Abstract
Animal studies indicate neurobehavioral anomalies after prenatal exposure to benzo[a]pyrene (BaP). In order to determine if BaP directly affects neurodevelopment, we compared its effects to those of the organophosphate insecticide, chlorpyrifos (CPF), in undifferentiated and differentiating neuronotypic PC12 cells, evaluating indices of cell replication, cell number, neurite outgrowth and phenotypic differentiation. Unlike CPF, BaP did not inhibit DNA synthesis in undifferentiated cells. In cells undergoing nerve growth factor-induced differentiation, CPF reduced cell numbers (assessed by DNA content) whereas BaP increased them, suggesting a delay in the transition between cell replication and differentiation. Indices of cell enlargement (total protein/DNA) and neurite outgrowth (membrane protein/DNA) also showed opposite effects of CPF (increases) and BaP (decreases). We directly confirmed BaP impairment of neurodifferentiation by measuring markers for the two neurotransmitter phenotypes expressed by PC12 cells: tyrosine hydroxylase (dopamine phenotype) and choline acetyltransferase (acetylcholine phenotype). BaP significantly reduced both markers in differentiating cells, with a preferentially greater effect on the acetylcholine phenotype. Our results indicate that low, nontoxic levels of BaP can impair neurodifferentiation, resulting in excess cell numbers at the expense of the emergence of neurotransmitter phenotypes. BaP thus has direct actions on developing neuronal cells that could contribute to the adverse neurodevelopmental effects seen with in vivo exposures.
Keywords: Benzo[a]pyrene, Chlorpyrifos, In vitro, Neurodevelopment, Organophosphate insecticides, PC12 cells, Pesticides, Polycyclic aromatic hydrocarbons
INTRODUCTION
Human exposure to polycyclic aromatic hydrocarbons is virtually ubiquitous due to their generation as combustion products and inclusion in food. Although a great deal of attention has been focused on their carcinogenic properties, it is increasingly evident that these agents are also neuroactive, raising the specter that they may contribute to the emerging, “silent pandemic” of neurodevelopmental disorders [13]. Recent studies indicate a positive correlation between fetal exposure to these agents and adverse consequences such as reduced childhood head circumference and impaired neurobehavioral development [23,24]. However, surprisingly little has been done to establish a mechanistic link between exposure to polycyclic aromatic hydrocarbons and disruption of brain development. For benzo[a]pyrene (BaP), the prototype of this chemical class, neural and behavioral effects have been noted after exposure in adulthood [28]. With prenatal exposures, BaP alters later behavioral performance [45] and affects the expression of neurotransmitter receptors [6]; the latter effect can also be recapitulated by exposing postmitotic cultured neurons in vitro [6]. However, fetal BaP exposure also influences the expression of nuclear transcription factors that mediate the onset of neuronal cell differentiation [14], suggesting that there may be far more widespread effects of this agent in the developing brain, ultimately contributing to neurobehavioral impairment [46].
One of the major limitations of in vivo studies in animal models is the potential for confounding effects mediated by actions of the toxicant on maternal nutrition, endocrine status, neonatal caretaking, or other factors that impact neurobehavioral development of the offspring. Accordingly, the current study was undertaken to explore the possibility that BaP acts directly on developing neuronal cells to disrupt neurodifferentiation. We utilized PC12 cells, a neuronotypic cell line derived from rat pheochromocytoma that has been widely exploited as a model of neuronal development [39]. Because they are transformed cells, the PC12 line enables the delineation of adverse effects aimed at mitotic activity, whereas cultured primary neurons do not maintain cell division and thus cannot detect adverse effects on this likely neurotoxic target. When PC12 cells are treated with nerve growth factor (NGF), they gradually cease dividing and instead undergo neurodifferentiation, with development of neuritic projections, electrical excitability, and neurotransmitter phenotypes for dopamine and acetylcholine [12,38,39]. In contrast, primary neurons provide a heterogeneous population of cell types, discoordinated staging of differentiation, and a large number of phenotypes. For those reasons, the PC12 model has been used to characterize essential features of the developmental neurotoxicity of a wide variety of chemicals, including pesticides, neurotoxic drugs, metals and organometals [1–3,5,7–11,16,17,19–22,25,26,29,34,38,40,42]. Our objective here was to evaluate the propensity of BaP to elicit developmental neurotoxicity, specifically in comparison to the organophosphate pesticide, chlorpyrifos (CPF), a known developmental neurotoxicant that has effects on PC12 cells that mirror its impact on brain development in vivo [30,31].
