Abstract
Cryptosporidium parvum oocysts were isolated from a child suffering from acute gastroenteritis and successfully passaged in a calf and mice (designated hereafter SNU-H1) in the Republic of Korea; its molecular genotype has been analyzed. The GAG microsatellite region was amplified by a polymerase chain reaction (PCR), with a 238 base pair product, which is commonly displayed in C. parvum. The isolate was shown to be a mixture of the genotypes 1 (anthroponotic) and 2 (zoonotic). To study its infectivity in animals, 2 calves and 3 strains of mice were infected with the SNU-H1; in these animals, the propagation of both genotypes was successful. In immunosuppressed (ImSP) BALB/c and C57BL/6 mice the number of oocysts decreased after day 10 post-infection (PI); but in ImSP ICR mice, they remained constant until day 27 PI. The results show that both the C. parvum genotypes 1 and 2 can be propagated in calves and ImSP mice.
Keywords: Cryptosporidium parvum, oocyst, human, calf, mouse, genotype
Cryptosporidium parvum, an enteric protozoan, is increasingly recognized as an important agent of gastrointestinal disease in a variety of animal species, including humans (Fayer and Ungar, 1986). In immunocompetent hosts, this protozoan generally causes mild and transient diarrhea, but in immunocompromised individuals, it can elicit severe, chronic, and intractable diarrhea which can be fatal (Fayer and Ungar, 1986). In the Republic of Korea, people in rural areas showed considerably high oocyst positive rates (Chai et al., 1996 & 2001; Yu et al., 2004).
Genetically, C. parvum are largely divided into 2 distinct groups; genotypes 1 and 2. The genotype 1 (transmitted anthroponotically), so far, has been known to be associated only with human and primate infections (Spano et al., 1998; Widmer et al., 2000), with the exception of a report of its presence in a dugong (Morgan et al., 2000). Recently, the genotype 1 was proposed to be a new species, C. hominis n. sp., based on biological and molecular data (Morgan et al., 2002). The genotype 2 (transmitted zoonotically) has been found to infect a wide range of mammals, including humans (McLauchlin et al., 2000). Despite worldwide reports, no information on the genotypes of C. parvum occurring in Korean people has been reported (Chai et al., 2001); isolates from humans have never been successfully propagated in the laboratory.
The major clinical characteristics of the genotype 1 include a longer duration of oocyst excretion and a greater number of oocysts produced, compared with the genotype 2 (Xiao et al., 2001). Although the transmission cycles of both genotypes may overlap (e. g., water-borne), mixtures of the genotypes 1 and 2 have rarely been identified in a single isolate from humans (Spano et al., 1998; McLauchlin et al., 2000; Widmer et al., 2002). With regard to mixtures of the genotypes 1 and 2 in human isolates, there have been no experimental studies on the biological characteristics, virulence, and laboratory maintenance. The present paper reports, for the first time in the Republic of Korea, a human isolate of C. parvum having the mixed genotypes 1 and 2 (designated hereafter SNU-H1), and a successful laboratory propagation of the SNU-H1 in a calf and 3 strains of mice.
The SNU-H1 was isolated from an 8-year-old boy, suffering from acute gastroenteritis due to cryptosporidiosis, at the Seoul National University Hospital, Seoul, Republic of Korea. The oocysts were purified by the discontinuous sucrose gradient method and confirmed by the modified acid-fast stain. Because the oocysts isolated from the patient were small in number, the first passage of this isolate was performed using specific-pathogen-free (SPF), immunosuppressed (ImSP), female C57BL/6 mice. The passage using these mice was successful, and the oocysts obtained from the first passage were used in the subsequent experiments.
To observe the animal infectivity, the SNU-H1 was experimentally infected to ICR, BALB/c, and C57BL/6 mice (SPF, 4-wk-old females) which were purchased from the Daehan Laboratory Animal Center, Ltd. (Jinju, Korea). Five mice in each group were supplied with food and water sterilized by irradiation and autoclaving. To increase the parasite load by immunosuppression of the host (Chai et al., 1990), the mice were each injected with 0.2 mg prednisolone intramuscularly every other day, from 7 days prior to infection, until the end of the experiment. Each mouse was infected orally with 106 oocysts, and the oocyst production in the feces was quantified by collection and pooling of the fecal pellets (Chai et al., 1999).
