Abstract
Background
HPT in MEN1 patients with ZES is caused by parathyroid hyperplasia. Surgery for parathyroid hyperplasia is tricky and difficult. Long-term outcome in ZES/MEN1 /HPT is not well known.
Methods
84 consecutive patients (49 F/35 M) with ZES/MEN1 /HPT underwent intial parathyroidectomy (PTX) and were followed at 1−3 yr intervals.
Results
Age at PTX was 36±2 yrs. Mean follow-up was 17±1 yrs. Prior to PTX, mean Ca=2.8 mmol/L (nl<2.5), PTHi=243 pg/ml (nl <65), and gastrin=6950 pg/ml (nl <100). 61% had nephrolithiasis. Each patient had parathyroid hyperplasia. 58% of patients had four parathyroid glands identified. 9/84 (11%) had 4 glands removed with immediate autograft, 40/84 (47%) 3−3.5 glands, while 35/84 (42%) <3 glands.removed. Persistent/recurrent HPT occurred in 42%/48% of patients with <3 glands, 12%/44% with 3−3.5 glands, and 0%/55% with 4 glands removed. Hypoparathyroidism occurred in 3%, 10% and 22%, respectively. The disease-free interval following surgery was significantly longer if >3 glands were removed. After surgery to correct the HPT, each biochemical parameter of ZES was improved and 20% of patients no longer had laboratory evidence of ZES.
Conclusions
HPT /MEN1/ZES is a severe form of parathyroid hyperplasia with a high rate of nephrolithiasis, persistent and recurrent HPT. Surgery to correct the hypercalcemia significantly ameliorates the ZES. Removal of less than 3 and ½ glands has an unacceptably high incidence of persistent HPT (42%), while 4 gland resection and transplant has an high rate of permanent hypoparathyroidism (22%). >3gland resection has a longer disease-free interval. 3 and ½ gland parathyroidectomy is the surgical procedure of choice for patients with HPT/MEN1/ ZES. Careful long-term follow-up is mandatory as a significant proportion will develop recurrent HPT.
Introduction
Primary hyperparathyroidism (HPT) is the most common clinical manifestation of Multiple Endocrine Neoplasia Type 1 (MEN-1)1, 2. Although there may be asymmetrical enlargement of the parathyroid glands causing the surgeon to mistaken the pathology as adenoma, it is always caused by hyperplasia or multiple abnormal parathyroid glands.3, 4 Surgery to control the HPT in MEN-1 patients requires either three and one-half gland parathyroidectomy or four-gland excision with autograft.5-8 Patients have a significant rate of persistent HPT with lesser procedures and a high-rate of recurrent HPT as well.5, 9-13 Careful long-term follow-up studies of these patients are few and the long-term results of surgery have been infrequently reported. We14 and others6, 15-17 have previously focused on the results of initial and reoperations18 in patients with MEN-1 and HPT. This study focuses on a more unique subgroup, those with MEN-1, HPT and ZES. Few previous studies have focused solely on this subgroup, even though ZES occurs in 30−70% of all patients with MEN1. 19
Zollinger-Ellison syndrome (ZES) is the most common functional pancreatic neuroendocrine syndrome associated with MEN-1.20, 21 It is generally caused by multiple endocrine tumors either within the pancreas or duodenum. Surgery to remove these tumors is seldom curative.20, 22 Further, in small numbers of cases some groups23, 42-47, 57-60 ,but not others46-49, 59, 61 , have reported an improvement in the laboratory parameters of ZES when surgery is performed to correct the hypercalcemia in MEN-1 patients with HPT and ZES.23 However, since patients with HPT, ZES and MEN-1 are even more rare than those with MEN-1 and HPT without ZES, no previous study has carefully examined the precise nature of the parathyroid disease in these patients.
This study carefully characterizes the primary hyperparathyroidism in patients with HPT, ZES and MEN-1. It reports the long-term results of initial surgery for the HPT in terms of correction of hypercalcemia and the parameters of ZES. The results suggest that the HPT in MEN-1 patients with ZES and HPT is more severe than previously reported in routine MEN1/HPT patients and that correction of the hypercalcemia dramatically effects the laboratory parameters of ZES.
Methods
Since 1980 at the National Institutes of Health (NIH), 84 consecutive patients with ZES23, 24, 25 HPT and MEN1 were included in this analysis.26 All patients had the diagnosis of HPT confirmed by biochemical studies and signs and symptoms of primary hyperparathyroidism were especially noted.19, 23, 27 Elevated serum levels of calcium and intact parathyroid hormone were measured to substantiate the diagnosis of HPT in all patients. 24 hour urinary levels of calcium excretion were also elevated. The diagnosis of ZES was based on acid secretory studies, measurement of fasting serum level of gastrin as well as the results of secretin and calcium provocative tests.24, 25, 28-31 Basal and maximal acid output (BAO, MAO) were determined for each patient using methods described previously.27 Doses of oral gastric antisecretory drug were determined as described previously.27 A detailed past history of disease was taken at first admission including symptoms related to ZES and past medical/surgical procedures as described previously26 . Time from onset of the disease to exploration was determined for all patients. The time of diagnosis of ZES was the time the diagnosis was first established by appropriate laboratory studies or when a physician established the diagnosis based on clinical presentation.
All patients had the diagnosis of Multiple Endocrine Neoplasia type 1 (MEN1). MEN1 was established by careful personal and family history, the presence of other tumors associated with MEN119, 32, and assessing plasma hormone levels (PTH [intact, mid-molecule], prolactin, insulin, proinsulin, glucagon), serum calcium (ionized, total) and glucose that may be abnormal in MEN1, All patients had primary hyperparathyroidism as diagnosed by elevated serum levels of calcium and parathyroid hormone. Surgery for primary hyperparathyroidism in patients with MEN1, HPT and ZES was done over a 35 year period between 1970 and 2005. The operating surgeon had the choice for the type of surgery that was performed. Some of the operations were done at other institutions and the exact protocol was not followed. Operations wre performed with the intent to identify all four parathyroid glands, and most consisted of either subtotal (three or 3.5 glands resected) parathyroidectomy 7,14 or a 4 gland parathyroidectomy with transplant23 of 20 2×1 mm fragments of parathyroid tissue into the non-dominant forearm. The cervical thymus was routinely excised in order to excise any supernumary fragments of parathyroid tissue. Intraoperative parathyroid hormone level determinations were not done.33 Thirty-nine of the patients had surgery performed initially at the NIH and 45 at outside institutions. For these latter patients the results of this surgery were carefully recorded paying special attention to the number of glands remove as well as total number identified and the preoperative biochemical and hormonal data. In that setting the surgeon may have failed to recognize the extent of HPT or the presence of MEN1 and may have removed less than 3 glands. For the outside surgical MEN1/ZES patients initially 29 patients had <3 glands removed, and 16 patients had 3−3.5 glands removed.
Postoperatively, NIH patients underwent evaluation for HPT and ZES immediately after surgery, within 3 to 6 months post-resection, and then every year thereafter. Yearly evaluations included measuring serum levels of calcium and PTH, acid secretory studies, fasting gastrin determinations, secretin provocative test, and assessment of other endocrine status (pituitary, adrenal function). Disease-free or cure is defined as a normal serum level of calcium and parathyroid hormone.29, 34-36 Persistent disease is defined as elevated serum levels of calcium and PTH immediately postoperatively. Recurrent disease is defined as an elevated serum level of calcium and PTH after a postoperative period of at least 6 months or more of normocalcemia.
The Fisher's exact test and the Mann-Whitney test were used for two-group comparisons.26 All continuous variables were reported as mean ± standard error of the mean. The probabilities of disease-free survival were calculated and plotted according to the Kaplan-Meier method and compared using the exact log rank test and the method of Rothman to determine the confidence intervals.37 Comparisons with the literature were performed using the normal distribution and calculating means plus 2 SD's to define the limits for p<0.05.
