Abstract
This study describes an evaluation of the sonographic, cholangiographic, pathological, and immunological findings, and the protective effect shown by rats reinfected with Clonorchis sinensis. Eight experimental rat groups were, namely, a normal control, a primary infection control, a reinfection I (reinfection 7 week after treatment following 3-week infection), a reinfection II (reinfection 2 week after treatment following 8-week infection), a reinfection III (exploration of the intrahepatic bile ducts 1 week after reinfection 4 week after treatment following 4-week infection), a superinfection, a secondary infection control, and an infection following immunization group. Sonographic and cholangiographic findings showed moderate or marked dilatation of the bile duct confluence in the primary infection control, reinfection II, and secondary infection control groups. Juvenile worms survived in the intrahepatic bile ducts 1 week after reinfection following treatment in the reinfection III group. It was concluded that reinfecting juvenile worms found during the first week following reinfection failed to survive or grow further. Anatomical, pathophysiological, or immunological changes may induce protection from reinfection in rats.
Keywords: Clonorchis sinensis, rats, pathology, ultrasonography, cholangiography, antibodies
INTRODUCTION
Clonorchis sinensis is the most important widely-distributed parasite of the human bile duct in East Asia, and is the most prevalent parasitic helminth in Korea (Rim, 1986; MHW & KAHP, 1997). Clonorchiasis is principally diagnosed by the detection of eggs in feces (Hong et al., 2003), but several other diagnostic tools such as skin testing, ELISA, and some radiologic modalities have been introduced (Chen et al., 1994; Choi D et al., 2004). Among these, sonography and ELISA are known to be feasible and effective in the field. Nevertheless, ELISA has a low sensitivity and shows cross reactions with other parasitic fluke infections (Hong et al., 1990).
The characteristic sonographic feature of diffuse dilatation of the intrahepatic bile ducts is an increased wall echogenicity, which well reflects the pathology of clonorchiasis (Lim et al., 1989; Ryu et al., 1993; Hong et al., 1994; Choi et al., 1999; Choi et al., 2004). A field study on human clonorchiasis proposed that sonography has a low sensitivity and specificity in diagnosis of clonorchiasis (Hong et al., 1998). The study asserted that the low sensitivity of sonography was due to a light worm burden in most cases and a low specificity due to residual pathology after cure. A few reports have indicated that biliary clonorchiasis produces an easily recognizable cholangiographic picture. These cholangiographic findings consist of characteristic filling defects in the dilated intrahepatic and extrahepatic ducts with tortuosity and duct wall irregularities (Choi et al., 1984; Lim, 1990).
In humans and rabbits, adult C. sinensis predominantly reside in the medium-sized or small-sized intrahepatic bile duct, and occasionally in the extrahepatic bile duct, gallbladder, and pancreatic duct. Unlike humans or rabbits, in rats the extrahepatic bile ducts are mainly involved. Moreover, the reinfection rate following treatment in rats was reported to be low, i.e., less than 5% (Chung et al., 2004). To the best of our knowledge, the rat is the only host which shows resistance to reinfection after treatment. In the present study, we evaluated the sonographic, cholangiographic, and pathological findings in rats which had been treated and then reinfected with C. sinensis, and counted recovered worms. Our findings may explain why reinfection is prevented in rats after treatment.
MATERIALS AND METHODS
Preparation of metacercariae of C. sinensis
The freshwater fish, Pseudorasbora parva, collected in the Nakdong-gang, were digested in a 0.5% pepsin solution, and C. sinensis metacercariae were isolated under a stereomicroscope.
Experimental rats
The experimental animal hosts were Sprague-Dawley rats. Animals in the normal control group (n = 5) were injected with saline and other animal groups (primary infection controls, n = 7; reinfection I, n = 21; reinfection II, n = 21; reinfection III, n = 6; superinfection, n = 4; secondary infection control, n = 14) were infected with 100 metacercariae of C. sinensis into the stomach through a gavage needle (Table 1). The rats in the reinfection groups were treated with praziquantel (Distocide, Shinpoong Pharmaceutical Co., Seoul, Korea) at 100 mg/kg for 3 days; and this was repeated if C. sinensis eggs were found in feces. This treatment was performed at 3 weeks (reinfection I group), at 8 weeks (reinfection II group) or 4 weeks (reinfection III group) after the primary infection, and animals were reinfected at 7 weeks (reinfection I group), 2 weeks (reinfection II group) or 4 weeks (reinfection III group) after treatment. Rats in the superinfection group were reinfected at 4 weeks after the primary infection. In a previous experimental study, the histopathological changes of acute C. sinensis infection during the first 2 weeks were found to be reversed by treatment, and most of these changes were established 4 weeks after infection (Lee et al., 1987). For these reasons, the above infection periods for the reinfection groups were chosen for the present study. Rats in all groups were kept in the animal room of the Seoul National University College of Medicine and fed with commercial diet pellets and piped water.