Our evaluations were based on established protocols for screening of developmental neurotoxicants with PC12 cells [25–27,32–34,38]. In undifferentiated cells, we assessed antimitotic activity through measurement of [3H]thymidine incorporation into DNA, along with measuring DNA content to evaluate potential effects on cell number, since each neuronotypic cell contains only a single nucleus [44]. In differentiating cells, we also measured DNA content, but then also evaluated effects on cell growth by monitoring the increase in cell size accompanying differentiation (total protein/DNA) as well as membrane outgrowth associated with the formation of neurites (membrane protein/DNA, membrane/total protein). Finally, we assayed the two enzymes delineating differentiation into the dopamine and acetylcholine phenotypes, tyrosine hydroxylase (TH) and choline acetyltransferase (ChAT), respectively.
MATERIALS AND METHODS
All of the techniques used in this study have appeared in previous papers and accordingly, only brief descriptions of procedures will be given.
Cell cultures
Because of the clonal instability of the PC12 cell line [12], the experiments were performed on cells that had undergone fewer than five passages. PC12 cells were grown as described earlier [8,27,38], in RPMI-1640 medium (Invitrogen, Carlsbad, CA) supplemented with 10% inactivated horse serum (Sigma Chemical Co., St. Louis, MO), 5% fetal bovine serum (Sigma), and 50 μg/ml penicillin streptomycin (Invitrogen); cells were incubated with 5% CO2 at 37°C. For studies in the undifferentiated state, the medium was changed 24h after seeding to include varying concentrations of BaP (Sigma) or, for comparison, 50 μM CPF (Chem Service, West Chester, PA). Because of the limited water solubility of BaP and CPF, all test agents were dissolved in dimethylsulfoxide to achieve a final concentration in the culture medium of 0.1%, which has no effect on replication or differentiation of PC12 cells [25,27,38]; control cultures contained the same concentration of dimethylsulfoxide. For studies in differentiating cells, 24h after seeding, the medium was changed to include 50 ng/mL of 2.5 S murine NGF (Invitrogen) and dimethylsulfoxide with or without the test agents, and were examined after 6 days of exposure, with medium changes (including test agents) every 48h. We chose the CPF concentration to elicit a robust response for each of the effects to be compared to the actions of BaP, but below the threshold for outright cytotoxicity or loss of viability [2,9,10,16,25–27,34,38]. For BaP, we evaluated a concentration range spanning nearly two orders of magnitude (0.3 to 10 μM) after preliminary experiments determined the appropriate range; although we used only a single CPF concentration for reference in the present studies, we have previously published full concentration-response information for CPF [16,25,33,34,38].
DNA synthesis
Undifferentiated cells were exposed to the test agents for 24h and then, for the final hour of exposure, the medium was changed to include the test agents along with 1 μCi/ml of [3H]thymidine (specific activity, 2 Ci/mmol; PerkinElmer Life Sciences, Waltham, MA). After 1h, the medium was aspirated and cells were harvested. DNA was precipitated and separated by established procedures [4,35]. We corrected incorporation values to the amount of DNA present in each culture to provide an index of DNA synthesis per cell [44].
DNA and protein ratios
Differentiating cells were harvested after 6 days of exposure, washed, and the DNA and protein fractions isolated and analyzed as described previously [25,27,38,41]. To prepare the cell membrane fraction, the homogenates were sedimented at 40,000 × g for 10 min and the pellet was washed and resedimented. Aliquots of the final resuspension were then assayed for membrane protein.
Enzyme activities
Differentiating cells were harvested after 6 days of exposure and were disrupted by homogenization in a ground-glass homogenizer fitted with a ground-glass pestle, using a buffer consisting of 154 mM NaCl and 10 mM sodium-potassium phosphate (pH 7.4). Aliquots were withdrawn for measurement of DNA [41]. ChAT assays were conducted by published techniques [18] using a substrate of 50 μM [14C]acetyl-coenzyme A (specific activity 60 mCi/mmol; PerkinElmer). Labeled acetylcholine was counted in a liquid scintillation counter and activity calculated as pmol synthesized per hour per μg DNA. TH activity was measured using [14C]tyrosine as a substrate and trapping the evolved 14CO2 after coupled decarboxylation [18,43]. Each assay contained 55 μM [1-14C]L-tyrosine (Moravek Biochewmicals, Inc., Brea, CA; specific activity, 51 mCi/mmol, diluted to 3.3 mCi/mmol with unlabeled tyrosine) as substrate and activity was calculated on the same basis as for ChAT.
Data analysis
All studies were performed with multiple batches of cells, with several independent cultures for each treatment in each batch. Results are presented as mean ± SE, with treatment comparisons carried out by analysis of variance (ANOVA) followed by Fisher’s protected least significant difference test for post hoc comparisons of individual treatments. In the initial test, we evaluated two ANOVA factors (treatment, cell batch) and found that the treatment effects were the same across the different batches of cells, although the absolute values differed from batch to batch. Accordingly, we normalized the results across batches prior to combining them for presentation. Significance was assumed at p < 0.05.
