Abstract
A 69-year-old Korean man was admitted to emergency room with complaints of abdominal pain, vomiting, and diarrhea. Laboratory tests revealed eosinophilia, anemia, hypoproteinemia, and hyponatremia. The gastric mucosa showed whitish mottled and slightly elevated lesions on the body angle of antrum. Microscopically, chronic gastritis with incomplete intestinal metaplasia was observed. Many adult worms, larvae, and eggs in cross sections were located in the crypts. Furthermore, the filariform larvae of Strongyloides stercoralis with a notched tail were detected through the culture.
Keywords: Strongyloides stercoralis, stomach, Korea
INTRODUCTION
Strongyloidiasis is prevalent throughout the tropical and temperate climates (Genta, 1989; Siddiqui and Berk, 2001), but its prevalence is low in Korea. Human is infected by third-stage filariform larvae of Strongyloides stercoralis. After penetration of the skin, they enter cutaneous vessels and are carried to the lung. They then migrate via the respiratory tree, are swallowed with sputum, and finally reach and mature into adult egg-laying females in the small intestine, especially in the duodenum and upper jejunum. The adult worm preferentially localizes in the intestine mucosal wall, producing eggs which develop rapidly into rhabdiform larvae observed in stool specimens (Beaver et al., 1984; Markell et al., 1999). Although S. stercoralis may infect many organs in hyperinfection (Igra-Siegman et al., 1981), the involvement of stomach is relatively rare. Herein, we report a patient with involvement of the gastric mucosa by S. stercoralis, and reviewed the literatures on strongyloidiasis which had previously been reported in Korea.
CASE RECORD
A 69-year-old Korean man, living at Hwasun-gun, Jeollanam-do, was admitted to emergency room of the Gwangju Hospital with complaints of abdominal pain, vomiting, and diarrhea on June 1, 2001. On admission, a physical examination revealed tenderness and bowel sounds in the abdomen. He passed diarrheic stools. Vital signs were as follows: blood pressure 100/50 mmHg, pulse rate 80/min, respiratory rate 20/min, and body temperature 36℃.
According to the past history, he had some episodes of abdominal pain and arthralgia in the knee joint, and was diagnosed as gastritis and degenerative arthritis at a private clinic. About 3 weeks ago, he experienced severe epigastric pain and arthralgia in the left leg and was admitted at the private clinic. At that time he was treated intravenously with prednisolone and orally with ranitidine.
Laboratory tests revealed eosinophilia, anemia, hypoproteinemia, and hyponatremia during hospital days. Diarrhea was not completely controlled until the fifth hospital day. No evidence of parasitic infection was found in routine stool examination with cellophane-thick smear technique. A chest radiograph showed features of chronic obstructive pulmonary disease in the both lungs, and a plain abdomen indicated mild gaseous dilatation of the bowel loop. A abdominal sonograph showed simple cyst in the left lobe of liver, distortion of pancreatic head contour, and dilatation of the common bile duct. A computed tomography showed simple cysts in the pancreas and mucinous duct ectasia in pancreatic head portion.
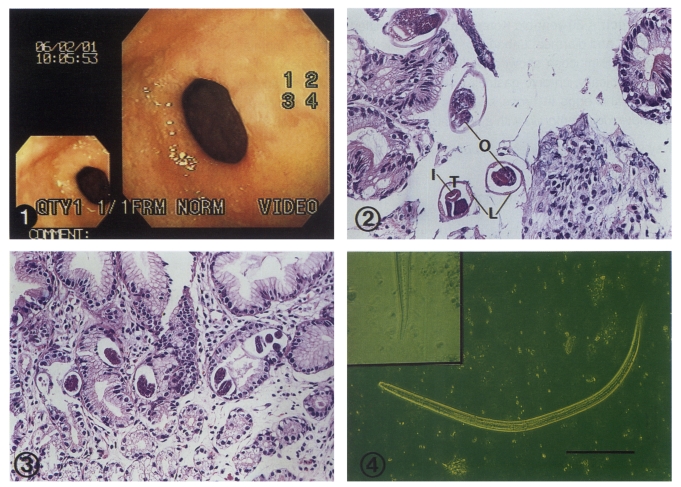
On upper gastrointestinal endoscopy, the esophagus, fundus, and body wall of stomach were grossly normal. The duodenum had also normal mucosal surface. However, in the body angle of antrum, the gastric mucosa showed whitish mottled and slightly elevated lesions (Fig. 1). The fiberoptic biopsy was performed at this region, and the formalin-fixed specimens showed four fragments of gray-white mucosal tissue, measuring 2 × 2 × 1 mm in dimensions.