Table 1.
Experimental protocols used for the eight experimental groups
a)No. are numbers of rats experimented. b)Inf., Infection. c)Sac., Sacrifice. d)s, sonography; b, blood sampling for antibody level evaluation; c, cholangiography; p, pathology; w, worm collection. e)Reinfection I group is group with reinfection after treatment following 3-week infection. f)Tx., Treatment. g)Reinf., Reinfection.
Preparation of C. sinensis antigen
Following the oral infection of rabbits with C. sinensis metacercariae, adult worms were recovered from livers at 8 weeks, and the worms were homogenized in phosphate-buffered saline. The supernatant obtained after high-speed centrifugation (15,000 rpm for 2 hours) was used as soluble crude antigen for the immunization group.
Immunization
Each rat of in immunization group (n = 14) was immunized by injecting crude antigen (0.1 mg) emulsified in an equal volume of Freund's adjuvant, via a subcutaneous route. Two more immunizations were repeated at 5 and 6 weeks after the first immunization. The rats were challenged with 100 metacercariae 1 week after the third immunizations.
Sonography
The rats in the experimental groups underwent sonography according to experimental scheme shown in Table 1. The rats were prepared for sonography by ether anesthesia, shaved on the upper abdomen, and dressed with jelly. An abdominal radiologist obtained sonograms of the liver and bile ducts using a 5-12 MHz linear probe on high-resolution ultrasound scanner (HDI 5000, Advanced Technology Laboratories, WA, USA). The degree of dilatation of the bile duct confluence was classified as mild (+, when dilatation of bile duct confluence was less than 1/3 of the thickness of the liver), moderate (++, greater than 1/3 but lesser than 1/2 of the thickness of the liver), and marked (+++, greater than 1/2 the thickness of the liver).
Cholangiographic examination
The rats of the experimental groups also underwent cholangiography according to the experimental scheme (Table 1). The rats were sacrificed by ether anesthesia and heart puncture. After making a midline incision, livers and bile ducts were exposed. Using a 26-gauge needle the bile duct was punctured, and 1 ml of intravenous contrast material (Iopamiro 300; Bracco, Milano, Italy) was injected using a tuberculin syringe. Cholangiograms were obtained using a digital subtraction angiography machine (V5000, Philips Medical Systems North America, CO, USA).
Collection of recovered adult worms
After sacrifice by deep anesthesia (Table 1), the adult worms were recovered from the extracted liver, bile ducts, and intestines under a stereomicroscope (Figs. 1 and 2). The protective effect was calculated using the numbers of recovered adult worms, as described below.
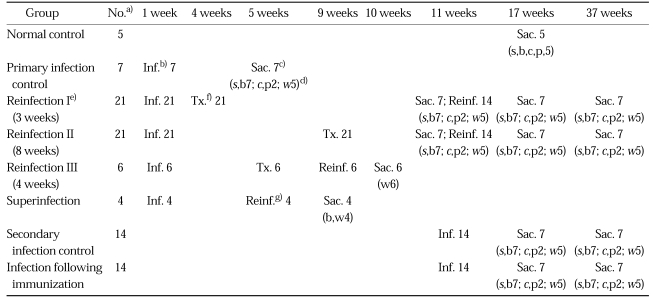
Fig. 1.
Extracted abdominal organs of a rat in the normal control group without C. sinensis infection, showing a string-like narrow extrahepatic bile duct (arrowheads). L, liver.
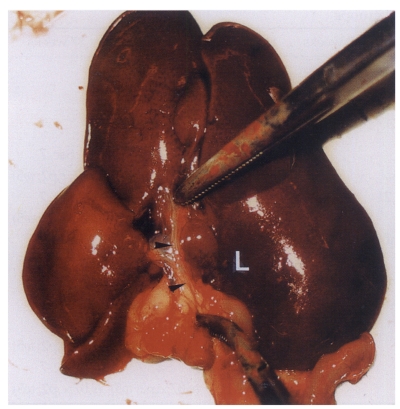
Fig. 2.