RESULTS
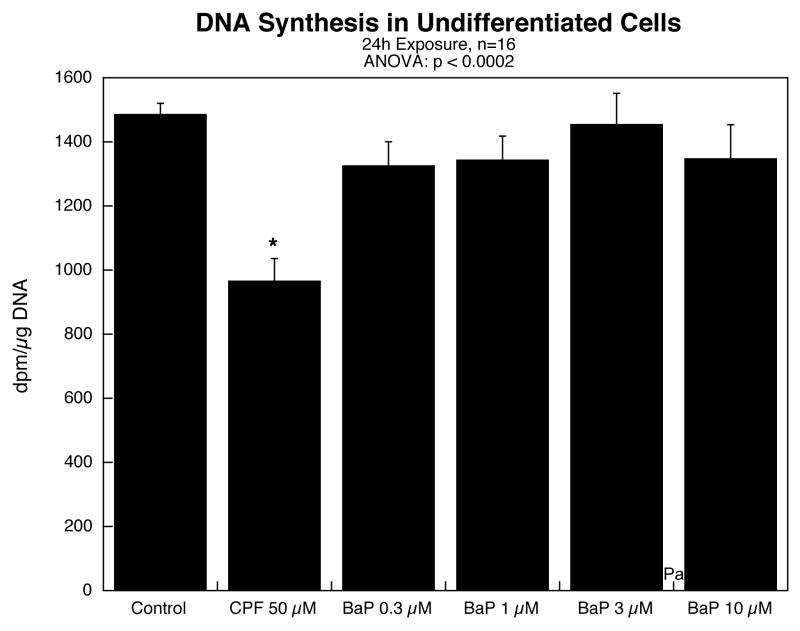
In undifferentiated cells, exposure to BaP for 24h in concentrations up to 10 μM failed to evoke any inhibition of DNA synthesis (Figure 1); the lack of effect was clearly distinguishable from the significant inhibition evoked by 50 μM CPF. Similarly, there was no effect of BaP on cell number, as determined from DNA content (data not shown).
Figure 1.
Effects of benzo[a]pyrene (BaP) in comparison to chlorpyrifos (CPF) on DNA synthesis in undifferentiated PC12 cells. Data represent means and standard errors. ANOVA across all treatments appears at the top of the panel and asterisks denote values that differ significantly from the control.
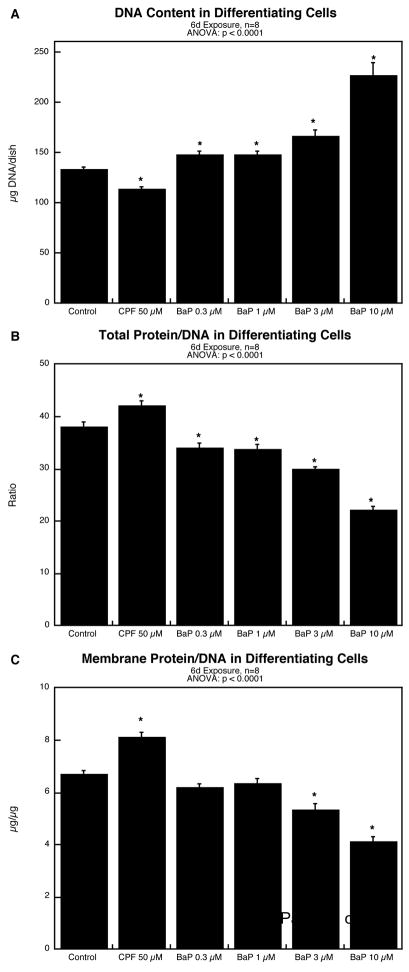
The results were quite different for cells undergoing NGF-induced differentiation. Exposure to BaP for six days evoked a concentration-dependent increase in DNA content, with significant effects evident even at 0.3 μM (Figure 2A); in contrast, CPF reduced the value. Indices of cell growth likewise showed significant effects of BaP in the opposite direction from those of CPF. BaP reduced the total protein/DNA ratio whereas CPF increased it (Figure 2B); again, there were significant effects of BaP even at 0.3 μM. The same pattern was seen for the membrane protein fraction, as evidenced by decreases in the membrane protein/DNA for BaP and increases for CPF (Figure 2C). The effects of BaP on the two protein fractions were equivalent, as there was no change in the membrane/total protein ratio (data not shown).
Figure 2.
Effects of benzo[a]pyrene (BaP) in comparison to chlorpyrifos (CPF) in differentiating PC12 cells after 6 days of NGF-induced differentiation: (A) DNA content; (B) total protein/DNA ratio; (C) membrane protein/DNA ratio. Data represent means and standard errors. ANOVA across all treatments appears at the top of each panel and asterisks denote values that differ significantly from the control.