Results
84 patients with MEN1, primary hyperparathyroidism, and ZES were studied (Table 1). 49 were female (58%). The mean age of onset of the MEN1 was 27 years, HPT 31 years and the ZES 33 years (Tables 1 and 2). Each patient had elevated serum levels of ionized calcium, total calcium and PTH with a mean level of 1.52 mmol/L (nl 1.17−1.31 mmol/L), 2.81 mmol/L (nl 2−2.5 mmol/L) and 2.43-fold normal, respectively (Table 2). Urinary levels of calcium excretion were also elevated with a mean level of 8.6 mmol for 24 hours. 62% had kidney stones. Bone density studies were done in 56 patients (67%) and 25 had decreased bone density (46%) (Table 2). Each patient had MEN1, and 81% had a family history consistent with MEN1, 63% had pituitary tumor and 34% carcinoid of either the bronchus (14%) or the thymus (7%)(Table 1). Each had concomitant ZES with elevated fasting levels of gastrin (8-fold increase over normal) and elevated basal acid output 38 mEq/h and maximal acid output 57 mEq/h (Tables 1 and 2). 79% had upper abdominal pain and indigestion, 65% had diarrhea and 48% had gastro-esophageal reflux symptoms. 56% had a peptic ulcer on endoscopy, while 20% had prior gastric surgery.
Table 1.
General characteristics of the MEN1/ZES patients studied
| Characteristic | Number (%) |
|---|---|
| No.of patients | 84 (100%) |
| Female gender | 49 (58%) |
| Age (yrs) | |
| Onset ZES | 32.9 ± 1.0 |
| Onset MEN1 | 26.7 ± 1.0 |
| MEN1 Diagnosis | 36.6 ± 1.3 |
| MEN1 features | |
| ZES | 84 (100%) |
| HPT | 84 (100%) |
| Pituitary tumor | 53 (63%) |
| Carcinoid tumor (1) | 29 (34%) |
| Bronchial | 12 (14%) |
| Thymic | 6 ( 7%) |
| Family History MEN1 | 68 (81%) |
| Presenting MEN1 feature | |
| ZES | 25 (30%) |
| HPT | 49 (58%) |
| Other (2) | 10 (12%) |
| Fasting gastrin (pg/ml) | |
| Median | 999 |
| [range] | [34−550,000] |
| BAO (mEq/hr) (3) | |
| Mean | 38.7 ± 4 |
| [range] | [3−144] |
| MAO (mEq/hr) (3) | |
| Mean | 56.9 ± 5.1 |
| [range] | [15−144] |
| ZES presenting symptom | |
| Pain | 66 (79%) |
| Diarrhea | 55 (65%) |
| Esophageal symptoms | 40 (48%) |
| Other (4) | 51 61%) |
| Peptic ulcer present | 47 (56%) |
| Previous GI surgery | |
| Gastric | 17 (20%) |
| PET resection | 9 (11%) |
| Other (5) | 5 (6%) |
Includes gastric, thymic bronchial carcinoids.
Includes pituitary disease, detection during screening, insulinoma
Includes data from patients without previous gastric acid reducing surgery (n=64)
Other presenting symptoms include bleeding, nausea, vomiting.
Other GI surgical procedures include oversew of perforated jejunal ulcer, cholecystectomy, esophageal leiomyoma resection, Nissen fundoplication.
Abbreviations: ZES, Zollinger-Ellison syndrome; MEN1, multiple endocrine neoplasia type 1; HPT, hyperparathyroidism; BAO, basal acid output; MAO, maximal acid output; PET, pancreatic endocrine tumor; GI, gastrointestinal.
Table 2.
Characteristics of the primary hyperparathyroidism of the 84 MEN1/ZES patients included in study.
| Characteristic |
|
Number (%) |
|---|---|---|
| Age (yrs) | ||
| Onset of HPT | 30.6 ± 1.1 | |
| First PTX | 36.0 ± 1.2 | |
| Last follow-up | 52.5 ± 1.4 | |
| Duration (yrs) | ||
| Onset HPT prior to 1st PTX | 5.4 ± 0.8 | |
| Onset MEN1 prior to 1st PTX | 9.3 ± 0.9 | |
| Onset HPT to last F/U | 21.9 ± 1.4 | |
| 1st PTX to Last F/U |
|
16.5 ± 1.2 |
| History renal colic |
|
52 (62%) |
| Onset ZES prior 1st PTX |
|
27 (32%) |
| Mean Preoperative Lab values (1st PTX) | ||
| Total calcium (mmol/L) (1) | 2.81 ± 0.07 | |
| Ionized calcium (mmol/L) (1) | 1.52 ± 0.02 | |
| PTH (mid) (fold normal) (2) | 1.88 ± 0.33-fold | |
| PTH (IRMA) (fold normal) (2) | 2.43 ± 0.28 | |
| Urinary calcium (mmol/24 hr) (3) | 8.63 ± 1.0 | |
| Fasting gastrin (fold increase) (median) (4) | 7.9 | |
| BAO (mEq/hr) |
|
38.2 ± 4.3 |
| Bone density, any Z score decreased ≥ 2SD (5) | 46% |
Normal serum total and ionized calcium levels are 2−2.5 mmol/L and 1.17−1.31 mmol/L, respectively. Data immediately before 1st PTX are from 69 patients.
Normal plasma PTH mid molecule and PTH IRMA values are 50−340 pg/mL and 10−65 before 9/94 and after 9/99. From 9/94−9/99 normal values were 9.4−49 pg/mL. Values are means from 52 patients taken immediately before the 1st PTX. PTH mid molecule levels are expressed as fold increase over the upper limit of normal to allow comparison of values from other labs.
Fasting serum gastrin levels are from 53 patients who were assessed immediately before and after PTX and expressed as fold increase over the upper limit of normal (i.e. 100 pg/mL) to allow comparison from different labs as described previously {24,25}.
Normal values of urinary calcium are 1.25−62.5 mmol/24 hours. To convert to mg/24 hours multiple by 40.
Bone density studies were performed in 56 patients and the result expressed as the percentage of patients showing any area evaluated with a Z score decreased at less 2SD for age matched controls.
Abbreviations: See Table 1 legend.
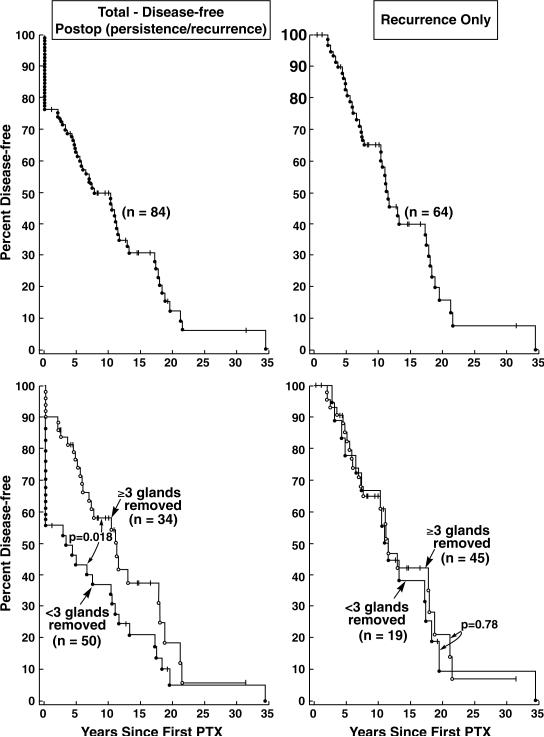
It was a mean of 9 years from the onset of MEN1 and 5 years from the onset of HPT to the time of initial neck surgery for HPT (Table 2). At the initial operation for HPT, 35 patients (42%) had less than 3 glands removed, 40 (47%) had between 3 and 3.5 removed, while 9 had a four gland resection with transplant (11%) (Table 3). The mean number of parathyroid glands removed was 2.8. The pathologist diagnosed parathyroid hyperplasia on every parathyroid gland that was removed. The mean follow-up after parathyroid surgery was 7.2 years and 34 (40%) were disease-free, 33 (39%) hypercalcemic, 17 (20%) hypocalcemic and 44% had an elevated PTH level. If less than 3 parathyroid glands were removed at the initial operation as occurred in 35 patients, the incidence of hypoparathyroidism was 3% and the nearly everyone developed either recurrent (46%) or persistent (43%) hypercalcemia for a total of 92%. If between 3 (n=4 patients) and 3.5 glands (n=36 patients) were removed, hypoparathyroidism was 10% and persistent (12%) or recurrent (44%) hyperparathyroidism for a total of 57%. If 4 or more glands were removed, persistent HPT was 0% and recurrent was 22%. Overall, in the 84 patients studied with 16 years follow-up, 24% had persistent disease and 46 had recurrent HPT, while 7% were hypoparathyroid (Table 4). The parathyroid disease-free survival for all patients from the original surgical procedure for HPT was 50% at 10 years (Figure 1 Upper Left Panel). If either 3 and ½ parathyroid glands or 4 glands were removed surgically, the disease-free survival was significantly improved (Figure 1 Lower Left Panel). In patients with recurrent HPT, the recurrence rate was 50% at ten years (Figure 1 Upper Right Panel) and there was no difference in time to recurrence between greater and less than 3 glands removed at the initial surgery (Figure 1 Lower Right Panel).
