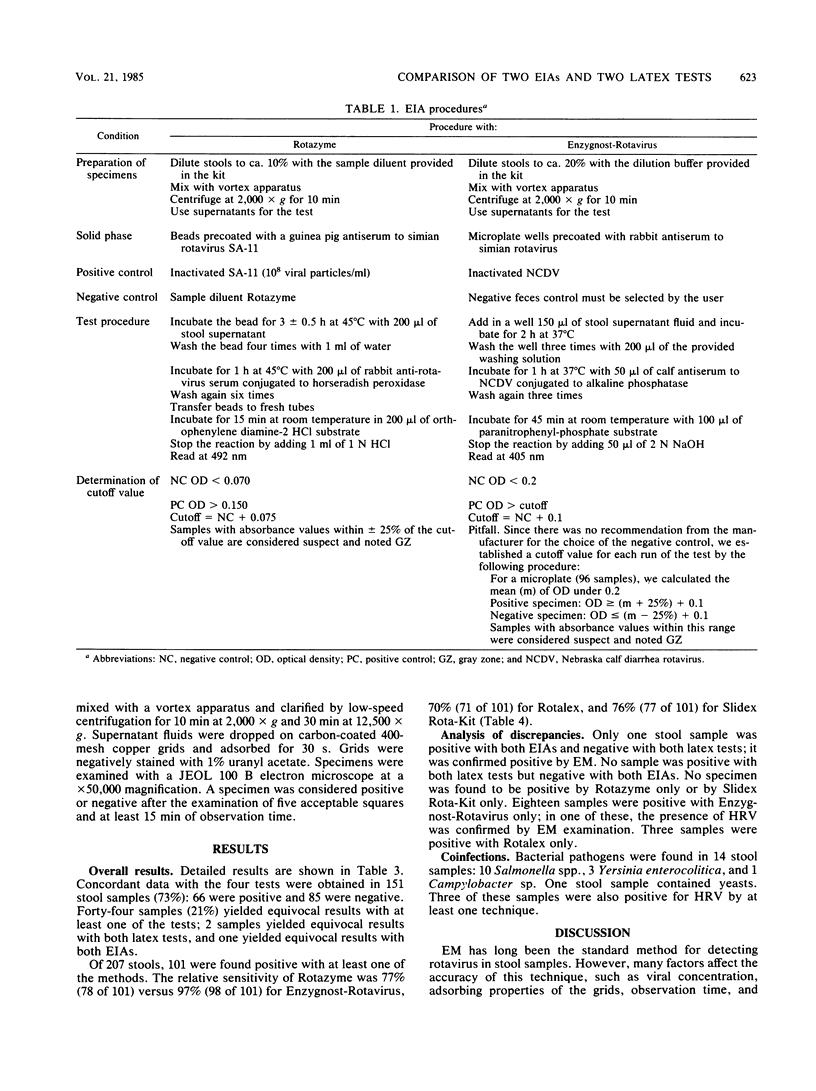
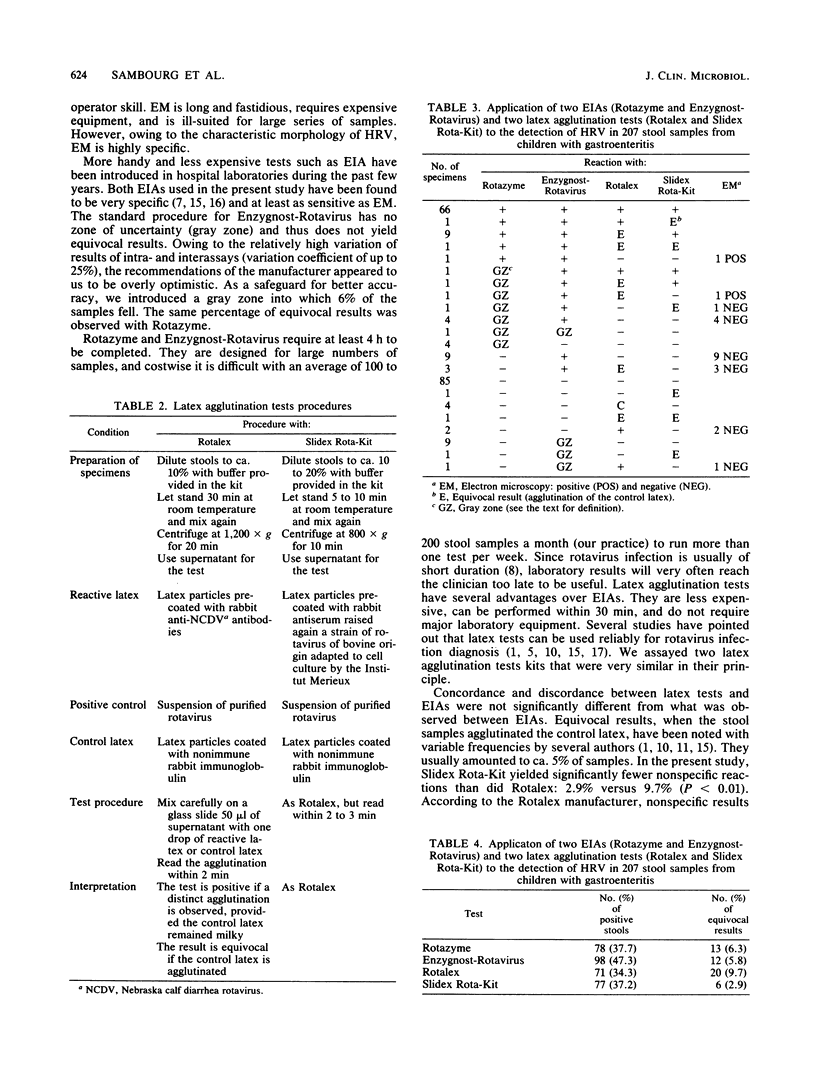
During February and March 1984, 207 fecal samples from infants and children with gastroenteritis were tested for rotavirus with four techniques: two enzyme immunoassays (Rotazyme; Abbott Laboratories, North Chicago, Ill., and Enzygnost-Rotavirus; Calbiochem-Behring, La Jolla, Calif.) and two latex agglutination tests (Rotalex; Orion Research, Inc., Cambridge, Mass., and Slidex Rota-Kit; Biomérieux). All stool samples were also tested for yeasts and bacterial pathogens. Electron microscopy was used to investigate discrepant results. We found 47% positive samples with Enzygnost-Rotavirus, 38% with Rotazyme, 37% with Slidex Rota-Kit, and 34% with Rotalex. No specimen was found positive by Rotazyme only or Slidex Rota-Kit only. On the contrary, 12 samples which were positive with Enzygnost-Rotavirus only and 3 which were positive with Rotalex only were not confirmed as positive by electron microscopy. Both enzyme immunoassays gave 6% equivocal results; Slidex Rota-Kit gave significantly fewer equivocal results than did Rotalex: 2.9% versus 9.7% (P less than 0.01). The sensitivity and specificity of latex tests compared favorably with that of enzyme immunoassays. Latex agglutination tests can be performed by unskilled personnel and are rapid and relatively cheap. They appear to be very suitable for routine laboratory work and may prove useful for large-scale screening in developing countries.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agius G., Baillargeau E., Castets M., Samb A. Détection rapide des rotavirus dans les selles. Intérêt d'un test au latex. Pathol Biol (Paris) 1984 Jan;32(1):56–58. [PubMed] [Google Scholar]
