Abstract
There is now compelling evidence that the excitatory amino acid neurotransmitter glutamate plays a pivotal role in drug addiction and alcoholism. As a result, there has been increasing interest in developing glutamate-based therapies for the treatment of addictive disorders. Receptors for glutamate are primarily divided into two classes: ionotropic glutamate receptors (iGluRs) that mediate fast excitatory glutamate transmission, and metabotropic glutamate receptors (mGluRs), which are G-protein coupled receptors that mediate slower, modulatory glutamate transmission. Most iGluR antagonists, while showing some efficacy in animal models of addiction, exhibit serious side effects when tested in humans. mGluR ligands, on the other hand, which have been advanced to testing in clinical trials for various medical conditions, have demonstrated the ability to reduce drug reward, reinforcement, and relapse-like behaviors in animal studies. mGluR ligands that have been shown to be primarily effective are Group I (mGluR1 and mGluR5) negative allosteric modulators and Group II (mGluR2 and mGluR3) orthosteric presynaptic autoreceptor agonists. In this review, we will summarize findings from animal studies suggesting that these mGluR ligands may be of potential benefit in reducing on-going drug self-administration and may aid in the prevention of relapse. The neuroanatomical distribution of mGluR1, mGluR2/3, and mGluR5 receptors and the pharmacological properties of Group I negative allosteric modulators and Group II agonists will also be overviewed. Finally, we will discuss the current status of mGluR ligands in human clinical trials.
Keywords: Metabotropic glutamate receptor, negative allosteric modulator, addiction, alcoholism, rodent models, self-administration, conditioned place preference, reinstatement, relapse
INTRODUCTION
Over the past two decades, however, a tremendous amount of evidence has accumulated suggesting that the excitatory amino acid neurotransmitter glutamate plays a pivotal role in drug addiction, drug self-administration and reward-related processes, and relapse [1–4]. Glutamate is the most abundant excitatory neurotransmitter in the central nervous system and governs many processes in the brain including fast and slow excitatory neurotransmission, control of basal neuronal activity, and synaptic plasticity.
Glutamate receptors fall into one of two categories: ligand-gated ion channels (i.e., ionotropic glutamate receptors, or iGluRs) which mediate fast excitatory neurotransmission, and G-protein coupled receptors (i.e., metabotropic glutamate receptors, or mGluRs) which mediate slower, modulatory neurotransmission. Numerous animal studies have shown that iGluR antagonists attenuate the rewarding and reinforcing effects of virtually all drugs of abuse, and can attenuate various forms of relapse-like behavior (see [4] for recent review). However, very few iGluR antagonists are without serious side effects in humans, which include memory loss, disorientation, and the production of symptoms of psychosis such as hallucinations and depersonalization. In light of these unwanted side effects, significant efforts in recent years have been undertaken to pharmacologically manipulate glutamate transmission with selective mGluRs ligands. Such ligands have been shown to not only be of potential benefit in the treatment of addiction, but other disorders of the central nervous system including chronic pain, Parkinson’s disease, depression, anxiety, epilepsy, and neurodegeneration (see refs [5–14] for reviews)
To date, eight different mGluR receptor subtypes have been cloned and characterized, and these receptors appear to have diverse neuroanatomical distributions as well as unique pharmacological and intracellular signaling properties [15–18]. The Group I family of mGluRs consists of mGluR1 and mGluR5 receptors, whereas the Group II family consists of mGluR2 and mGluR3 and the Group III family consists of mGluR4, mGluR6, mGluR7 and mGluR8.
Addiction is a multifaceted chronically relapsing disorder characterized by excessive drug intake, repeated unsuccessful attempts and stopping or reducing drug use, narrowing of the behavioral repertoire towards drug-seeking and self-administration, the emergence of symptoms of tolerance and withdrawal, and continued drug intake despite negative consequences [19]. Although animal models of addiction such as the intravenous drug self-administration, reinstatement, and conditioned place preference paradigms closely resemble several aspects of addiction in humans, none of these paradigms completely encompasses all aspects of the human condition. There is a great need to develop or improve animal models of addiction so that they provide reliable measures of drug craving, voluntary abstinence, relapse following protracted withdrawal, devaluation of natural reinforcers in lieu of drug reinforcement, and impaired decision-making [20–23]. Nonetheless, currently employed animal models of addiction have yielded a vast amount of information on the neural substrates of drug reward and reinforcement, drug-seeking and relapse-like behaviors, as well as the neuroadaptations that accompany chronic drug exposure.
Studies employing the intravenous drug self-administration, reinstatement, and conditioned place preference paradigms have revealed that the mGluR ligands that show the greatest therapeutic potential as anti-addiction agents include mGluR1 and mGluR5 negative allosteric modulators and mGluR2/3 orthosteric agonists. The purpose of the present review is to summarize the existing preclinical literature on the effects of these mGluR ligands on drug and ethanol reward, reinforcement, and relapse-like behaviors. Following a brief overview of glutamatergic transmission, the neuroanatomical distribution of these receptors will be presented, followed by a summary of the pharmacological properties of mGluR1/5 antagonists and mGluR2/3 agonists. Next, following a brief overview of widely used animal models of addiction, we will then summarize the existing literature from animal studies on the efficacy of mGluR ligands in animal models of drug addiction and alcoholism. The review will conclude with an update on the status of mGluR ligands being tested in human clinical trials for other medical conditions, and a discussion of the possible clinical utility of mGluR ligands in the treatment of addiction.
GLUTAMATERGIC NEUROTRANSMISSION
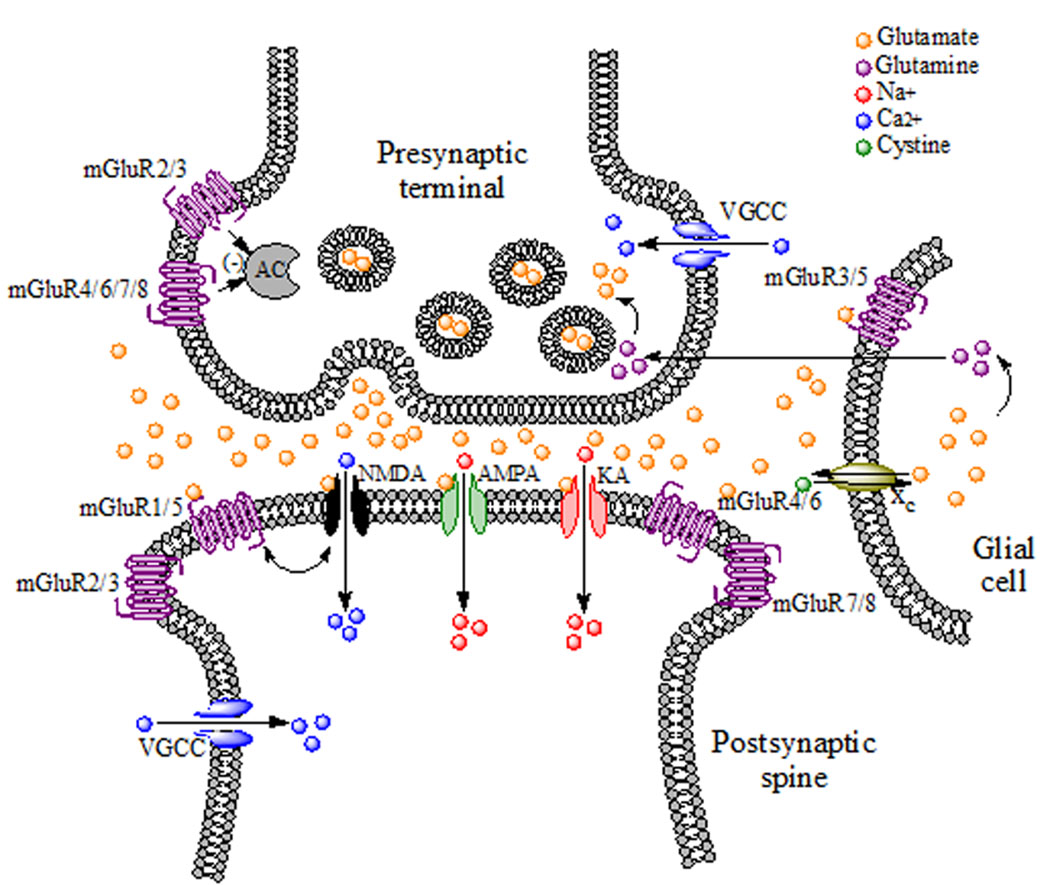
Glutamate is the most abundant excitatory neurotransmitter in the brain, mediating as much as 70% of synaptic transmission within the central nervous system and reaching concentrations in the synaptic cleft as high as the mid-micromolar range. A diagram of a typical glutamatergic synapse is shown in Fig. 1. Glutamate is packaged into synaptic vesicles in the presynaptic terminal by vesicular glutamate transporters. Once released into the synaptic cleft, glutamate can bind to one of three different types of ionotropic glutamate receptors (iGluRs) located on the head of the postsynaptic dendritic spine: the N-methyl-D-aspartate (NMDA) receptor, the α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) receptor, and the kainic acid (kainate, KA) receptor. iGluRs are ligand-gated ion channels that mediate fast excitatory neurotransmission [24–27].
Fig. 1.
The glutamatergic synapse. When an action potential arrives at the terminal, glutamate is released by exocytosis into the synaptic cleft where it binds to and activates iGluRs (NMDA, AMPA and KA receptors) localized on the postsynaptic neuron, which results in cation influx and subsequent activation of VGCCs that propagate the action potential. Glutamate can also be released into the extracellular space via nonexocytotic mechanisms such as cystine-glutamate-exchanger (xc) located on glial cells. Whether released from the presynaptic terminal or neighboring glial cells, extracellular glutamate binds and activates not only iGluRs but also postsynaptic mGluRs in the perisynaptic annulus. Glutamate release from the presynaptic terminal is negatively regulated by Group II or III mGluR autoreceptors, which are negatively coupled to adenylyl cyclase (AC). On the postsynaptic neuron, there are bidirection interactions between Group I mGluRs and NMDA receptors. In glia, glutamate is converted to glutamine, which is then transported back to the presynaptic terminal and converted back to glutamate.
NMDA receptors are heterotetrameric protein complexes composed of at least one NR1 subunit (for which there are at least 8 splice variants) and a combination of NR2A-D and NR3A or 3B subunits [28–30]. In addition to being stimulated by glutamate, amino acids such as D-serine and glycine act as co-agonists at the NMDA receptor. The NR2 subunits contain the glutamate binding domain, whereas the NR1 subunit contains the glycine-binding domain. Under resting conditions, the NMDA receptor channel pore is blocked by Mg2+ ions, but once sufficient membrane depolarization has been established (i.e., by opening of AMPA receptor channels), the Mg2+ block is removed, allowing the influx of cations (primarily Ca2+ ions, but the NMDA receptor is also permeable to K+ and Na+ ions). Once thought to be exclusively located on neurons, NMDA receptors have recently been shown to be expressed on glial cells including microglia, astrocytes and oligodendrocytes [30]. NMDA receptor subunits have also been found to exist on presynaptic terminals [31]. The NMDA receptor has been extensively implicated in mediating neural plasticity as well as learning and memory processes [32–34].
AMPA receptors are also heterotetrameric protein complexes composed of various subunits termed GluR1–4 (also termed GluRA-D) and GluRδ1 and 2 [28]. The mRNAs encoding AMPA subunits can be edited or alternatively spliced to form variants such as the flip and flop isoforms. Each GluR subunit contains a binding site for glutamate. Once activated, AMPA receptors are permeable to various cations including Ca2+, Na+ and K+, although the majority of AMPA receptors in the brain contain GluR2 subunits, which render the channel impermeable to Ca2+. It is believed that both NMDA and AMPA receptors are necessary for the induction of many forms of synaptic plasticity such as long-term potentiation (LTP) and long-term depression (LTD) [35–41].
Like NMDA and AMPA receptors, kainic acid (kainate, KA) receptors are also tetrameric protein complexes composed of various subunits. These subunits are termed GluR5–7 and KA1 and 2 [28]. KA receptors can form homomeric tetramers composed entirely of GluR5, 6 or 7 subunits or heteromeric complexes containing GluR5 or KA subunits. KA receptors are permeable to Na+ and K+ ions and, like NMDA and AMPA receptors, contribute to excitatory postsynaptic currents. The role of KA receptors in synaptic plasticity is less well-defined, however, but KA receptors have been found to be localized presynaptically where they can modulate neurotransmitter release [42].
In addition to the iGluRs, glutamate can also bind to mGluRs, which are located either in the perisynaptic annulus or on presynaptic terminals. mGluRs are seven transmembrane domain G-protein coupled receptors (GPCRs) that mediate slower, modulatory glutamatergic transmission. mGluRs can be divided into three distinct groups, based on their pharmacological and signal transduction properties. Group I mGluR receptors (mGluR1 and mGluR5) activate the Gαq class of G-proteins, which stimulate one of several phospholipases (including phospholipase C), resulting in phosphoinositide (PI) hydrolysis and the formation of lipid signaling intermediates such as inositol triphosphate (IP3) and diacylglycerol (DAG), which in turn can activate various intracellular messengers including protein kinase C (PKC) [15, 17, 43]. Activation of Group I mGluR receptors also mobilizes calcium release from IP3 receptor-mediated intracellular stores, which can in turn activate other intracellular messengers such as calcium/calmodulin-dependent kinase II (CaMKII). Group I mGluRs, particularly mGluR5, are positively coupled to NMDA receptor function via PKC, and are structurally linked to these receptors as well as IP3-gated intracellular Ca2+ stores via the Homer family of proteins [44–48]. Group I mGluRs are rarely found presynaptically. Group II (mGluR2 and mGluR3) and Group III (mGluR4, mGluR6, mGluR7, and mGluR8) mGluRs, on the other hand, activate the Gαi class of G-proteins and are negatively coupled to adenylyl cyclase (AC) activity, and upon stimulation result in decreased intracellular levels of cyclic adenosine monophosphate (cAMP). Presynaptically localized Group II and Group III mGluRs, particularly mGluR2 and mGluR3, are thought to represent the classical inhibitory autoreceptor mechanism that suppresses excess glutamate release from the presynaptic terminal [49].
Glutamate is cleared from the extracellular environment by a family of sodium-dependent excitatory amino acid transporters (EAATs)[50]. This family of EAATs provides numerous mechanisms to prevent an excessive accumulation of extracellular glutamate, which if high enough concentrations are reached, can result in excitotoxicity. Once inside glial cells, glutamate is converted to glutamine by glutamine synthetase, where it is secreted back outside the glia and taken up by the presynaptic terminal for conversion back to glutamate by glutaminase. Conversely, glutamate can be transported from within glial cells to the extracellular environment by the cystine-glutamate exchanger (xc), and some investigators have proposed that this mechanism is critically involved in regulating extracellular glutamate levels [51–54].
NEUROANATOMICAL DISTRIBUTION OF GROUP I AND GROUP II mGluR RECEPTORS
The neuroanatomical localization of Group I and Group II mGluRs in the rodent brain, as assessed by immunohistochemical or in situ hybridization techniques, has revealed overlapping yet distinct patterns of expression of these receptors [18, 55–68] (see Table 1 for details). High levels of mGluR1 expression are found in the olfactory bulb, thalamus, hippocampus (excluding the CA1 region), lateral septum, superior colliculus and cerebellum. Moderate levels are found in the dorsal striatum, hypothalamus, pallidum, ventral midbrain, and cerebral cortex, and low levels are observed in the amygdala, medial septum, nucleus accumbens and brainstem.