Table 3.
Parathyroidectomy characteristics and results in 84 patients with MEN1/ZES.
| Characteristic |
|
|
Number (% of patients) |
|---|---|---|---|
| 1st PTX. # glands removed | |||
| <3 | 35 (42%) | ||
| 3−3.5 | 40 (47%) | ||
| 4 | 9 (11%) | ||
| mean number |
|
|
2.8 ± 0.1 |
| Parathyroid graft | |||
| No. patients receiving immediate graft |
|
|
9 (11%) |
| Yrs F/U | |||
| <5 | 33 (39%) | ||
| 5−9.9 | 30 (37%) | ||
| 10−19.9 | 15 (18%) | ||
| ≥20 | 6 (7%) | ||
| mean number |
|
|
7.2 ± 0.7 |
| Parathyroid status last F/U | |||
| Normocalcemic | 34 (40%) | ||
| Hypercalcemic | 33 (39%) | ||
| Hypocalcemic | 17 (20%) | ||
| Taking Vitamin D/Calcium |
|
|
13 (15%) |
| Mean Last Lab values (1),(2) | |||
| Total calcium (mmol/L) | 2.29 ± 0.03 | ||
| Ionized calcium (mmol/L) | 1.28 ± 0.02 | ||
| PTH (mid) (fold increased) | 0.80 ± 0.28-fold | ||
| PTH (IRMA) | 93 ± 19 | ||
| Urinary calcium (mmol/24 hr) | 3.91 ± 0.4 | ||
| Fasting gastrin (fold increased) (median) | 4.97-fold | ||
| BAO (mEq/hr) | 13.5 ± 3.2 |
Normal values present in Table 2 legend.
Total and ionized calcium levels from 80 patients were averaged, PTH levels from 70 patients, urinary calcium from 47 patients, fasting gastrin levels from 72 patients and BAO measurements from 27 patients.
Table 4.
Results of Parathyroidectomy in 84 MEN1/ZES patients.
| No. glands removed | # pts | Age 1st PTX (yrs) | Yrs F/U post 1st PTX | Persistent HPT (%) | Recurrent HPT (%) | Hypopara (%) | Normocalcemia No Ca/VitD (%) | Time to recurrence (yrs) |
|---|---|---|---|---|---|---|---|---|
| <3 | 35 | 33 ± 1.6 | 20.7 ± 1.9 | 15 (43%) | 16(46%) | 1 (3%) | 2 (6%) | 12.0 ± 2.0 |
| 3−3.5 | 40 | 38.0 ± 1.7 | 14.5 ± 1.5 | 5 (12%) | 18 (45%) | 4 (10%) | 14 (35%) | 10.4 ± 1.6 |
| ≥4 | 9 | 36.7 ± 4.4 | 9.9 ± 1.5 | 0 (0%) | 5 (55%)(1) | 2 (22%) | 2 (22%) | 6.0 ± 0.8 |
| Total | 84 | 36.0 ± 1.2 | 16.5 ± 1.2 | 20 (24%) | 39 (46%) | 7 (7%) | 18 (21%) | 10.7 ± 1.2 |
Four of the 5 recurrences were in the graft.
Abbreviations. See Table 1 legend. pts, patients; F/u, follow-up; hypopara, hypoparathyroidism; Ca./VitD, taking calcium and/or vitamin D
Figure 1.
The disease-free survival (left panels) and recurrence rate (right panels) following initial surgery for hyperparathyroidism in MEN-1 patients with HPT and ZES. The two upper panels show the overall percent who are either free of disease (left panel) or with recurrent HPT (right panel) at follow-up in years. The lower two panels show the same data divided as to whether >3 or <3 glands were removed. It demonstrates that the disease-free survival (but not the recurrence rate) was greater if 3 or more than 3 parathyroid glands were removed compared to <3 glands removed (p=0.018).
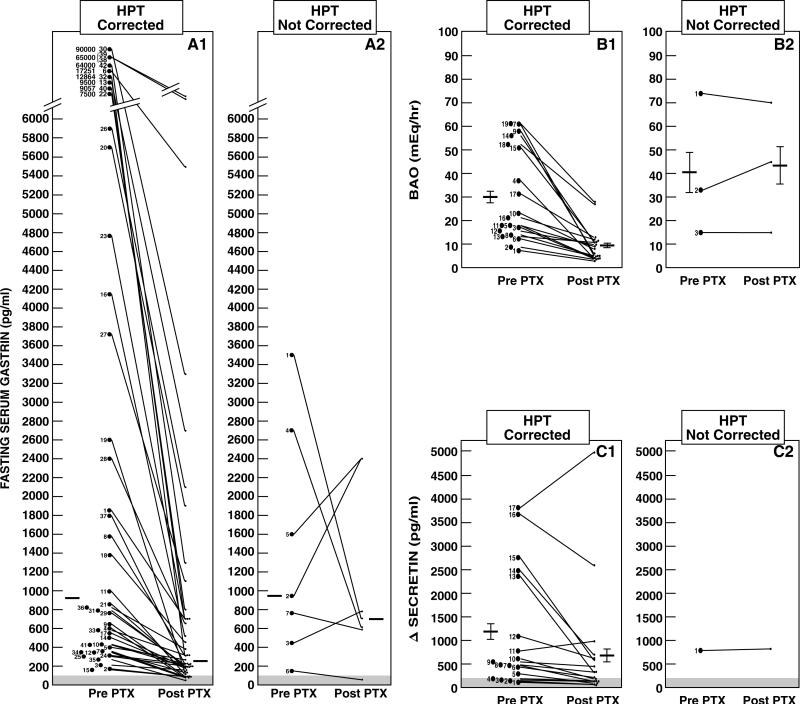
Fasting serum levels of gastrin did not affect the parameters of HPT including ZES onset, age at first surgery for HPT, and surgical parameters (Table 5). However, when the biochemical parameters of HPT are corrected with surgery, the biochemical parameters of ZES are dramatically improved (Figure 2). When HPT was corrected surgically, serum levels of gastrin, BAO and delta gastrin level with secretin each significantly improved. Those who underwent parathyroid surgery without correction of hypercalcemia had significantly less changes in their biochemical parameters of ZES post PTX (Figure 2). Specifically, the means±SEM decrease in fasting serum gastrin in the patients with successful PTX was 70 ± 3% versus 35 ± 25% in patients with an unsuccessful PTX, which was significantly different (p=0.023). Furthermore, in a recent study we sampled fasting serum gastrin on serial days from ZES patients and showed with our assays intra- and inter-assay variations that a significant decrease occurred (p<0.05) only with changes greater than 60% (abou-saif . add referenceCancer 98:249−261, 2003). In our study 28% of the patients with an unsuccessful PTX showed such a change on post PTX follow-up, whereas 74% of the patients with a successful PTX showed such a change, which was significantly different (p=0.030). The biochemical parameters decreased to such a degree in a number of patients after successful PTX that 20% of patients with MEN1, HPT and ZES no longer had any biochemical evidence of ZES following surgery to treat HPT without removing any pancreatic or duodenal neuroendocrine tumor.