Extracted abdominal organs of a rat in the primary infection control group 4 weeks after C. sinensis infection, showing marked dilatation of the bile duct confluence (arrows) and of the extrahepatic bile duct (arrowheads). L, liver.
Protection rate (%) = [1 - (number of worms in challenged rat / mean number of worms in control rats)] × 100
Histopathological examination
After sacrifice by deep anesthesia (Table 1), livers and bile ducts were removed and fixed in Carnoy fixative. The tissues were then sliced and prepared for paraffin embedding. After staining with hematoxylineosin, an experienced parasitologist assessed the degree of mucosal hyperplasia of the bile ducts, periductal fibrosis and periductal inflammation.
Immunological examination
After blood sampling according to experimental scheme (Table 1), the antibody levels in sera were calculated by ELISA. Antigen was diluted 1:400, sera diluted 1:800, and the conjugate (ImmunoPure®Gout, Pierce, IL, USA) diluted 1:25,000. Diaminobenzidine was used as the substrate for the color reaction and absorbance was read at 490 nm using an ELISA reader (Emax®Precision Microplate Reader, Molecular Devices Corp., CA, USA).
Statistical analysis
The statistical analyses were performed by using SPSS program (SPSS Inc., IL, USA). Fisher's exact test, the Mann-Whitney test, and the Wilcoxon rank sum test were used to test for significance, with a threshold p value of 0.05.
RESULTS
Sonographic findings
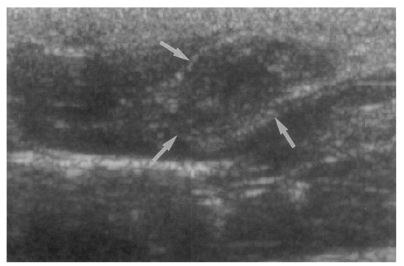
Sonography showed no dilatation of the bile duct confluence in the normal control group (Fig. 3). Sonograms showed moderate or marked dilatation of the bile duct confluence in the primary infection control group, the secondary infection control group, and the reinfection II group (Fig. 4, Table 2). A small number (1 of 7 in all groups) of animals showed mild dilatation of bile duct confluence in these groups (Fig. 5, Table 2). Dilated extrahepatic bile ducts were also observed. In the reinfection I group and the infection following immunization group, the degree of dilatation of the bile duct confluence was usually moderate (in n = 4 and n = 5, respectively). Marked dilatation was less frequent in these groups (n = 2 in each group).
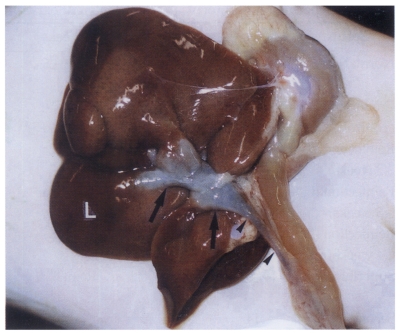
Fig. 3.
Sonogram of a rat in the normal control group without C. sinensis infection, showing no dilatation of the bile duct confluence.
Fig. 4.
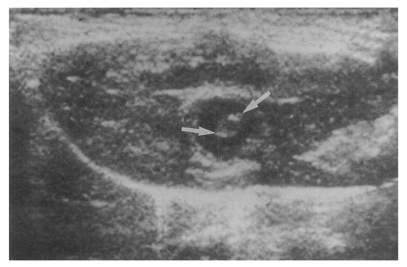
Sonogram of a rat in the primary infection control group 4 weeks after C. sinensis infection, showing marked dilatation of the bile duct confluence (arrows) with high echogenicity, reflecting worm burden and desquamated materials.
Table 2.
Degree of dilatation of the bile duct confluence of rats by sonography
a)-, No dilatation of the bile duct confluence. b)+, Mild dilatation. c)++, Moderate dilatation. d)+++, Marked dilatation.
Fig. 5.
Sonogram of a rat in the primary infection control group 4 weeks after C. sinensis infection, showing mild dilatation of the bile duct confluence (arrows).
