Abstract
It was recently reported that female mice lacking a functional vomeronasal organ (VNO) displayed male-typical sexual behavior indiscriminately toward female and male conspecifics. These results have been cited as showing that a circuit controlling male-typical sex behavior exists in both sexes, with its activation in females being tonically inhibited by VNO signaling, independent of adult sex hormones. We further assessed this hypothesis while controlling the endocrine status of female mice in which VNO function was surgically disrupted. In experiment 1, VNO-lesioned (VNOx) female mice showed no more mounting or pelvic-thrusting behavior toward an estrous female or a castrated, urine-swabbed male (presented simultaneously) than sham-operated (VNOi) females. This was true when subjects were either ovary-intact or ovariectomized and treated with estradiol, estradiol plus progesterone, or testosterone. In experiment 2, female mice given accessory olfactory bulb lesions or a sham lesion displayed equivalent frequencies of male sex behaviors when given testosterone after ovariectomy. In experiment 3, VNOx and VNOi females displayed equivalent frequencies of male sex behaviors toward an estrous female or a castrated male (presented in separate tests), again, when given testosterone after ovariectomy. Our results confirm early reports that adult testosterone can stimulate appreciable male-typical sex behavior in female mice. However, we failed to corroborate the recent claim that VNO signaling normally inhibits the activity of neural circuitry controlling the expression of male-typical mating behavior by female mice.
Introduction
Pheromone detection by the vomeronasal organ (VNO) contributes to several aspects of social communication and neuroendocrine function in female mice, including pregnancy block (Bruce, 1959), puberty acceleration (Vandenbergh, 1973), synchronization of estrus (Whitten, 1959), and facilitation of lordosis behavior (Keller et al., 2006). Recently, Kimchi et al. (2007) used two methods to disable VNO signaling in female mice: genetic ablation of the transient receptor potential cation channel C2 (TRPC2−/−) and surgical removal of the VNO (VNOx). Significant increases were reported in male-typical sex behavior, including mounting and pelvic thrusting, displayed indiscriminately by ovary-intact TRPC2−/− as well as ovary-intact VNOx females toward male and female stimulus animals. They proposed that all female mice normally possess the neural circuitry that controls both male- and female-typical sexual behavior and that VNO inputs tonically inhibit the male-typical circuit. Kimchi et al. (2007) also argued that the ability of disrupted VNO signaling to stimulate male-typical sexual behavior does not depend on the adult endocrine state of the female, although they did report significantly elevated plasma-free testosterone levels in ovary-intact TRPC2−/− females.
The Kimchi et al. (2007) results were cited (Shah and Breedlove, 2007; Spors and Sobel, 2007) as shedding new light on the brain mechanism controlling a sexually dimorphic behavior and challenging the traditional view that testosterone, acting perinatally, organizes circuits in the CNS that are structurally and functionally unique to the male sex. It should be noted that the Kimchi et al. (2007) results are also consistent with the view that the VNO itself is sexually differentiated in its ability to inhibit the expression of male sex behaviors.
Because previous workers (Edwards and Burge, 1971; Södersten, 1972) reported that adult administration of testosterone stimulated appreciable male-typical sex behavior in female rodents, we further explored possible interactions between VNO signaling and adult sex steroid actions in the regulation of male sex behavior in female mice. First, we studied male-typical sexual behavior displayed toward an estrous female versus a castrated, urine-swabbed male (presented simultaneously) in VNOx and sham-operated (VNOi) females that were ovary-intact or after ovariectomy followed by treatment with estradiol (E2), E2 plus progesterone, or testosterone. We used VNOx females rather than TRPC2−/− mutants because TRPC2−/− independent VNO signaling has been reported in female mice (Kelliher et al., 2006), and developmental deficits may result from the permanent lack of VNO signaling. Kimchi et al. (2007) argued that blood clots formed in the nasal sinuses after VNO removal surgery can block main olfactory function, reducing the display of male sex behavior. We therefore confirmed that all VNOx subjects could discriminate between urinary volatiles from mice of different sexes—a task that requires a functional main olfactory system. Second, we further avoided this issue by assessing the behavioral effects of lesions of the accessory olfactory bulb (AOBx), again in tests with male and female stimulus mice being presented simultaneously. Much of the data reported by Kimchi et al. (2007) involved behavioral tests of TRPC2−/− or VNOx female mice in which a stimulus female or male was presented in separate tests. Therefore, in experiment 3, we compared behavior directed by VNOx and VNOi females toward an estrous female or a castrated male when presented in separate tests.