Table 5.
Effect of gastrin levels on HPT parameters/treatment
| Fasting gastrin level (median) (pg/mL) | |||
|---|---|---|---|
| Parameter | Parameter absent | Parameter present | P value |
| ZES onset prior HPT | 680 | 1300 | 0.17 |
| Family History MEN1 | 648 | 1055 | 0.80 |
| Age PTX >35 | 1050 | 998 | 0.81 |
| HPT recur after PTX | 1060 | 600 | 0.24 |
| HPT persist after PTX | 784 | 1094 | 0.19 |
| > 4 glands removed | 909 | 1030 | 0.68 |
| HPT present last F/U | 1131 | 600 | 0.32 |
| Graft hyperfunctioning | 1060 | 398 | 0.25 |
Figure 2.
Effect of parathyroidectomy on fasting gastrin level, BAO and Δ secretin in MEN1/ZES patients without or without the hypercalcemia corrected postparathyroidectomy. Results are shown from 50 patients of whom HPT was corrected in 43 and not corrected in 7 patients. Panels A1/A2 show the mean fasting gastrin levels pre- and post-PTX in 49 patients. The horizontal line shows the median value pre- and post-PTX which were 925 and 247 pg/mL for the corrected HPT group and 842 and 740 pg/mL for the uncorrected group. Eight patients had gastrin levels return to the normal range (i.e. <100 pg/mL)(shaded area) post PTX. Panels B1/B2 shows the effect on the BAO in 22 patients. The horizontal line is the mean±SEM and for the HPT corrected group (n=19) was 30.2 ± 4.5 and 9.5 ± 1.6 mEq/hr and for the uncorrected HPT group (n=3) was 40.6 ± 17.4 and 43.3 ±15.9 mEq/hr. Panels C1/C2 show results of the Δ secretin in 18 patients. The horizontal line is the mean ± SEM which was 1175 ± 294 and 745 ± 286 for the HPT corrected group Eight patients demonstrated a negative secretin test post PTX using the criterion of ≥200 pg/mL increase postsecretin (shaded area) and 3 patients using the recently proposed criterion of ≥ 120 pg/mL increase. 24, 25
Laboratory, clinical and operative findings were analyzed in an attempt to determine factors that may predict the long-term disease-free survival of HPT in patients with HPT, ZES and MEN1 (Table 6). The diagnosis of ZES as the initial manifestation of MEN1 is associated with a decreased probability of HPT being present at last follow-up (9% vs 43%, p=0.0006). If a germline mutation in the Menin gene was detected, there is an increased probability that HPT is present at last follow-up (100% vs 79%, p=0.0069). If HPT was diagnosed greater than 28.5 years of age, there was a decreased likelihood of recurrence (39% vs 61%,) (p=0.045). Finally, if serum calcium levels were increased greater than 110% normal, there was a 78% chance of HPT at last follow-up versus 28% in those with lesser elevations (p=0.0020) (Table 6).
Table 6.
Prognostic factors for correction of HPT
| |
At last F/U: |
|
|
|---|---|---|---|
| Chararacteristic | Hypercalcemia (n=33) | No hypercalcemia (n=51) | P value (3) |
| I. ZES features |
|
|
|
| ZES 1st MEN1 symptom |
9% |
43% |
0.0006 |
| Liver metastases present |
24% |
24% |
0.39 |
| Yrs ZES onset last F/U>18 |
45% |
51% |
0.57 |
| |
|
|
|
| II. MEN1 features |
|
|
|
| Age onset MEN1 >27.5 yrs old |
27% |
45% |
0.078 |
| Positive family history MEN1 |
85% |
78% |
0.33 |
| Germline MEN1 mutation present (1) |
100% |
79% |
0.0069 |
| |
|
|
|
| III. HPT Feature |
|
|
|
| Age HPT detected >28.5 yrs |
39% |
61% |
0.045 |
| Yrs onset HPT last F/U >19.5 |
45% |
51% |
0.39 |
| |
|
|
|
| IV. PTX Feature |
|
|
|
| Age PTX>35 yrs |
42% |
53% |
0.24 |
| Yrs last F/u to PTX>15 |
61% |
43% |
0.090 |
| PTX ≥3−3.5 glds |
33%% |
59% |
0.31 |
| PTX result | |||
| Persistent HPT | 36% | 16% | 0.029 |
| Recurrent HPT |
64% |
35% |
0.010 |
| Yrs to recur after PTX>10 |
38% |
67% |
0.072 |
| V. Lab value prior PTX (2) | |||
| Ca total increased >110% |
78% |
28% |
0.0020 |
| PTH increased > 160% | 80 | 36% | 0.102 |
The presence of germline mutations was assessed in 70 patients including 28/31 (85%) of the HPT present group and 42/51 (84%) of the HPT absent group.
Preoperative calcium values and PTH values prior to the first PTX were available from 46 and 27 patients.
P values were calculated using the Fishers Exact test with median values for continuous variables.
Discussion
Even though up to 70% of all patients with MEN1 develop ZES in some studies19, 38 and almost one-half of the patients in an average of 14 series19; few, if any, prior studies have focused on parathyroid surgery only in MEN1 patients with HPT and ZES (MEN1/HPT/ZES). These patients have a number of unique features from patients with MEN1/HPT without ZES. First, in previous studies of parathyroid surgery in MEN1 patients the percentage of patients with ZES was not distinguished, was low in percentage of all patients in the study, as well as in the total number of MEN1/ZES included in all these series (i.e., 5 series, mean 14 ±4 patients, 26 ±4 % of total)1, 6, 14, 39, 40. Second, MEN1/HPT patients in these other surgical series were often detected by screening for MEN1 and hence the MEN1/HPT was usually detected at a different time in the natural history of the disease than most MEN1/HPT/ZES patients 19, 41. Third, up to 30% of MEN1/ZES patients present with symptoms of the ZES and the HPT may only be recognized later19, 28. Therefore, the timing of the initial PTX as well as its place in the natural history of the MEN1 may differ substantially from patients without ZES. Fourth, the occurrence of the ZES may have an effect on the timing of the PTX in an MEN1 patient, because previous studies in small numbers of patients show, in some studies 23, 42-45, but not others44, 46-49, the PTX with correction of the hypercalcemia, may have an ameliorating effect on the acid secretion, responsiveness to anti-secretetory drugs, fasting gastrin levels and secretin provocative testing . The current study includes a large number of MEN1/HPT/ZES patients (n=84) who were prospectively studied.
A number of our results support the conclusion that the primary hyperparathyroidism in patients with MEN1/HPT/ZES is more severe and more difficult to treat than reported in MEN1/HPT patients. First, a higher percentage of our patients had a history of neprolithiasis at the time of the initial PTX than reported in the literature in MEN1/HPT patients (62%- our study vs 40±6%, P<0.05, 7 series)10, 14, 16, 40, 50-52. This finding suggests our patients had more aggressive disease at the time of surgery. Second, in our patients the average serum intact PTH level at the initial PTX was increased to a greater extent than reported in the MEN/HPT patients in the literature (our patients-2.4 fold increase vs 1.67 ± 0.38-fold in the literature (n=8 series), p<0.05)1, 9, 39, 40, 53-55. These results further support the conclusion that our patients had a more severe form of HPT at the time of the initial PTX. Third, our patients had a mean lower age at the initial PTX than reported in the literature for series of MEN1/HPT patients (ours-36 yrs vs 40.5 ± 1.8 yrs, p<0.05)(10 series)1, 6, 7, 10, 14, 50, 53, 54, 56. These results demonstrated that the increased severity of the HPT seen in our patients was not due to a later time of presentation in the natural history of the MEN1 of the patients, but instead due to an increased severity of the HPT itself. Fourth, our surgical results demonstrate our recurrence rate after subtotal parathyroidectomy (3−3.5 glands) was 2.6-fold higher than that reported in the literature for MEN1/HPT patients (our patients-44% vs literature-16.8%, p<0.00001, 15 series, Table 7). Finally, bone density was decreased in 47% of patients which is also consistent with more severe HPT.