Two newborn calves were purchased from a farm and transported to the laboratory of the National Veterinary Research and Quarantine Services within a few hrs after birth. To reduce the possibility of contamination with exogenous genotypes of C. parvum, only the parasites propagated in the SPF mice were used to infect the 2 calves. Each newborn calf was infected orally with 2 x 107 oocysts, and the oocyst production in the feces was measured by collection and pooling of the fecal pellets (Guk et al., 2003).
The oocysts were purified by the method previously described (Guk et al., 2003). DNA extraction was performed following the procedure previously described (Caccio et al., 2000). The purified DNA was stored at 4℃. Amplification of a locus containing the GAG microsatellite (GenBank accession number: G35358) was performed using the forward primer, 5'-CTAAAAATGGTGGAGAATATTC, and the reverse primer, 5'-CAACAAAATCTATATCCTC, to obtain the expected 238 base pair (bp) product. The PCR mix consisted of 1x buffer (50 mM KCl, 10 mM Tris-HCl, pH 9.0, 0.1% Triton X-100), 1.5 mM MgCl2, 200 µM each dNTP, 50 pmol of each primer, 2.5 units of Taq polymerase (Promega, Madison, Wisconsin), and 1~5 µl of the purified DNA in a final volume of 50 µl. The PCR was performed as follows: after an initial denaturation for 5 min at 94℃ a set of 40 cycles was run, each consisting of 30 sec at 94℃ 30 sec at 50℃ and 60 sec at 72℃ followed by a final extension of 5 min at 72℃ The PCR products were separated by electrophoresis on a 1.2% agarose gel.
On the basis of the sequence difference between the genotypes 1 and 2, previously designed human-specific and animal-specific reverse primers were used (Morgan et al., 1997). The human-specific primer, CPHR (5'-CCTCTTTCCAATTAAAGTTGATG-3'), was designed to amplify a 411 bp product from the human isolates of C. parvum. The animal-specific primer, CPCR (5'-TCCAAATTATTGTAACCTGGAAG-3'), was designed to amplify a 312 bp product from the animal isolates. The forward primer, 021F (5'-GGTACTGGATAGATAGTGGA-3'), was annealed into both the human and animal isolates (Morgan et al., 1997). The PCR mix consisted of 1x buffer (50 mM KCl, 10 mM Tris-HCl, pH 9.0, 0.1% Triton X-100), 1.5 mM MgCl2, 200µM each dNTP, 1.75 pmol of CP-HR and 2.5 pmol of CP-CR, 0.5 units of Taq polymerase (Promega), and 1~5µl of the purified DNA in a final volume of 50µl. The PCR was performed as follows: after an initial denaturation for 2 min at 94 C, a set of 45 cycles was run, each consisting of 30 sec at 94℃, 30 sec at 58℃, and 30 sec at 72℃, followed by a final extension of 5 min at 72℃. The PCR products were separated by electrophoresis on a 1.2% agarose gel.
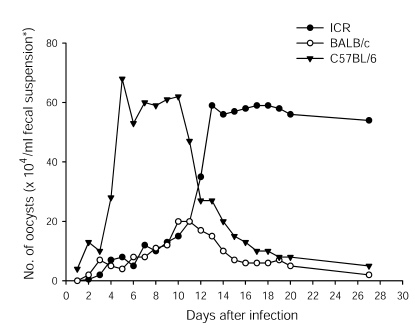
All 3 ImSP mouse strains appeared to be successfully infected with the SNU-H1, a C. parvum human isolate, with excretion of oocysts in the feces from days 2-3 post-infection (PI) (Fig. 1). Prolonged infections were observed in ImSP ICR mice, but not in ImSP BALB/c and C57BL/6 mice. ImSP BALB/c mice excreted only a small number of oocysts over a shorter period, with no oocysts detected at day 27 PI. ImSP C57BL/6 mice excreted a large number of oocysts from day 5 to 12 PI, but the oocyst numbers decreased thereafter. In ImSP ICR mice, until day 10 PI, the numbers of oocysts excreted were less than in ImSP C57BL/6 mice, but were elevated abruptly after day 10 PI and peaked at day 13 PI; the peak value was maintained longer than in the other mouse strains (Fig. 1).