Table 1.
Neuroanatomical distribution of Group I and Group II metabotropic glutamate receptors in the rodent brain
| Structure | mGluR1 | mGluR5 | mGluR2/3 |
|---|---|---|---|
| Olfactory bulb | +++ | +++ | ++ |
| Cerebral cortex | ++ | ++ | ++ |
| Dorsal Striatum | ++ | +++ | ++ |
| Nucleus Accumbens | + | +++ | ++ |
| Pallidum | +++ | ++ | + |
| Septum | +++ | +++ | +/0 |
| Hippocampus | ++ | +++ | ++ |
| Thalamus | +++ | + | + |
| Hypothalamus | + | + | 0 |
| Amygdala | + | ++ | ++ |
| Ventral Midbrain | +++ | + | + |
| Superior colliculus | ++ | +++ | + |
| Inferior colliculus | 0 | +++ | + |
| Cerebellum | +++ | +/0 | ++ |
Symbols: 0, not detectable; +, low levels of expression; ++, moderate levels of expression; +++, high levels of expression
In contrast to expression patterns of mGluR1, the expression of mGluR5 is highly concentrated in forebrain and limbic structures. High levels of mGluR5 expression are found in the olfactory bulb, anterior olfactory nuclei, olfactory tubercle, dorsal striatum, nucleus accumbens, lateral septum, hippocampal formation (CA1–CA3 regions and dentate gyrus), and inferior colliculus. More moderate levels of mGluR5 expression are observed in the cerebral cortex (with expression being more dense in superficial as compared to deeper layers), amygdala and caudal portions of the spinal trigeminal nucleus. The presence of mGluR5 is notably low or absent in regions of the hypothalamus, medial septum, ventral midbrain, pons and medulla, and the cerebellum only demonstrates a small amount of mGlu5 mRNA expression in Golgi cells.
To a moderate degree, the expression patterns of mGluR2/3 receptors in the rodent brain parallel those of mGluR5, although the overall abundance of mGluR2/3 receptors appears slighty reduced as compared with that of mGluR5 [5, 18]. Expression levels of mGluR2/3 receptors are high in the olfactory bulb and hippocampus, and moderate in the dorsal striatum, nucleus accumbens, amygdala, anterior thalamic nuclei, cerebral cortex and cerebellum. Low levels of mGluR2/3 are found in the pallidum, colliculi, ventral midbrain and hypothalamus.
Studies employing electron microscopy techniques have revealed that the vast majority of mGluR1 and mGlu5 receptors (i.e., >90%) appear to be located postsynaptically on the perisynaptic annulus of dendritic spines ([69]). There is, however, some evidence that these receptors can indeed be localized to axon terminals in regions such as the hippocampus [68] and cerebral cortex [70, 71]. The function of presynaptic Group I mGluRs is unknown, but a recent study [72] demonstrated that they cooperatively interact with presynaptic NMDA receptors in a similar fashion to that observed in the postsynaptic membrane. mGluR2/3 receptors are highly expressed in glutamatergic presynaptic terminals where they function as inhibitory autoreceptors, but are also found on postsynaptic membranes as well.
Finally, although it is clear that high levels of Group I and Group II mGluRs are expressed in neurons, these receptors have also been detected in glial cells such as astrocytes of both human and rat origin [73–81], where they demonstrate similar signaling properties as observed in neurons (i.e., increases in PI hydrolysis upon stimulation) [82]. The precise function of mGlu5 receptors in astrocytes is not currently known but may have a role in regulating ion channel function [83], glutamate transporter expression [80], reactive gliosis [81, 84] and calcium signaling [85].
SELECTIVE GROUP I NEGATIVE ALLOSTERIC MODULATORS AND GROUP II AGONISTS
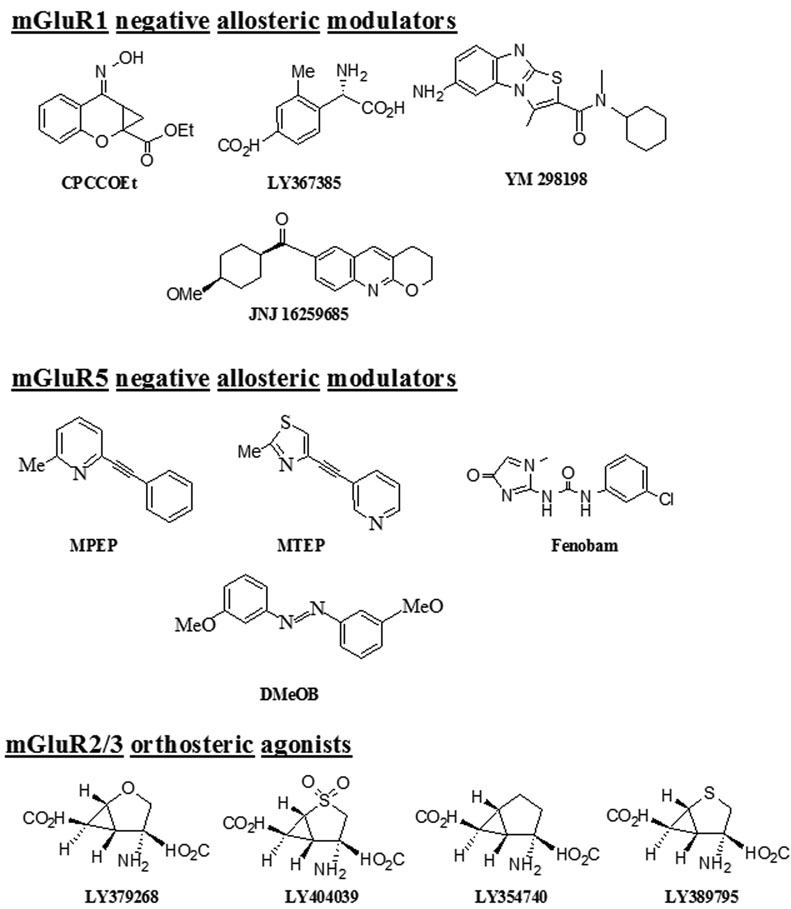
In the following section, an overview of mGluR ligands (mGluR1 and mGluR5 negative allosteric modulators, and mGluR2/3 agonists) that are commonly used in preclinical research on addiction and other CNS disorders is presented. The structures of these compounds are shown in Fig. 2, and their chemical names and abbreviations are presented in Table 2.
Fig. 2.
Chemical structures of Group I mGluR NAMs and Group II orthosteric agonists. See Table 2 for chemical names and abbreviations. Note that the structures of EMQMCM, an mGluR1 antagonist, and ADX10059, a negative allosteric modulator (NAM) of mGluR5 receptors, have not been published and are therefore not shown in this figure.
Table 2.
Chemical names and abbreviations of commonly used Group I mGluR antagonists and Group II agonists
| Ligand | Chemical Name | Mode of Action |
|---|---|---|
| CPCCOEt | 7-(hydroxyimino)cyclopropa[b]chromen-1a-carboxylate ethyl ester | mGluR1 antagonist (allosteric) |
| LY367385 | (S)-(+)-α-amino-4-carboxy-2-methylbenzeneacetic acid, (+)-2-methyl-4-carboxyphenylglycine |
mGluR1 antagonist (allosteric) |
| EMQMCM | (3-ethyl-2-methyl-quinolin-6-yl)-(4-methoxy-cyclohexyl)-methanone methane sulfonate | mGluR1 antagonist (allosteric) |
| JNJ16259685 | (3,4-dihydro-2H-pyrano[2,3]b-quinolin-7-yl)(cis-4-methoxycyclohexyl) methanone | mGluR1 antagonist (allosteric) |
| YM298198 | 6-Amino-N-cyclohexyl-N,3-dimethylthiazolo[3,2-a]benzimi dazole-2-carboxamide | mGluR1 antagonist (allosteric) |
| MPEP | 2-methyl-6-(phenylethynyl)pyridine | mGluR5 antagonist (allosteric) |
| MTEP | 3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]pyridine | mGluR5 antagonist (allosteric) |
| Fenobam | N-(3-chlorophenyl)-N-(4,5-dihydro-1-methyl-4-oxo-1H-imidazole-2-yl)urea] | mGluR5 antagonist (allosteric) |
| DMeOB | (3-Methoxyphenyl)methylene]hydrazone-3-methoxybenzaldehyde | mGluR5 NAM |
| LY379268 | (1R,4R,5S,6R)-4-amino-2-oxabicyclo[3.1.0]hexane-4,6-dicarboxylic acid | mGluR2/3 agonist (orthosteric) |
| LY404039 | (−)-(1R,4S,5S,6S)-4-amino-2-sulfonylbicyclo[3.1.0]hexane-4,6-dicarboxylic acid | mGluR2/3 agonist (orthosteric) |
| LY354740 | (1S,2S,5R,6S)-2-aminobicyclo[3.1.0]hexane-2,6-dicarboxylate monohydrate | mGluR2/3 agonist (orthosteric) |
| LY389795 | (−)-4-Amino-2-thiabicyclo-[3.1.0]hexane-4,6-dicarboxylate | mGluR2/3 agonist (orthosteric) |
While the term “antagonist” has been widely used when applied to ligands that inhibit the function of specific mGluRs (i.e., mGluR1 and mGluR5), it is more appropriate to refer to such ligands as “negative allosteric modulators” (NAMs), since they inhibit the function of the receptor at a site that is distal to the actual orthosteric ligand binding domain of the receptor, and only in the presence of the orthosteric ligand [6, 8, 11, 86–88]. Thus, many pharmacological inhibitors of Group I mGluRs are referred to in the literature as “antagonists”, while the term NAM is more technically correct. In addition, there is recent evidence to suggest that Group I NAMs may actually function as inverse agonists, since in cell-based assays they can inhibit the basal (constitutive) activity of Group I mGluRs in the absence of any orthosteric agonist or even when the glutamate binding domain has been removed or mutated [89–100]. Thus, it is possible that in near future the functional terminology if mGluR ligands may be changed yet again to reflect the inverse agonistic properties of these ligands.
mGluR1 NEGATIVE ALLOSTERIC MODULATORS
One of the first selective mGluR1 NAMs to be developed was CPCCOEt [101, 102]. CPCCOEt exhibited an IC50 value of 6.5 µM for inhibition of glutamate-stimulated increases in intracellular calcium in cells expressing the human mGluR1b receptor, and did not displace [3H]glutamate binding, suggesting that it acted at an allosteric site separate from the glutamate-binding domain of the receptor. Another mGluR1 NAM, LY367385, was also characterized at approximately the same time [103]. LY367385 exhibited an IC50 value of 8.8 µM for blockade of quisqualate-stimulated PI hydrolysis. Both CPCCOEt and LY367385 have been reported to be systemically active [104, 105].
Since the development of LY367385 and CPCCOEt, selective mGluR1 NAMs with greater potency that retain systemic bioavailability have been synthesized. One of these compounds is YM298198 [106], which has selective affinity for mGluR1 receptors in the low nanomolar range (Ki=19 nM, IC50=16 nM). Another mGluR1 NAM, JNJ16259685, has also been recently developed which selectively inhibits the activity of mGluR1 receptors at subnanomolar concentrations (Ki=0.34 nM, IC50 = 0.55 nM) [107, 108]. Remarkably, when administered subcutaneously, less than 0.1 mg/kg is needed to occupy central mGluR1 receptors.
mGluR5 NEGATIVE ALLOSTERIC MODULATORS
In the late 1990s, researchers at SIBIA Neurosciences developed two compounds that were some of the first mGluR5-selective receptor NAMs to be characterized [109]. These compounds, 6-methyl-2-(phenylazo)-3-pyrindol (SIB-1757) and (E)-2-methyl-6-(2-phenylethenyl)pyridine (SIB-1893) were originally isolated by high throughput screening assays for their ability to alter basal or glutamate-stimulated IP3 accumulation in various cell lines transfected with human cDNA for each of the 8 mGluR receptor subtypes. Since SIB-1757 and SIB-1893 had IC50 concentration in the low micromolar range, researchers began to modify their structure with hopes of finding more potent mGluR5 NAMs. In 1999, Gasparini and colleagues published the structure and initial characterization of 2-methyl-6-(phenylethynyl)-pyridine (MPEP) [110], which exhibited relative high solubility in aqueous solutions and good brain penetrance and potency. MPEP inhibits quisqualate-stimulated PI hydrolysis at an IC50 value of 36 nM (100 times less than that of SIB-1757 and SIB-1893) and displays no agonist or antagonist activity at other mGluR receptor subtypes at concentrations up to 30 µM. After peripheral administration in rats, MPEP dose-dependently occupies mGluR5 receptors in the brain (as measured by displacement of the selective mGluR5 radioligand [3H]methoxymethyl-MTEP) within 5 minutes of administration, with maximal occupation observed at doses of 10 mg/kg i.p or higher [111]. In addition, >60% mGluR5 occupancy is observed 2 hr after peripheral administration [111, 112], indicating that its effects are somewhat long-lasting, although shorter durations of receptor occupancy have been observed in mice as compared to rats [112].
Unfortunately, several reports have been published that have brought the selectivity of MPEP for mGluR5 receptors into question. First, when applied to cultured rat cortical neurons at concentrations of 20 and 200 µM, O’Leary and colleagues [113, 114] found that MPEP significantly reduced NMDA receptor-mediated neurotoxicity via a direct action on NMDA receptors. Consistent with this, it was reported that MPEP displays antagonistic activity in cells expressing the NMDA receptor containing NR2B subunits at an IC50 value of 18 µM [115]. This study also demonstrated that MPEP can inhibit monoamine oxidase A (MAO-A) activity at an IC50 value of 8 µM [115]. Non-specific agonist-like effects of MPEP and SIB-1893 have also been reported in cells expressing recombinant human mGlu4 receptors [116]. A recent report also demonstrated that MPEP displaced binding of [3H]nisoxetine (a selective norepinephrine transporter (NET) inhibitor) and inhibited norepinephrine uptake with in LLCPK cells transfected with various human monoamine transporters [117]. However, as in the aforementioned studies, only concentrations of MPEP in the micromolar range exerted these effects or norepinephrine uptake. In addition, these findings have not been replicated in neuronal preparations. Nonetheless, this latter study by Heidbreder and colleagues also demonstrated that intraperitoneal (i.p.) administration of MPEP (30 mg/kg) increased extracellular levels of norepinephrine while decreasing firing rates of locus coeruleus neurons. These effects are intriguing, as they provide a neurochemical basis by which MPEP produces antidepressant-like effects in rodents [5, 118, 119]; however, it remains to be determined if MPEP-induced elevations of extracellular norepinephrine levels are mediated by a direct action of MPEP on neuronal NETs. Taken together, it appears that, like most neurotransmitter receptor antagonists, the selectivity of MPEP for mGluR5 appears to diminish at concentrations that far exceed its IC50 value (36 nM).