Table 7.
Persistent and recurrent disease in current series and literature of MEN1 patients with HPT treated with subtotal parathyroidectomy (3−3.5 glds)
| Author (ref) | yr | No of pts | Postop persistent disease (%) | Postop recurrent disease (%) | # RECURR. | Years follow-up (mean/median) |
|---|---|---|---|---|---|---|
| Current study | 2007 | 41 | 12 | 44* | 18 | 7.9 |
| Previous series | ||||||
| Prinz 11 | 1981 | 9 | 11 | 12 | 1 | 9.5 |
| Rizzoli 56 | 1985 | 20 | 15 | 15 | 3 | 5.5 |
| Malmeus 5 | 1986 | 6 | 0 | 33 | 2 | 5 |
| Samaan 51 | 1989 | 8 | 12 | 14 | 1 | 5.5 |
| Kraimps 10 | 1992 | 14 | 14 | 36 | 5 | 8 |
| Hellman 72 | 1992 | 34 | 0 | 9 | 3 | 9 |
| Obara 17 | 1992 | 9 | 0 | 11 | 1 | 5.4 |
| O'Riordan 16 | 1993 | 54 | 0 | 16 | 9 | 10 |
| Thompson 73 | 1995 | 34 | 6 | 9 | 3 | 5.4 |
| Burgess 74 | 1998 | 37 | 8 | 19 | 7 | 8 |
| Hellman 53 | 1998 | 9 | 22 | 44 | 4 | 6 |
| Dotzenrath 40 | 2001 | 25 | nd | 12 | 3 | 5.2 |
| Kaczirek 52 | 2002 | 4 | 25 | 0 | 0 | 5.8 |
| Hubbard 7 | 2006 | 21 | 0 | 5 | 1 | 5.2 |
| Lee 6 | 2006 | 6 | 0 | 17 | 1 | 7.2 |
| Previous series | ||||||
| Mean | 19.33 | 8.20 | 16.80 | 6.71 | ||
| sd | 14.75 | 8.39 | 11.98 | 1.71 | ||
| SUM | 290.00 | 44 |
P<0.00001 compared to recurrence rate for all literature cases (44/290=15%).
To attempt to identify clinical or laboratory factors that might be predictive for which patients demonstrate more aggressive HPT with a higher recurrence rate, we correlated a number of these factors with the surgical outcome. Four factors that were particularly important and three favoring a higher recurrence rate were; the occurrence of ZES as the initial symptom, the presence of higher calcium levels pre-PTX and the presence of a germline MEN1 mutation; whereas a lower recurrence rate was favored by older age of detection of HPT (>28.5 yrs old). In one study54 an important predictor of whether the PTX would control the HPT long-term was the absence of a family history of MEN1. Because a family history is associated with a germline MEN1 mutation detected19, this is consistent with our findings. A second study 27 also shows that patients19 with a positive family history had a more severe form of HPT which is consistent with our findings. These results suggest these factors will be important on recurrence rates post-PTX in future studies.
The effect of PTX on the behavior of the gastrinoma in patients with MEN1/HPT/ZES including fasting gastrin levels, basal acid ouput and secretin provocative testing is controversial in the literature44. Whereas some previous studies on small numbers of cases23, 42-47, 57-60 report that PTX in MEN1/HPT/ZES patients can markedly decrease the fasting gastrin levels, basal acid output and/or secretin-stimulated gastrin response, a number of other studies report either no effect or a minimal effect on these measures of gastrinoma function46-49, 59, 61. Our study convincingly demonstrates the marked ameliorating effect of a successful PTX on these parameters, because we were able to study a larger number of patients and correlate the changes in gastrinoma function with the outcome of the PTX. (Figure 2). In fact, some patients (20%) with MEN1/HPT/ZES no longer had biochemical evidence of ZES with surgery not directed at the pancreatic neuroendocrine tumor, but to remove only the abnormal parathyroid glands. These data are similar to what we previously reported in 198723; however, in that study a higher proportion of patients were cured. The prior study had fewer patients with shorter follow-up which may explain the increase in cure-rate. These findings are even more important when we remember that surgery to remove the gastrinoma in MEN1 patients seldom affects the parameters of ZES19, 20, 26, 62. In fact, ZES in these patients is seldom cured by surgery to remove the gastrinoma because the tumors are often multiple within the duodenum and pancreas, as well as frequently associated with lymph node metastases21, 62-64. This further supports the strategy that parathyroid surgery should be performed first prior to any abdominal surgery for ZES.
Previously it is reported that the HPT in patients with MEN1 is always hyperplasia and the operation of choice is either 3 and ½ gland parathyroidectomy or four gland parathyroidectomy with transplant.11-13, 16, 17 The current study corroborates the presence of multiple gland disease in MEN1/HPT/ZES patients and the need for extirpation of at least 3 or more glands. In fact, when this was accomplished patients had a longer disease-free interval, but not a lower incidence of recurrent hypercalcemia (Figure 1). Recurrent HPT is explained by the pathophysiology of the HPT in these patients, while persistent HPT is explained by the extent of the surgery. Recurrent HPT depends on the subsequent growth of residual abnormal parathyroid glands, while persistent HPT is more common if less than 3 glands are excised. From our results the four-gland resection and transplant is not recommended because there was a high graft failure rate and therefore an unacceptable rate of hypoparathyroidism. Furthermore, most studies of MEN1/HPT patients5, 6, 33, 53, 65, 66 demonstrate a low rate (0−13% of patients ), whereas a few studies report a high rate (50−66%)67, 68 of autonomous hyperfunction with time in the transplanted parathyroid glands resulting in recurrent hyperparathyroidism. We found in the MEN1/HPT/ZES patients this was not an infrequent outcome, occurring in 44% of our patients with a 4-gland resection and an implant. This finding supports the contention that the parathyroid disease in patients with MEN1/HPT/ZES is severe. Recurrent HPT due to the graft has previously been felt to make re-operation easier 39; however, forearm re-operations have been more difficult than previously anticipated (personal observation). Large amounts of forearm muscle may need to excised in order to remove the embedded abnormal parathyroid tissue. Therefore, it appears best to leave a small amount of parathyroid tissue within the neck and mark it with a clip so that, if necessary, it can subsequently be found and either excised or trimmed.
In summary, our studies support the conclusion that patients with MEN-1 HPT and ZES represent a unique subgroup of patients with a more virulent form of primary hyperparathyroidism. In these patients there is no ideal surgical procedure to control the hypercalcemia and ameliorate the symptoms. This is because the HPT is caused by diffuse hyperplasia of all the parathyroid glands even though at times it may be asymmetric. Currently, the three and one half gland parathyroidectomy is recommended as the best surgical procedure. It is associated with a prolonged disease-free interval in many patients and a low incidence of hypoparathyroidism. Furthermore, three and one half gland resection corrects the hypercalcemia and impacts favorably on the biochemical parameters of ZES. However, our study demonstrates these patients need particularly careful follow-up as they have a high recurrence rate.
Table 8.