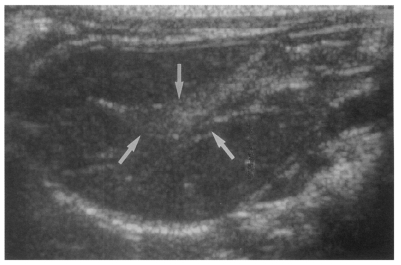
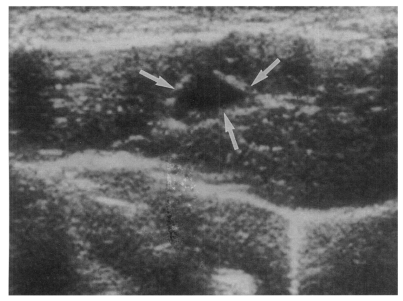
High echogenicity closely packed at the dilated bile duct confluence was seen in all rats in the primary or secondary infection control groups; however, this was seldom noted in the reinfection I (n = 1) and reinfection II groups (n = 0) (p = 0.005 and p = 0.001, respectively) (Fig. 6). The echogenic mass was found to be composed of clusters of worms and desquamated materials by pathological examination. Small echogenic foci were noted in the dilated bile duct confluence in the infection following immunization group. These also represented worms and desquamated materials (Fig. 7). In the reinfection I and II groups, the degree of dilatation of the bile duct confluence on the last follow-up sonograms at 26 weeks after reinfection were noted to be slightly milder than those at 6 weeks after reinfection, but this was not significant (p = 0.266 and p = 0.431, respectively) (Table 2).
Fig. 6.
Sonogram of a rat in the reinfection I group 6 weeks after reinfection (13 weeks after treatment), showing an empty dilated bile duct confluence (arrows). Neither worms nor desquamated materials were found.
Fig. 7.
Sonogram of a rat in the infection following immunization group 6 weeks after C. sinensis challenge, showing a few small echogenic foci (arrows), indicating worms or desquamated materials in the dilated bile duct.
Cholangiographic findings
Cholangiography could not be performed in the normal control group because the bile ducts were too fine to puncture with a 26-gauge needle (Fig. 1). Cholangiograms showed moderate or marked dilatation of the bile duct confluence and of the proximal extrahepatic bile duct in the primary infection control group, the secondary infection control group, the reinfection I group, the reinfection II group, and the infection following immunization group. By contrast, the distal extrahepatic bile duct had a normal or near normal caliber (Fig. 8). In the primary and secondary infection control groups, and the infection following immunization group, multiple irregular filling defects were demonstrated on cholangiograms, indicating worms or desquamated materials (Fig. 9). These filling defects were hardly seen in the reinfection I and II groups. In these groups, the degree of dilatation of the bile duct at 26 weeks after reinfection (33 or 28 weeks after treatment, respectively) was slightly milder than that at 6 weeks after reinfection (13 or 8 weeks after treatment, respectively).
Fig. 8.
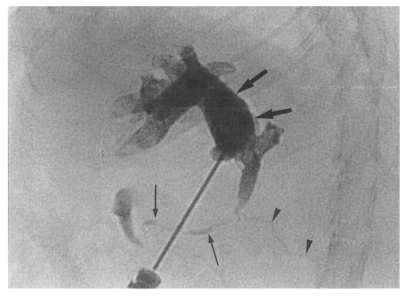
Cholangiogram of a rat in the secondary infection control group 6 weeks after C. sinensis infection, showing marked dilatation of the bile duct confluence and of the proximal extrahepatic bile duct (thick arrows). Note the normal caliber distal extrahepatic bile duct (thin arrows). Pancreatic duct (arrowheads).
Fig. 9.
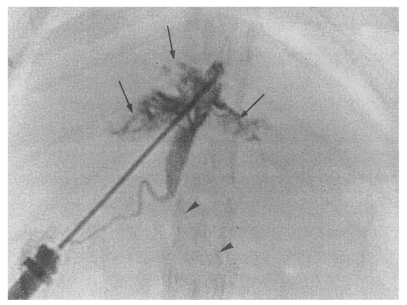
Cholangiogram of a rat in the primary infection control group 4 weeks after infection, showing moderate dilatation of the bile duct confluence and of the proximal extrahepatic bile duct. A normally appearing distal extrahepatic bile duct is also shown. Note the multiple irregular filling representing worms or desquamated material (arrows). Pancreatic duct (arrowheads).