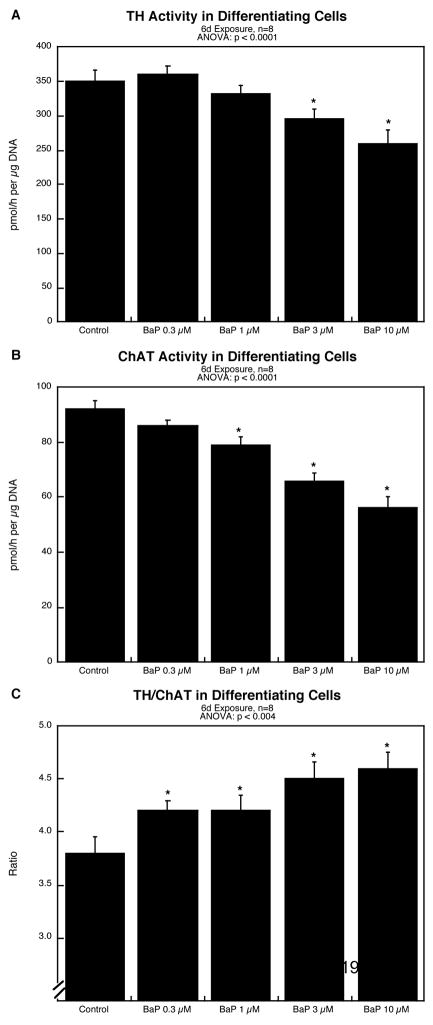
BaP exposure during differentiation suppressed the emergence of neuronal phenotypes, as evidenced by significant reductions in TH (Figure 3A) and ChAT (Figure 3B). The effect on the cholinergic phenotype was greater than that on the dopaminergic type, resulting in an increase in the TH/ChAT ratio (Figure 3C); again, even the lowest BaP concentration produced a significant increase in the ratio.
Figure 3.
Effects of BaP on differentiation into dopamine and acetylcholine phenotypes after 6 days of NGF-induced differentiation: (A) tyrosine hydroxylase activity, (B) choline acetyltransferase activity, (C) TH/ChAT ratio. Data represent means and standard errors. ANOVA across all treatments appears at the top of each panel and asterisks denote values that differ significantly from the control.
DISCUSSION
Our results provide some of the first evidence for a direct impairment of neurodifferentiation by BaP, with more potent effects than those of CPF, a known neuroteratogen. Unlike CPF, which affects both neuronal cell replication and differentiation, BaP was devoid of effects on the mitotic index or cell numbers in undifferentiated PC12 cells. With the onset of differentiation, CPF evoked a corresponding loss of cell numbers, as evidenced by a lowering of DNA content in the cultures, whereas BaP evoked substantial elevations, even at concentrations two orders of magnitude below that of CPF. An increase in cell number can come about in three ways: (a) enhancement of cell replication, (b) suppression of normally-occurring cell loss, or (c) interference with the transition from cell replication to neurodifferentiation. The first possibility is effectively ruled out by the fact that BaP did not evoke any increase in the mitotic index. Although a “beneficial” effect on cell loss seems unlikely, we nevertheless performed additional determinations to distinguish that possibility from the more likely outcome of impaired neurodifferentiation.
The total protein/DNA ratio, an index of net cell size, showed a clear-cut deficit in differentiating cells exposed to BaP, whereas CPF, which impairs cell replication without suppressing cell growth, evoked an increase. A reduction in cell size can come about either through general growth retardation (smaller cells) or through interference with the development of neuritic projections that accompany neurodifferentiation. Although the membrane protein/DNA ratio will fall for both cases, a cell that is simply smaller, will have an increased membrane/total protein ratio because of its higher surface-to-volume ratio. It is therefore important to note that BaP evoked equivalent changes in membrane and total protein (no change in the ratio), indicating that the reduction in overall cell size reflected a parallel loss of membrane area, i.e. impairment of the neurite formation that is characteristic of neurodifferentiation. Finally, we performed a definitive test as to whether BaP suppresses neurodifferentiation, examining the increase in TH and ChAT activities that accompanies the emergence of the dopaminergic and cholinergic phenotypes. Here again, BaP evoked clear-cut deficits in both markers. Superimposed on this shared effect, we obtained evidence for a selective impairment of the cholinergic phenotype, since BaP evoked a net increase in the TH/ChAT ratio, reflecting a significantly greater impairment for ChAT than for TH. One likely target for impairment of neurodifferentiation is the pivotal role played by NGF, both in this model system and in the developing nervous system in vivo. Interference with the effect of NGF could readily contribute to a delay in the fall-off of DNA synthesis, deficits in cell growth, reduced neurite formation and impaired differentiation into neurotransmitter phenotypes. Nevertheless, this cannot provide a total explanation for the observed outcomes, since a straightforward effect on NGF sensitivity should not provide selective effects on ChAT vs. TH, and should actually cause reductions in cell numbers because of increased apoptosis. Again, future work should focus on whether NGF or other neurotrophic factors are a target for the effects of BaP on neurodevelopment.
