Abstract
The results of the NICE-SUGAR (Normoglycaemia in Intensive Care Evaluation Survival Using Glucose Algorithm Regulation) trial were released last March. The primary outcome variable, 90-day mortality, was actually increased in patients randomly assigned to intensive insulin therapy, as compared with an intermediate target range for blood glucose. These findings, reflecting data collected in a set of more than 6,000 patients, clearly refute the external validity of tight glucose control. Future research will probably focus on several questions raised by the divergent results reported from investigations in the field of glucose control in the critically ill.
On Tuesday, 24 March 2009 at 10:05 hours, the Erasmus Room of the Exhibition and Congress Centre of Brussels was overcrowded. Attendees from all over the world had gathered for a well planned and widely announced event. Professor Simon Finfer, from the Royal North Shore Hospital of Sydney, Australia was about to release the results of the NICE-SUGAR (Normoglycaemia in Intensive Care Evaluation Survival Using Glucose Algorithm Regulation) trial, the largest clinical study conducted in critical care medicine to date. At the end of his presentation, the article was published and available on the website of the New England Journal of Medicine [1].
NICE-SUGAR was designed to test whether tight glucose control by intensive insulin therapy (TGCIIT; n = 3,010 evaluable patients) increases 90-day survival as compared with less strict glucose control (n = 3,012 evaluable patients). The issue of TGCIIT has been among the most popular and passionate areas of debate and discussion since 2001, when the landmark Leuven I study [2] was published. Several investigators [3-6] and the Leuven medical ICU team [7] had already assessed the effects of TGCIIT in various settings and conditions. These trials failed to reproduce the impressive improvement in survival reported in the Leuven I study [2]. It is unsurprising (in view of the now presented NICE-SUGAR findings) that two recent meta-analyses [8,9] concluded simply that tight glucose control is not associated with significantly reduced hospital mortality. Criticisms of each of the individual studies were raised, including inadequate statistical power and the use of various degrees of glucose control, all lower in the subsequent trials [3-7] than in the initial Leuven I study [2]. Therefore, the NICE-SUGAR trial was eagerly awaited by the intensive care medicine community worldwide.
The sample size of NICE-SUGAR was calculated to detect a 3.8% absolute difference in mortality (treatment effect reported in the Leuven I trial) with a power of 90%, assuming a baseline mortality of 30% [2]. NICE-SUGAR was conducted in a network of intensive care units that had previously collaborated and included patients in large-scale trials. A web-based electronic algorithm was used to adapt the insulin infusion rate. Under these conditions (optimal for successful performance of a multicentre trial) the primary outcome variable, namely 90-day mortality, was found to be increased from 24.9% in the conventional/control group arm to 27.5% in the intensive treatment arm, which is in complete contrast to the findings of the Leuven I trial. These findings allow us to address certain issues and provide some answers, but they also raise new questions.
The main issue considered by NICE-SUGAR – whether the Leuven I trial has external validity – is clearly addressed in the negative, in contrast to previous hopes and beliefs. Possible reasons for the lack of external validity are multiple and include major differences in the amount of intravenous glucose infused, the frequency of use of enteral nutrition and possibly a lower 'commitment' to TGCIIT by centres other than Leuven. Nonetheless, NICE-SUGAR probably succeeded in separating the levels of glycaemia reached in the two experimental groups, even though the interquartile ranges of the values are not stated in the report [2]. Whatever the reason for the disparity between the results of the Leuven I trial and other studies, some standards of care will be changed. The Endocrine Society has already issued a statement [10], just after the publication of the results of NICE-SUGAR, advocating the need for more nuanced recommendations on glucose control. Likewise, other official bodies (for instance, the Joint Commission on Accreditation of Healthcare Organizations, the Institute for Healthcare Improvement and the Volunteer Hospital Organization) that issued recommendations on tight glucose control in critically ill patients will need to re-consider their position.