In an effort to reduce the off-target actions of MPEP and improve its potency and selectivity for mGluR5 receptors, Cosford and colleagues synthesized a series of MPEP analogues [115], one of which was 3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]pyridine (MTEP). MTEP appears to be about twice as potent as MPEP in displacing radioligand binding from mGluR5 receptors in rat cortical membranes (Ki = 16 nM vs. 32 nM for MPEP). In addition, MTEP retains selectivity for mGluR5 receptors over mGluR1 receptors at concentrations up to 100 µM, and unlike MPEP, displays no antagonist activity at NR2Bcontaining NMDA receptors at concentrations up to 300 µM [115]. However, MTEP does exert some antagonist activity at MAO-A with an IC50 value of 30 µM. In vivo experiments have shown that the ability of MTEP (16 mg/kg s.c.) to reduce anxiety-like behaviors is absent in mice lacking mGluR5 receptors [120], providing further evidence that MTEP is indeed selective for mGluR5. Other in vivo studies have shown that MTEP reaches CSF concentrations in the brain following a systemic oral administration of 30 mg/kg that are 5 times higher than those obtained after the same dose of MPEP (1 µM for MTEP vs. 0.2 µM for MPEP), although concentration in individual brain structures such as the hippocampus were not different [115]. These data suggest that MTEP has either greater bioavailability after oral administration and/or greater solubility in CSF than MPEP. MTEP also appears to be more potent than MPEP at occupying mGluR5 receptors in vivo, with a dose of 10 mg/kg i.p. MPEP needed to occupy >75% of brain mGluR5 receptors, as opposed to only 3 mg/kg i.p. needed for MTEP [112]. As is the case for MPEP, MTEP appears to be shorter-acting in mice than in rats, with occupancy of >75% of brain mGluR5 receptors being observed for 2 hrs at the aforementioned systemic doses in rats, while this level of receptor occupancy only lasted 15–30 min for these compounds in mice [112]. Thus, MTEP appears to be a more potent, selective and orally bioavailable mGluR5 NAM than MPEP.
In the early 1980’s, a clinical report was published indicating that fenobam, a nonbenzodiazepine compound whose mechanism of action was unknown at the time, demonstrated clinical efficacy in reducing symptoms of anxiety in humans in the early 1980’s [121–123]. However, due to reports of adverse side effects by some patients, further clinical testing was discontinued. However, recently it has come to light that fenobam is actually a selective mGluR5 antagonist with moderate potency and efficacy (Ki=295 nM, IC50=134 nM) [91].
A novel family of benzaldazine compounds has been recently described that possesses distinct allosteric modulatory effects on mGluR5 receptor activity [124]. One of these compounds is (3-methoxyphenyl)methylene]hydrazone-3-methoxybenzaldehyde (DMeOB), an mGluR5 NAM that inhibits glutamate-stimulated Ca2+ in mGluR5 transfected cells with an IC50 value of 3 µM without altering [3H]quisqualate binding to the glutamate binding domain of the receptor. The structure of this compound is shown in Fig. (2).
mGluR2/3 AGONISTS
Most synthetic mGluR2/3 agonists that are used in preclinical addiction research are conformationally constrained analogues of glutamate that act at the glutamate binding site on the receptor. One of the first mGluR2/3 agonists to developed was LY354740 [125, 126], which exhibits an EC50 value of approx 5 and 24 nM in inhibiting forskolin-stimulated cAMP production in cells expressing human mGluR2 and mGluR3, respectively. Later, LY389795 and LY379268 were developed [127], which displayed slightly increased potency as compared to LY354740 in inhibiting forskolin-stimulated cAMP formation (EC50 values approximately 3 and 5 nM at human mGluR2 and mGluR3 receptors, respectively, for LY379268; EC50 values approximately 4 and 8 nM at human mGluR2 and mGluR3 receptors, respectively, for LY389795). More recently, the synthesis and in vitro and in vivo characterization of LY404039 were published [128, 129]. The potency of LY404039 for inhibiting cAMP formation is similar to that observed for LY354740. All of these compounds exhibit excellent brain penetrance and selectivity for mGluR2/3 over other mGluR subtypes.
ANIMAL MODELS OF DRUG ADDICTION
In the latter half of the 20th century, numerous animal models of drug addiction were developed and have proved invaluable in elucidating the neural substrates of addictive behaviors. One of the most widely used methods to study drug addiction in animals is the intravenous self-administration paradigm. In this paradigm, an animal is trained to perform an operant task such as a nose poke or lever press in order to receive an intravenous infusion of a drug solution via an indwelling venous catheter. In the case of ethanol, each operant response results in the presentation of a dilute ethanol solution (usually 8–12% v/v) in a receptacle located near the lever or nose-poke orifice, where the animal can consume it orally. In the progressive ratio paradigm of drug self-administration, the behavioral demand (i.e., number of lever presses) placed on the animal in order for it to receive a single drug reinforcer is gradually increased until the animal stops responding (called the “breakpoint”). Many researchers use environmental cues such as stimulus lights or auditory tones that are paired with the drug presentation or delivery to allow for the formation of Pavlovian drug-stimulus associations, which can subsequently be used in second-order schedules of reinforcement (i.e., responding for the drug-associated stimulus in the absence of primary drug reinforcement) as well as in reinstatement of drug-seeking behavior, a widely used model of relapse. In the reinstatement model, following stabilization of drug self-administration patterns, the animal is subjection to extinction procedures where the drug is no longer available. After extinction criteria have been met, the animal is exposed to drug-associated cues, drug priming, or stressors, which generally cause a reinstatement of operant responding that previously resulted in drug delivery.
Another widely used animal model of drug addiction is the conditioned place preference (CPP) paradigm, where passively administration of a drug is repeatedly paired with a unique contextual environment, and over time the animal exhibits a preference for the drug-paired environment over an environment that has been paired with a neutral pharmacological stimulus (i.e., saline). The CPP paradigm has provided substantial information on the neural substrates of the rewarding effects of drugs of abuse [130–132]. A disadvantage of the CPP technique, however, is that it does not directly measure drug-seeking behavior, but rather the motivation for secondary reinforcers (i.e., drug-associated environments).
A third experimental paradigm used to model drug addiction in animals the sensitization paradigm, whereby repeated passive administration of a drug of abuse results in a progressive and long-lasting increase in the behavioral response to the drug (i.e., increased motor hyperactivity in response to repeated exposure to psychostimulants). It has been theorized that this increased behavioral response reflects an increase in the incentive salience of the drug [133, 134]. However, given that most human drug abusers develop a significant degree of tolerance to the effects of the abused substance, the relevance of the locomotor sensitization to drug addiction in humans is often subject to debate.
EFFECTS OF GROUP I mGluR NEGATIVE ALLOSTERIC MODULATORS AND GROUP II mGluR AGONISTS ON DRUG REINFORCEMENT, REWARD, AND OTHER DRUG-RELATED BEHAVIORS
mGluR1 NEGATIVE ALLOSTERIC MODULATORS
Although high concentrations of mGluR1 receptors and not typically found in forebrain and limbic regions known to be involved in addictive behaviors (see Table 1), a handful of studies have shown significant effects of selective mGluR1 NAMs on drug- or ethanol-related behaviors. For example, in rats trained to self-administer nicotine intravenously, it was shown that the selective mGluR1 NAM EMQMCM at a dose of 5 mg/kg inhibited cue and nicotine-induced reinstatement of nicotine-seeking behavior [135]. However, higher doses of this compound were shown to inhibit cue-induced reinstatement of food-seeking, indicating that this compound at higher doses may have general inhibitory effects on appetitive responding. Nonetheless, these findings demonstrate a potential role for mGluR1 receptors in relapse-like behaviors related to nicotine addiction, which are especially important in light of findings that nicotine exposure up-regulates the expression of mGluR1 mRNA in addiction-related brain regions such as the ventral tegmental area (VTA) and amygdala [136]. There also reports that EMQMCM inhibits the expression of behavioral (i.e., locomotor) sensitization to both morphine and cocaine [137, 138]. Clearly, additional studies are needed to further characterize the ability of selective mGluR1 NAMs on the drug reward, reinforcement and relapse with respect to other abused substances such as cocaine, methamphetamine and heroin.
Of the few studies that have been published on the effects of mGluR1 antagonists on ethanol-related behaviors, there exists some degree of controversy. Hodge and colleagues showed that the mGluR1 NAM CPCCOEt at doses up to 10 mg/kg i.p. had no effect on oral ethanol self-administration in ethanol-preferring P rats and C57BL/6J mice [139, 140]. In contras., Lominac et al. [105] showed that in C57BL/6J mice, similar doses of CPCCOEt reduced ethanol reinforcement, consumption, and expression of ethanol CPP while facilitating the motor-impairing effects of ethanol. Lominac and colleagues also showed that CPCCOEt suppressed acute ethanol-stimulated increases in extracellular levels of dopamine and glutamate in the nucleus accumbens, while potentiating the effects of acute ethanol on extracellular GABA in this region. The reasons underlying the discordant effects of CPCCOEt on ethanol reinforcement and consumption between these reports are currently unclear, but are likely due to numerous procedural differences between these studies, as discussed in [105]. More recently, Hodge and colleagues have demonstrated that the highly potent mGluR1 NAM JNJ16259685 reduces operant ethanol self-administration at doses of 0.1, 0.3 and 1 mg/kg i.p. [141] as well as breakpoints for ethanol reinforcement in a progressive ratio paradigm [142]. However, the high dose of JNJ16259685 also reduced sucrose-reinforced responding, and the authors noted that this compound also decreased horizontal locomotor activity [141]. This, motor side effects of this particular compound may have contributed to the reductions in ethanol self-administration and breakpoints for ethanol reinforcement.
mGluR5 NEGATIVE ALLOSTERIC MODULATORS
Perhaps the most influential publication suggesting a role of mGluR5 receptors in addiction was published by Chiamulera and colleagues in 2001 [143]. These investigators demonstrated that mice carrying a targeted deletion of the mGluR5 receptor gene do not demonstrate hyperlocomotion in response to acute administration of various doses of cocaine (10, 20 or 40 mg/kg i.p.), and fail to acquire intravenous self-administration of cocaine. This phenomenon was not due to a deficit the ability to learn an operant task, as lever pressing for food was unaltered in these mice. These investigators further confirmed a role for mGluR5 receptors in cocaine self-administration by demonstrating that intravenous administration of MPEP also dose-dependently reduced cocaine self-administration, without producing non-specific motor effects (as indicated by a lack of alteration in the rate of lever pressing). It was also shown that mGluR5-deficient mice exhibited normal increases in extracellular dopamine levels in the nucleus accumbens following an acute injection of cocaine, and that radioligand binding to the dopamine transporter and D1 and D2 dopamine receptors was unaltered in the brains of these mice, all of which indicate normal mesolimbic dopamine function. This paper provided the first compelling evidence that either genetic or pharmacological inhibition of mGluR5 receptor function reduces cocaine self-administration as well as cocaine-induced hyperlocomotion, suggesting that inhibition of mGluR5 function may prove useful in treating cocaine addiction in humans. More recent studies have shown that mice lacking mGluR5 also exhibit reduced ethanol consumption, but in contrast to cocaine, exhibit increased behavioral sensitivity to ethanol [144, 145].
Using pharmacological methods, others have demonstrated that mGluR5 NAMs such as MPEP and MTEP attenuate intravenous self-administration of cocaine, nicotine, and heroin [146–154] and reduce voluntary ethanol consumption in a variety of rodent strains [105, 140, 142, 155–159] without altering food reinforcement [146, 148, 149, 152]. MPEP also reduces breakpoints for cocaine, nicotine and ethanol reinforcement under progressive ratio schedules of reinforcement [142, 160]; however, attenuation of breakpoints for food reinforcement by MPEP have also been reported [160], suggesting that MPEP may also reduce the reinforcing efficacy of natural rewards under conditions of increased behavioral demands. MPEP has also been shown to prevent reinstatement of cocaine, nicotine or ethanol-seeking behavior induced by acute drug priming [148, 151] or drug-associated environmental cues [149, 155, 161, 162]. MPEP also enhances the sedative/hypnotic properties of ethanol [163], and mGluR5 receptors appear to interact with adenosine A2A receptors in their ability to regulate ethanol self-administration [164]. A recent microinjection study revealed that local infusion of MPEP into the nucleus accumbens core also attenuates cue-induced reinstatement of cocaine-seeking behavior [165].
MPEP at doses up to 9 mg/kg has also been shown to elevated thresholds for intracranial electrical self-stimulation [150, 166], indicating that negative allosteric modulation of mGluR5 receptors negatively influences brain reward function, which may underlie the ability of mGluR5 antagonists to suppress active drug self-administration. However, evidence against this hypothesis comes from reports that MPEP does not alter nicotine- or cocaine-induced lowering of brain reward stimulation thresholds [147, 166] or food reinforcement. An alternative explanation for the ability of mGluR5 NAMs to reduce drug or ethanol self-administration is that mGluR5 NAMs alter the discriminative stimulus (i.e., subjective) effects of various drugs of abuse, as has been demonstrated for cocaine [151], nicotine [167] and ethanol [168, 169].
Consistent with the findings of Chiamulera and colleagues [143], data from our laboratory have confirmed that pharmacological inhibition of mGluR5 receptor function with MPEP can reduce or completely prevent the hyperlocomotion produced by acute administration of cocaine or D-amphetamine in DBA/2J mice [170]. These findings were later replicated by another group of investigators in rats [171]. However, reductive effects of MPEP on amphetamine-induced locomotor activity were not observed in a different mouse strain (OF1/IC) after oral administration of similar doses of MPEP [172], suggesting that pharmacokinetic, bioavailability and/or genetic factors may influence the effects of mGluR5 NAMs on amphetamine-induced hyperlocomotion. Despite the findings that MPEP attenuates acute cocaine-stimulated hyperlocomotion, it has been shown that MTEP (at doses up to 10 mg/kg i.p.) does not prevent the expression of sensitization to the locomotor stimulant properties of repeatedly administered cocaine [137], but does prevent the expression of locomotor sensitization to morphine [138]. Thus, neuroadaptations produced by repeated cocaine exposure may eliminate the ability of mGluR5 NAMs to attenuate the behavioral sensitizing effects of cocaine.
Work from our laboratory has also demonstrated that MPEP, at doses up to 20 mg/kg i.p., prevents the development of cocaine CPP in mice [173], suggesting that mGluR5 receptors are also involved in the acute conditioned rewarding effects of cocaine. When used as the conditioning drug, our group and others have found that MPEP alone does not produce CPP or CPA in mice or rats [173–175], indicating that MPEP does not possess intrinsic rewarding or aversive effects. However, we found that MPEP did not inhibit the development of CPP to other drugs of abuse such as amphetamine, morphine, nicotine or ethanol [173], suggesting that the ability of MPEP to inhibit the development of cocaine CPP was not due to the production of a learning deficit, resulting in an inability of the animal to associate the subjective effects of the drug with the conditioning environment. However, others have found that MPEP attenuates the expression of CPP for amphetamine [176], ethanol [105] and morphine [174, 175], but interestingly, not cocaine [171]. Together, these data suggest that mGluR5 receptors are involved in some, but not all, aspects of the conditioned rewarding effects of various drugs of abuse. With respect to cocaine and ethanol, there appears to be a disconnect between the role of mGluR5 receptors in the development versus expression of drug CPP. These data underscore the notion that pharmacological manipulations of CPP can yield results that are highly divergent from those obtained using operant self-administration paradigms.