Materials and Methods
Subjects
Eighty-eight female and 30 male sexually naive Swiss Webster mice (Charles River Laboratories) were purchased at 8–12 weeks of age and housed in same-sex groups under a reversed 12 h light/dark photoperiod (lights off at 9:00 A.M.). Food and water were provided ad libitum. All procedures were approved by the Boston University Animal Care and Use Committee and were in accordance with National Institutes of Health guidelines. During the first week, female subjects in experiments 2 and 3 underwent bilateral ovariectomy using 2% isoflurane anesthesia and were given 1 week to recover. Females used in experiment 1 were ovariectomized after an initial set of behavior tests given while they were ovary-intact, as described below. The endocrine state of ovariectomized female subjects was manipulated in several ways. SILASTIC capsules (inner diameter, 1.57 mm; outer diameter, 2.41 mm; length, 5 mm) packed with E2 (diluted 1:1 with cholesterol) were implanted subcutaneously at the back of the neck 7 d before the onset of behavioral testing. SILASTIC capsules of this length and thickness produce circulating E2 levels within the range of values observed in estrous female mice (Bakker et al., 2002). To induce behavioral estrus, injections of progesterone (P; 500 μg, s.c.) were administered 2–4 h before testing to ovariectomized females previously implanted with E2 capsules. For behavioral tests conducted at the end of experiments 1–3, the E2 capsules were removed and each subject was given daily injections of testosterone propionate (TP; 3 mg/kg, s.c.) for a minimum of 7 d before the start of testing. Daily injections of TP continued while tests were being conducted. To verify that serum testosterone levels in ovariectomized female subjects given this dose of TP were comparable to serum testosterone levels of testes-intact males, trunk blood was collected from nine ovariectomized female mice given TP injections for 7 consecutive days (samples collected 24 h after the last TP injection) and five testes-intact adult male mice. Serum testosterone concentrations were measured using a Coat-A-Count Total Testosterone in vitro Diagnostic Test Kit (Siemens). Testosterone values for testes-intact males (5.97 ± 1.97 ng/ml) did not differ significantly from values for ovariectomized TP-treated females (7.42 ± 0.86 ng/ml).
VNO removal surgery
Female subjects used in experiments 1 and 3 underwent either bilateral surgical removal of the VNO or sham surgery (Pankevich et al., 2004). VNO removal was performed under ketamine (100 mg/kg) and xylazine (20 mg/kg) anesthesia. Subjects were positioned supine in a head holder, and the jaw was held open using retractors. A midline incision was made in the soft palate extending from the first palatal ridge to the incisors, and the underlying vomer bone was exposed by blunt dissection. For subjects receiving sham surgery, the incision was closed with absorbable suture and the wound was sealed with Vetbond tissue adhesive (Henry Schein). For VNOx subjects, the rostral end of the VNO was exposed by drilling, the caudal vomer bone was cut, and the VNO was removed bilaterally with a twisting motion. The cavity was packed with gel foam, closed with absorbable suture, and sealed with Vetbond. To control bleeding during the procedure, gentle suction was applied using a blunted 18 gauge needle attached to a vacuum. Animals were given daily injections of analgesic (carprofen; 5 mg/kg, s.c.) and antibiotic (Baytril; 5 mg/kg, s.c.) for 2 and 3 d, respectively, after the procedure, and subjects were closely monitored for difficulty breathing. Subjects were allowed 2 weeks to recover before behavioral testing.
AOB lesion surgery
Subjects used in experiment 2 underwent bilateral AOB lesions or sham surgery. Subjects were deeply anesthetized under ketamine/xylazine whereupon their heads were fixed in a stereotaxic apparatus (David Kopf Instruments); animals received continuous 1% isoflurane anesthesia throughout the entire procedure. A midline incision was made to expose the skull, and two small holes were drilled above the left and right olfactory bulbs. The dura was penetrated by passing 200 μA of current through a tungsten microelectrode (500 μm diameter with tapered tip; FHC) attached to a Grass Lesion Maker, with the ground electrode attached to the subject's tail. The electrode was then lowered at a 42° angle at a point 0.8 mm anterior and 0.9 mm lateral to the intersection of the midline and the inferior cerebral vein. A 300 μA current was passed for 30 s at depths of −2.4, −2.0, and −1.6 mm below the surface of the dura in each hemisphere. For sham-operated subjects, the electrode was lowered to each depth, but no current was passed. The skull holes were then filled with gel foam, and the incision was closed with absorbable suture.