- Bishop R. F., Davidson G. P., Holmes I. H., Ruck B. J. Virus particles in epithelial cells of duodenal mucosa from children with acute non-bacterial gastroenteritis. Lancet. 1973 Dec 8;2(7841):1281–1283. doi: 10.1016/s0140-6736(73)92867-5. [DOI] [PubMed] [Google Scholar]
- Brandt C. D., Kim H. W., Rodriguez W. J., Arrobio J. O., Jeffries B. C., Stallings E. P., Lewis C., Miles A. J., Chanock R. M., Kapikian A. Z. Pediatric viral gastroenteritis during eight years of study. J Clin Microbiol. 1983 Jul;18(1):71–78. doi: 10.1128/jcm.18.1.71-78.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryden A. S., Davies H. A., Thouless M. E., Flewitt T. H. Diagnosis of rotavirus infection by cell culture. J Med Microbiol. 1977 Feb;10(1):121–125. doi: 10.1099/00222615-10-1-121. [DOI] [PubMed] [Google Scholar]
- Cheung E. Y., Hnatko S. I., Gunning H., Wilson J. Comparison of Rotazyme and direct electron microscopy for detection of rotavirus in human stools. J Clin Microbiol. 1982 Sep;16(3):562–563. doi: 10.1128/jcm.16.3.562-563.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haikala O. J., Kokkonen J. O., Leinonen M. K., Nurmi T., Mäntyjärvi R., Sarkkinen H. K. Rapid detection of rotavirus in stool by latex agglutination: comparison with radioimmunoassay and electron microscopy and clinical evaluation of the test. J Med Virol. 1983;11(2):91–97. doi: 10.1002/jmv.1890110202. [DOI] [PubMed] [Google Scholar]
- Hammond G. W., Ahluwalia G. S., Hazelton P. R. Detection of human rotaviruses in fecal specimens by a commercial latex-agglutination test. J Infect Dis. 1984 Jun;149(6):1021–1021. doi: 10.1093/infdis/149.6.1021. [DOI] [PubMed] [Google Scholar]