Fig. 1.
Kinetics of Cryptosporidium parvum oocyst excretion in immunosuppressed ICR, BALB/c, and C57BL/6 mice infected with the human isolate naturally mixed genotypes 1 and 2. The fecal suspension (*) consisted of a 1: 1 volume of the feces pooled from 5 mice and 2.5% potassium dichromate solution.
The infection and propagation of the SNU-H1 was successful in one of the 2 calves, which were infected with 2 x 107 oocysts harvested from the first passage. From day 4 PI, severe watery diarrhea was observed in the infected calf. A great number of oocysts were excreted from day 5 to 9 PI. After day 9 PI, the oocyst excretion decreased, and the calf recovered from the severe diarrhea. In contrast, the other newborn calf died on day 2 PI for an unknown reason.
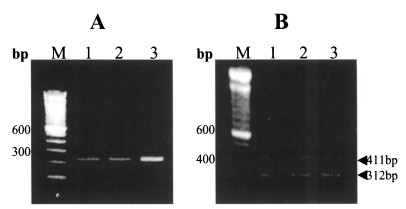
When the locus containing the microsatellite (tandem repeats of the GAG trinucleotide) of the SNU-H1 was amplified by PCR, a 238 bp fragment was obtained (Fig. 2-A), which indicates that the SNU-H1 belongs to C. parvum (Caccio et al., 2000). To identify its specific genotype, the previously confirmed method of Morgan et al. (1997) was applied. Analyses of the oocysts isolated from the original specimen from the patient, as well as oocysts from the first passage in ImSP C57BL/6 mice and second passage in the calf, showed amplified fragments of both 411 bp (genotype 1-specific) and 312 bp (genotype 2-specific) (Fig. 2-B).
Fig. 2.
PCR analysis of the SNU-H1 human isolate with subsequent passages in different animal hosts using 1.2% agarose gels stained with ethidium bromide. A. PCR using the C. parvum-specific primer. A 238 bp fragment was amplified in all samples. B. Genotype-specific PCR using the genotype 1- and 2-specific primers. In all samples, 312 bp and 411 bp fragments were amplified. Lane M, DNA size marker; lane 1, the human isolate SNU-H1; lane 2, 1st passage of the SNU-H1 in immunosuppressed C57BL/6 mice; lane 3, 2nd passage in a calf inoculated with the 1st passage.
The results of the present study have shown that the SNU-H1 is a mixture of the genotypes 1 and 2 of C. parvum. Moreover, this human isolate was successfully propagated in 3 mouse strains and a calf, and both genotypes were preserved. The DNA extracted from the oocysts collected after several passages showed no changes in the genetic profiles from that of the original isolate.
With regard to the animal susceptibility to C. parvum infection, no reports are currently available on the natural infections of animals (other than man) with the genotype 1, with the exception of a report on a dugong (Morgan et al., 2000). However, the successful experimental propagation of the genotype 1 has been achieved using animal models, such as captive primates, gnotobiotic piglets, lambs, and calves (Widmer et al., 2000; Giles et al., 2001; Widmer et al., 2002; Akiyoshi et al., 2002). The present study agrees that calves are susceptible to an experimental infection with the genotype 1.
Mice have never previously been included in the animals susceptible to the experimental infection with the genotype 1. Even the gamma-interferon knock-out mice and rodents showed no susceptibility to infection with the oocysts of the genotype 1 (Widmer et al., 2000; Akiyoshi et al., 2002). The present study, however, showed that immunosuppressed mice by steroid injection could be infected with both the genotypes 1 and 2. Therefore, immunosuppressed mice are suggested to be useful in evaluating the infectivity of the genotype 1.