Preparation of stimulus animals and urine donors
Five male mice underwent bilateral castration using 2% isoflurane anesthesia and were allowed 1 week to recover. Two additional cohorts of five sexually naive, testes-intact males used in mating tests were housed individually. Six female stimulus animals were ovariectomized using 2% isoflurane anesthesia and given SILASTIC E2 implants as described above. For urine collection and all mating tests requiring an estrous stimulus female, P (500 μg, s.c.) was administered 2–4 h before urine collection or behavioral testing to induce behavioral estrus. All urinary stimuli were collected by scruffing mice over a funnel and applying gentle pressure to the abdomen. Urine from five to six donors was pooled according to sex and endocrine status, aliquotted (60 μl samples), and stored at −80°C.
Behavioral tests
Male-typical sexual behavior.
Subjects were individually housed in plastic cages (29 × 18 × 13 cm) 48 h before the start of behavioral testing. All testing was done during the dark phase of the light–dark photoperiod under dim yellow light. For all behavioral tests, female subjects were coded so that the investigator was blind to the surgical status of each animal. A subset of subjects, including both lesioned and sham-operated females as well as control males, was videotaped during each type of mating test to document examples of the observed behaviors.
Two types of mating tests were conducted to assess male-typical sexual behavior in female mice under a variety of endocrine conditions (see below for sequences of endocrine manipulation). In the first testing paradigm, used in experiments 1 and 2, a castrated male mouse with intact male urine (20 μl) swabbed on the anogenital region and an estrous female mouse were introduced simultaneously into each subject's home cage. Each subject was tested for 30 min on two occasions, and the investigator recorded on a score sheet (Baum et al., 1994; Pankevich et al., 2004) the incidence and time of occurrence of anogenital investigations, mounts, and pelvic thrusts displayed toward each stimulus animal. Separate additional cohorts of testes-intact male mice were also tested for 30 min on six occasions in experiment 1 and on two occasions in experiment 2 to provide a comparison of male-typical levels of each behavior directed toward each type of social stimulus. Differences between lesioned and sham-operated groups in the percentage of subjects mounting either stimulus animal under a particular endocrine treatment were assessed using the Fisher exact probability test. Mann–Whitney U tests were used to analyze differences in mount and pelvic-thrusting frequency between lesioned and sham-operated subjects. Nonparametric Wilcoxon signed-rank tests were used to make within-groups comparisons to assess possible effects of endocrine status on mount and pelvic-thrusting frequencies.
In experiment 3, a second testing paradigm was introduced to assess male-typical sexual behavior in females in which two 30 min tests were given on consecutive days under each endocrine condition. In the first test, a castrated male mouse with intact male urine (20 μl) swabbed on the anogenital region was introduced individually, and in the second test, an estrous female mouse was introduced individually into each subject's home cage. The investigator again recorded on a score sheet the incidence and time of occurrence of anogenital investigations, mounts, and pelvic thrusts displayed toward the stimulus male or female under a series of endocrine conditions (see below). Additionally, to further facilitate comparison of male-typical sexual behavior in VNOx and VNOi female mice in our study to the data reported in Kimchi et al. (2007), these tests were repeated in TP-primed subjects while the total mount time displayed by subjects in addition to the number of anogenital investigations, mounts, and pelvic thrusts were recorded. Differences between lesioned and sham-operated groups in the percentage of animals mounting either stimulus animal under a particular endocrine treatment were calculated using the Fisher exact probability test. For tests with multiple endocrine conditions, differences between groups in the number of mounts and pelvic thrusts were assessed using two-way repeated-measures ANOVAs followed by Student–Newman–Keuls post hoc tests. Differences between sham-operated and lesioned groups for the final two tests in which subjects continued to be treated with TP were assessed using two-tailed Student's t tests.
Habituation/dishabituation tests of olfactory discrimination.
