Abstract
The metacercariae of Acanthotrema felis Sohn et al., 2003 (Digenea: Heterophyidae) were discovered in a species of the brackish water fish, Acanthogobius flavimanus, in the Republic of Korea. They were experimentally fed to kittens, and adult flukes were harvested 7 days later. The adults were morphologically characterized by the presence of a bipartite seminal vesicle, the ventral sucker associated with a ventrogenital sac enclosing 3 sclerites (2 long and pointed, and 1 short and thumb-like), and an unarmed gonotyl. The adult flukes were identified as A. felis Sohn et al., 2003, and the brackish water fish A. flavimanus has been verified as one of its second intermediate hosts.
Keywords: Acanthotrema felis, heterophyid, metacercaria, cat, intermediate host, brackish water fish
The genus Acanthotrema (Digenea: Heterophyidae) is a group of minute intestinal trematodes parasitizing the small intestine of fish-eating birds and mammals. This genus was erected by Travassos (1928) with the type species, Acanthotrema acanthotrema. Due to morphological similarities, it was synonymized with Stictodora Looss, 1899 (Martin, 1950; Witenberg, 1953; Cable et al., 1960; Yamaguti, 1971). However, it was distinguished from Stictodora, based on the number and morphology of the sclerotized armatures in the ventrogenital sac (Sogandares-Bernal and Walton, 1965; Pearson, 1973). Including the new species, A. felis Sohn et al., 2003, reported recently in the Republic of Korea, there are now a total of eight species in Acanthotrema (Sohn et al., 2003). The new species is known to occur in the small intestine of stray cats in the southwestern parts of the Republic of Korea.
Several species of the brackish water fish are known to carry the metacercariae of Acanthotrema species (Martin, 1950; Martin and Kuntz, 1954; Velasquez, 1973; Kinsella and Heard, 1974). With regard to A. felis, however, the fish intermediate host is yet unknown. In order to determine its fish intermediate host, the goby Acanthogobius flavimanus, one of the most popular species of the brackish water fish in the Republic of Korea, was subjected to examination for the metacercariae of A. felis.
A total of 20 gobies, A. flavimanus, were purchased from a local market in Haenam-gun, Jeonranam-do in March and April, 1999. They were brought to the laboratory under refrigeration, and artificially digested in a pepsin-HCl solution. The digested material was filtered through a sieve and washed several times with 0.85% physiological saline. Trematode metacercariae were collected from the sediment using a stereomicroscope. Twenty metacercariae were observed and measured using a light microscope. In order to obtain adult flukes, two kittens, which appeared to be free from intestinal helminth infections by fecal examination, were each orally fed about 50 metacercariae, and sacrificed on day 7 post-infection (PI). The small intestines were resected, opened along the mesenteric border, and washed several times with 0.85% saline. The worms were collected from the intestinal content using a stereomicroscope. They were washed several times with saline, fixed in 10% neutral formalin, stained with Semichon's acetocarmine, mounted in Canada balsam, and identified using a light microscope. All measurements are given in micrometers, unless otherwise stated.
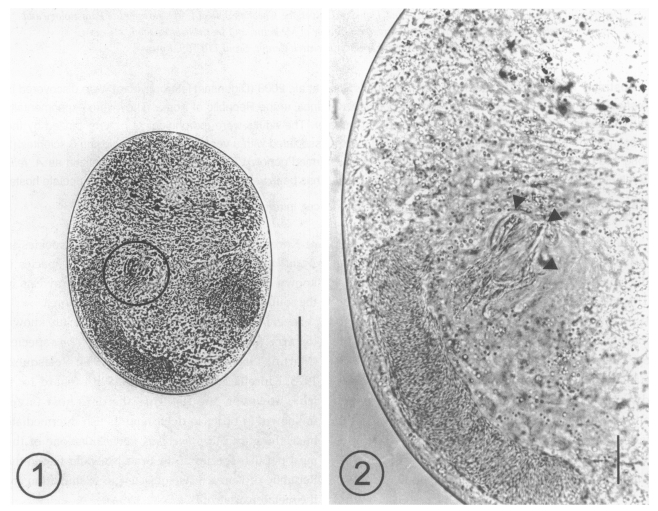
A total of 116 metacercariae of Acanthotrema sp. were collected from 7 A. flavimanus, with an average number of 16.6 per fish. The metacercariae were 216 - 254 (233 in average) long and 152-185 (167) wide (n = 10), yellowish brown, and elliptical, with a thin and transparent cyst wall (Fig. 1). They were equipped with an oral sucker and pharynx, and characterized by the presence of a ventro-genital sac, which enclosed fork-like sclerites (Fig. 2).