There are a handful of studies indicating that mGluR5 NAMs may be beneficial in the treatment of opiate withdrawal symptoms. Both MPEP and MTEP were shown to ameliorate the behavioral signs of withdrawal from morphine [138, 177, 178]. However MPEP was recently shown to actually exacerbate the somatic signs of nicotine withdrawal [152], and reduces handling-induced convulsions induced by ethanol withdrawal in female but not male mice [145, 179]. Thus, mGluR5 NAMs may be of potential therapeutic benefit in the treatment of symptoms of opiate withdrawal but may be of limited use in amelioration of nicotine or ethanol withdrawal symptoms.
mGluR2/3 AGONISTS
Stimulation of inhibitory presynaptic mGluR2/3 autoreceptors, which results in decreased release of glutamate from the presynaptic terminal, is another pharmacological method to dampen glutamate transmission. One of the first demonstrations that mGluR2/3 agonists might possess therapeutic efficacy in the treatment of drug addiction was published in 1997 by Helton and colleagues, who showed that LY354740 suppressed behavioral signs of nicotine withdrawal in rats [180]. However, it was not until several years later when it was demonstrated that mGluR2/3 agonists reduce active drug reinforcement and drug-seeking behavior. In 2004, Baptista and colleagues showed that LY379268 (at doses up to 3 mg/kg i.p.) suppressed self-administration of cocaine but not a conventional reinforcer (sweetened condensed milk) in rats [181]. These effects on intravenous cocaine self-administration have subsequently been replicated by other investigators in both rats and squirrel monkeys [182, 183]. However, while Baptista et al. showed that LY379268 also attenuated cue-induced reinstatement of cocaine-seeking behavior, this compound also suppressed reinstatement of seeking of the conventional reinforcer. This study suggests that LY379268 has selective effects on cocaine reinforcement but its effects on natural reinforcers become apparent under cue-induced reinstatement conditions.
It has since been shown that mGluR2/3 agonists also attenuate the reinforcing effects of nicotine and ethanol [184, 185] as well as cue-, stress- or drug-primed reinstatement [183, 184, 186]. Interestingly, however, Rodd and colleagues [187] demonstrated that a different mGluR2/3 agonist, LY404039, suppressed cue-induced reinstatement of ethanol-seeking behavior but not active ethanol self-administration. The reason for the lack of effect on LY404039 on active ethanol self-administration are unclear, but may be due to the use of a high ethanol-preferring P rat strain as opposed to outbred rats used in other studies. Systemic administration of LY379268 has also been shown to attenuate the phenomenon of “incubation” of cocaine craving [188], which is characterized by a progressive increase in the magnitude of cue-induced reinstatement over time following extinction [189, 190].
Some of the aforementioned effects of mGluR2/3 agonists on drug and ethanol reinforcement or reinstatement must be interpreted with caution, however, since higher doses (3–5 mg/kg i.p.) of LY379268 have been shown to decrease spontaneous locomotor activity [184], food reinforcement [182] but see [186] as well as responding for conventional reinforcers such as sweetened solutions [187].
Studies imploying intracerebral microinjection techniques have revealed some of the neural circuitry involved in regulation of drug-reinforcement or reinstatement of drug-seeking behavior by Group II mGluRs. Infusion of LY379268 into the ventral tegmental area attenuates nicotine self-administration [185] as well as contextual cue-induced reinstatement of heroin-seeking [191]. Infusion of this ligand into the nucleus accumbens core attenuates cocaine, nicotine, and food reinforcement [182, 185], while infusion into the nucleus accumbens core or shell reduces context-induced reinstatement of heroin seeking without affecting sucrose seeking [192]. Finally, it was recently demonstrated that infusions of LY379268 into the central nucleus of the amygdala attenuates the “incubation” of cocaine craving [188]. Thus, not surprisingly, regions of the extended amygdala and mesolimbic reward circuitry are critical for regulation of drug-seeking and relapse-like behaviors by mGluR2/3 agonists.
DELETERIOUS EFFECTS OF GROUP I mGluR ANTAGONISTS ON LEARNING AND MEMORY – A STUMBLING BLOCK IN THE ROAD TO THERAPEUTIC APPLICATION?
As depicted in Fig. 1, Group I mGluR receptors, particular mGluR5, are structurally linked to NMDA receptors by various scaffolding proteins (such as Homer, Shank and GKAP), and positively modulate NMDA receptor function (reviewed elsewhere in [48, 193, 194]). In accord with this well-established positive coupling between Group I mGluRs and NMDA receptors, there is a wealth of evidence suggesting that these receptors play an important role in NMDA-dependent synaptic plasticity and learning and memory processes [33, 195]. As a result, numerous studies have shown that pharmacological inhibition of Group I mGluR function or genetic deletion of the mGluR1 or mGluR5 receptor gene produces performance decrements in various learning and memory tasks in rodents such as the Morris water maze [196, 197], radial arm maze [198–203], 5-choice serial reaction time task [204, 205], contextual and auditory conditioning paradigms [196, 206–211], spatial alternation tasks [212, 213], and passive/inhibitory avoidance tasks [214–217]. However, there are reports that inhibition of mGluR5 function has no effect on performance in a spatial reward-finding task [174, 218], the acquisition of food reinforcement [143] or in the development of the conditioned rewarding effects of morphine, ethanol, nicotine or amphetamine in the CPP paradigm [173]. Thus, inhibition of Group I mGluR receptor function does not eliminate all forms of learning and memory, and many observed effects may be dependent on the type of paradigm used or the particular phase of the task examined (acquisition, expression, consolidation, etc.).
Along these lines, some investigators have reported that mGluR1 NAMs actually enhance performance on some learning or short-term memory or conditioning tasks [219–221]. For example, EMQMCM has been shown to improve working memory and reduce impulsive choice-making in rodents [222], providing support for the notion that mGluR1 NAMs may be potential cognitive enhancing agents. However, given the number of other studies showing deleterious effects of mGluR5 NAMs on learning and memory tasks in rodents, it is possible that such ligands, if ever brought to clinical testing in humans for the treatment of addiction, may cause dose-related unwanted side effects such as amnesia.
SUMMARY AND CONCLUDING REMARKS
The data from the animal studies reviewed in this article strongly suggest that attenuation of glutamatergic neurotransmission by Group I mGluR NAMs or Group II mGluR agonists may be novel and effective therapeutics in the treatment of addiction to cocaine, opiates, nicotine, or ethanol. Perhaps one of the most attractive aspects of these ligands is their apparent beneficial effects in preclinical models of anxiety, depression and chronic pain, conditions that are often co-morbid with substance use disorders.
However, to date there are no clinical studies on the efficacy of Group I mGluR NAMs or Group II mGluR agonists in treating addictive disorders. This lack of clinical data makes it difficult to speculate what the precise therapeutic scope of use of such ligands would antagonists might be in human drug addicts. Would such ligands be most efficacious in primarily reducing basal drug intake or the more problematic phenomenon of relapse? If the latter is the case, will such ligands be most effective in preventing relapse triggered by drug-associated environmental cues, stress, the drug itself, or spontaneous relapse? Will mGluR ligands reduce the incidence drug craving, which is primarily a subjective measure that is difficult to model in animals? Could such drugs be used in in-patient populations during acute detoxification as well as in outpatient populations with psychosocial support networks? Are there any demographic characteristics, biomarkers, or pharmacogenetic screens that might be used to predict responsiveness to these mGluR ligands?
The answers to these questions may be generated sometime in the near future. Addex Pharmaceuticals (Geneva, Switzerland) has recently completed Phase IIa clinical trials on its mGluR5 NAM ADX10059 for the treatment of migraine, anxiety and gastroesophageal reflux disease, and is currently conducting Phase I clinical trials on another mGluR5 NAM, ADX48621, for depression and anxiety [223]. Similarly, Eli Lilly (Indianpolis, IN) has conducted clinical studies with positive outcomes on its mGluR2/3 agonist LY354740 in the treatment of anxiety [224, 225] and its LY404309 pro-drug LY2140023 in the treatment of schizophrenia [226]. Should these compounds eventually gain approval by the Food and Drug Administration, there is a strong scientifically-based rationale for testing these ligands in the treatment of drug addiction and alcoholism.
Other unanswered questions about the potential use of mGluR ligands in the treatment addiction pertain to their neurobiological actions. Does the mere acute suppression of glutamate transmission by inhibition of postsynaptic Group I mGluRs or stimulation of presynaptic mGluR2/3 autoreceptors mediate the ability of mGluR ligands to attenuate drug reinforcement and relapse? Or are there neuroadaptations at the cellular and molecular levels (i.e., changes in intracellular enzyme activity, gene expression, or synaptic plasticity) induced by these compounds that underlie their potential efficacy? For example, while numerous studies have shown that mGluR5 NAMs reduce ethanol consumption, data from the author’s laboratory has shown that the epsilon isoform of protein kinase C is an important downstream signaling target that is required for the ability of mGluR5 NAMs to reduce ethanol intake [158]. Further research is needed to identify cellular and molecular signaling components that may be essential for the potential anti-addictive properties of Group I NAMs and Group II agonists. In addition, the neuroanatomical loci where these drugs act to produce their potential beneficial effects have only partially been characterized and found to be located within the mesolimbic dopamine reward circuitry [165, 182, 191, 192]. Additional neuroanatomical mapping of the effects of these mGluR ligands on drug self-administration and reinstatement is needed to further characterize the neural circuitry involved in regulation of addictive behaviors by mGluR ligands.
KEY LEARNING OBJECTIVES
To provide the reader with an overview of glutamatergic neurotransmission and the classification of ionotropic and metabotropic glutamate receptors
To summarize the neuroanatomical distribution of Group I and Group II metabotropic glutamate receptors in the brain
To review animal studies suggesting potential promise for metabotropic glutamate receptor ligands in the treatment of drug addiction and alcoholism
FUTURE RESEARCH QUESTIONS
Will Group I mGluR antagonists and Group II mGluR agonists be beneficial in the treatment of drug addiction and alcoholism in humans?
Will such ligands be devoid of serious side effects?
Will such ligands reduce subjective measures of drug or ethanol craving, which are empirically difficult to measure in animal models?
Will such ligands promote abstinence even following the discontinuation of the medication?
ACKNOWLEDGEMENTS
M.F.O. is supported by a Public Health Service grants AA013852 from the National Institute on Alcohol Abuse and Alcoholism and DA024355 from the National Institute on Drug Abuse. The author has no conflicts of interest to declare.
REFERENCES
- 1.Tzschentke TM, Schmidt WJ. Glutamatergic mechanisms in addiction. Mol Psychiatry. 2003;8:373–382. doi: 10.1038/sj.mp.4001269. [DOI] [PubMed] [Google Scholar]
- 2.Kalivas PW. Glutamate systems in cocaine addiction. Curr Opin Pharmacol. 2004;4:23–29. doi: 10.1016/j.coph.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 3.Kalivas PW, Volkow N, Seamans J. Unmanageable motivation in addiction: a pathology in prefrontal-accumbens glutamate transmission. Neuron. 2005;45:647–650. doi: 10.1016/j.neuron.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 4.Gass JT, Olive MF. Glutamatergic substrates of drug addiction and alcoholism. Biochem Pharmacol. 2008;75:218–265. doi: 10.1016/j.bcp.2007.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spooren W, Ballard T, Gasparini F, Amalric M, Mutel V, Schrieber R. Insight into the function of Group I and Group II metabotropic glutamate (mGlu) receptors: behavioural characterization and implications for the treatment of CNS disorders. Behav Pharmacol. 2003;14:257–277. doi: 10.1097/01.fbp.0000081783.35927.8f. [DOI] [PubMed] [Google Scholar]
- 6.Kew JNC. Positive and negative allosteric modulation of metabotropic glutamate receptors: emerging therapeutic potential. Pharmacol Ther. 2004;104:233–244. doi: 10.1016/j.pharmthera.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 7.Marek GJ. Metabotropic glutamate 2/3 receptors as drug targets. Curr Opin Pharmacol. 2004;4:18–22. doi: 10.1016/j.coph.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 8.Ritzen A, Mathiesen JM, Thomsen C. Molecular pharmacology and therapeutic prospects of metabotropic glutamate receptor allosteric modulators. Basic Clin Pharmacol Toxicol. 2005;97:202–213. doi: 10.1111/j.1742-7843.2005.pto_156.x. [DOI] [PubMed] [Google Scholar]
- 9.Slassi A, Isaac M, Edwards L, et al. Recent advances in non-competitive mGlu5 receptor antagonists and their potential therapeutic applications. Curr Top Med Chem. 2005;5:897–911. doi: 10.2174/1568026054750236. [DOI] [PubMed] [Google Scholar]
- 10.Swanson CJ, Bures M, Johnson MP, Linden AM, Monn JA, Schoepp DD. Metabotropic glutamate receptors as novel targets for anxiety and stress disorders. Nat Rev Drug Discov. 2005;4:131–144. doi: 10.1038/nrd1630. [DOI] [PubMed] [Google Scholar]