Summary of findings showing MEN1/ZES patients have a more severe form of HPT than MEN1 patients alone and predictive factors for severity.
| I. | Increased severity of HPT |
| a. Higher frequency of nephrolithiases at presentation[62% vs 40 ±6%,(7 series,literature), p<0.05](1) | |
| b. Higher serum PTH levels at presentation[2.4-fold normal vs 1.67 ± 0.38,(8 series,literature), p<0.05](1) | |
| c. Higher relapse rate post subtotal PTX[44 % vs 17%, P<0.00001, (15 series literature) ](2) | |
| II. | Predictive factors for increcreased recuurence post subtotal PTX(3) |
| a. ZES initial clinical manifestation of MEN1 (p=0.0006) | |
| b. Presence of MEN1 germline mutation (P=0.0069) | |
| c. HPT diagnosed <28.5 yrs of age (p=0.045) | |
| d. Serum calcium levels at presentation >110% normal (P=0.0020) |
References
- 1.Arnalsteen LC, Alesina PF, Quiereux JL, et al. Long-term results of less than total parathyroidectomy for hyperparathyroidism in multiple endocrine neoplasia type 1. Surgery. 2002;132(6):1119–24. doi: 10.1067/msy.2002.128607. discussion 1124−5. [DOI] [PubMed] [Google Scholar]
- 2.Burgess JR, David R, Parameswaran V, et al. The outcome of subtotal parathyroidectomy for the treatment of hyperparathyroidism in multiple endocrine neoplasia type 1. Arch Surg. 1998;133(2):126–9. doi: 10.1001/archsurg.133.2.126. [DOI] [PubMed] [Google Scholar]
- 3.Marx SJ, Simonds WF, Agarwal SK, et al. Hyperparathyroidism in hereditary syndromes: special expressions and special managements. J Bone Miner Res. 2002;17(Suppl 2):N37–43. [PubMed] [Google Scholar]
- 4.Marx SJ MJ, Campbell G, Aurbach GD, Spiegel AM, Norton JA. Heterogeneous size of the parathyroid glands in familial multiple endocrine neoplasia type 1. Clin Endocrinol. 1991;35:521–526. doi: 10.1111/j.1365-2265.1991.tb00938.x. [DOI] [PubMed] [Google Scholar]
- 5.Malmaeus J BL, Johansson H, et al. Parathyroid surgery in the multiple endocrine neoplasia type i syndrome: choice of surgical procedure. World J Surg. 1986;10:668–672. doi: 10.1007/BF01655552. [DOI] [PubMed] [Google Scholar]
- 6.Lee CH, Tseng LM, Chen JY, et al. Primary hyperparathyroidism in multiple endocrine neoplasia type 1: individualized management with low recurrence rates. Ann Surg Oncol. 2006;13(1):103–9. doi: 10.1245/ASO.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 7.Hubbard JG, Sebag F, Maweja S, Henry JF. Subtotal parathyroidectomy as an adequate treatment for primary hyperparathyroidism in multiple endocrine neoplasia type 1. Arch Surg. 2006;141(3):235–9. doi: 10.1001/archsurg.141.3.235. [DOI] [PubMed] [Google Scholar]
- 8.Hubbard JG, Sebag F, Maweja S, Henry JF. Primary hyperparathyroidism in MEN 1--how radical should surgery be? Langenbecks Arch Surg. 2002;386(8):553–7. doi: 10.1007/s00423-002-0275-0. [DOI] [PubMed] [Google Scholar]
- 9.Katai M, Sakurai A, Ikeo Y, Hashizume K. Primary hyperparathyroidism in patients with multiple endocrine neoplasia type 1: comparison with sporadic parathyroid adenomas. Horm Metab Res. 2001;33(8):499–503. doi: 10.1055/s-2001-16944. [DOI] [PubMed] [Google Scholar]
- 10.Kraimps JL DQ- Y, Demeure M, Clark OH. Hyperparathyroidsim in multiple endocrine neoplasia syndrome. Surgery. 1992;112(6):1080–1086. [PubMed] [Google Scholar]
- 11.Prinz RA GO, Sellu D, Lynn JA. Subtotal parathyroidectomy for primary chief cell hyperplasia of the multiple endocrine neoplasia type 1 syndrome. Ann Surg. 1981;193(1):26–29. doi: 10.1097/00000658-198101000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Proye C, Carnaille B, Quievreux JL, et al. Late outcome of 304 consecutive patients with multiple gland enlargement in primary hyperparathyroidism treated by conservative surgery. World J Surg. 1998;22(6):526–9. doi: 10.1007/s002689900430. discussion 529−30. [DOI] [PubMed] [Google Scholar]
- 13.Proye C. [Surgical treatment of primary hyperparathyroidism during MEN1 and other genetically determined forms]. Ann Ital Chir. 2003;74(4):417–22. [PubMed] [Google Scholar]
- 14.Elaraj DM SM, Libutti SK, Norton JA, Bartlett DL, Pingpank JF, Gibril F, Weinstein L, Jensen RT, Marx SJ, Alexander HR. Results of initial operations for hyperparathyroidsim in patients with multiple endocrine neoplasia type 1. Surgery. 2003;134(6):858–864. doi: 10.1016/s0039-6060(03)00406-9. [DOI] [PubMed] [Google Scholar]
- 15.Malone JP, Srivastava A, Khardori R. Hyperparathyroidism and multiple endocrine neoplasia. Otolaryngol Clin North Am. 2004;37(4):715–36, viii. doi: 10.1016/j.otc.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 16.O'Riordain DS OBT, Grant CS, Weaver A, Gharib H, Van Heerden JA. Surgical management of primary hyperparathyroidism in multiple endocrine neoplasia types 1 and 2. Surgery. 1993;114:1031–1039. [PubMed] [Google Scholar]
- 17.Obara T FY, Ito Y. Primary hyperparathyroidsim in patients with multiple endocrine neoplasia type 1: experience by a single surgical team in Japan. Henry Ford Hosp Med J. 1992;40(3−4):191–194. [PubMed] [Google Scholar]
- 18.Kivlen M, Bartlett DL, Libutti SK, Skarulis MC, Marx SJ, Simonds WF, Weinstein LS, Jensen RT, McCart JA, Naik AM, Kranda KC, Brennan MF, Norton JA, Fraker DL, Alexander HR. Reoperation for hyperparathyroidism in multiple endocrine neoplasia type 1. Surgery. 2001;130(2):991–8. doi: 10.1067/msy.2001.118379. [DOI] [PubMed] [Google Scholar]
- 19.Gibril F, Schumann M, Pace A, Jensen RT. Multiple endocrine neoplasia type 1 and Zollinger-Ellison syndrome: a prospective study of 107 cases and comparison with 1009 cases from the literature. Medicine (Baltimore) 2004;83(1):43–83. doi: 10.1097/01.md.0000112297.72510.32. [DOI] [PubMed] [Google Scholar]
- 20.Norton JA, Jensen RT. Current surgical management of Zollinger-Ellison syndrome (ZES) in patients without multiple endocrine neoplasia-type 1 (MEN1). Surg Oncol. 2003;12(2):145–51. doi: 10.1016/s0960-7404(03)00035-5. [DOI] [PubMed] [Google Scholar]
- 21.Thompson NW. Current concepts in the surgical management of multiple endocrine neoplasia type 1 pancreatic-duodenal disease. Results in the treatment of 40 patients with Zollinger-Ellison syndrome, hypoglycaemia or both. J Intern Med. 1998;243(6):495–500. doi: 10.1046/j.1365-2796.1998.00307.x. [DOI] [PubMed] [Google Scholar]
- 22.Meko JB, Norton JA. Management of patients with Zollinger-Ellison syndrome. Annu Rev Med. 1995;46:395–411. doi: 10.1146/annurev.med.46.1.395. [DOI] [PubMed] [Google Scholar]
- 23.NortonJA CM, Doppman JL, Maton PN, Gardner JD, Jensen RT. Effect of parathyroidecomy in patients with hyperparathyroidism and Zollinger-Ellison syndrome and multiple endocrine neoplasia type 1: a prospective study. Surgery. 1987;102:958–996. [PubMed] [Google Scholar]