Figs. 1-2.
Fig. 1. A metacercaria of Acanthotrema felis isolated from a goby, Acanthogobius flavimanus, showing the sclerotized pieces (slightly left and middle). Bar = 50 µm. Fig. 2. Magnification of the ventrogenital sac area which is enclosing the sclerotized pieces (arrowheads). Bar = 50 µm.
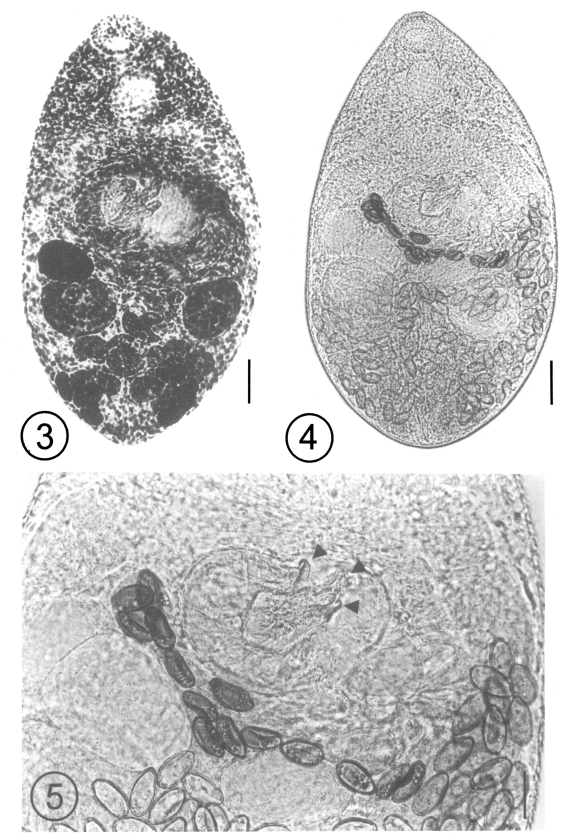
After the experimental infection of the two kittens, 3 adult (Figs. 3 and 4) and 2 young flukes were recovered from the small intestine of one kitten on day 7 PI, and the worms were identified as A. felis, based on the following morphological features and measurements (n = 3, adults): body small, claviform, averaging 440.0 (425.0 - 455.0) long, 265.0 (250.0 - 292.5) wide. Oral sucker 40.8 (37.5 - 45.0) long, 51.3 (50.0 - 52.5) wide. Prepharynx short. Pharynx subglobular, 46.7 (45.0 - 47.5) long, 37.5 (35.0 - 42.5) wide. Esophagus 5.0 (2.5 - 7.5) long; intestinal ceca terminate close to posterior extremity. Ventral sucker 82.5 (75.0 - 90.0) long, 65 (62.5 - 70.0) wide, parenchymatous, associated with muscular ventrogenital sac, enclosing 3 sclerotized pieces. Two sclerites long, pointed; one sclerite short, thumb-like with 15 (14 - 16) minute triangular spines on base margin of sclerite complex (Fig. 5). Gonotyl thin, unspined, included in ventrogenital sac. Two testes slightly oblique; right testis 79.2 (75.0 - 87.5) long, 61.7 (55.0 - 67.5) wide; left testis 95.0 (87.5 - 102.5) long, 72.5 (65.0 - 77.5) wide. Seminal vesicle bipartite, 62.5 (60.0 - 65.0) long, 50.8 (45.0 - 57.5) wide. Ovary submedian, 66.7 (65.0 - 70.0) long, 45.0 (40.0 - 50.0) wide. Seminal receptacle 48.3 (37.5 - 60.0) long, 41.7 (30.0 - 50.0) wide. Vitellaria in post-testicular fields. Eggs small, 26.3 (25.0 - 27.5) long, 14.1 (12.5 - 15.0) wide.
Figs. 3-5.
Fig. 3. An adult fluke of Acanthotrema felis recovered from a kitten at day 7 post-infection (PI) with the metacercariae, acetocarmine stained. Bar = 27 µm. Fig. 4. A fresh, unstained, specimen of an adult Acanthotrema felis recovered from a kitten at day 7 PI. Bar = 50 µm. Fig. 5. Magnification of the ventrogenital sac enclosing the 3 sclerotized pieces (arrowheads). Two of the pieces are long and pointed, and the other is short and thumb-like. Bar = 20 µm.