- 11.Marino MJ, Conn PJ. Glutamate-based therapeutic approaches: allosteric modulators of metabotropic glutamate receptors. Curr Opin Pharmacol. 2006;6:98–102. doi: 10.1016/j.coph.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 12.Palucha A. Are compounds acting at metabotropic glutamate receptors the answer to treating depression? Expert Opin Investig Drugs. 2006;15:1545–1553. doi: 10.1517/13543784.15.12.1545. [DOI] [PubMed] [Google Scholar]
- 13.Palucha A, Pilc A. Metabotropic glutamate receptor ligands as possible anxiolytic and antidepressant drugs. Pharmacol Ther. 2007;115:116–147. doi: 10.1016/j.pharmthera.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 14.Witkin JM, Marek GJ, Johnson BG, Schoepp DD. Metabotropic glutamate receptors in the control of mood disorders. CNS Neurol Disord Drug Targets. 2007;6:87–100. doi: 10.2174/187152707780363302. [DOI] [PubMed] [Google Scholar]
- 15.Conn PJ, Pin J-P. Pharmacology and functions of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol. 1997;37:205–237. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- 16.Pin JP, Duvoisin R. The metabotropic glutamate receptors: structure and functions. Neuropharmacology. 1995;34:1–26. doi: 10.1016/0028-3908(94)00129-g. [DOI] [PubMed] [Google Scholar]
- 17.Hermans E, Challiss RA. Structural, signalling and regulatory properties of the group I metabotropic glutamate receptors: prototypic family C G-protein-coupled receptors. Biochem J. 2001;359:465–484. doi: 10.1042/0264-6021:3590465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shigemoto R, Mizuno N. In: Handbook of Chemical Neuroanatomy: Metabotropic Glutamate Receptors: Immunocytochemical and In Situ Hybridization Analyses. Ottersen OP, Storm-Mathisen J, editors. London: Elsevier; 2000. pp. 63–98. [Google Scholar]
- 19.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th Edition. Washington DC: American Psychiatric Press; 1994. [Google Scholar]
- 20.Olmstead MC. Animal models of drug addiction: where do we go from here? Q J Exp Psychol. 2006;59:625–653. doi: 10.1080/17470210500356308. [DOI] [PubMed] [Google Scholar]
- 21.Kalivas PW, Peters J, Knackstedt L. Animal models and brain circuits in drug addiction. Mol Interv. 2006;6:339–344. doi: 10.1124/mi.6.6.7. [DOI] [PubMed] [Google Scholar]
- 22.Epstein DH, Preston KL, Stewart J, Shaham Y. Toward a model of drug relapse: an assessment of the validity of the reinstatement procedure. Psychopharmacology. 2006;189:1–16. doi: 10.1007/s00213-006-0529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sanchis-Segura C, Spanagel R. Behavioural assessment of drug reinforcement and addictive features in rodents: an overview. Addict Biol. 2006;11:2–38. doi: 10.1111/j.1369-1600.2006.00012.x. [DOI] [PubMed] [Google Scholar]
- 24.Lerma J, Morales M, Vicente MA, Herreras O. Glutamate receptors of the kainate type and synaptic transmission. Trends Neurosci. 1997;20:9–12. doi: 10.1016/S0166-2236(96)20055-4. [DOI] [PubMed] [Google Scholar]
- 25.Cull-Candy S, Brickley S, Farrant M. NMDA receptor subunits: diversity, development and disease. Curr Opin Neurobiol. 2001;11:327–335. doi: 10.1016/s0959-4388(00)00215-4. [DOI] [PubMed] [Google Scholar]
- 26.Cull-Candy S, Kelly L, Farrant M. Regulation of Ca2+-permeable AMPA receptors: synaptic plasticity and beyond. Curr Opin Neurobiol. 2006;16:288–297. doi: 10.1016/j.conb.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 27.Papadia S, Hardingham GE. The dichotomy of NMDA receptor signaling. Neuroscientist. 2007;13:572–579. doi: 10.1177/10738584070130060401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev. 1999;51:7–62. [PubMed] [Google Scholar]
- 29.Stephenson FA. Structure and trafficking of NMDA and GABAA receptors. Biochem Soc Trans. 2006;34:877–881. doi: 10.1042/BST0340877. [DOI] [PubMed] [Google Scholar]
- 30.Paoletti P, Neyton J. NMDA receptor subunits: function and pharmacology. Curr Opin Pharmacol. 2007;7:39–47. doi: 10.1016/j.coph.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 31.Garcia-Junco-Clemente P, Linares-Clemente P, Fernandez-Chacon R. Active zones for presynaptic plasticity in the brain. Mol Psychiatry. 2005;10:185–200. doi: 10.1038/sj.mp.4001628. [DOI] [PubMed] [Google Scholar]
- 32.Castellano C, Cestari V, Ciamei A. NMDA receptors and learning and memory processes. Curr Drug Targets. 2001;2:273–283. doi: 10.2174/1389450013348515. [DOI] [PubMed] [Google Scholar]
- 33.Riedel G, Platta B, Micheaub J. Glutamate receptor function in learning and memory. Behav Brain Res. 2003;140:1–47. doi: 10.1016/s0166-4328(02)00272-3. [DOI] [PubMed] [Google Scholar]
- 34.Perez-Otano I, Ehlers MD. Homeostatic plasticity and NMDA receptor trafficking. Trends Neurosci. 2005;28:229–238. doi: 10.1016/j.tins.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 35.Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 36.Malenka RC, Nicoll RA. Long-term potentiation--a decade of progress? Science. 1999;285:1870–1874. doi: 10.1126/science.285.5435.1870. [DOI] [PubMed] [Google Scholar]
- 37.Song I, Huganir RL. Regulation of AMPA receptors during synaptic plasticity. Trends Neurosci. 2002;25:578–588. doi: 10.1016/s0166-2236(02)02270-1. [DOI] [PubMed] [Google Scholar]
- 38.Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 39.Cooke SF, liss TV. Plasticity in the human central nervous system. Brain. 2006;129:1659–1673. doi: 10.1093/brain/awl082. [DOI] [PubMed] [Google Scholar]
- 40.Liu SJ, Zukin RS. Ca2+-permeable AMPA receptors in synaptic plasticity and neuronal death. Trends Neurosci. 2007;30:126–134. doi: 10.1016/j.tins.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 41.Derkach VA, Oh MC, Guire ES, Soderling TR. Regulatory mechanisms of AMPA receptors in synaptic plasticity. Nat Rev Neurosci. 2007;8:101–113. doi: 10.1038/nrn2055. [DOI] [PubMed] [Google Scholar]
- 42.Huettner JE. Kainate receptors and synaptic transmission. Prog Neurobiol. 2003;70:387–407. doi: 10.1016/s0301-0082(03)00122-9. [DOI] [PubMed] [Google Scholar]
- 43.Coutinho V, Knopfel T. Metabotropic glutamate receptors: electrical and chemical signaling properties. Neuroscientist. 2002;8:551–561. doi: 10.1177/1073858402238514. [DOI] [PubMed] [Google Scholar]
- 44.Brakeman PR, Lanahan AA, O'Brien R, et al. Homer: a protein that selectively binds metabotropic glutamate receptors. Nature. 1997;386:284–288. doi: 10.1038/386284a0. [DOI] [PubMed] [Google Scholar]
- 45.Tu JC, Xiao B, Naisbitt S, et al. Coupling of mGluR/Homer and PSD-95 complexes by the Shank family of postsynaptic density proteins. Neuron. 1999;23:583–592. doi: 10.1016/s0896-6273(00)80810-7. [DOI] [PubMed] [Google Scholar]
- 46.Xiao B, Tu JC, Worley PF. Homer: a link between neural activity and glutamate receptor function. Curr Opin Neurobiol. 2000;10:370–374. doi: 10.1016/s0959-4388(00)00087-8. [DOI] [PubMed] [Google Scholar]
- 47.de Bartolomeis A, Iasevoli F. The Homer family and the signal transduction system at glutamatergic postsynaptic density: potential role in behavior and pharmacotherapy. Psychopharmacol Bull. 2003;37:51–83. [PubMed] [Google Scholar]
- 48.Szumlinski KK, Kalivas PW, Worley PF. Homer proteins: implications for neuropsychiatric disorders. Curr Opin Neurobiol. 2006;16:251–257. doi: 10.1016/j.conb.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 49.Schoepp DD. Unveiling the functions of presynaptic metabotropic glutamate receptors in the central nervous system. J Pharmacol Exp Ther. 2001;299:12–20. [PubMed] [Google Scholar]
- 50.Shigeri Y, Seal RP, Shimamoto K. Molecular pharmacology of glutamate transporters, EAATs and VGLUTs. Brain Res Rev. 2004;45:250–265. doi: 10.1016/j.brainresrev.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 51.Melendez RI, Vuthiganon J, Kalivas PW. Regulation of extracellular glutamate in the prefrontal cortex: focus on the cystine glutamate exchanger and group I metabotropic glutamate receptors. J Pharmacol Exp Ther. 2005;314:139–147. doi: 10.1124/jpet.104.081521. [DOI] [PubMed] [Google Scholar]
- 52.McBean GJ. Cerebral cystine uptake: a tale of two transporters. Trends Pharmacol Sci. 2002;23:299–302. doi: 10.1016/s0165-6147(02)02060-6. [DOI] [PubMed] [Google Scholar]
- 53.Baker DA, Shen H, Kalivas PW. Cystine/glutamate exchange serves as the source for extracellular glutamate: modifications by repeated cocaine administration. Amino Acids. 2002;23:161–162. doi: 10.1007/s00726-001-0122-6. [DOI] [PubMed] [Google Scholar]
- 54.Moran MM, McFarland K, Melendez RI, Kalivas PW, Seamans JK. Cystine/glutamate exchange regulates metabotropic glutamate receptor presynaptic inhibition of excitatory transmission and vulnerability to cocaine seeking. J Neurosci. 2005;25:6389–6393. doi: 10.1523/JNEUROSCI.1007-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Martin LJ, Blackstone CD, Huganir RL, Price DL. Cellular localization of a metabotropic glutamate receptor in rat brain. Neuron. 1992;9:259–270. doi: 10.1016/0896-6273(92)90165-a. [DOI] [PubMed] [Google Scholar]
- 56.Shigemoto R, Nakanishi S, Mizuno N. Distribution of the mRNA for a metabotropic glutamate receptor (mGluR1) in the central nervous system: an in situ hybridization study in adult and developing rat. J Comp Neurol. 1992;322:121–135. doi: 10.1002/cne.903220110. [DOI] [PubMed] [Google Scholar]
- 57.Gorcs TJ, Penke B, Boti Z, Katarova Z, Hamori J. Immunohistochemical visualization of a metabotropic glutamate receptor. Neuroreport. 1993;4:283–286. doi: 10.1097/00001756-199303000-00014. [DOI] [PubMed] [Google Scholar]
- 58.Fotuhi M, Sharp AH, Glatt CE, et al. Differential localization of phosphoinositide-linked metabotropic glutamate receptor (mGluR1) and the inositol 1,4,5-trisphosphate receptor in rat brain. J Neurosci. 1993;13:2001–2012. doi: 10.1523/JNEUROSCI.13-05-02001.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hampson DR, Theriault E, Huang XP, et al. Characterization of two alternatively spliced forms of a metabotropic glutamate receptor in the central nervous system of the rat. Neuroscience. 1994;60:325–336. doi: 10.1016/0306-4522(94)90246-1. [DOI] [PubMed] [Google Scholar]
- 60.Tanabe Y, Nomura A, Masu M, Shigemoto R, Mizuno N, Nakanishi S. Signal transduction, pharmacological properties, and expression patterns of two rat metabotropic glutamate receptors, mGluR3 and mGluR4. J Neurosci. 1993;13:1372–1378. doi: 10.1523/JNEUROSCI.13-04-01372.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ohishi H, Shigemoto R, Nakanishi S, Mizuno N. Distribution of the messenger RNA for a metabotropic glutamate receptor, mGluR2, in the central nervous system of the rat. Neuroscience. 1993;53:1009–1018. doi: 10.1016/0306-4522(93)90485-x. [DOI] [PubMed] [Google Scholar]
- 62.Ohishi H, Shigemoto R, Nakanishi S, Mizuno N. Distribution of the mRNA for a metabotropic glutamate receptor (mGluR3) in the rat brain: an in situ hybridization study. J Comp Neurol. 1993;335:252–266. doi: 10.1002/cne.903350209. [DOI] [PubMed] [Google Scholar]
- 63.Petralia RS, Wang YX, Niedzielski AS, Wenthold RJ. The metabotropic glutamate receptors, mGluR2 and mGluR3, show unique postsynaptic, presynaptic and glial localizations. Neuroscience. 1996;71:949–976. doi: 10.1016/0306-4522(95)00533-1. [DOI] [PubMed] [Google Scholar]
- 64.Ohishi H, Neki A, Mizuno N. Distribution of a metabotropic glutamate receptor, mGluR2, in the central nervous system of the rat and mouse: an immunohistochemical study with a monoclonal antibody. Neurosci Res. 1998;30:65–82. doi: 10.1016/s0168-0102(97)00120-x. [DOI] [PubMed] [Google Scholar]
- 65.Tamaru Y, Nomura S, Mizuno N, Shigemoto R. Distribution of metabotropic glutamate receptor mGluR3 in the mouse CNS: differential location relative to pre- and postsynaptic sites. Neuroscience. 2001;106:481–503. doi: 10.1016/s0306-4522(01)00305-0. [DOI] [PubMed] [Google Scholar]
- 66.Abe T, Sugihara H, Nawa H, Shigemoto R, Mizuno N, Nakanishi S. Molecular characterization of a novel metabotropic glutamate receptor mGluR5 coupled to inositol phosphate/Ca2+ signal transduction. J Biol Chem. 1992;267:13361–13368. [PubMed] [Google Scholar]
- 67.Shigemoto R, Nomura S, Ohishi H, Sugihara H, Nakanishi S, Mizuno N. Immunohistochemical localization of a metabotropic glutamate receptor, mGluR5, in the rat brain. Neurosci Lett. 1993;163:53–57. doi: 10.1016/0304-3940(93)90227-c. [DOI] [PubMed] [Google Scholar]
- 68.Romano C, Sesma MA, McDonald CT, O'Malley K, Van den Pol AN, Olney JW. Distribution of metabotropic glutamate receptor mGluR5 immunoreactivity in rat brain. J Comp Neurol. 1995;355:455–469. doi: 10.1002/cne.903550310. [DOI] [PubMed] [Google Scholar]
- 69.Lujan R, Roberts JD, Shigemoto R, Ohishi H, Somogyi P. Differential plasma membrane distribution of metabotropic glutamate receptors mGluR1α, mGluR2 and mGluR5, relative to neurotransmitter release sites. J Chem Neuroanat. 1997;13:219–241. doi: 10.1016/s0891-0618(97)00051-3. [DOI] [PubMed] [Google Scholar]
- 70.Paquet M, Smith Y. Group I metabotropic glutamate receptors in the monkey striatum: subsynaptic association with glutamatergic and dopaminergic afferents. J Neurosci. 2003;23:7659–7669. doi: 10.1523/JNEUROSCI.23-20-07659.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Muly EC, Maddox M, Smith Y. Distribution of mGluR1α and mGluR5 immunolabeling in primate prefrontal cortex. J Comp Neurol. 2003;467:521–535. doi: 10.1002/cne.10937. [DOI] [PubMed] [Google Scholar]
- 72.Luccini E, Musante V, Neri E, et al. Functional interactions between presynaptic NMDA receptors and metabotropic glutamate receptors co-expressed on rat and human noradrenergic terminals. Br J Pharmacol. 2007;151:1087–1094. doi: 10.1038/sj.bjp.0707280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Condorelli DF, Dell'Albani P, Corsaro M, et al. Metabotropic glutamate receptor expression in cultured rat astrocytes and human gliomas. Neurochem Res. 1997;22:1127–1133. doi: 10.1023/a:1027317319166. [DOI] [PubMed] [Google Scholar]