Although PC12 cells provide an advantage for identifying direct mechanisms of toxicant exposure on neurodevelopment, they share the limitations common to all cell culture systems, namely difficulties in assessing neuronal-glial interactions or architectural aspects of brain region development, maternal-fetal or neonatal pharmacokinetics and related issues of bioavailability, dose and bioeffective concentrations. The translation of in vitro to in vivo exposure is especially problematic, given the fact that the PC12 model involves transformed cells that are inherently more resistant to toxicants than are primary neurons and that effects need to observed over a much shorter period in cells as compared to long-term exposures for brain development in vivo. It is thus important to note that we observed effects at the same submicromolar concentrations as those achieved with BaP exposures in fetal rats that lead to adverse effects on brain development [6].
Our findings are thus indicative of a highly selective effect of BaP on neuronal development, namely impairment of neurodifferentiation, leading to excessive accumulation of cells at the expense of both neurite formation and the emergence of neurotransmitter phenotypes. If the same effects occur with in vivo BaP exposures, each of these effects has important ramifications for brain development. First, the proper modeling of brain architecture requires the elimination of “exuberant” neurons so as to permit the formation and reinforcement of proper synaptic connections. Impairment of neuronal cell loss, as seen here, is thus likely to produce widespread interference with the development of neural circuitry. Second, the guidance of axonal projections to their proper targets requires carefully-coordinated interactions of developing neurons, glia and trophic factors, and accordingly, the appropriate timing of neurodifferentiation is a critical event. Here, we found a substantial delay in the transition from cell replication to neurodifferentiation and a corresponding impairment of neurite formation and expression of neurotransmitter phenotypes. Again, this could potentially lead to widespread “miswiring” of neural circuits. Finally, we saw a selective effect on the cholinergic phenotype as distinct from the dopaminergic phenotype, an outcome that BaP shares with a number of other environmental toxicants that are known to disrupt brain development and to evoke corresponding behavioral deficits [15–17,32,34,36,37]. Future studies should thus focus on behaviors linked to these phenotypes as likely targets for the consequences of developmental exposure to BaP.
In conclusion, our studies indicate that BaP can disrupt neuronal cell differentiation, providing mechanistic links to the epidemiological findings for human fetal and childhood exposures to polycyclic aromatic hydrocarbons that show an impact on neurobehavioral development [24]. Accordingly, animal studies of BaP exposure that show corresponding synaptic and behavioral deficits are likely to involve direct actions on brain development, beyond any indirectly-mediated effects on the maternal-fetal unit, endocrine status, or other contributing factors.
Acknowledgments
Research was supported by NIH ES10356. The authors thanks Ian Ryde and Nicola Wrench for technical assistance. The authors do not have any conflicts of interest. However, TAS has provided expert witness testimony in the past three years at the behest of the following law firms: The Calwell Practice (Charleston WV), Frost Brown Todd (Charleston WV), Snyder Weltchek & Snyder (Baltimore MD), Finnegan Henderson Farabow Garrett & Dunner (Washington DC), Frommer Lawrence Haug (Washington DC), Carter Law (Peoria IL), Corneille Law (Madison WI), Angelos Law (Baltimore MD), Kopff, Nardelli & Dopf (New York NY), and Gutglass Erickson Bonville & Larson (Madison WI).