- 24.Berna MJ, Hoffmann KM, Long SH, et al. Serum gastrin in Zollinger-Ellison syndrome: II. Prospective study of gastrin provocative testing in 293 patients from the National Institutes of Health and comparison with 537 cases from the literature. evaluation of diagnostic criteria, proposal of new criteria, and correlations with clinical and tumoral features. Medicine (Baltimore) 2006;85(6):331–64. doi: 10.1097/MD.0b013e31802b518c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berna MJ, Hoffmann KM, Serrano J, et al. Serum gastrin in Zollinger-Ellison syndrome: I. Prospective study of fasting serum gastrin in 309 patients from the National Institutes of Health and comparison with 2229 cases from the literature. Medicine (Baltimore) 2006;85(6):295–330. doi: 10.1097/01.md.0000236956.74128.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Norton JA, Alexander HR, Fraker DL, et al. Comparison of surgical results in patients with advanced and limited disease with multiple endocrine neoplasia type 1 and Zollinger-Ellison syndrome. Ann Surg. 2001;234(4):495–505. doi: 10.1097/00000658-200110000-00009. discussion 505−6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gibril F, Jensen RT. Zollinger-Ellison syndrome revisited: diagnosis, biologic markers, associated inherited disorders, and acid hypersecretion. Curr Gastroenterol Rep. 2004;6(6):454–63. doi: 10.1007/s11894-004-0067-5. [DOI] [PubMed] [Google Scholar]
- 28.Benya RV, Metz DC, Venzon DJ, et al. Zollinger-Ellison syndrome can be the initial endocrine manifestation in patients with multiple endocrine neoplasia-type I. Am J Med. 1994;97(5):436–44. doi: 10.1016/0002-9343(94)90323-9. [DOI] [PubMed] [Google Scholar]
- 29.Fishbeyn V, Norton JA, Benya RV, Pisegna JR, Venzon DJ, Metz D, Jensen RT. Assessment and prediction of long-term cure in patients with Zollinger-Ellison syndrome: the best approach. Ann Intern Med. 1993;119:199–206. doi: 10.7326/0003-4819-119-3-199308010-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roy PK, Venzon DJ, Feigenbaum KM, et al. Gastric secretion in Zollinger-Ellison syndrome. Correlation with clinical expression, tumor extent and role in diagnosis--a prospective NIH study of 235 patients and a review of 984 cases in the literature. Medicine (Baltimore) 2001;80(3):189–222. doi: 10.1097/00005792-200105000-00005. [DOI] [PubMed] [Google Scholar]
- 31.Roy PK, Venzon DJ, Shojamanesh H, et al. Zollinger-Ellison syndrome. Clinical presentation in 261 patients. Medicine (Baltimore) 2000;79(6):379–411. doi: 10.1097/00005792-200011000-00004. [DOI] [PubMed] [Google Scholar]
- 32.Hoffmann KM, Gibril F, Entsuah LK, et al. Patients with multiple endocrine neoplasia type 1 with gastrinomas have an increased risk of severe esophageal disease including stricture and the premalignant condition, Barrett's esophagus. J Clin Endocrinol Metab. 2006;91(1):204–12. doi: 10.1210/jc.2005-1349. [DOI] [PubMed] [Google Scholar]
- 33.Tonelli F, Spini S, Tommasi M, et al. Intraoperative parathormone measurement in patients with multiple endocrine neoplasia type I syndrome and hyperparathyroidism. World J Surg. 2000;24(5):556–62. doi: 10.1007/s002689910091. discussion 562−3. [DOI] [PubMed] [Google Scholar]
- 34.Alexander HR, Bartlett DL, Venzon DJ, et al. Analysis of factors associated with long-term (five or more years) cure in patients undergoing operation for Zollinger-Ellison syndrome. Surgery. 1998;124(6):1160–6. doi: 10.1067/msy.1998.92010. [DOI] [PubMed] [Google Scholar]
- 35.Norton J, Fraker DL, Alexander HR, Venzon DJ, Doppman JL, Serrano J, Goebel SU, Peghini P, Roy PK, Gibril F, Jensen RT. Surgery to cure the Zollinger-Ellison syndrome. N Engl J Med. 1999;341:635–644. doi: 10.1056/NEJM199908263410902. [DOI] [PubMed] [Google Scholar]
- 36.Norton J, Doppman JL, Jensen RT. Curative resection in Zollinger-Ellison syndrome: results of a 10-year prospective study. Ann Surg. 1992;215:8–18. doi: 10.1097/00000658-199201000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Norton JA, Alexander HR, Fraker DL, et al. Does the use of routine duodenotomy (DUODX) affect rate of cure, development of liver metastases, or survival in patients with Zollinger-Ellison syndrome? Ann Surg. 2004;239(5):617–25. doi: 10.1097/01.sla.0000124290.05524.5e. discussion 626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eberle F, Grun R. Multiple Endocrine Neoplasia Type 1 (MEN1). Ergeb Inn Med Kinderheilkd. 1981;46:76–149. [PubMed] [Google Scholar]
- 39.Lambert LASS, Lee JE, Perrier ND, Truong M, Wallace MJ, Hoff AO, Gagel RF, Evans DB. Surgical treatment of hyperparathyroidsim in patients with Multiple Endocrine Neoplasia Type 1. Arch Surg. 2005;140:374–382. doi: 10.1001/archsurg.140.4.374. [DOI] [PubMed] [Google Scholar]
- 40.Dotzenrath C, Cupisti K, Goretzki PE, et al. Long-term biochemical results after operative treatment of primary hyperparathyroidism associated with multiple endocrine neoplasia types I and IIa: is a more or less extended operation essential? Eur J Surg. 2001;167(3):173–8. doi: 10.1080/110241501750099294. [DOI] [PubMed] [Google Scholar]
- 41.Thakker R. Multiple Endocrine Neoplasia type 1. Endocrinol Metab Clin North Am. 2000;29:541–567. doi: 10.1016/s0889-8529(05)70150-x. [DOI] [PubMed] [Google Scholar]
- 42.Trudeau W, McGuigan JE. Effects of calciumon serum gastrin levels in the Zollinger-Ellison syndrome. N Engl J Med. 1969;281:862–866. doi: 10.1056/NEJM196910162811602. [DOI] [PubMed] [Google Scholar]
- 43.McCarthy D, Peiken SR, Lopatin RN, Long BW, Spiegel A, Marx S, Brennan MF. Hyperparathyroidism a reversible cause of cimetidine resistant gastric hypersecretion. Br Med J. 1979;1:1765–1766. doi: 10.1136/bmj.1.6180.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gogel K, Buckman MT, Cadieux T, McCarthy DM. Gastric secretion and hormonal interactions in Multiple Endocrine Neoplasia Type 1. Arch Intern Med. 1985;145:855–859. [PubMed] [Google Scholar]
- 45.Jensen RT. Management of the Zollinger-Ellison syndrome in patients with multiple endocrine neoplasia type 1. J Intern Med. 1998;243(6):477–88. doi: 10.1046/j.1365-2796.1998.00281.x. [DOI] [PubMed] [Google Scholar]
- 46.Kerr G, Smith R. Hypercalaemia and gastric hypersecretion in the familial endocrine-adenoma syndrome. Lancet. 1967;1:1074–1077. doi: 10.1016/s0140-6736(67)92649-9. [DOI] [PubMed] [Google Scholar]
- 47.Turbey W, Passaro E., Jr Hyperparathyroidism in the Zollinger-Ellison syndrome: influence of hypercalcemia on clinical course. Arch Surg. 1972;105:62–66. doi: 10.1001/archsurg.1972.04180070060012. [DOI] [PubMed] [Google Scholar]