- 74.van den Pol AN, Romano C, Ghosh P. Metabotropic glutamate receptor mGluR5 subcellular distribution and developmental expression in hypothalamus. J Comp Neurol. 1995;362:134–150. doi: 10.1002/cne.903620108. [DOI] [PubMed] [Google Scholar]
- 75.Yamaguchi S, Nakanishi S. Regional expression and regulation of alternative forms of mRNAs derived from two distinct transcription initiation sites of the rat mGluR5 gene. J Neurochem. 1998;71:60–68. doi: 10.1046/j.1471-4159.1998.71010060.x. [DOI] [PubMed] [Google Scholar]
- 76.Schools GP, Kimelberg HK. mGluR3 and mGluR5 are the predominant metabotropic glutamate receptor mRNAs expressed in hippocampal astrocytes acutely isolated from young rats. J Neurosci Res. 1999;58:533–543. doi: 10.1002/(sici)1097-4547(19991115)58:4<533::aid-jnr6>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 77.Blumcke I, Behle K, Malitschek B, et al. Immunohistochemical distribution of metabotropic glutamate receptor subtypes mGluR1b, mGluR2/3, mGluR4a and mGluR5 in human hippocampus. Brain Res. 1996;736:217–226. doi: 10.1016/0006-8993(96)00697-x. [DOI] [PubMed] [Google Scholar]
- 78.Biber K, Laurie DJ, Berthele A, et al. Expression and signaling of group I metabotropic glutamate receptors in astrocytes and microglia. J Neurochem. 1999;72:1671–1680. doi: 10.1046/j.1471-4159.1999.721671.x. [DOI] [PubMed] [Google Scholar]
- 79.Berthele A, Platzer S, Laurie DJ, et al. Expression of metabotropic glutamate receptor subtype mRNA (mGluR1–8) in human cerebellum. Neuroreport. 1999;10:3861–3867. doi: 10.1097/00001756-199912160-00026. [DOI] [PubMed] [Google Scholar]
- 80.Aronica E, Gorter JA, Ijlst-Keizers H, et al. Expression and functional role of mGluR3 and mGluR5 in human astrocytes and glioma cells: opposite regulation of glutamate transporter proteins. Eur J Neurosci. 2003;17:2106–2118. doi: 10.1046/j.1460-9568.2003.02657.x. [DOI] [PubMed] [Google Scholar]
- 81.Mudo G, Trovato-Salinaro A, Caniglia G, Cheng Q, Condorelli DF. Cellular localization of mGluR3 and mGluR5 mRNAs in normal and injured rat brain. Brain Res. 2007;1149:1–13. doi: 10.1016/j.brainres.2007.02.041. [DOI] [PubMed] [Google Scholar]
- 82.Balazs R, Miller S, Romano C, de Vries A, Chun Y, Cotman CW. Metabotropic glutamate receptor mGluR5 in astrocytes: pharmacological properties and agonist regulation. J Neurochem. 1997;69:151–163. doi: 10.1046/j.1471-4159.1997.69010151.x. [DOI] [PubMed] [Google Scholar]
- 83.Gebremedhin D, Yamaura K, Zhang C, Bylund J, Koehler RC, Harder DR. Metabotropic glutamate receptor activation enhances the activities of two types of Ca2+-activated K+ channels in rat hippocampal astrocytes. J Neurosci. 2003;23:1678–1687. doi: 10.1523/JNEUROSCI.23-05-01678.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Miller S, Kesslak JP, Romano C, Cotman CW. Roles of metabotropic glutamate receptors in brain plasticity and pathology. Ann N Y Acad Sci. 1995;757:460–474. doi: 10.1111/j.1749-6632.1995.tb17506.x. [DOI] [PubMed] [Google Scholar]
- 85.D'Ascenzo M, Fellin T, Terunuma M, et al. mGluR5 stimulates gliotransmission in the nucleus accumbens. Proc Natl Acad Sci U S A. 2007;104:1995–2000. doi: 10.1073/pnas.0609408104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gasparini F, Kuhn R, Pin JP. Allosteric modulators of group I metabotropic glutamate receptors: novel subtype-selective ligands and therapeutic perspectives. Curr Opin Pharmacol. 2002;2:43–49. doi: 10.1016/s1471-4892(01)00119-9. [DOI] [PubMed] [Google Scholar]
- 87.Johnson MP, Nisenbaum ES, Large TH, Emkey R, Baez M, Kingston AE. Allosteric modulators of metabotropic glutamate receptors: lessons learnt from mGlu1, mGlu2 and mGlu5 potentiators and antagonists. Biochem Soc Trans. 2004;32:881–887. doi: 10.1042/BST0320881. [DOI] [PubMed] [Google Scholar]
- 88.Pin JP, Acher F. The metabotropic glutamate receptors: structure, activation mechanism and pharmacology. Curr Drug Targets CNS Neurol Disord. 2002;1:297–317. doi: 10.2174/1568007023339328. [DOI] [PubMed] [Google Scholar]
- 89.Pagano A, Ruegg D, Litschig S, et al. The non-competitive antagonists 2-methyl-6-(phenylethynyl)pyridine and 7-hydroxyiminocyclopropan[b]chromen-1a-carboxylic acid ethyl ester interact with overlapping binding pockets in the transmembrane region of group I metabotropic glutamate receptors. J Biol Chem. 2000;275:33750–33758. doi: 10.1074/jbc.M006230200. [DOI] [PubMed] [Google Scholar]
- 90.Muhlemann A, Diener C, Fischer C, Piussi J, Stucki A, Porter RH. Constitutive activity modulation of human metabotropic glutamate 5a receptors in HEK293 cells: a role for key amino-terminal cysteine residues. Br J Pharmacol. 2005;144:1118–1125. doi: 10.1038/sj.bjp.0706152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Porter RH, Jaeschke G, Spooren W, et al. Fenobam: a clinically validated non-benzodiazepine anxiolytic is a potent, selective and non-competitive mGlu5 receptor antagonist with inverse agonist activity. J Pharmacol Exp Ther. 2005;315:711–721. doi: 10.1124/jpet.105.089839. [DOI] [PubMed] [Google Scholar]
- 92.Goudet C, Gaven F, Kniazeff J, et al. Heptahelical domain of metabotropic glutamate receptor 5 behaves like rhodopsin-like receptors. Proc Natl Acad Sci U S A. 2004;101:378–383. doi: 10.1073/pnas.0304699101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fourgeaud L, Bessis AS, Rossignol F, Pin JP, Olivo-Marin JC, Hemar A. The metabotropic glutamate receptor mGluR5 is endocytosed by a clathrin-independent pathway. J Biol Chem. 2003;278:12222–12230. doi: 10.1074/jbc.M205663200. [DOI] [PubMed] [Google Scholar]
- 94.Ango F, Prezeau L, Muller T, et al. Agonist-independent activation of metabotropic glutamate receptors by the intracellular protein Homer. Nature. 2001;411:962–965. doi: 10.1038/35082096. [DOI] [PubMed] [Google Scholar]
- 95.Suzuki G, Kimura T, Satow A, et al. Pharmacological characterization of a new, orally active and potent allosteric metabotropic glutamate receptor 1 antagonist, 4-[1-(2-fluoropyridin-3-yl)-5-methyl-1H-1,2,3-triazol-4-yl]-N-isopropyl-N- methyl-3,6-dihydropyridine-1(2H)-carboxamide (FTIDC) J Pharmacol Exp Ther. 2007;321:1144–1153. doi: 10.1124/jpet.106.116574. [DOI] [PubMed] [Google Scholar]
- 96.Malherbe P, Kratochwil N, Muhlemann A, et al. Comparison of the binding pockets of two chemically unrelated allosteric antagonists of the mGlu5 receptor and identification of crucial residues involved in the inverse agonism of MPEP. J Neurochem. 2006;98:601–615. doi: 10.1111/j.1471-4159.2006.03886.x. [DOI] [PubMed] [Google Scholar]
- 97.Mundell SJ, Pula G, McIlhinney RA, Roberts PJ, Kelly E. Desensitization and internalization of metabotropic glutamate receptor 1a following activation of heterologous Gq/11-coupled receptors. Biochemistry. 2004;43:7541–7551. doi: 10.1021/bi0359022. [DOI] [PubMed] [Google Scholar]
- 98.Pula G, Mundell SJ, Roberts PJ, Kelly E. Agonist-independent internalization of metabotropic glutamate receptor 1a is arrestin- and clathrin-dependent and is suppressed by receptor inverse agonists. J Neurochem. 2004;89:1009–1020. doi: 10.1111/j.1471-4159.2004.02387.x. [DOI] [PubMed] [Google Scholar]
- 99.Carroll FY, Stolle A, Beart PM, et al. BAY36-7620: a potent non-competitive mGlu1 receptor antagonist with inverse agonist activity. Mol Pharmacol. 2001;59:965–973. [PMC free article] [PubMed] [Google Scholar]
- 100.Prezeau L, Gomeza J, Ahern S, et al. Changes in the carboxyl-terminal domain of metabotropic glutamate receptor 1 by alternative splicing generate receptors with differing agonist-independent activity. Mol Pharmacol. 1996;49:422–429. [PubMed] [Google Scholar]
- 101.Hermans E, Nahorski SR, Challiss RA. Reversible and non-competitive antagonist profile of CPCCOEt at the human type 1α metabotropic glutamate receptor. Neuropharmacology. 1998;37:1645–1647. doi: 10.1016/s0028-3908(98)00132-4. [DOI] [PubMed] [Google Scholar]
- 102.Litschig S, Gasparini F, Rueegg D, et al. CPCCOEt, a noncompetitive metabotropic glutamate receptor 1 antagonist, inhibits receptor signaling without affecting glutamate binding. Mol Pharmacol. 1999;55:453–461. [PubMed] [Google Scholar]
- 103.Clark BP, Baker SR, Goldsworthy J, Harris JR, Kingston AE. (+)-2-Methyl-4-carboxyphenylglycine ( LY367385) selectively antagonises metabotropic glutamate mGluR1 receptors. Bioorg Med Chem Lett. 1997;7:2777–2780. [Google Scholar]
- 104.Chapman AG, Yip PK, Yap JS, et al. Anticonvulsant actions of LY 367385 ((+)-2-methyl-4-carboxyphenylglycine) and AIDA ((RS)-1-aminoindan-1,5-dicarboxylic acid) Eur J Pharmacol. 1999;368:17–24. doi: 10.1016/s0014-2999(99)00014-x. [DOI] [PubMed] [Google Scholar]
- 105.Lominac KD, Kapasova Z, Hannun RA, Patterson C, Middaugh LD, Szumlinski KK. Behavioral and neurochemical interactions between group I mGluR antagonists and ethanol: potential insight into their anti-addictive properties. Drug Alcohol Depend. 2006;85:142–156. doi: 10.1016/j.drugalcdep.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 106.Kohara A, Toya T, Tamura S, et al. Radioligand binding properties and pharmacological characterization of 6-amino-N-cyclohexyl-N,3-dimethylthiazolo[3,2-a]benzimidazole-2-carboxamid e (YM-298198), a high-affinity, selective, and noncompetitive antagonist of metabotropic glutamate receptor type 1. J Pharmacol Exp Ther. 2005;315:163–169. doi: 10.1124/jpet.105.087171. [DOI] [PubMed] [Google Scholar]
- 107.Lavreysen H, Wouters R, Bischoff F, et al. JNJ16259685, a highly potent, selective and systemically active mGlu1 receptor antagonist. Neuropharmacology. 2004;47:961–972. doi: 10.1016/j.neuropharm.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 108.Mabire D, Coupa S, Adelinet C, et al. Synthesis, structure-activity relationship, and receptor pharmacology of a new series of quinoline derivatives acting as selective, noncompetitive mGlu1 antagonists. J Med Chem. 2005;48:2134–2153. doi: 10.1021/jm049499o. [DOI] [PubMed] [Google Scholar]
- 109.Varney MA, Cosford ND, Jachec C, et al. SIB-1757 and SIB-1893: selective, noncompetitive antagonists of metabotropic glutamate receptor type 5. J Pharmacol Exp Ther. 1999;290:170–181. [PubMed] [Google Scholar]
- 110.Gasparini F, Lingenhohl K, Stoehr N, et al. 2-Methyl-6-(phenylethynyl)-pyridine (MPEP), a potent, selective and systemically active mGlu5 receptor antagonist. Neuropharmacology. 1999;38:1493–1503. doi: 10.1016/s0028-3908(99)00082-9. [DOI] [PubMed] [Google Scholar]
- 111.Anderson JJ, Rao SP, Rowe B, et al. [3H]Methoxymethyl-3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]pyridine binding to metabotropic glutamate receptor subtype 5 in rodent brain: in vitro and in vivo characterization. J Pharmacol Exp Ther. 2002;303:1044–1051. doi: 10.1124/jpet.102.040618. [DOI] [PubMed] [Google Scholar]
- 112.Anderson JJ, Bradbury MJ, Giracello DR, et al. In vivo receptor occupancy of mGlu5 receptor antagonists using the novel radioligand [3H]-methoxy-5-(pyridin-2-ylethynyl)pyridine) Eur J Pharmacol. 2003;473:35–40. doi: 10.1016/s0014-2999(03)01935-6. [DOI] [PubMed] [Google Scholar]
- 113.O'Leary DM, Movsesyan V, Vicini S, Faden AI. Selective mGluR5 antagonists MPEP and SIB-1893 decrease NMDA or glutamate-mediated neuronal toxicity through actions that reflect NMDA receptor antagonism. Br J Pharmacol. 2000;131:1429–1437. doi: 10.1038/sj.bjp.0703715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Movsesyan VA, O'Leary DM, Fan L, et al. mGluR5 antagonists 2-methyl-6-(phenylethynyl)-pyridine and (E)-2-methyl-6-(2-phenylethenyl)-pyridine reduce traumatic neuronal injury in vitro and in vivo by antagonizing N-methyl-D-aspartate receptors. J Pharmacol Exp Ther. 2001;296:41–47. [PubMed] [Google Scholar]
- 115.Cosford ND, Tehrani L, Roppe J, et al. 3-[(2-Methyl-1,3-thiazol-4-yl)ethynyl]-pyridine: a potent and highly selective metabotropic glutamate subtype 5 receptor antagonist with anxiolytic activity. J Med Chem. 2003;46:204–206. doi: 10.1021/jm025570j. [DOI] [PubMed] [Google Scholar]
- 116.Mathiesen JM, Svendsen N, Brauner-Osborne H, Thomsen C, Ramirez MT. Positive allosteric modulation of the human metabotropic glutamate receptor 4 (hmGluR4) by SIB-1893 and MPEP. Br J Pharmacol. 2003;138:1026–1030. doi: 10.1038/sj.bjp.0705159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Heidbreder CA, Bianchi M, Lacroix LP, et al. Evidence that the metabotropic glutamate receptor 5 antagonist MPEP may act as an inhibitor of the norepinephrine transporter in vitro and in vivo. Synapse. 2003;50:269–276. doi: 10.1002/syn.10261. [DOI] [PubMed] [Google Scholar]
- 118.Spooren WP, Gasparini F, Salt TE, Kuhn R. Novel allosteric antagonists shed light on mglu5 receptors and CNS disorders. Trends Pharmacol Sci. 2001;22:331–337. doi: 10.1016/s0165-6147(00)01694-1. [DOI] [PubMed] [Google Scholar]
- 119.Kuhn R, Pagano A, Stoehr N, et al. In vitro and in vivo characterization of MPEP, an allosteric modulator of the metabotropic glutamate receptor subtype 5: Review article. Amino Acids. 2002;23:207–211. doi: 10.1007/s00726-001-0130-6. [DOI] [PubMed] [Google Scholar]
- 120.Brodkin J, Bradbury M, Busse C, Warren N, Bristow LJ, Varney MA. Reduced stress-induced hyperthermia in mGluR5 knockout mice. Eur J Neurosci. 2002;16:2241–2244. doi: 10.1046/j.1460-9568.2002.02294.x. [DOI] [PubMed] [Google Scholar]
- 121.Itil TM, Seaman PA, Huque M, et al. The clinical and quantitative EEG effects and plasma levels of fenobam (McN-3377) in subjects with anxiety: an open rising dose tolerance and efficacy study. Curr Ther Res. 1978;24:708–724. [Google Scholar]
- 122.Pecknold JC, McClure DJ, Appeltauer L, Wrzesinski L, Allan T. Treatment of anxiety using fenobam (a nonbenzodiazepine) in a double-blind standard (diazepam) placebo-controlled study. J Clin Psychopharmacol. 1982;2:129–133. [PubMed] [Google Scholar]
- 123.Pecknold JC, McClure DJ, Appeltauer L. Fenobam in anxious outpatients. Curr Ther Res. 1980;27:119–123. [Google Scholar]
- 124.O'Brien JA, Lemaire W, Chen TB, et al. A family of highly selective allosteric modulators of the metabotropic glutamate receptor subtype 5. Mol Pharmacol. 2003;64:731–740. doi: 10.1124/mol.64.3.731. [DOI] [PubMed] [Google Scholar]