Worm recovery
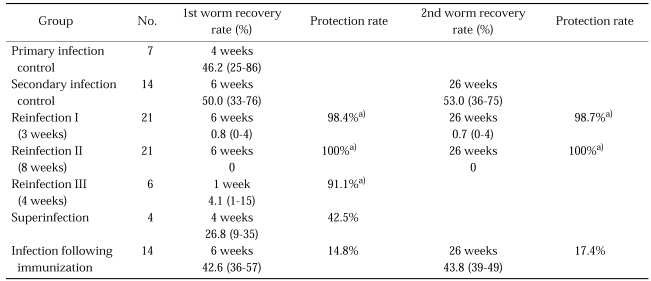
In the primary infection control group, 25-86 (mean, 46.2) worms were collected from rats infected with 100 metacercariae. In the secondary infection control group, 33-76 (mean, 50.0) worms and 36-75 (mean, 53.0) worms were collected at 6 weeks and 26 weeks after infection, respectively. In the reinfection I group, 0-4 (mean, 0.8) and 0-4 (mean, 0.7) worms were collected at 6 weeks and 26 weeks after reinfection, respectively. Therefore, the protection rates were 98.4% and 98.7% at 6 weeks and 26 weeks after reinfection, respectively (p = 0.005 and p = 0.003, respectively). In the reinfection II group, no worm was collected at 6 and 26 weeks after reinfection, i.e., a protection rate of 100% (p = 0.002 and p = 0.002, respectively). Of 42 rats in the reinfection I and II groups, only two rats treated at 3 weeks after primary infection (reinfection I group) were actually infected by the secondary infection. On the other hand, in the reinfection III group, there were 1-15 (mean, 4.1) juvenile worms in the intrahepatic bile ducts at 1 week after reinfection, a protection rate of 91.1% (p = 0.007). In the superinfection group, 9-35 (mean, 26.8) worms were collected at 4 weeks reinfection, a protection rate of 42.5% (p = 0.067). And, in the infection following immunization group, 36-57 (mean, 42.6) worms and 39-49 (mean, 43.8) worms were collected at 6 weeks and 26 weeks after infection, giving protection rates of 14.8% and 17.4%, respectively (p = 0.831 and p = 1.000) (Table 3). The numbers of worms recovered from each rat were correlated with echogenicity within the dilated bile ducts by sonography and with filling defects by cholangiography.
Table 3.
Worm recovery and protection rates by experimental groups
a)In these groups, the worm recovery results (protection rates) show the significant protective effect against C. sinensis reinfection (p < 0.05).
Histopathological findings
Microscopically, hematoxylin-eosin stained sections of the resected extrahepatic bile ducts showed the features of active and chronic clonorchiasis, i.e., mucosal hyperplasia of the extrahepatic bile duct, ductal wall thickening, slight periductal fibrosis and periductal inflammation in the primary and secondary infection control groups, and the infection following immunization group (Fig. 10). Worms were found in the bile ducts of all rats (Fig. 10), and obvious desquamation of the duct epithelium was observed in two rats. In the reinfection groups, mucosal hyperplasia of the extrahepatic bile duct, ductal wall thickening, and slight periductal fibrosis were noted; however, periductal inflammation was considerably milder than that in the other groups. In these groups, no significant difference was observed between rats reinfected at 6 and at 26 weeks.
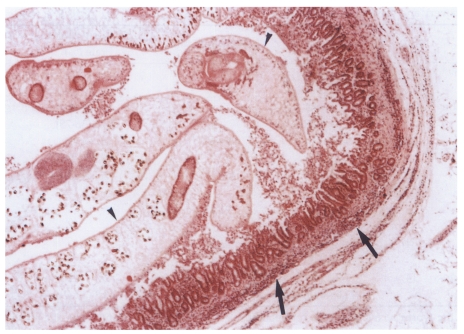
Fig. 10.
Histopathological photograph of a rat in the primary infection control group 4 weeks after C. sinensis infection, showing some adult worms (arrowheads) in the bile duct associated with papillary and adenomatous hyperplasia of bile duct mucosa, ductal wall thickening, minimal periductal fibrosis, and periductal inflammation (arrows).