Abbreviations
- ANOVA
analysis of variance
- BaP
benzo[a]pyrene
- ChAT
choline acetyltransferase
- CPF
chlorpyrifos
- NGF
nerve growth factor
- TH
tyrosine hydroxylase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abreu-Villaça Y, Seidler FJ, Qiao D, Slotkin TA. Modeling the developmental neurotoxicity of nicotine in vitro: cell acquisition, growth and viability in PC12 cells. Dev Brain Res. 2005;154:239–246. doi: 10.1016/j.devbrainres.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 2.Bagchi D, Bagchi M, Hassoun EA, Stohs SJ. In vitro and in vivo generation of reactive oxygen species, DNA damage and lactate dehydrogenase leakage by selected pesticides. Toxicology. 1995;104:129–140. doi: 10.1016/0300-483x(95)03156-a. [DOI] [PubMed] [Google Scholar]
- 3.Bagchi D, Bhattacharya G, Stohs SJ. In vitro and in vivo induction of heat shock (stress) protein (Hsp) gene expression by selected pesticides. Toxicology. 1996;112:57–68. doi: 10.1016/0300-483x(96)03350-1. [DOI] [PubMed] [Google Scholar]
- 4.Bell JM, Whitmore WL, Slotkin TA. Effects of α-difluoromethylornithine, a specific irreversible inhibitor of ornithine decarboxylase, on nucleic acids and proteins in developing rat brain: critical perinatal periods for regional selectivity. Neuroscience. 1986;17:399–407. doi: 10.1016/0306-4522(86)90255-1. [DOI] [PubMed] [Google Scholar]
- 5.Benters J, Schafer T, Beyersmann D, Hechtenberg S. Agonist-stimulated calcium transients in PC12 cells are affected differentially by cadmium and nickel. Cell Calcium. 1996;20:441–446. doi: 10.1016/s0143-4160(96)90007-x. [DOI] [PubMed] [Google Scholar]
- 6.Brown LA, Khousbouei H, Goodwin JS, Irvin-Wilson CV, Ramesh A, Sheng L, McCallister MM, Jiant GCT, Aschner M, Hood DB. Down-regulation of early ionotrophic glutamate receptor subunit developmental expression as a mechanism for observed plasticity deficits following gestational exposure to benzo(a)pyrene. Neurotoxicology. 2007;28:965–978. doi: 10.1016/j.neuro.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crumpton T, Atkins DS, Zawia NH, Barone S., Jr Lead exposure in pheochromocytoma (PC12) cells alters neural differentiation and Sp1 DNA-binding. Neurotoxicology. 2001;22:49–62. doi: 10.1016/s0161-813x(00)00008-5. [DOI] [PubMed] [Google Scholar]
- 8.Crumpton TL, Seidler FJ, Slotkin TA. Developmental neurotoxicity of chlorpyrifos in vivo and in vitro: effects on nuclear transcription factor involved in cell replication and differentiation. Brain Res. 2000;857:87–98. doi: 10.1016/s0006-8993(99)02357-4. [DOI] [PubMed] [Google Scholar]
- 9.Crumpton TL, Seidler FJ, Slotkin TA. Is oxidative stress involved in the developmental neurotoxicity of chlorpyrifos? Dev Brain Res. 2000;121:189–195. doi: 10.1016/s0165-3806(00)00045-6. [DOI] [PubMed] [Google Scholar]
- 10.Das KP, Barone S. Neuronal differentiation in PC12 cells is inhibited by chlorpyrifos and its metabolites: is acetylcholinesterase inhibition the site of action? Toxicol Appl Pharmacol. 1999;160:217–230. doi: 10.1006/taap.1999.8767. [DOI] [PubMed] [Google Scholar]
- 11.Flaskos J, McLean WG, Hargreaves AJ. The toxicity of organophosphate compounds towards cultured PC12 cells. Toxicol Lett. 1994;70:71–76. doi: 10.1016/0378-4274(94)90146-5. [DOI] [PubMed] [Google Scholar]
- 12.Fujita K, Lazarovici P, Guroff G. Regulation of the differentiation of PC12 pheochromocytoma cells. Environ Health Perspect. 1989;80:127–142. doi: 10.1289/ehp.8980127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grandjean P, Landrigan PJ. Developmental neurotoxicity of industrial chemicals. Lancet. 2006;368:2167–2178. doi: 10.1016/S0140-6736(06)69665-7. [DOI] [PubMed] [Google Scholar]
- 14.Hood DB, Nayyar T, Ramesh A, Greenwood M, Inyang F. Modulation in the developmental expression profile of Sp1 subsequent to transplacental exposure of fetal rats to desorbed benzo[a]pyrene following maternal inhalation. Inhal Toxicol. 2000;12:511–535. doi: 10.1080/089583700402897. [DOI] [PubMed] [Google Scholar]
- 15.Jameson RR, Seidler FJ, Qiao D, Slotkin TA. Adverse neurodevelopmental effects of dexamethasone modeled in PC12 cells: identifying the critical stages and concentration thresholds for the targeting of cell acquisition, differentiation and viability. Neuropsychopharmacology. 2006;31:1647–1658. doi: 10.1038/sj.npp.1300967. [DOI] [PubMed] [Google Scholar]