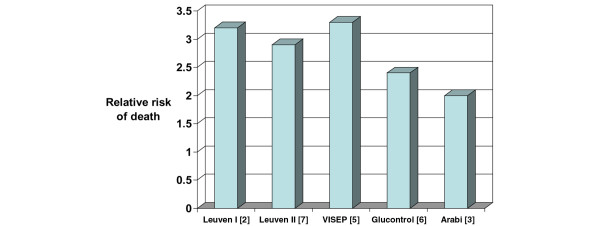
The new questions raised by NICE-SUGAR probably include the actual validity of the concept of 80 to 110 mg/dl (4.4 to 6.1 mmol/l) as 'normoglycaemia' or even desirable glycaemia during critical illness [11]. Another key but unresolved and poorly investigated issue is the possible nonglycaemic effects of insulin in the late divergence in the cumulative survival curves observed both in the Leuven studies [2,7] and in NICE-SUGAR [1], albeit in opposite directions. Other pending questions raised include the risks and potentially harmful effects of high variability in glucose levels, which are probably influenced by TGCIIT [12-14]. Finally, the absence of risks for hypoglycaemia, although not studied specifically in NICE-SUGAR, is questionable when the mortality rate of patients who experienced hypoglycaemia was systematically two to three times higher than in nonhypoglycaemic patients (Figure 1). The effects of hypoglycaemia can be particularly harmful in brain-injured patients [15,16].
Figure 1.
Relative risk for death in patients with hypoglycaemia. Shown are the relative mortality rates in patients included in prospective studies of tight glucose control by intensive insulin therapy who experienced hypoglycaemia versus those who did not. Mortality rate was increased by a factor of 2 to 3.3 among patients who experienced hypoglycaemia.
With these uncertainties in mind, the only target for blood glucose that can currently be recommended will probably be in the intermediate range, even in the absence of direct evidence. An intermediate level will probably allow safer although effective glucose control [17].
Abbreviations
NICE-SUGAR: Normoglycaemia in Intensive Care Evaluation Survival Using Glucose Algorithm Regulation; TGCIIT: tight glucose control by intensive insulin therapy.
Competing interests
The author declares that they have no competing interests.
References
- NICE-SUGAR Study Investigators. Finfer S, Chittock DR, Su SY, Blair D, Foster D, Dhingra V, Bellomo R, Cook D, Dodek P, Henderson WR, Hebert PC, Heritier S, Heyland DK, McArthur C, McDonald E, Mitchell I, Myburgh JA, Norton R, Potter J, Robinson BG, Ronco JJ. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360:1346–1349. doi: 10.1056/NEJMe0901507. [DOI] [PubMed] [Google Scholar]
- Berghe G Van den, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, Vlasselaers D, Ferdinande P, Lauwers P, Bouillon R. Intensive insulin therapy in the critically ill patients. N Engl J Med. 2001;345:1359–67. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- Arabi YM, Dabbagh OC, Tamim HM, Al-Shimemeri AA, Memish ZA, Haddad SH, Sved SJ, Giridhar HR, Rishu AH, Al-Daker MO, Kahoul SH, Britts RJ, Sakkijha MH. Intensive versus conventional insulin therapy: a randomized controlled trial in medical and surgical critically ill patients. Crit Care Med. 2008;36:3190–3197. doi: 10.1097/CCM.0b013e31818f21aa. [DOI] [PubMed] [Google Scholar]