Acanthotrema felis was described based on the specimens recovered from the small intestine of stray cats in the Republic of Korea (Sohn et al., 2003). Its second intermediate host was unknown. In the present study, A. flavimanus, a species of the brackish water fish, was found to carry the metacercariae of A. felis, which were identified by obtaining the adult flukes from an experimental infection.
There was no much difficulty in the taxonomic identification of the adult specimens obtained from the experimental kitten. The trematode genera which have a ventral sucker associated with a ventrogenital sac, as seen in our specimens, include Stictodora, NeoStictodora, Parastictodora, Sobolephya, Acanthotrema, Galactosomum, Cercarioides, and Knipowitschiatrema (Pearson, 1973). However, those having a ventral sucker armed with spines less than 150 in number and each longer than 12 µm, are Acanthotrema or Stictodora (Pearson, 1973). Species of Acanthotrema have less than 12 spines or sclerotizations on the ventrogenital sac, a bipartite seminal vesicle, and no prostatic bulb, whereas species of Stictodora have more than 12 spines, a 3-chambered seminal vesicle and a prostatic bulb (Pearson, 1973). Our specimens were characterized by the presence of a bipartite seminal vesicle, and the ventral sucker associated with a ventrogenital sac, which enclosed 3 large sclerites, i.e., 2 long and pointed and 1 short and thumb-like, which were armed with minute spines at their basal portions. They belonged to Acanthotrema (Sohn et al., 2003).
There are currently 8 species of Acanthotrema (Lafuente et al., 2000; Sohn et al., 2003), including A. acanthotrema Travassos, 1928, A. hancocki (Martin, 1950) Lafuente et al., 2000, A. martini (Sogandares-Bernal, 1959) Lafuente et al., 2000, A. armata Lafuente et al., 2000, A. tridactyla (Martin and Kuntz, 1955) Sohn et al., 2003, A. tanayensis (Velasquez, 1973) Sohn et al., 2003, A. cursitans (Kinsella and Heard, 1974) Sohn et al., 2003, and A. felis Sohn et al., 2003. For the diagnosis of Acanthotrema species, the morphology and number of the sclerotized armatures in the ventrogenital sac is the most important key (Lafuente et al., 2000; Sohn et al., 2003). By having 3 sclerites, A. felis most closely resembles A. acanthotrema and A. tridactyla, but differs from them in the shape of the sclerites; 1 of which is thumb-like in A. felis (Sohn et al., 2003), whereas all 3 sclerites are long and pointed in the latter 2 species (Tavassos, 1928; Martin and Kuntz, 1955). The 3 species also differ in terms of the presence and position of minute spines on the sclerites, which are near the basal portions of the 3 sclerites in A. felis, but near the tips of only the 2 sclerites in A. acanthotrema, or the tips of the 3 sclerites in A. tridactyla (Martin and Kuntz, 1955).
The second intermediate hosts of the species of Acanthotrema have been reported to be the brackish water fishes; Gillichthys mirabilis and Fundulus parvipinnis for A. hancocki (Martin, 1950), Aphanius fasciatus for A. tridactyla (Martin and Kuntz, 1955), Ophiocara aporos for A. tanayensis (Velasquez, 1973) and Fundulus confluentis, F. similis and F. grandis for A. cursitans (Kinsella and Heard, 1974). Additionally, in the case of A. felis, a species of the brackish water fish, A. flavimanus, has been confirmed to act as a second intermediate host.
A wide range of definitive hosts has been reported for Acanthotrema species. Kittens have been shown to be the experimental definitive host not only for A. felis but also for A. tanayensis (Velasquez, 1973) and A. tridactyla (Abdul-Salam et al., 2000). Other mammals, including laboratory rodents, were reported to be the natural and experimental definitive hosts for A. cursitans (Kinsella and Heard, 1974). Avian species, including their chicks, were shown to be the definitive hosts for A. martini (Sogandares-Bernal, 1959), A. acanthotrema (Travassos, 1928), A. armata (Roca et al., 1999; Lafuente et al., 2000), A. tridactyla (Martin and Kuntz, 1955) and A. hancocki (Martin, 1950). The possibility of human infection by A. felis cannot be ruled out.