- 16.Jameson RR, Seidler FJ, Qiao D, Slotkin TA. Chlorpyrifos affects phenotypic outcomes in a model of mammalian neurodevelopment: critical stages targeting differentiation in PC12 cells. Environ Health Perspect. 2006;114:667–672. doi: 10.1289/ehp.8750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lassiter TL, MacKillop EA, Ryde IT, Seidler FJ, Slotkin TA. Is fipronil safer than chlorpyrifos? Comparative developmental neurotoxicity modeled in PC12 cells. Brain Res Bull. 2009;78:313–322. doi: 10.1016/j.brainresbull.2008.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lau C, Seidler FJ, Cameron AM, Navarro HA, Bell JM, Bartolome J, Slotkin TA. Nutritional influences on adrenal chromaffin cell development: comparison with central neurons. Pediatr Res. 1988;24:583–587. doi: 10.1203/00006450-198811000-00009. [DOI] [PubMed] [Google Scholar]
- 19.Li WW, Casida JE. Organophosphorus neuropathy target esterase inhibitors selectively block outgrowth of neurite-like and cell processes in cultured cells. Toxicol Lett. 1998;98:139–146. doi: 10.1016/s0378-4274(98)00116-7. [DOI] [PubMed] [Google Scholar]
- 20.Matsuoka M, Igisu H. Induction of c-fos expression by tributylin in PC12 cells: involvement of intracellular Ca2+. Environ. Toxicol Pharmacol. 1996;2:373–380. doi: 10.1016/s1382-6689(96)00074-9. [DOI] [PubMed] [Google Scholar]
- 21.Nagata K, Huang CS, Song JH, Narahashi T. Direct actions of anticholinesterases on the neuronal nicotinic acetylcholine receptor channels. Brain Res. 1997;769:211–218. doi: 10.1016/s0006-8993(97)00707-5. [DOI] [PubMed] [Google Scholar]
- 22.Parran DK, Barone S, Jr, Mundy WR. Methylmercury decreases NGF-induced TrkA autophosphorylation and neurite outgrowth in PC12 cells. Dev Brain Res. 2003;141:71–81. doi: 10.1016/s0165-3806(02)00644-2. [DOI] [PubMed] [Google Scholar]
- 23.Perera FP, Rauh V, Tsai WY, Kinney P, Barr CDD, Bernert T, Garfinkel R, Tu YH, Diaz D, Dietrich J, Whyatt RM. Effects of transplacental exposure to environmental pollutants on birth outcomes in a multiethnic population. Environ Health Perspect. 2003;111:201–205. doi: 10.1289/ehp.5742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perera FP, Rauh V, Whyatt RM, Tsai WY, Tang D, Diaz D, Hoepner L, Barr D, Tu YH, Camann D, Kinney P. Effect of prenatal exposure to airborne polycyclic aromatic hydrocarbons on neurodevelopment in the first 3 years of life among inner-city children. Environ Health Perspect. 2006;114:1287–1292. doi: 10.1289/ehp.9084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qiao D, Seidler FJ, Slotkin TA. Developmental neurotoxicity of chlorpyrifos modeled in vitro: comparative effects of metabolites and other cholinesterase inhibitors on DNA synthesis in PC12 and C6 cells. Environ Health Perspect. 2001;109:909–913. doi: 10.1289/ehp.01109909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qiao D, Seidler FJ, Slotkin TA. Oxidative mechanisms contributing to the developmental neurotoxicity of nicotine and chlorpyrifos. Toxicol Appl Pharmacol. 2005;206:17–26. doi: 10.1016/j.taap.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 27.Qiao D, Seidler FJ, Violin JD, Slotkin TA. Nicotine is a developmental neurotoxicant and neuroprotectant: stage-selective inhibition of DNA synthesis coincident with shielding from effects of chlorpyrifos. Dev Brain Res. 2003;147:183–190. doi: 10.1016/s0165-3806(03)00222-0. [DOI] [PubMed] [Google Scholar]
- 28.Saunders CR, Ramesh A, Shockley DC. Modulation of neurotoxic behavior in F-344 rats by temporal disposition of benzo(a)pyrene. Toxicol Lett. 2002;129:33–45. doi: 10.1016/s0378-4274(01)00467-2. [DOI] [PubMed] [Google Scholar]
- 29.Shafer TJ. Effects of Cd2+, Pb2+ and CH3Hg+ on high voltage-activated calcium currents in pheochromocytoma (PC12) cells: potency, reversibility, interactions with extracellular Ca2+ and mechanisms of block. Toxicol Lett. 1998;99:207–221. doi: 10.1016/s0378-4274(98)00225-2. [DOI] [PubMed] [Google Scholar]