Prior to the present study, natural infections of humans, or animals, with a mixture of the genotypes 1 and 2 of C. parvum have rarely been reported (Widmer et al., 2002). The present study confirms the presence of a mixed human infection with the genotypes 1 and 2 in the Republic of Korea. However, mixed human infections with the genotypes 1 and 2 seem to be a common characteristic in the Republic of Korea; for example, in a recent survey of Koreans infected with C. parvum, 6 of 7 positive samples were identified to be mixed-infected with both the genotypes 1 and 2 (unpublished observation). The present study, together with this communication, suggests that there may be unique transmission patterns of C. parvum in the Republic of Korea. A peculiar epidemiological feature reported was that, unlike the general tendency for cryptosporidial infections to occur predominantly among children, it was highly prevalent among aged people (Chai et al., 2001). Further studies are required to understand the transmission cycles of the mixed genotypes 1 and 2 in the Republic of Korea.
It is of considerable interest to note that the passage of the human genotype 1 to gnotobiotic piglets frequently results in the emergence of the genotype 2 (Widmer et al., 2002). Two possibilities were suggested for the reason; the genotype 2 subpopulations could have either accidentally contaminated, or originally resided in the genotype 1, with the resultant genotype 2 overgrowing the genotype 1. In the present study, we used the isolate from a natural mixed infection with the genotypes 1 and 2, and both genotypes were preserved with no changes in the genetic profiles during several passages in different animal hosts.
In the experimental genetic cross between 2 distinct isolates of the genotype 2, mixed infections produced a recombinant progeny, and it is suggested that outcrossing between genotypes may occur in nature (Feng et al., 2002). Experimental co-infections have shown that the development of different genetic subpopulations is not synchronous, and it may explain the rarity of mixed infections with different genotypes (Widmer et al., 2002).
In the present study, the oocyst shedding patterns of the mixed genotypes 1 and 2 were significantly different between the mouse strains. A delayed-onset, but a prolonged excretion of oocysts was observed in ImSP ICR mice, whereas ImSP C57BL/6 mice excreted a large number of oocysts during the early stages of the infection. ImSP BALB/c mice shed the smallest number of oocysts. Further studies are required to elucidate the immunophysiological mechanisms responsible for these differences.
ACKNOWLEDGMENTS
We are grateful to Dr. Sung-Won Kang, National Veterinary Research and Quarantine Services, Republic of Korea, for his help with the C. parvum propagation from the calve.
Footnotes
This work was supported by a grant from Seoul National University College of Medicine, Republic of Korea (2001).
References
- 1.Akiyoshi DE, Feng X, Buckholt MA, Widmer G, Tzipori S. Genetic analysis of a Cryptosporidium parvum human genotype 1 isolate passaged through different host species. Infect Immun. 2002;70:5670–5675. doi: 10.1128/IAI.70.10.5670-5675.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Caccio S, Homan W, Camilli R, Traldi G, Kortbeek T, Pozio E. A microsatellite marker reveals population heterogeneity within human and animal genotypes of Cryptosporidium parvum. Parasitology. 2000;120:237–244. doi: 10.1017/s0031182099005508. [DOI] [PubMed] [Google Scholar]
- 3.Chai JY, Shin SM, Yun CK, Yu JR, Lee SH. Experimental activation of cryptosporidiosis in mice by immunosuppression. Korean J Parasitol. 1990;28:31–37. doi: 10.3347/kjp.1990.28.1.31. [DOI] [PubMed] [Google Scholar]
- 4.Chai JY, Lee SH, Guk SM, Lee SH. An epidemiological survey of Cryptosporidium parvum infection in randomly selected inhabitants of Seoul and Chollanam-do. Korean J Parasitol. 1996;34:113–119. doi: 10.3347/kjp.1996.34.2.113. [DOI] [PubMed] [Google Scholar]