Immunological findings
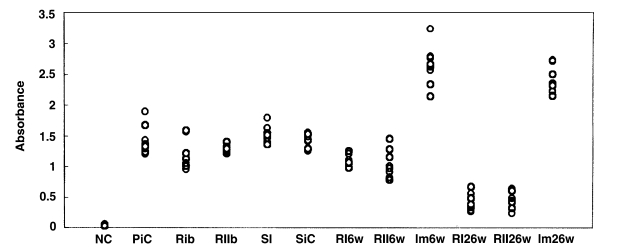
Rats in each group were examined by ELISA for anti-C. sinensis IgG antibody in serum. In the primary infection control group, the absorbance levels (antibody levels) were 1.21-1.89 (mean, 1.43) at 4 weeks after infection (Fig. 11). In the secondary infection control group, the antibody levels were 1.25-1.55 (mean, 1.42) at 6 weeks after infection. Before reinfection, antibody levels were 0.95-1.57 (mean, 1.19; p = 0.052) and 1.22-1.4 (mean, 1.28; p = 0.207) in the reinfection I (treatment following 3-week infection) and reinfection II groups (treatment following 8-week infection), respectively. In the reinfection I group, antibody levels were 0.98-1.25 (mean, 1.1; p = 0.001) and 0.26-0.56 (mean, 0.40; p < 0.001) at 6 weeks and 26 weeks after reinfection, respectively. And, in the reinfection II group, antibody levels of 0.78-1.28 (mean, 1.04; p = 0.006) and 0.24-0.64 (mean, 0.47; p < 0.001) were recorded at 6 weeks and 26 weeks after reinfection. Antibody levels were 1.36-1.79 (mean, 1.52; p = 0.128) in the superinfection group, but 2.13-3.23 (mean, 2.63; p < 0.001) and 2.14-2.72 (mean, 2.39; p < 0.001) in the infection following immunization group at 6 weeks and 26 weeks after reinfection, respectively.
Fig. 11.
Absorbance levels (at 490 nm) of IgG antibody by ELISA to C. sinensis crude antigen. NC, normal control; PiC, primary infection control; RIb, reinfection I group before reinfection (treatment following 3-week infection); RIIb, reinfection II group before reinfection (treatment following 8-week infection); SI, superinfection; SiC, secondary infection control, 6-week follow-up after infection; RI6w, reinfection I group, 6-week follow-up after reinfection; RII6w, reinfection II group, 6-week follow-up after reinfection; Im6w, immunization group, 6-week follow-up after reinfection; RI26w, reinfection I group, 26-week follow-up after reinfection; RII26w, reinfection II group, 26-week follow-up after reinfection; Im26w, immunization, 26-week follow-up after reinfection.
DISCUSSION
The present findings confirm that rats are resistant to C. sinensis reinfection after cure. Contrary to the absence of a protective effect against C. sinensis reinfection in almost all definitive hosts, the rat is the only confirmed animal host to possess near complete protection (Chung et al., 2004). The protection rate depends upon the numbers of infecting worms, and protection was found to be strong in rats infected with 100 metacercariae. According to the results of the present study, the protection afforded by immunizing with crude or purified antigens (glutathione S-transferase and cysteine protease) was insufficient, which suggests that the protection developed by rats may be due to a mechanism other than immune reactions.
The results of the present study also demonstrate a close correlation between the radiologic (sonographic and cholangiographic) findings and the numbers of worms recovered. Sonographic findings of the bile ducts have been confirmed to be well correlated with the pathology of clonorchiasis (Lim et al., 1989; Ryu et al., 1993; Hong et al., 1994), and it is known that the diffuse dilatation of the intrahepatic bile ducts had a good diagnostic value. Some other features also increase ductal wall echogenicity and floating echogenicities in the gall bladder (Ryu et al., 1993; Choi et al., 1999). A recent high-resolution sonographic study revealed that sonography is sensitive enough for the diagnosis of rabbit clonorchiasis (Lim et al., 1999).
However, no report has been issued on the sonographic findings of clonorchiasis in rats. The present study first reports that dilatation of the bile duct confluence is associated with high echogenicity by highresolution sonography, and well reflects pathological features. No rat showed such a finding unless rat was infected by C. sinensis. The present study shows that sonography is a good noninvasive radiologic tool to determine the presence of worms in rats, particularly in cases with a moderate or heavy worm burden. In the present study, sonography showed moderate or marked dilatation of the bile duct confluence and of the extrahepatic bile duct in rats with an active (present) or a previous infection. However, the hyperechoic mass formed by worms and desquamated materials in dilated duct was found only in rats with an active infection.
The relatively mild dilatation of the bile duct confluence by sonography resulted from a mild pathological changes after a short infection period, as in reinfection I group, or a mild burden of worms, as in the infection following immunization group. In the reinfection I and II groups, the degree of dilatation of the bile duct confluence on the final follow-up sonograms at 26 weeks after reinfection was noted to be slightly milder than that at 6 weeks after reinfection, which implies a resolution of the bile duct pathology without C. sinensis infection. However, on histopathological findings, no significant difference with respect to mucosal hyperplasia of the extrahepatic bile duct, ductal wall thickening, slight periductal fibrosis, and periductal inflammation was observed between rats at 6 and 26 weeks after reinfection. Also no significant difference was found between rats in the reinfection I group and those in the reinfection II group, despite the different infection periods (3 weeks and 8 weeks, respectively) and different intervals between treatment and reinfection (7 weeks and 2 weeks, respectively).