Figs. 1-4.
A case of gastric strongyloidiasis diagnosed by endoscopic biopsy and fecal cultivation. Fig. 1. The gastric mucosa shows whitish mottled and slightly elevated lesions on the body angle of antrum. Fig. 2. Numerous sections of S. stercoralis adult worms in gastric biopsy are observed. The internal organs seen in the cross section are the intestine (I), the ovary (O), the lateral chord (L), and the tegument (T) composed of cuticle and weak muscle layer. × 300. Fig. 3. Many larvae and eggs in different stages of maturation are located in the crypts. Eggs in the crypt have basophilic granular mass or developing larvae within a thin egg shell. × 200. Fig. 4. The filariform larva of Strongyloides stercoralis obtained through Harada-Mori filter paper strip culture shows the typical notched tail under inverted light microscope. Bar = 100 µm. (inset: × 400) × 200.
Microscopically, chronic gastritis with incomplete intestinal metaplasia and occasional scattered eosinophils were observed. The adult worms appeared to be freed from gastric mucosal tissue during histologic processing. Also, histopathological examination of the gastric mucosa showed numerous cross sections of adult worms, eggs, and rhabditiform larvae developing in the gastric crypts (Fig. 2 & 3). The females in cross sections were up to 45 µm in diameter. The body wall composed of cuticle and weak muscle layer. The internal structure recognized in cross sections were the lateral chord, the intestine, the uterus, and the ovary. Because the ovary was convoluted, it appeared as single or double (Fig. 2). Eggs in the crypts were basophilic granular mass or larvae within a thin egg shell, measuring 58 by 33 µm. Hatched rhabditoid larvae were located in the mucosa near the lumen, and measured up to 13 µm in diameter (Fig. 3).
The patient was treated with 400 mg of albendazole a day for 3 days, and his abdominal pain improved and he was discharged on June 18. However, about 4 weeks later, the patient revisited hospital complaining of right hip joint pain. Rhabditoid larvae were found in stool examination with formalin-ether sedimentation technique. Fecal specimens were cultured by both the Harada-Mori filter paper strip culture technique and filter paper/slant culture technique (Petri dish)(Garcia, 2001), and the larvae recovered from culture tubes and dishes were measured up to 518 µm in length and 16 µm in diameter, and showed a notch appearance in the tail under inverted light microscope (Fig. 4). Based on these results, therefore, they were identified as the third-stage filariform larvae of S. stercoralis. Consequently, the second course of albendazole therapy was recommended for 5 days at home, because he wanted to be discharged. The third stool examination was requested from the out-patient clinic, and the larva of S. stercoralis was no longer detected from both stool examination and culture.
DISCUSSION
S. stercoralis is a common parasite of the intestinal tract, especially in tropical and subtropical areas (Genta, 1989; Siddiqui and Berk, 2001). In Korea, it has a low prevalence and several authors have occasionally reported it's presence on stool examination (Lee et al., 1994; Hong and Han, 1999). Excluding some papers published during the period of Josun Colonial Government, total 36 cases of human strongyloidiasis since 1945 have been recorded in the literature. The present case, therefore, constitutes the 37th documented case of strongyloidiasis in Korea.
The parasitic females of S. stercoralis usually live buried in the crypts of human proximal small intestine, producing eggs which develop rapidly into rhabditoid larvae in the mucosa. Extraintestinal infection can involve the lung, liver, spleen, pancreas, thyroid, kidney, brain, and meninges in hyperinfection (Igra-Siegman et al., 1981), however, cases of gastric involvement have relatively rarely been reported. Although the stomach is not an ideal site for S. stercoralis, reduced gastric acid secretion might favor infection and invasion of the stomach (Giannella et al., 1973). It has been suggested that the organisms reach the stomach of patient via consequent sputum swallowing or retrograde migration from the proximal small intestine. Of the 37 reported cases in Korea, gastric involvement has been histologically diagnosed in 7 patients including the present case (Kim et al., 1989; Lee et al., 1997; Yoon et al., 1997; Lee et al., 1999a, 1999b; Yun et al., 2001).