- 30.Slotkin TA. Cholinergic systems in brain development and disruption by neurotoxicants: nicotine, environmental tobacco smoke, organophosphates. Toxicol Appl Pharmacol. 2004;198:132–151. doi: 10.1016/j.taap.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 31.Slotkin TA. Developmental neurotoxicity of organophosphates: a case study of chlorpyrifos. In: Gupta RC, editor. Toxicity of Organophosphate and Carbamate Pesticides. Elsevier Academic Press; San Diego: 2005. pp. 293–314. [Google Scholar]
- 32.Slotkin TA, MacKillop EA, Melnick RL, Thayer KA, Seidler FJ. Developmental neurotoxicity of perfluorinated chemicals modeled in vitro. Environ Health Perspect. 2008;116:716–722. doi: 10.1289/ehp.11253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Slotkin TA, MacKillop EA, Ryde IT, Seidler FJ. Ameliorating the developmental neurotoxicity of chlorpyrifos: a mechanisms-based approach in PC12 cells. Environ Health Perspect. 2007;115:1306–1313. doi: 10.1289/ehp.10194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Slotkin TA, MacKillop EA, Ryde IT, Tate CA, Seidler FJ. Screening for developmental neurotoxicity using PC12 cells: comparisons of organophosphates with a carbamate, an organochlorine and divalent nickel. Environ Health Perspect. 2007;115:93–101. doi: 10.1289/ehp.9527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Slotkin TA, Persons D, Slepetis RJ, Taylor D, Bartolome J. Control of nucleic acid and protein synthesis in developing brain, kidney, and heart of the neonatal rat: effects of α-difluoromethylornithine, a specific, irreversible inhibitor of ornithine decarboxylase. Teratology. 1984;30:211–224. doi: 10.1002/tera.1420300209. [DOI] [PubMed] [Google Scholar]
- 36.Slotkin TA, Seidler FJ. Developmental neurotoxicants target neurodifferentiation into the serotonin phenotype: chlorpyrifos, diazinon, dieldrin and divalent nickel. Toxicol Appl Pharmacol. 2008;233:211–219. doi: 10.1016/j.taap.2008.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Slotkin TA, Seidler FJ. Transcriptional profiles reveal similarities and differences in the effects of developmental neurotoxicants on differentiation into neurotransmitter phenotypes in PC12 cells. Brain Res Bull. 2009;78:211–225. doi: 10.1016/j.brainresbull.2008.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Song X, Violin JD, Seidler FJ, Slotkin TA. Modeling the developmental neurotoxicity of chlorpyrifos in vitro: macromolecule synthesis in PC12 cells. Toxicol Appl Pharmacol. 1998;151:182–191. doi: 10.1006/taap.1998.8424. [DOI] [PubMed] [Google Scholar]
- 39.Teng KK, Greene LA. Cultured PC12 cells: a model for neuronal function and differentiation. In: Celis JE, editor. Cell Biology: A Laboratory Handbook. Academic Press; San Diego: 1994. pp. 218–224. [Google Scholar]
- 40.Tian X, Sun X, Suszkiw JB. Upregulation of tyrosine hydroxylase and downregulation of choline acetyltransferase in lead-exposed PC12 cells: the role of PKC activation. Toxicol Appl Pharmacol. 2000;167:246–252. doi: 10.1006/taap.2000.8996. [DOI] [PubMed] [Google Scholar]
- 41.Trauth JA, Seidler FJ, Slotkin TA. An animal model of adolescent nicotine exposure: effects on gene expression and macromolecular constituents in rat brain regions. Brain Res. 2000;867:29–39. doi: 10.1016/s0006-8993(00)02208-3. [DOI] [PubMed] [Google Scholar]
- 42.Tuler SM, Hazen AA, Bowen JM. Release and metabolism of dopamine in a clonal line of pheochromocytoma (PC12) cells exposed to fenthion. Fund Appl Toxicol. 1989;13:484–492. doi: 10.1016/0272-0590(89)90284-4. [DOI] [PubMed] [Google Scholar]
- 43.Waymire JC, Bjur R, Weiner N. Assay of tyrosine hydroxylase by coupled decarboxylation of dopa formed from 1-14C-L-tyrosine. Anal Biochem. 1971;43:588–600. doi: 10.1016/0003-2697(71)90291-0. [DOI] [PubMed] [Google Scholar]
- 44.Winick M, Noble A. Quantitative changes in DNA, RNA and protein during prenatal and postnatal growth in the rat. Dev Biol. 1965;12:451–466. doi: 10.1016/0012-1606(65)90009-6. [DOI] [PubMed] [Google Scholar]
- 45.Wormley D, Chirwa S, Harris E, Nayyar T, Wu J, Hood DB. Inhaled benzo(a)-pyrene impairs long-term potentiation in rat dentate gyrus: reduced capacity for long-term potentiation in the F1 generation. Cell Mol Biol. 2004;50:715–721. [PubMed] [Google Scholar]
- 46.Wormley D, Ramesh A, Hood DB. Environmental contaminant-mixture effects on CNS development, plasticity, and behavior. Toxicol Appl Pharmacol. 2004;197:49–65. doi: 10.1016/j.taap.2004.01.016. [DOI] [PubMed] [Google Scholar]