- 125.Schoepp DD, Johnson BG, Wright RA, et al. LY354740 is a potent and highly selective group II metabotropic glutamate receptor agonist in cells expressing human glutamate receptors. Neuropharmacology. 1997;36:1–11. doi: 10.1016/s0028-3908(96)00160-8. [DOI] [PubMed] [Google Scholar]
- 126.Monn JA, Valli MJ, Massey SM, et al. Design, synthesis, and pharmacological characterization of (+)-2-aminobicyclo[3.1.0]hexane-2,6-dicarboxylic acid ( LY354740): a potent, selective, and Page 44 orally active group 2 metabotropic glutamate receptor agonist possessing anticonvulsant and anxiolytic properties. J Med Chem. 1997;40:528–537. doi: 10.1021/jm9606756. [DOI] [PubMed] [Google Scholar]
- 127.Monn JA, Valli MJ, Massey SM, et al. Synthesis, pharmacological characterization, and molecular modeling of heterobicyclic amino acids related to (+)-2-aminobicyclo[3.1.0] hexane-2,6-dicarboxylic acid ( LY354740): identification of two new potent, selective, and systemically active agonists for group II metabotropic glutamate receptors. J Med Chem. 1999;42:1027–1040. doi: 10.1021/jm980616n. [DOI] [PubMed] [Google Scholar]
- 128.Rorick-Kehn LM, Johnson BG, Burkey JL, et al. Pharmacological and pharmacokinetic properties of a structurally novel, potent, and selective metabotropic glutamate 2/3 receptor agonist: in vitro characterization of agonist (−)-(1R,4S,5S,6S)-4-amino-2-sulfonylbicyclo[3.1.0]-hexane-4,6-dicarboxylic acid ( LY404039) J Pharmacol Exp Ther. 2007;321:308–317. doi: 10.1124/jpet.106.110809. [DOI] [PubMed] [Google Scholar]
- 129.Rorick-Kehn LM, Johnson BG, Knitowski KM, et al. In vivo pharmacological characterization of the structurally novel, potent, selective mGlu2/3 receptor agonist LY404039 in animal models of psychiatric disorders. Psychopharmacology. 2007;193:121–136. doi: 10.1007/s00213-007-0758-3. [DOI] [PubMed] [Google Scholar]
- 130.Tzschentke TM. Measuring reward with the conditioned place preference paradigm: a comprehensive review of drug effects, recent progress and new issues. Prog Neurobiol. 1998;56:613–672. doi: 10.1016/s0301-0082(98)00060-4. [DOI] [PubMed] [Google Scholar]
- 131.Bardo MT, Bevins RA. Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology. 2000;153:31–43. doi: 10.1007/s002130000569. [DOI] [PubMed] [Google Scholar]
- 132.Tzschentke TM. Measuring reward with the conditioned place preference (CPP) paradigm: update of the last decade. Addict Biol. 2007;12:227–462. doi: 10.1111/j.1369-1600.2007.00070.x. [DOI] [PubMed] [Google Scholar]
- 133.Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- 134.Robinson TE, Berridge KC. The psychology and neurobiology of addiction: an incentive-sensitization view. Addiction. 2000;95 Suppl 2:S91–S117. doi: 10.1080/09652140050111681. [DOI] [PubMed] [Google Scholar]
- 135.Dravolina OA, Zakharova ES, Shekunova EV, Zvartau EE, Danysz W, Bespalov AY. mGlu1 receptor blockade attenuates cue- and nicotine-induced reinstatement of extinguished nicotine self-administration behavior in rats. Neuropharmacology. 2007;52:263–269. doi: 10.1016/j.neuropharm.2006.07.023. [DOI] [PubMed] [Google Scholar]
- 136.Kane JK, Hwang Y, Konu O, Loughlin SE, Leslie FM, Li MD. Regulation of Homer and group I metabotropic glutamate receptors by nicotine. Eur J Neurosci. 2005;21:1145–1154. doi: 10.1111/j.1460-9568.2005.03945.x. [DOI] [PubMed] [Google Scholar]
- 137.Dravolina OA, Danysz W, Bespalov AY. Effects of group I metabotropic glutamate receptor antagonists on the behavioral sensitization to motor effects of cocaine in rats. Psychopharmacology. 2006;187:397–404. doi: 10.1007/s00213-006-0440-1. [DOI] [PubMed] [Google Scholar]
- 138.Kotlinska J, Bochenski M. Comparison of the effects of mGluR1 and mGluR5 antagonists on the expression of behavioral sensitization to the locomotor effect of morphine and the morphine withdrawal jumping in mice. Eur J Pharmacol. 2007;558:113–118. doi: 10.1016/j.ejphar.2006.11.067. [DOI] [PubMed] [Google Scholar]
- 139.Schroeder JP, Overstreet DH, Hodge CW. The mGluR5 antagonist MPEP decreases operant ethanol self-administration during maintenance and after repeated alcohol deprivations in alcohol-preferring (P) rats. Psychopharmacology. 2005;179:262–270. doi: 10.1007/s00213-005-2175-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hodge CW, Miles MF, Sharko AC, et al. The mGluR5 antagonist MPEP selectively inhibits the onset and maintenance of ethanol self-administration in C57BL/6J mice. Psychopharmacology. 2006;183:429–438. doi: 10.1007/s00213-005-0217-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Besheer J, Faccidomo S, Grondin JJ, Hodge CW. Effects of mGlu1-receptor blockade on ethanol self-administration in inbred alcohol-preferring rats. Alcohol. 2008;42:13–20. doi: 10.1016/j.alcohol.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Besheer J, Faccidomo S, Grondin JJ, Hodge CW. Regulation of motivation to self-administer ethanol by mGluR5 in alcohol-preferring (P) rats. Alcohol Clin Exp Res. 2008;32:209–221. doi: 10.1111/j.1530-0277.2007.00570.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chiamulera C, Epping-Jordan MP, Zocchi A, et al. Reinforcing and locomotor stimulant effects of cocaine are absent in mGluR5 null mutant mice. Nat Neurosci. 2001;4:873–874. doi: 10.1038/nn0901-873. [DOI] [PubMed] [Google Scholar]
- 144.Bird MK, Kirchhoff J, Djouma E, Lawrence AJ. Metabotropic glutamate 5 receptors regulate sensitivity to ethanol in mice. Int J Neuropsychopharmacol. doi: 10.1017/S1461145708008572. in press. [DOI] [PubMed] [Google Scholar]
- 145.Blednov YA, Harris RA. Metabotropic glutamate receptor 5 (mGluR5) regulation of ethanol sedation, dependence and consumption: relationship to acamprosate actions. Int J Neuropsychopharmacol. doi: 10.1017/S1461145708008584. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Paterson NE, Semenova S, Gasparini F, Markou A. The mGluR5 antagonist MPEP decreased nicotine self-administration in rats and mice. Psychopharmacology. 2003;167:257–264. doi: 10.1007/s00213-003-1432-z. [DOI] [PubMed] [Google Scholar]
- 147.Kenny PJ, Paterson NE, Boutrel B, et al. Metabotropic glutamate 5 receptor antagonist MPEP decreased nicotine and cocaine self-administration but not nicotine and cocaine-induced facilitation of brain reward function in rats. Ann N Y Acad Sci. 2003;1003:415–418. doi: 10.1196/annals.1300.040. [DOI] [PubMed] [Google Scholar]
- 148.Tessari M, Pilla M, Andreoli M, Hutcheson DM, Heidbreder CA. Antagonism at metabotropic glutamate 5 receptors inhibits nicotine- and cocaine-taking behaviours and prevents nicotine-triggered relapse to nicotine-seeking. Eur J Pharmacol. 2004;499:121–133. doi: 10.1016/j.ejphar.2004.07.056. [DOI] [PubMed] [Google Scholar]
- 149.Bespalov AY, Dravolina OA, Sukhanov I, et al. Metabotropic glutamate receptor (mGluR5) antagonist MPEP attenuated cue- and schedule-induced reinstatement of nicotine self-administration behavior in rats. Neuropharmacology. 2005;49 Suppl 1:167–178. doi: 10.1016/j.neuropharm.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 150.Kenny PJ, Boutrel B, Gasparini F, Koob GF, Markou A. Metabotropic glutamate 5 receptor blockade may attenuate cocaine self-administration by decreasing brain reward function in rats. Psychopharmacology. 2005;179:247–254. doi: 10.1007/s00213-004-2069-2. [DOI] [PubMed] [Google Scholar]
- 151.Lee B, Platt DM, Rowlett JK, Adewale AS, Spealman RD. Attenuation of behavioral effects of cocaine by the metabotropic glutamate receptor 5 antagonist 2-methyl-6-(phenylethynyl)-pyridine in squirrel monkeys: comparison with dizocilpine. J Pharmacol Exp Ther. 2005;312:1232–1240. doi: 10.1124/jpet.104.078733. [DOI] [PubMed] [Google Scholar]
- 152.Liechti ME, Markou A. Interactive effects of the mGlu5 receptor antagonist MPEP and the mGlu2/3 receptor antagonist LY341495 on nicotine self-administration and reward deficits associated with nicotine withdrawal in rats. Eur J Pharmacol. 2006;554:164–174. doi: 10.1016/j.ejphar.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.van der Kam EL, De Vry J, Tzschentke TM. Effect of 2-methyl-6-(phenylethynyl) pyridine on intravenous self-administration of ketamine and heroin in the rat. Behav Pharmacol. 2007;18:717–724. doi: 10.1097/FBP.0b013e3282f18d58. [DOI] [PubMed] [Google Scholar]
- 154.Palmatier MI, Liu X, Donny EC, Caggiula AR, Sved AF. Metabotropic glutamate 5 receptor (mGluR5) antagonists decrease nicotine seeking, but do not affect the reinforcement enhancing effects of nicotine. Neuropsychopharmacology. doi: 10.1038/sj.npp.1301623. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Backstrom P, Bachteler D, Koch S, Hyytia P, Spanagel R. mGluR5 antagonist MPEP reduces ethanol-seeking and relapse behavior. Neuropsychopharmacology. 2004;29:921–928. doi: 10.1038/sj.npp.1300381. [DOI] [PubMed] [Google Scholar]
- 156.McMillen BA, Crawford MS, Kulers CM, Williams HL. Effects of a metabotropic, mglu5, glutamate receptor antagonist on ethanol consumption by genetic drinking rats. Alcohol Alcohol. 2005;40:494–497. doi: 10.1093/alcalc/agh200. [DOI] [PubMed] [Google Scholar]
- 157.Cowen MS, Djouma E, Lawrence AJ. The metabotropic glutamate 5 receptor antagonist 3-[(2-methyl-1,3-thiazol-4-yl)ethynyl)-pyridine reduces ethanol self-administration in multiple strains of alcohol-preferring rats and regulates olfactory glutamatergic systems. J Pharmacol Exp Ther. 2005;315:590–600. doi: 10.1124/jpet.105.090449. [DOI] [PubMed] [Google Scholar]
- 158.Olive MF, McGeehan AJ, Kinder JR, et al. The mGluR5 antagonist 6-methyl-2-(phenylethynyl)pyridine decreases ethanol consumption via a protein kinase Cε-dependent mechanism. Mol Pharmacol. 2005;67:349–355. doi: 10.1124/mol.104.003319. [DOI] [PubMed] [Google Scholar]
- 159.Cowen MS, Krstew E, Lawrence AJ. Assessing appetitive and consummatory phases of ethanol self-administration in C57BL/6J mice under operant conditions: regulation by mGlu5 receptor antagonism. Psychopharmacology. 2007;190:21–29. doi: 10.1007/s00213-006-0583-0. [DOI] [PubMed] [Google Scholar]
- 160.Paterson NE, Markou A. The metabotropic glutamate receptor 5 antagonist MPEP decreased break points for nicotine, cocaine and food in rats. Psychopharmacology. 2005;179:255–261. doi: 10.1007/s00213-004-2070-9. [DOI] [PubMed] [Google Scholar]
- 161.Iso Y, Grajkowska E, Wroblewski JT, et al. Synthesis and structure-activity relationships of 3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]pyridine analogues as potent, noncompetitive metabotropic glutamate receptor subtype 5 antagonists; search for cocaine medications. J Med Chem. 2006;49:1080–1100. doi: 10.1021/jm050570f. [DOI] [PubMed] [Google Scholar]
- 162.Backstrom P, Hyytia P. Ionotropic and metabotropic glutamate receptor antagonism attenuates cue-induced cocaine seeking. Neuropsychopharmacology. 2006;31:778–786. doi: 10.1038/sj.npp.1300845. [DOI] [PubMed] [Google Scholar]
- 163.Sharko AC, Hodge CW. Differential modulation of ethanol-induced sedation and hypnosis by metabotropic glutamate receptor antagonists in C57BL/6J mice. Alcohol Clin Exp Res. 2008;32:67–76. doi: 10.1111/j.1530-0277.2007.00554.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Adams CL, Cowen MS, Short JL, Lawrence AJ. Combined antagonism of glutamate mGlu5 and adenosine A2A receptors interact to regulate alcohol-seeking in rats. Int J Neuropsychopharmacol. 2008;11:229–241. doi: 10.1017/S1461145707007845. [DOI] [PubMed] [Google Scholar]
- 165.Backstrom P, Hyytia P. Involvement of AMPA/kainate, NMDA, and mGlu5 receptors in the nucleus accumbens core in cue-induced reinstatement of cocaine seeking in rats. Psychopharmacology. 2007;192:571–580. doi: 10.1007/s00213-007-0753-8. [DOI] [PubMed] [Google Scholar]
- 166.Harrison AA, Gasparini F, Markou A. Nicotine potentiation of brain stimulation reward reversed by DHβE and SCH 23390, but not by eticlopride, LY 314582 or MPEP in rats. Psychopharmacology. 2002;160:56–66. doi: 10.1007/s00213-001-0953-6. [DOI] [PubMed] [Google Scholar]
- 167.Murray JE, Bevins RA. Behavioral and neuropharmacological characterization of nicotine as a conditional stimulus. Eur J Pharmacol. 2007;561:91–104. doi: 10.1016/j.ejphar.2007.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Besheer J, Hodge CW. Pharmacological and anatomical evidence for an interaction between mGluR5- and GABAA α1-containing receptors in the discriminative stimulus effects of ethanol. Neuropsychopharmacology. 2005;30:747–757. doi: 10.1038/sj.npp.1300616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Besheer J, Stevenson RA, Hodge CW. mGlu5 receptors are involved in the discriminative stimulus effects of self-administered ethanol in rats. Eur J Pharmacol. 2006;551:71–75. doi: 10.1016/j.ejphar.2006.08.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Mcgeehan AJ, Janak PH, Olive MF. Effect of the mGluR5 antagonist 6-methyl-2-(phenylethynyl)pyridine (MPEP) on the acute locomotor stimulant properties of cocaine, d-Page 49 amphetamine, and the dopamine reuptake inhibitor GBR12909 in mice. Psychopharmacology. 2004;174:266–273. doi: 10.1007/s00213-003-1733-2. [DOI] [PubMed] [Google Scholar]
- 171.Herzig V, Schmidt WJ. Effects of MPEP on locomotion, sensitization and conditioned reward induced by cocaine or morphine. Neuropharmacology. 2004;47:973–984. doi: 10.1016/j.neuropharm.2004.07.037. [DOI] [PubMed] [Google Scholar]
- 172.Spooren WP, Vassout A, Neijt HC, et al. Anxiolytic-like effects of the prototypical metabotropic glutamate receptor 5 antagonist 2-methyl-6-(phenylethynyl)pyridine in rodents. J Pharmacol Exp Ther. 2000;295:1267–1275. [PubMed] [Google Scholar]
- 173.Mcgeehan AJ, Olive MF. The mGluR5 antagonist MPEP reduces the conditioned rewarding effects of cocaine but not other drugs of abuse. Synapse. 2003;47:240–242. doi: 10.1002/syn.10166. [DOI] [PubMed] [Google Scholar]
- 174.Popik P, Wrobel M. Morphine conditioned reward is inhibited by MPEP, the mGluR5 antagonist. Neuropharmacology. 2002;43:1210–1217. doi: 10.1016/s0028-3908(02)00309-x. [DOI] [PubMed] [Google Scholar]