There are many high risk factors of strongyloidiasis, including old age, underlying chronic lung disease, orticosteroid treatment, and antacid treatment. (Berk et al., 1987). In Korea, the incidence of S. stercoralis infection was the highest among over 50 years of age (32 of 33 cases, 97%) and the youngest of gastric involvement was 64-year-old (Kim et al., 1989). Male was significantly dominant (25 of 33 cases, 76%). These data suggest that much more chances of infection were encountered by aged males than females. It has also been reported that immunosuppressive therapy increased the possibility of an infection with this parasite and that achlorhydria (often brought about by treatment with histamine-2 blockers or proton pump inhibitors) may facilitate gastric strongyloidiasis (Wurtz et al., 1994). In our case, a probable risk factor might have been that the patient simultaneously received corticosteroid and antacid therapy. He had been a heavy drinker for many years and roentgenographic findings also showed a change of chronic obstructive pulmonary disease in the both lungs.
The laboratory diagnosis of strongyloides is usually made by the finding of rhabditoid larvae in the fecal specimens, however, a routine stool examination may fail to find larvae, when the intestinal worm burden is very low and the output of larvae is minimal. It also needs to be remembered that worm may not be found in a cursory examination of a small quantity of feces. In our case, we could not find any larvae at the first routine stool examination. To improve chances of finding parasites, repeated examinations of stool specimens should be done (Nielsen and Mojon, 1987). Sometimes, parasite larvae are first found in gastric or small intestinal biopsy specimens taken for reasons other than strongyloidiasis; in these circumstances, there is less confidence in specificity of identification. Therefore, more reliable special culture technique such as Harada-Mori filter paper strip culture method or filter paper/slant culture technique are essential to differentiate S. stercoralis infection from other intestinal nematode infection (Garcia, 2001).
Eosinophilia is common in strongyloidiasis, ranging about 25 to 35 percent in acute cases and six to eight percent in chronic cases (Berkmen and Rabinowitz, 1972). However, it has previously been reported that eosinophil counts in strongyloidiasis tend to be lower in some immunosuppressive conditions, such as corticosteroid administration (Purtilo et al., 1974; Berger et al., 1980), and it's absence in patients indicated a poor prognosis. Cases of gastric strongyloidiasis reported in this country showed eosinophilia 6 to 29 percent (Kim et al., 1989; Lee et al., 1997; Lee et al., 1999a; Lee et al., 1999b), while two cases did not. It was found that the patients without eosinophilia received long-term corticosteroid therapies on admission for treatment of uveitis and rheumatoid arthritis (Yoon et al., 1997; Yun et al., 2001).
Although the severity of infection influences the extent of the clinical disorder, main gastrointestinal symptomes of strongyloidiasis include diarrhea, abdominal discomport, nausea, and anorexia (Berk et al., 1987). In our patient, The microscopic finding was a gastritis with intestinal metaplasia and a large number of parasites in the gastric pyloric region. Adult female worms inhabited the crypt of the stomach where eggs were deposited and larvae hatched. They might cause abdominal pain and diarrhea in the present patient. Unless heavily infected, the clinical symptoms and signs reported in strongyloidiasis are generally not so severe and frequently nonspecific characters. For this reason, the infection is easily overlooked by patients and even by physicians. In the case of gastric strongyloidiasis reported in Korea, the chief complaint frequently includes epigastric pain, nausea, and vomiting. Although these are nonspecific symptoms in strongyloidiasis, the possibility of gastric involvement should be carefully considered by physicians.