Among the human-infecting heterophyid flukes, Heterophyes nocens, Heterophyopsis continua, Pygidiopsis summa, Stellantchasmus falcatus, Stictodora fuscata and S. lari are contracted by eating raw flesh of the brackish water fish such as the shad, perch, mullet and goby (Chai and Lee, 2002). As a result of this study, A. felis is added to the group of heterophyid flukes that can be contracted by eating the raw flesh of the goby.
References
- 1.Abdul-Salam J, Sreelatha BS, Ashkanani H. Surface ultrastructure of Stictodora tridactyla (Trematoda: Heterophyidae) from Kuwait Bay. Parasitol Int. 2000;49:1–7. doi: 10.1016/s1383-5769(00)00027-1. [DOI] [PubMed] [Google Scholar]
- 2.Cable RM, Connor RS, Balling JW. Digenetic trematodes of Puerto Rican shore birds. Scientific Survey of Porto Rico and the Virgin Islands. Proc New York Acad Sci. 1960;17:187–255. [Google Scholar]
- 3.Chai JY, Lee SH. Food-borne intestinal trematodeinfections in the Republic of Korea. Parasitol Int. 2002;51:129–154. doi: 10.1016/s1383-5769(02)00008-9. [DOI] [PubMed] [Google Scholar]
- 4.Kinsella JM, Heard RW., 3rd Morphology and life cycle of Stictodora cursitans n. comb. (Trematoda: Heterophyidae) from mammals in Florida salt marshes. Trans Am Microsc Soc. 1974;93:408–412. [PubMed] [Google Scholar]
- 5.Lafuente M, Roca V, Carbonell E. Description of Acanthotrema armata n. sp. (Trematoda: Heterophyidae) from Larus audouinii (Aves: Laridae), with an amended diagnosis of the genus Acanthotrema Travassos, 1928. Syst Parasitol. 2000;45:131–134. doi: 10.1023/a:1006293611598. [DOI] [PubMed] [Google Scholar]
- 6.Martin WE. ParaStictodora hancocki n. gen., n. sp. (Trematoda: Heterophyidae), with observation on its life cycle. J Parasitol. 1950;36:360–370. [PubMed] [Google Scholar]
- 7.Martin WE, Kuntz RE. Some Egyptian heterophyid trematodes. J Parasitol. 1955;41:374–382. [PubMed] [Google Scholar]
- 8.Pearson JC. A revision of the subfamily Haplorchinae Looss, 1899 (Trematoda: Heterophyidae) II. Genus Galactostomum. Philos Trans R Soc Lond B(Biol Sci) 1973;266:341–447. doi: 10.1098/rstb.1973.0052. [DOI] [PubMed] [Google Scholar]
- 9.Roca V, Lafuente M, Carbonell E. Helminth communities in Audouin's gulls, Larus audouinii from Chafarinas Islands (western Mediterranean) J Parasitol. 1999;85:984–986. [PubMed] [Google Scholar]
- 10.Sogandares-Bernal F. Four trematodes from the Black Skimmer, Rynchops nigra Linn. (Aves: Rynchopidae), in Gasparilla Sound, Florida, including the description of a new genus and two new species. Quart J Florida Acad Sci. 1959;22:125–132. [Google Scholar]
- 11.Sogandares-Bernal F, Walton DA. Stictodora lariformicola n. sp. (Trematoda; Heterophyidae) from Floridian piscivorous birds. Proc Helminth Soc Washington. 1965;32:115–117. [Google Scholar]
- 12.Sohn WM, Han ET, Chai JY. Acanthotrema felis n. sp. (Digenea: Heterophyidae) from the small intestine of stray cats in the Republic of Korea. J Parasitol. 2003;89:154–158. doi: 10.1645/0022-3395(2003)089[0154:AFNSDH]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 13.Travassos L. Nouveau trematode parasite de Laridae. Comptes Rendues de la Societe Biologique du Bresil. 1928;99:884–885. [Google Scholar]
- 14.Velasquez CC. Observations on some Heterophyidae (Trematoda: Digenea) encysted in Philippine fishes. J Parasitol. 1973;59:77–84. [PubMed] [Google Scholar]
- 15.Yamaguti S. Synopsis of digenetic trematodes of vertebrates. Vol. I. Tokyo, Japan: Keigaku Publishing Co; 1971. p. 1,074. [Google Scholar]