- 175.Aoki T, Narita M, Shibasaki M, Suzuki T. Metabotropic glutamate receptor 5 localized in the limbic forebrain is critical for the development of morphine-induced rewarding effect in mice. Eur J Neurosci. 2004;20:1633–1638. doi: 10.1111/j.1460-9568.2004.03609.x. [DOI] [PubMed] [Google Scholar]
- 176.Herzig V, Capuani EM, Kovar KA, Schmidt WJ. Effects of MPEP on expression of food-, MDMA- or amphetamine-conditioned place preference in rats. Addict Biol. 2005;10:243–249. doi: 10.1080/13556210500223272. [DOI] [PubMed] [Google Scholar]
- 177.Palucha A, Branski P, Pilc A. Selective mGlu5 receptor antagonist MTEP attenuates naloxone-induced morphine withdrawal symptoms. Pol J Pharmacol. 2004;56:863–866. [PubMed] [Google Scholar]
- 178.Rasmussen K, Martin H, Berger JE, Seager MA. The mGlu5 receptor antagonists MPEP and MTEP attenuate behavioral signs of morphine withdrawal and morphine-withdrawal-induced activation of locus coeruleus neurons in rats. Neuropharmacology. 2005;48:173–180. doi: 10.1016/j.neuropharm.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 179.Olive MF, Becker HC. Effects of the mGluR2/3 agonist LY379268 and the mGluR5 antagonist MPEP on handling-induced convulsions during ethanol withdrawal in mice. Alcohol. 2008;42:191–197. doi: 10.1016/j.alcohol.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Helton DR, Tizzano JP, Monn JA, Schoepp DD, Kallman MJ. LY354740: a metabotropic glutamate receptor agonist which ameliorates symptoms of nicotine withdrawal in rats. Neuropharmacology. 1997;36:1511–1516. doi: 10.1016/s0028-3908(97)00170-6. [DOI] [PubMed] [Google Scholar]
- 181.Baptista MAS, Martin-Fardon R, Weiss F. Preferential effects of the metabotropic glutamate 2/3 receptor agonist LY379268 on conditioned reinstatement versus primary reinforcement: comparison between cocaine and a potent conventional reinforcer. J Neurosci. 2004;24:4723–4727. doi: 10.1523/JNEUROSCI.0176-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Peters J, Kalivas PW. The group II metabotropic glutamate receptor agonist, LY379268, inhibits both cocaine- and food-seeking behavior in rats. Psychopharmacology. 2006;186:143–149. doi: 10.1007/s00213-006-0372-9. [DOI] [PubMed] [Google Scholar]
- 183.Adewale AS, Platt DM, Spealman RD. Pharmacological stimulation of group II metabotropic glutamate receptors reduces cocaine self-administration and cocaine-induced reinstatement of drug seeking in squirrel monkeys. J Pharmacol Exp Ther. 2006;318:922–931. doi: 10.1124/jpet.106.105387. [DOI] [PubMed] [Google Scholar]
- 184.Backstrom P, Hyytia P. Suppression of alcohol self-administration and cue-induced reinstatement of alcohol seeking by the mGlu2/3 receptor agonist LY379268 and the mGlu8 receptor agonist (S)-3,4-DCPG. Eur J Pharmacol. 2005;528:110–118. doi: 10.1016/j.ejphar.2005.10.051. [DOI] [PubMed] [Google Scholar]
- 185.Liechti ME, Lhuillier L, Kaupmann K, Markou A. Metabotropic glutamate 2/3 receptors in the ventral tegmental area and the nucleus accumbens shell are involved in behaviors relating to nicotine dependence. J Neurosci. 2007;27:9077–9085. doi: 10.1523/JNEUROSCI.1766-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Zhao Y, Dayas CV, Aujla H, Baptista MAS, Martin-Fardon R, Weiss F. Activation of group II metabotropic glutamate receptors attenuates both stress and cue-induced ethanol-seeking and modulates c-fos expression in the hippocampus and amygdala. J Neurosci. 2006;26:9967–9974. doi: 10.1523/JNEUROSCI.2384-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Rodd ZA, McKinzie DL, Bell RL, et al. The metabotropic glutamate 2/3 receptor agonist LY404039 reduces alcohol-seeking but not alcohol self-administration in alcohol-preferring (P) rats. Behav Brain Res. 2006;171:207–215. doi: 10.1016/j.bbr.2006.03.032. [DOI] [PubMed] [Google Scholar]
- 188.Lu L, Uejima JL, Gray SM, Bossert JM, Shaham Y. Systemic and central amygdala injections of the mGluR2/3 agonist LY379268 attenuate the expression of incubation of cocaine craving. Biol Psychiatry. 2007;61:591–598. doi: 10.1016/j.biopsych.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 189.Grimm JW, Hope BT, Wise RA, Shaham Y. Incubation of cocaine craving after withdrawal. Nature. 2001;412:141–142. doi: 10.1038/35084134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Lu L, Grimm JW, Hope BT, Shaham Y. Incubation of cocaine craving after withdrawal: a review of preclinical data. Neuropharmacology. 2004;47 Suppl 1:214–226. doi: 10.1016/j.neuropharm.2004.06.027. [DOI] [PubMed] [Google Scholar]
- 191.Bossert JM, Liu SY, Lu L, Shaham Y. A role of ventral tegmental area glutamate in contextual cue-induced relapse to heroin seeking. J Neurosci. 2004;24:10726–10730. doi: 10.1523/JNEUROSCI.3207-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Bossert JM, Gray SM, Lu L, Shaham Y. Activation of group II metabotropic glutamate receptors in the nucleus accumbens shell attenuates context-induced relapse to heroin seeking. Neuropsychopharmacology. 2006;31:2197–2209. doi: 10.1038/sj.npp.1300977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Marino MJ, Conn PJ. Direct and indirect modulation of the N-methyl D-aspartate receptor: potential for the development of novel antipsychotic therapies. Curr Drug Targets CNS Neurol Disord. 2002;1:1–16. doi: 10.2174/1568007023339544. [DOI] [PubMed] [Google Scholar]
- 194.Conn PJ. Physiological roles and therapeutic potential of metabotropic glutamate receptors. Ann N Y Acad Sci. 2003;1003:12–21. doi: 10.1196/annals.1300.002. [DOI] [PubMed] [Google Scholar]
- 195.Simonyi A, Schachtman TR, Christoffersen GR. The role of metabotropic glutamate receptor 5 in learning and memory processes. Drug News Perspect. 2005;18:353–361. doi: 10.1358/dnp.2005.18.6.927927. [DOI] [PubMed] [Google Scholar]
- 196.Lu YM, Jia Z, Janus C, et al. Mice lacking metabotropic glutamate receptor 5 show impaired learning and reduced CA1 long-term potentiation (LTP) but normal CA3 LTP. J Neurosci. 1997;17:5196–5205. doi: 10.1523/JNEUROSCI.17-13-05196.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Steckler T, Oliveira AF, Van Dyck C, et al. Metabotropic glutamate receptor 1 blockade impairs acquisition and retention in a spatial Water maze task. Behav Brain Res. 2005;164:52–60. doi: 10.1016/j.bbr.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 198.Ohno M, Watanabe S. Enhanced N-methyl-D-aspartate function reverses working memory failure induced by blockade of group I metabotropic glutamate receptors in the rat hippocampus. Neurosci Lett. 1998;240:37–40. doi: 10.1016/s0304-3940(97)00922-1. [DOI] [PubMed] [Google Scholar]
- 199.Ballard TM, Woolley ML, Prinssen E, Huwyler J, Porter R, Spooren W. The effect of the mGlu5 receptor antagonist MPEP in rodent tests of anxiety and cognition: a comparison. Psychopharmacology. 2005;179:218–229. doi: 10.1007/s00213-005-2211-9. [DOI] [PubMed] [Google Scholar]
- 200.Homayoun H, Stefani MR, Adams BW, Tamagan GD, Moghaddam B. Functional interaction between NMDA and mGlu5 receptors: effects on working memory, instrumental learning, motor behaviors, and dopamine release. Neuropsychopharmacology. 2004;29:1259–1269. doi: 10.1038/sj.npp.1300417. [DOI] [PubMed] [Google Scholar]
- 201.Naie K, Manahan-Vaughan D. Regulation by metabotropic glutamate receptor 5 of LTP in the dentate gyrus of freely moving rats: relevance for learning and memory formation. Cereb Cortex. 2004;14:189–198. doi: 10.1093/cercor/bhg118. [DOI] [PubMed] [Google Scholar]
- 202.Manahan-Vaughan D, Braunewell KH. The metabotropic glutamate receptor, mGluR5, is a key determinant of good and bad spatial learning performance and hippocampal synaptic plasticity. Cereb Cortex. 2005;15:1703–1713. doi: 10.1093/cercor/bhi047. [DOI] [PubMed] [Google Scholar]
- 203.Naie K, Manahan-Vaughan D. Pharmacological antagonism of metabotropic glutamate receptor 1 regulates long-term potentiation and spatial reference memory in the dentate gyrus of freely moving rats via N-methyl-D-aspartate and metabotropic glutamate receptor-dependent mechanisms. Eur J Neurosci. 2005;21:411–421. doi: 10.1111/j.1460-9568.2005.03864.x. [DOI] [PubMed] [Google Scholar]
- 204.Semenova S, Markou A. The effects of the mGluR5 antagonist MPEP and the mGluR2/3 antagonist LY341495 on rats' performance in the 5-choice serial reaction time task. Neuropharmacology. 2007;52:863–872. doi: 10.1016/j.neuropharm.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Quarta D, Naylor CG, Morris HV, Patel S, Genn RF, Stolerman IP. Different effects of ionotropic and metabotropic glutamate receptor antagonists on attention and the attentional properties of nicotine. Neuropharmacology. 2007;53:421–430. doi: 10.1016/j.neuropharm.2007.05.023. [DOI] [PubMed] [Google Scholar]
- 206.Aiba A, Chen C, Herrup K, Rosenmund C, Stevens CF, Tonegawa S. Reduced hippocampal long-term potentiation and context-specific deficit in associative learning in mGluR1 mutant mice. Cell. 1994;79:365–375. doi: 10.1016/0092-8674(94)90204-6. [DOI] [PubMed] [Google Scholar]
- 207.Schulz B, Fendt M, Gasparini F, Lingenhohl K, Kuhn R, Koch M. The metabotropic glutamate receptor antagonist 2-methyl-6-(phenylethynyl)-pyridine (MPEP) blocks fear conditioning in rats. Neuropharmacology. 2001;41:1–7. doi: 10.1016/s0028-3908(01)00036-3. [DOI] [PubMed] [Google Scholar]
- 208.Fendt M, Schmid S. Metabotropic glutamate receptors are involved in amygdaloid plasticity. Eur Neurosci. 2002;15:1535–1541. doi: 10.1046/j.1460-9568.2002.01988.x. [DOI] [PubMed] [Google Scholar]
- 209.Rodrigues SM, Bauer EP, Farb CR, Schafe GE, LeDoux JE. The group I metabotropic glutamate receptor mGluR5 is required for fear memory formation and long-term potentiation in the lateral amygdala. J Neurosci. 2002;22:5219–5229. doi: 10.1523/JNEUROSCI.22-12-05219.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Gravius A, Barberi C, Schafer D, Schmidt WJ, Danysz W. The role of group I metabotropic glutamate receptors in acquisition and expression of contextual and auditory fear conditioning in rats - a comparison. Neuropharmacology. 2006;51:1146–1155. doi: 10.1016/j.neuropharm.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 211.Zou D, Huang J, Wu X, Li L. Metabotropic glutamate subtype 5 receptors modulate fear-conditioning induced enhancement of prepulse inhibition in rats. Neuropharmacology. 2007;52:476–486. doi: 10.1016/j.neuropharm.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 212.Balschun D, Wetzel W. Inhibition of group I metabotropic glutamate receptors blocks spatial learning in rats. Neurosci Lett. 1998;249:41–44. doi: 10.1016/s0304-3940(98)00388-7. [DOI] [PubMed] [Google Scholar]
- 213.Balschun D, Wetzel W. Inhibition of mGluR5 blocks hippocampal LTP in vivo and spatial learning in rats. Pharmacol Biochem Behav. 2002;73:375–380. doi: 10.1016/s0091-3057(02)00847-x. [DOI] [PubMed] [Google Scholar]
- 214.Gravius A, Pietraszek M, Schafer D, Schmidt WJ, Danysz W. Effects of mGlu1 and mGlu5 receptor antagonists on negatively reinforced learning. Behav Pharmacol. 2005;16:113–121. doi: 10.1097/00008877-200503000-00007. [DOI] [PubMed] [Google Scholar]
- 215.Gravius A, Pietraszek M, Schmidt WJ, Danysz W. Functional interaction of NMDA and group I metabotropic glutamate receptors in negatively reinforced learning in rats. Psychopharmacology. 2006;185:58–65. doi: 10.1007/s00213-005-0249-3. [DOI] [PubMed] [Google Scholar]
- 216.Salinska E. The role of group I metabotropic glutamate receptors in memory consolidation and reconsolidation in the passive avoidance task in 1-day-old chicks. Neurochem Int. 2006;48:447–452. doi: 10.1016/j.neuint.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 217.Simonyi A, Serfozo P, Shelat PB, Dopheide MM, Coulibaly AP, Schachtman TR. Differential roles of hippocampal metabotropic glutamate receptors 1 and 5 in inhibitory avoidance learning. Neurobiol Learn Mem. 2007;88:305–311. doi: 10.1016/j.nlm.2007.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Petersen S, Bomme C, Baastrup C, Kemp A, Christoffersen GRJ. Differential effects of mGluR1 and mGluR5 antagonism on spatial learning in rats. Pharmacol Biochem Behav. 2002;73:381–389. doi: 10.1016/s0091-3057(02)00829-8. [DOI] [PubMed] [Google Scholar]
- 219.Maciejak P, Taracha E, Lehner M, et al. Hippocampal mGluR1 and consolidation of contextual fear conditioning. Brain Res Bull. 2003;62:39–45. doi: 10.1016/j.brainresbull.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 220.Christoffersen GR, Christensen LH, Hammer P, Vang M. The class I metabotropic glutamate receptor antagonist, AIDA, improves short-term and impairs long-term memory in a spatial task for rats. Neuropharmacology. 1999;38:817–823. doi: 10.1016/s0028-3908(99)00008-8. [DOI] [PubMed] [Google Scholar]
- 221.Christoffersen GR, Christensen LH, Harrington NR, Macphail EM, Riedel G. Task-specific enhancement of short-term, but not long-term, memory by class I metabotropic glutamate receptor antagonist 1-aminoindan-1,5-dicarboxylic acid in rats. Behav Brain Res. 1999;101:215–226. doi: 10.1016/s0166-4328(98)00156-9. [DOI] [PubMed] [Google Scholar]
- 222.Sukhotina IA, Dravolina OA, Novitskaya Y, Zvartau EE, Danysz W, Bespalov AY. Effects of mGlu1 receptor blockade on working memory, time estimation, and impulsivity in rats. Psychopharmacology. 2008;196:211–220. doi: 10.1007/s00213-007-0953-2. [DOI] [PubMed] [Google Scholar]
- 223. [accessed January 15, 2008]; http://www.addexpharma.com.
- 224.Schoepp DD, Wright RA, Levine LR, Gaydos B, Potter WZ. LY354740, an mGlu2/3 receptor agonist as a novel approach to treat anxiety/stress. Stress. 2003;6:189–197. doi: 10.1080/1025389031000146773. [DOI] [PubMed] [Google Scholar]
- 225.Dunayevich E, Erickson J, Levine L, Landbloom R, Schoepp DD, Tollefson GD. Efficacy and tolerability of an mGlu2/3 agonist in the treatment of generalized anxiety disorder. Neuropsychopharmacology. doi: 10.1038/sj.npp.1301531. in press. [DOI] [PubMed] [Google Scholar]
- 226.Patil ST, Zhang L, Martenyi F, et al. Activation of mGlu2/3 receptors as a new approach to treat schizophrenia: a randomized Phase 2 clinical trial. Nat Med. 2007;13:1102–1107. doi: 10.1038/nm1632. [DOI] [PubMed] [Google Scholar]