- 5.Chai JY, Guk SM, Han HK, Yun CK. Role of intraepithelial lymphocytes in mucosal immune responses of mice experimentally infected with Cryptosporidium parvum. J Parasitol. 1999;85:234–239. [PubMed] [Google Scholar]
- 6.Chai JY, Kim NY, Guk SM, et al. High prevalence and seasonality of cryptosporidiosis in a small rural village occupied predominantly by aged people in the Republic of Korea. Am J Trop Med Hyg. 2001;65:518–522. doi: 10.4269/ajtmh.2001.65.518. [DOI] [PubMed] [Google Scholar]
- 7.Fayer R, Ungar BLP. Cryptosporidium spp. and cryptosporidiosis. Microbiol Rev. 1986;50:458–483. doi: 10.1128/mr.50.4.458-483.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feng X, Rich SM, Tzipori S, Widmer G. Experimental evidence for genetic recombination in the opportunistic pathogen Cryptosporidium parvum. Mol Biochem Parasitol. 2002;119:55–62. doi: 10.1016/s0166-6851(01)00393-0. [DOI] [PubMed] [Google Scholar]
- 9.Giles M, Webster KA, Marshall JA, Catchpole J, Goddard TM. Experimental infection of a lamb withCryptosporidium parvum genotype 1. Vet Rec. 2001;149:523–525. doi: 10.1136/vr.149.17.523. [DOI] [PubMed] [Google Scholar]
- 10.Guk SM, Yong TS, Chai JY. Role of murine intestinal intraepithelial lymphocytes and lamina propria lymphocytes against primary and challenge infections with Cryptosporidium parvum. J Parasitol. 2003;89:270–275. doi: 10.1645/0022-3395(2003)089[0270:ROMIIL]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 11.McLauchlin J, Amar C, Pedraza-Diaz S, Nichols GL. Molecular epidemiological analysis of Cryptosporidium spp. in the United Kingdom: results of genotyping Cryptosporidium spp. in 1,705 fecal samples from humans and 105 fecal samples from livestock animals. J Clin Microbiol. 2000;38:3984–3990. doi: 10.1128/jcm.38.11.3984-3990.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morgan UM, Constantine CC, Forbes DA, Thompson RCA. Differentiation between human and animal isolates of Cryptosporidium parvum using a rDNA sequencing and direct PCR analysis. J Parasitol. 1997;83:825–830. [PubMed] [Google Scholar]
- 13.Morgan UM, Fall A, Ward LA, et al. Cryptosporidium hominis n. sp. (Apicomplexa: Cryptosporidiidae) from Homo sapiens. J Eukaryot Microbiol. 2002;49:433–440. doi: 10.1111/j.1550-7408.2002.tb00224.x. [DOI] [PubMed] [Google Scholar]
- 14.Morgan UM, Xiao L, Hill BD, et al. Detection of the Cryptosporidium parvum "Human" genotype in Dugong (Dugong dugon) J Parasitol. 2000;86:1352–1354. doi: 10.1645/0022-3395(2000)086[1352:DOTCPH]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 15.Spano F, Putignani L, Crisanti A, et al. Multilocus genotypic analysis of Cryptosporidium parvum isolates from different hosts and geographical origins. J Clin Microbiol. 1998;36:3255–3259. doi: 10.1128/jcm.36.11.3255-3259.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Widmer G, Akiyoshi D, Buckholt MA, et al. Animal propagation and genomic survey of a genotype 1 isolate of Cryptosporidium parvum. Mol Biochem Parasitol. 2000;108:187–197. doi: 10.1016/s0166-6851(00)00211-5. [DOI] [PubMed] [Google Scholar]
- 17.Widmer G, Lin L, Kapur V, Feng X, Abrahamsen MS. Genomics and genetics of Cryptosporidium parvum: the key to understanding cryptosporidiosis. Microbes Infect. 2002;4:1081–1090. doi: 10.1016/s1286-4579(02)01632-5. [DOI] [PubMed] [Google Scholar]
- 18.Xiao L, Bern C, Limor J, et al. Identification of 5 types of Cryptosporidium parasites in children in Lima, Peru. J Infect Dis. 2001;183:492–497. doi: 10.1086/318090. [DOI] [PubMed] [Google Scholar]
- 19.Yu JR, Lee JK, Seo M, et al. Prevalence of cryptosporidiosis among the villagers and domestic animals in several rural areas of Korea. Korean J Parasitol. 2004;42:1–6. doi: 10.3347/kjp.2004.42.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]