Cholangiograms showed moderate or marked dilatation of the bile duct confluence and of the proximal extrahepatic bile duct in rats with active or a previous infection, whereas the distal extrahepatic bile duct had a near normal caliber. In rats with active infection, worms or desquamated materials showing multiple irregular filling defects were demonstrated. As was the case for sonography, filling defects were hardly noted on cholangiograms in the reinfection groups. The finding of moderate or marked dilatation of the proximal extrahepatic bile duct persisted after C. sinensis treatment. This cholangiographic feature showed fusiform dilatation of the extrahepatic bile duct resembling that of a choledochal cyst, Todani's classification 1C (Todani et al., 1977). All of the cholangiograms demonstrated the long common channels of the bile duct and pancreatic duct. Such an anatomical change may have influenced the niche afforded by the bile ducts. Some pathophysiologic changes such as stagnated bile, an increased concentration of regurgitated pancreatic juice in the dilated extrahepatic bile duct, or other unknown factors may be hazardous to newly infected juvenile worms, and result in low reinfection rates (Oguchi et al., 1988; Lim, 1991).
In rats, the infected metacercariae of C. sinensis excyst in the duodenum, and juvenile worms crawl through the ampulla and extrahepatic bile duct and arrive in the intrahepatic bile ducts. Two to three weeks after infection, the juvenile worms descend to the extrahepatic bile duct and mature to become adults. Most of the adult worms reside at the bile duct confluence and at the proximal extrahepatic bile duct (Hong et al., 1993; Kim, 1995). The present study found only small numbers (mean, 4.1) of juvenile worms in the intrahepatic bile ducts at 1 week after reinfection in the reinfection III group. However, considering difficulty was experienced detecting juvenile worms in the intrahepatic bile ducts; many juvenile worms probably survive the intrahepatic bile ducts after reinfection. Actually, the calculation of protection rate in the reinfection III group may be meaningless. There remains a problem as to how to explain the worm recovery results in the superinfection group. Many superinfected juvenile worms survived with preexisting adult worms in the dilated extrahepatic bile duct. Therefore, it is cautiously suggested that preexisting adult worms helped the juvenile worms survive by secreting some material that alleviates the toxic effects of regurgitated pancreatic juice or some other hazardous environmental factor.
According to the present immunological results, the immunization group showed the highest level of serum IgG antibody. The antibody levels in the treatment groups were lower than those in the primary or secondary infection control groups. Considering these findings and the worm recovery results, humoral immune reactions probably plays a limited role in protection of reinfection.
Further studies are needed to evaluate any hazardous impact of pathophysiologic changes in the dilated bile ducts of cured rats, such as stagnated bile, increased concentration of regurgitated pancreatic juice, hyperplastic mucosa, or others. Also, there remains a possibility that protection to reinfection may be caused by acquired immunity (Chung et al., 2004). Nevertheless, the present study observed that the sonography of rats with active C. sinensis infection showed high echogenicity, which represented worms within the dilated bile duct, and found that reinfection of cured rats was prohibited during the settlement of juvenile worms in the dilated extrahepatic bile duct after primary migration into the intrahepatic bile duct.
Footnotes
This work was supported by the Grant for the Reform of University Education under the BK21 Project of Seoul National University in 2001-2003.