References
- 1.Beaver PC, Jung RC, Cupp EW. Clinical Parasitology. 9th ed. Philadelphia, USA: Lea & Febiger; 1984. pp. 253–268. [Google Scholar]
- 2.Berger R, Kraman S, Paciotti M. Pulmonary stronglyoidiasis complicating therapy with corticosteroids. Report of a case with secondary bacterial infections. Am J Trop Med Hyg. 1980;29:31–34. doi: 10.4269/ajtmh.1980.29.31. [DOI] [PubMed] [Google Scholar]
- 3.Berk SL, Verghese A, Alvarez S, Hall K, Smith B. Clinical and epidemiologic features of strongyloidiasis. A prospective study in rural Tennessee. Arch Intern Med. 1987;147:1257–1261. [PubMed] [Google Scholar]
- 4.Berkmen YM, Rabinowitz J. Gastrointestinal manifestations of the stongyloidiasis. Am J Roentgenol Radium Ther Nucl Med. 1972;115:306–311. doi: 10.2214/ajr.115.2.306. [DOI] [PubMed] [Google Scholar]
- 5.Garcia LS. Diagnostic Medical Parasitology. 4th ed. Washington DC, USA: ASM press; 2001. pp. 787–789. [Google Scholar]
- 6.Genta RM. Global prevalence of strongyloidiasis: critical review with epidemiologic insights into the prevention of disseminated disease. Rev Infect Dis. 1989;11:755–767. doi: 10.1093/clinids/11.5.755. [DOI] [PubMed] [Google Scholar]
- 7.Giannella RA, Broitman SA, Zamcheck N. Influence of gastric acidity on bacterial and parasitic enteric infections. Ann Intern Med. 1973;78:271–276. doi: 10.7326/0003-4819-78-2-271. [DOI] [PubMed] [Google Scholar]
- 8.Hong SJ, Han JH. A case of Strongyloides stercoralis infection. Korean J Parasitol. 1999;37:117–120. doi: 10.3347/kjp.1999.37.2.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Igra-Siegman Y, Kapila R, Sen P, Kaminski ZC, Louria DB. Syndrome of hyperinfection with Strongyloides stercoralis. Rev Infect Dis. 1981;3:397–407. doi: 10.1093/clinids/3.3.397. [DOI] [PubMed] [Google Scholar]
- 10.Kim YK, Kim H, Park YC, et al. A case of hyperinfection with Strongyloides stercoralis in an immunosuppressed patient. Korean J Intern Med. 1989;4:165–170. doi: 10.3904/kjim.1989.4.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee SH, Kho WG, Kim OK, et al. A case of stongyloidiasis associated with acute hemorrhagic gastritis. Korean J Gastrointest Endosc. 1999a;19:73–80. (in Korean) [Google Scholar]
- 12.Lee MK, Kim YK, Hwang IS, et al. A case of Strongyloides stercoralis infection associated with long-term administration of steroid in a patient with alcoholic liver disease. Korean J Gastrointest Endosc. 1997;17:675–679. (in Korean) [Google Scholar]
- 13.Lee SH, Lee KT, Lee JH, et al. A case of gastric strongyloidiasis diagnosed by endoscopic biopsy. Korean J Gastrointest Endosc. 1999b;19:249–253. (in Korean) [Google Scholar]
- 14.Lee SK, Shin BM, Khang SK, et al. Nine cases of strongyloidiasis in Korea. Korean J Parasitol. 1994;32:49–52. doi: 10.3347/kjp.1994.32.1.49. (in Korean) [DOI] [PubMed] [Google Scholar]
- 15.Markell EK, John DT, Krotoski WA. Markell and Voge's Medical Parasitology. 8th ed. Philadelphia, USA: W.B. Saunders Company; 1999. pp. 287–292. [Google Scholar]
- 16.Nielsen PB, Mojon M. Improved diagnosis of Strongyloides stercoralis by seven consecutive stool specimens. Zentralbl Bakteriol Mikrobiol Hyg A. 1987;263:616–618. doi: 10.1016/s0176-6724(87)80207-9. [DOI] [PubMed] [Google Scholar]
- 17.Purtilo DT, Meyers WM, Connor DH. Fatal strongyloidiasis in immunosuppressed patients. Am J Med. 1974;56:488–493. doi: 10.1016/0002-9343(74)90481-1. [DOI] [PubMed] [Google Scholar]
- 18.Siddiqui AA, Berk SL. Diagnosis of Strongyloides stercoralis infection. Clin Infect Dis. 2001;33:1040–1047. doi: 10.1086/322707. [DOI] [PubMed] [Google Scholar]
- 19.Yoon CH, Kim HO, Kim MY, et al. A case of Strongyloides stercoralis infection of stomach in association with meningitis. Korean J Med. 1997;52:550–553. (in Korean) [Google Scholar]
- 20.Yun HR, Yoo DH, Lee HS, et al. Fatal strongyloides hyperinfection in a patient with rheumatoid arthritis. Clin Exp Rheumatol. 2001;19:224. [PubMed] [Google Scholar]
- 21.Wurtz R, Mirot M, Fronda G, Peters C, Kocka F. Short report: gastric infection by Strongyloides stercoralis. Am J Trop Med Hyg. 1994;51:339–340. doi: 10.4269/ajtmh.1994.51.339. [DOI] [PubMed] [Google Scholar]