- De La Rosa GD, Donado JH, Restrepo AH, Quintero AM, Gonzalez LG, Saldarriaga NE, Bedoya M, Toro JM, Velasquez JB, Valencia JC, Arango CM, Aleman PH, Vasquez EM, Chavarriaga JC, Yepes A, Pulido W, Cadavid CA, Grupo de Investigacion en Cuidado intensivo: GICI-HPTU Strict glycaemic control in patients hospitalised in a mixed medical and surgical intensive care unit: a randomised clinical trial. Crit Care. 2008;12:R120. doi: 10.1186/cc7017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunkhorst FM, Engel C, Bloos F, Meier-Hellmann A, Ragaller M, Weiler N, Moerer O, Gruendling M, Oppert M, Grond S, Olthoff D, Jaschinski U, John S, Rossaint R, Welte T, Schaefer M, Kern P, Kuhnt E, Kiehntopf M, Hartoq C, Natanson C, Loeffler M, Reinhart K, German Competence Network Sepsis (SepNet) Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med. 2008;358:125–139. doi: 10.1056/NEJMoa070716. [DOI] [PubMed] [Google Scholar]
- Devos P, Preiser JC, Melot C. Impact of tight glucose control by intensive insulin therapy on ICU mortality and the rate of hypoglycaemia: final results of the Glucontrol study. Intensive Care Med. 2007;33:S189. [Google Scholar]
- Berghe G Van den, Wilmer A, Hermans G, Meersseman W, Wouters PJ, Milants I, Van Wijngaerden E, Bobbaers H, Bouillon R. Intensive insulin therapy in the medical ICU. N Engl J Med. 2006;354:449–461. doi: 10.1056/NEJMoa052521. [DOI] [PubMed] [Google Scholar]
- Wiener RS, Wiener DC, Larson RJ. Benefits and risks of tight glucose control in critically ill adults: a meta-analysis. JAMA. 2008;300:933–944. doi: 10.1001/jama.300.8.933. [DOI] [PubMed] [Google Scholar]
- Griesdale DEG, de Souza RJ, van Dam RM, Heyland DK, Cook DJ, Malhotra A, Dhaliwal R, Henderson WR, Chittock DR, Finfer S, Talmor D. Intensive insulin therapy and mortality among critically ill patients: a meta-analysis including NICE-SUGAR study data. CMAJ. 2009;180:821–827. doi: 10.1503/cmaj.090206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Endocrine Society Statement to Providers on the Report Published in the New England Journal of Medicine on NICE-SUGAR http://www.endo-society.org/advocacy/legislative/letters/upload/NICE-SUGAR-Position-Statement-FINAL.pdf
- Preiser JC. Restoring normoglycaemia: not so harmless. Crit Care. 2008;12:116. doi: 10.1186/cc6787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali NA, O'Brien JM, Jr, Dungan K, Phillips G, Marsh CB, Lemeshow S, Connors AF, Jr, Preiser JC. Glucose variability is independently associated with increased mortality in patients with severe sepsis. Crit Care Med. 2008;36:2316–2321. doi: 10.1097/CCM.0b013e3181810378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egi M, Bellomo R, Reade MC. Is reducing variability of blood glucose the real but hidden target of intensive insulin therapy? Crit Care. 2009 doi: 10.1186/cc7755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali NA, Krinsley JS, Preiser JC. Glucose variability in critically ill patients. In: Vincent JL, editor. The Yearbook of Intensive Care and Emergency Medicine. Berlin, Heidelberg, New York: Springer; 2009. pp. 728–737. [Google Scholar]
- Billotta F, Giovannini F, Caramia R, Rosa G. Glycemia management in neurocritical care patients: a review. J Neurosurg Anesthesiol. 2009;21:2–9. doi: 10.1097/ANA.0b013e31818f8a5c. [DOI] [PubMed] [Google Scholar]
- Oddo M, Schmidt JM, Carrera E, Badjatia N, Connolly ES, Presciutti M, Ostapkovich ND, Levine JM, Le Roux P, Mayer SA. Impact of tight glycemic control on cerebral glucose metabolism after severe brain injury: a microdialysis study. Crit Care Med. 2008;36:3233–3238. doi: 10.1097/CCM.0b013e31818f4026. [DOI] [PubMed] [Google Scholar]
- Krinsley J, Preiser JC. Moving beyond tight glucose control to safe effective glucose control. Crit Care. 2008;12:149. doi: 10.1186/cc6889. [DOI] [PMC free article] [PubMed] [Google Scholar]