- Hasegawa A., Matsuno S., Inouye S., Kono R., Tsurukubo Y., Mukoyama A., Saito Y. Isolation of human rotaviruses in primary cultures of monkey kidney cells. J Clin Microbiol. 1982 Aug;16(2):387–390. doi: 10.1128/jcm.16.2.387-390.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes I. H. Viral gastroenteritis. Prog Med Virol. 1979;25:1–36. [PubMed] [Google Scholar]
- Kapikian A. Z., Kim H. W., Wyatt R. G., Cline W. L., Arrobio J. O., Brandt C. D., Rodriguez W. J., Sack D. A., Chanock R. M., Parrott R. H. Human reovirus-like agent as the major pathogen associated with "winter" gastroenteritis in hospitalized infants and young children. N Engl J Med. 1976 Apr 29;294(18):965–972. doi: 10.1056/NEJM197604292941801. [DOI] [PubMed] [Google Scholar]
- Morinet F., Ferchal F., Colimon R., Pérol Y. Comparison of six methods for detecting human rotavirus in stools. Eur J Clin Microbiol. 1984 Apr;3(2):136–140. doi: 10.1007/BF02014331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein A. S., Miller M. F. Comparison of an enzyme immunoassay with electron microscopic procedures for detecting rotavirus. J Clin Microbiol. 1982 May;15(5):938–944. doi: 10.1128/jcm.15.5.938-944.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanekata T., Yoshida Y., Okada H. Detection of rotavirus in faeces by latex agglutination. J Immunol Methods. 1981;41(3):377–385. doi: 10.1016/0022-1759(81)90199-x. [DOI] [PubMed] [Google Scholar]
- Truant A. L., Chonmaitree T. Incidence of rotavirus infection in different age groups of pediatric patients with gastroenteritis. J Clin Microbiol. 1982 Sep;16(3):568–569. doi: 10.1128/jcm.16.3.568-569.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward R. L., Knowlton D. R., Pierce M. J. Efficiency of human rotavirus propagation in cell culture. J Clin Microbiol. 1984 Jun;19(6):748–753. doi: 10.1128/jcm.19.6.748-753.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]