- 48.Dent R, James JH, Want CA, Deftos LJ, Talamo R, Fischer JE. Hyperparathyroidsim: gastric acid secretion and gastrin. Ann Surg. 1972;176:360–369. doi: 10.1097/00000658-197209000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stevens M, Thirlby RC, Richardson CT. Lack of effect of parathyroidectomy or calcium blockade on serum gastrin concentration and gastric acid secretion in a patient with hyperparathyroidism and Zollinger-Ellison syndrome. Surgery. 1987;101:108–113. [PubMed] [Google Scholar]
- 50.Van Heerden J, Kent RB, Sizemore GW, Grant CS, ReMine WH. Primary hyperparathyoidisim in patients with multiple endocrine neoplasia syndromes: Surgical experience. Arch Surg. 1983;118:533–536. doi: 10.1001/archsurg.1983.01390050017003. [DOI] [PubMed] [Google Scholar]
- 51.Samaan NA QS, Ordonez NG, Choksi UA, Sellin RV, Hickey RC. Multiple endocrine neoplasia type 1. Cancer. 1989;64:741–752. doi: 10.1002/1097-0142(19890801)64:3<741::aid-cncr2820640329>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 52.Kaczirek K, Prager G, Schindl M, et al. Multiple endocrine neoplasia 1--current recommendations for diagnosis and treatment. Wien Klin Wochenschr. 2002;114(7):258–66. [PubMed] [Google Scholar]
- 53.Hellman P, Skogseid B, Oberg K, et al. Primary and reoperative parathyroid operations in hyperparathyroidism of multiple endocrine neoplasia type 1. Surgery. 1998;124(6):993–9. [PubMed] [Google Scholar]
- 54.Goudet P, Cougard P, Verges B, et al. Hyperparathyroidism in multiple endocrine neoplasia type I: surgical trends and results of a 256-patient series from Groupe D'etude des Neoplasies Endocriniennes Multiples Study Group. World J Surg. 2001;25(7):886–90. doi: 10.1007/s00268-001-0046-z. [DOI] [PubMed] [Google Scholar]
- 55.Langer P, Wild A, Schilling T, et al. [Multiple endocrine neoplasia type 1. Surgical therapy of primary hyperparathyroidism]. Chirurg. 2004;75(9):900–6. doi: 10.1007/s00104-004-0838-4. [DOI] [PubMed] [Google Scholar]
- 56.Rizzoli R GJ, Marx SJ. Primary hyperparathyroidsim in familial multiple endocrine neoplasia type 1. Am J Med. 1985;78(3):467–474. doi: 10.1016/0002-9343(85)90340-7. [DOI] [PubMed] [Google Scholar]
- 57.Dutta P, Wallace MR, Wrong OM, Taylor S, Welbourn RB. Familial multiple endocrine adenopathy (primary hyperparathyroidism and Zollinger-Ellison syndrome) in two siblings. Proc R Soc Med. 1968;61:658–660. [PMC free article] [PubMed] [Google Scholar]
- 58.Christiansen J, Aagaaard P. Parathyroid adenoma and gastric acid secretion. Scan J Gastroenterol. 1972;7:445–449. doi: 10.3109/00365527209180768. [DOI] [PubMed] [Google Scholar]
- 59.Wilson S, Singh RB, Kalkhoff RK, Go VLW. Does hyperparathyroidsim cause hypergastrinemia? Surgery. 1976;80:231–237. [PubMed] [Google Scholar]
- 60.Lamers C, Van Tongeren JHM. Serum gastrin response to acute and chronic hypercalcemia in man: studies on the value of calcium stimulated serum gastrin levels in the diagnosis of Zollinger-Ellison syndrome. Eur J Clin Invest. 1977;7:315–317. doi: 10.1111/j.1365-2362.1977.tb01611.x. [DOI] [PubMed] [Google Scholar]
- 61.Thompson M, Sanders DJ, Grund ER. The relationship of the serum gastrin and calcium concentrations in patients with multiple endocrine neoplasia type 1. Br J Surg. 1976;63:779–783. doi: 10.1002/bjs.1800631012. [DOI] [PubMed] [Google Scholar]
- 62.Norton JA, Jensen RT. Resolved and unresolved controversies in the surgical management of patients with Zollinger-Ellison syndrome. Ann Surg. 2004;240(5):757–73. doi: 10.1097/01.sla.0000143252.02142.3e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sheppard B, Norton JA, Doppman JL, Maton PN, Gardner JD, Jensen RT. Management of islet cell tumors in patients with multiple endocrine neoplasia; a prospective study. Surgery. 1989;106:1108–1117. [PubMed] [Google Scholar]
- 64.MacFarlane M, Fraker DL, Alexander HR, Norton JA, Jensen RT. A prospective study of surgical resection of duodenal and pancreatic gastrinomas in multiple endocrine neoplasia type 1. Surgery. 1995;101:738–745. doi: 10.1016/s0039-6060(05)80102-3. [DOI] [PubMed] [Google Scholar]
- 65.Feldman AL, Sharaf RN, Skarulis MC, et al. Results of heterotopic parathyroid autotransplantation: a 13-year experience. Surgery. 1999;126(6):1042–8. doi: 10.1067/msy.2099.101580. [DOI] [PubMed] [Google Scholar]
- 66.Cohen M, Dilley WG, Wells SA, Jr, Moley JF, Doherty GM, Sicard GA, Skinner MA, Norton JA, DeBenedetti MK, Lairmore TC. Long-term functionality of cryopreserved parathyroid autografts: a 13-year prospective analysis. Surgery. 2005;138:1033–1040. doi: 10.1016/j.surg.2005.09.029. [DOI] [PubMed] [Google Scholar]
- 67.Wells S, Farndon JR, Dale JK, Leight GS, Dilley WG. Long-term evaluation of patients with primary parathyroid hyperplasia managed by total parathyroidectomy and heterotopic autotransplantation. Ann Surg. 1980;192:451–458. doi: 10.1097/00000658-198010000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Malette L, Blevins T, Jordan PHNGP. Autogeneous parathyroid grafts for generalized primary parathyroid hyperplasia. Surgery. 1987;101:738–745. [PubMed] [Google Scholar]
- 69.Gibril f, Venzon DJ, Ojeauburu JV, Bashir S, Jensen RT. Prospective study of the natural history of gastrinoma in patients with MEN1: definition of an aggressive and non-aggressive form. J Clin Endocrinol Metab. 2001;86:5282–5293. doi: 10.1210/jcem.86.11.8011. [DOI] [PubMed] [Google Scholar]
- 70.Norton JA, Melcher ML, Gibril F, Jensen RT. Gastric carcinoid tumors in multiple endocrine neoplasia-1 patients with Zollinger-Ellison syndrome can be symptomatic, demonstrate aggressive growth, and require surgical treatment. Surgery. 2004;136(6):1267–74. doi: 10.1016/j.surg.2004.06.057. [DOI] [PubMed] [Google Scholar]
- 71.Triponez F, Dosseh D, Goudet P, Cougard P, Bauters C, Murat A, Cadiot G, Niccoli-Sire P, Chayvialle JA, Calender A, Proye CA. Epidemiology data on 108 MEN 1 patients from the GTE with isolated nonfunctioning tumors of the pancreas. Ann Surg. 2006;243:265–272. doi: 10.1097/01.sla.0000197715.96762.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hellman P SB, Juhlin C, Akerstrom G, Rastad J. Findings and long-term results of parathyroid surgery in the multiple endocrine neoplasia type 1. World J Surg. 1992;16:718–723. doi: 10.1007/BF02067367. [DOI] [PubMed] [Google Scholar]
- 73.Thompson NW. The surgical management of hyperparathyroidism and endocrine disease of the pancreas in the multiple endocrine neoplasia type 1 patient. J Intern Med. 1995;238(3):269–80. doi: 10.1111/j.1365-2796.1995.tb00934.x. [DOI] [PubMed] [Google Scholar]
- 74.Burgess JR DR, Parameswaran V, Greenaway TM, Shepherd JJ. The outcome of subtotal parathroidectomy for the treatment of hyperparathyroidism in multiple endocrine neoplasia type 1. Arch Surg. 1998;133:126–129. doi: 10.1001/archsurg.133.2.126. [DOI] [PubMed] [Google Scholar]