References
- 1.Chen M, Lu Y, Hua X, Mott KE. Progress in assessment of morbidity due to Clonorchis sinensis infection: a review of recent literature. Trop Dis Bull. 1994;91:R7–R65. [Google Scholar]
- 2.Choi D, Lim JH, Kim SK, Kim EY, Lee M, Hong ST. Long-lasting sonographic and histopathological findings in cured clonorchiasis of rabbits. Korean J Parasitol. 1999;37:77–83. doi: 10.3347/kjp.1999.37.2.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choi D, Hong ST, Lim JH, et al. Sonographic findings of active Clonorchis sinensis infection. J Clin Ultrasound. 2004;32:17–23. doi: 10.1002/jcu.10216. [DOI] [PubMed] [Google Scholar]
- 4.Choi TK, Wong KP, Wong J. Cholangiographic appearance in clonorchiasis. Br J Radiol. 1984;57:681–684. doi: 10.1259/0007-1285-57-680-681. [DOI] [PubMed] [Google Scholar]
- 5.Chung BS, Zhang H, Choi MH, et al. Development of resistance to reinfection by Clonorchis sinensis in rats. Korean J Parasitol. 2004;42:19–26. doi: 10.3347/kjp.2004.42.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hong ST, Choi MH, Kim CH, Chung BS, Ji Z. The Kato-Katz method is reliable for diagnosis of Clonorchis sinensis infection. Diagn Microbiol Infect Dis. 2003;47:345–347. doi: 10.1016/s0732-8893(03)00113-5. [DOI] [PubMed] [Google Scholar]
- 7.Hong ST, Huh S, Kho WG, et al. Changes in histopathological and serological findings of the liver after treatment in rabbit clonorchiasis. Seoul J Med. 1990;31:117–127. [Google Scholar]
- 8.Hong ST, Kho WG, Kim WH, Chai JI, Lee SH. Turnover of biliary epithelial cells in Clonorchis sinensis infected rats. Korean J Parasitol. 1993;31:83–89. doi: 10.3347/kjp.1993.31.2.83. [DOI] [PubMed] [Google Scholar]
- 9.Hong ST, Park KH, Seo M, Choi BI, Chai JY, Lee SH. Correlation of sonographic findings with histopathological changes of the bile ducts in rabbits infected with Clonorchis sinensis. Korean J Parasitol. 1994;32:223–230. doi: 10.3347/kjp.1994.32.4.223. [DOI] [PubMed] [Google Scholar]
- 10.Hong ST, Yoon K, Lee M, et al. Control of clonorchiasis by repeated praziquantel treatment and low diagnostic efficacy of sonography. Korean J Parasitol. 1998;36:249–254. doi: 10.3347/kjp.1998.36.4.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim J. Image analytical observation on the growth and development of Clonorchis sinensis in rats. Korean J Parasitol. 1995;33:281–288. doi: 10.3347/kjp.1995.33.4.281. [DOI] [PubMed] [Google Scholar]
- 12.Lim JH. Radiologic findings of clonorchiasis. AJR. 1990;155:1001–1008. doi: 10.2214/ajr.155.5.2120925. [DOI] [PubMed] [Google Scholar]
- 13.Lim JH. Oriental cholangiohepatitis: pathologic, clinical, and radiologic features. AJR. 1991;157:1–8. doi: 10.2214/ajr.157.1.2048504. [DOI] [PubMed] [Google Scholar]
- 14.Lim JH, Choi D, Hong ST, et al. Usefulness of highresolution sonography in early diagnosis of rabbit clonorchiasis. J Korean Soc Med Ultrasound. 1999;18:161–166. [Google Scholar]
- 15.Lim JH, Ko YT, Lee DH, Kim SY. Clonorchiasis: sonographic findings in 59 proved cases. AJR. 1989;152:761–764. doi: 10.2214/ajr.152.4.761. [DOI] [PubMed] [Google Scholar]
- 16.Ministry of Health and Welfare and Korean Association of Health Promotion. Precalence of intestinal parasitic infections in Korea - The Sixth Report. Seoul, Korea: 1997. [Google Scholar]
- 17.Oguchi Y, Okada A, Nakamura T, et al. Histopathologic studies of congenital dilatation of the bile duct as related to an anomalous junction of the pancreaticobiliary ductal system: clinical and experimental studies. Surgery. 1988;103:168–173. [PubMed] [Google Scholar]
- 18.Rim HJ. The current pathobiology and chemotherapy of clonorchiasis. Korean J Parasitol. 1986;24(suppl):1–141. doi: 10.3347/kjp.1986.24.suppl.1. [DOI] [PubMed] [Google Scholar]
- 19.Ryu KN, Lim JH, Cho YJ, Yang MH. Comparative study of radiologic-pathological findings of experimental clonorchiasis in rabbits. J Korean Radiol Soc. 1993;29:1–8. [Google Scholar]
- 20.Todani T, Watanabe Y, Narusue M, Tabuchi K, Okajima K. Congenital bile duct cysts: Classification, operative procedures, and review of thirty-seven cases including cancer arising from choledochal cyst. Am J Surg. 1977;134:263–269. doi: 10.1016/0002-9610(77)90359-2. [DOI] [PubMed] [Google Scholar]