To assess subjects' ability to detect and discriminate volatile urinary odors from a testes-intact male versus an estrous female or a castrated male, as well as to verify that any residual blood clots in the nasal sinuses from VNO removal surgery did not render the animals anosmic, home-cage habituation/dishabituation tests of urinary odor discrimination (Baum and Keverne, 2002) were given to all female subjects. Testing was conducted by pipetting 20 μl of distilled water, intact male urine, or estrous female urine onto filter paper secured to a plastic weigh boat, and placing the weigh boat in a clean food hopper outside of the home cage. A wire mesh was placed between the filter paper and the cage top so that only volatile odorants were available at body level. Subjects were first presented with three consecutive 2 min presentations of distilled water, followed by three consecutive presentations each of intact male and estrous female urine, respectively. Each presentation was separated by a 1 min intertrial interval. Using an iPAQ Pocket PC (Hewlett-Packard) and Noldus Observer Software, the investigator recorded the amount of time each subject spent sniffing with its nose at the wire mesh (∼8 mm from the urine stimulus) during each 2 min trial. In experiments 1 and 2, this procedure was repeated with the stimuli moved inside of the home cage allowing direct nasal access to volatile and nonvolatile urinary odors, and both inside and outside of the cage using testes-intact male versus castrated male urine. In experiment 3, stimuli were presented both outside and inside of the home cage using testes-intact male versus estrous female urine. Two-tailed Wilcoxon signed-rank tests were used to assess within-group differences in investigation times between the third presentation of a stimulus and the first presentation of the subsequent stimulus. Between groups differences were assessed using Mann–Whitney U tests.
Odor preference tests.
In experiment 3, two 5 min odor preference tests were conducted in each subject's home cage. The first preference test involved pipetting 20 μl of testes-intact male urine or estrous female urine onto filter paper secured to a plastic weigh boat and placing the two stimuli next to each other in a clean food hopper outside of the home cage. A wire mesh was placed between the filter paper and the cage top to prevent direct nasal access to the stimuli, thus only volatile urinary odorants were available at body level. Time spent sniffing each stimulus at the wire mesh (∼8 mm from the urinary stimulus) was recorded using a Pocket PC and Noldus Observer Software. In the second odor preference test, the two stimuli were placed side-by-side inside the home cage, allowing direct nasal access to both volatile and nonvolatile components of the urine. Time spent in nasal contact with each stimulus was recorded. One-tailed Student's t tests were used to assess differences in investigation times directed toward each stimulus.
Lordosis behavior tests.
In experiments 1 and 2, we assessed female-typical lordosis behavior in a series of four tests, each lasting for a total of 10 mounts received from a stimulus male or a maximum of 20 min. Before lordosis testing, subjects were group-housed for 4 d, and each test was conducted in the home cage of a sexually experienced testes-intact male mouse. The lordosis quotient was calculated for each subject (number of mounts to which lordosis was shown divided by the total number of mounts received), and averages over the four tests were compared between lesioned and sham-operated groups using one-tailed Student's t tests.
During lordosis testing, we noticed that group-housed ovariectomized females of mixed lesion status (four to five per cage), when primed with E2+P, mounted each other vigorously. We evaluated this behavior systematically by recording the number of mounts toward cage mates by each subject in two 30 min tests. Differences in the proportion of lesioned and sham-operated subjects showing mounts were assessed by Fisher exact probability tests.
Histological confirmation of VNO and AOB lesions
At the conclusion of behavioral testing, all subjects were anesthetized with sodium pentobarbital and underwent transcardiac perfusion with 0.1 m PBS, followed by 4% paraformaldehyde in 0.1 m PBS. All brains were removed and postfixed in 4% paraformaldehyde for 4 h and then cryoprotected in 30% sucrose in 0.1 m PBS for 48 h. Olfactory bulbs were subsequently blocked, frozen in OCT (Tissue-Tek), and stored at −80°C. In experiments 1 and 3, snouts were removed immediately after perfusion, cleared of all soft tissue, and soaked for 30 min in rapid decalcifier (Apex Engineering Products). Decalcified snouts were then soaked overnight in 30% sucrose, at which time a 1:1 mixture of 30% sucrose and OCT was suctioned into the nasal passages. Snouts were then incubated for 4 h in the 1:1 solution and were finally frozen in OCT and stored at −80°C.
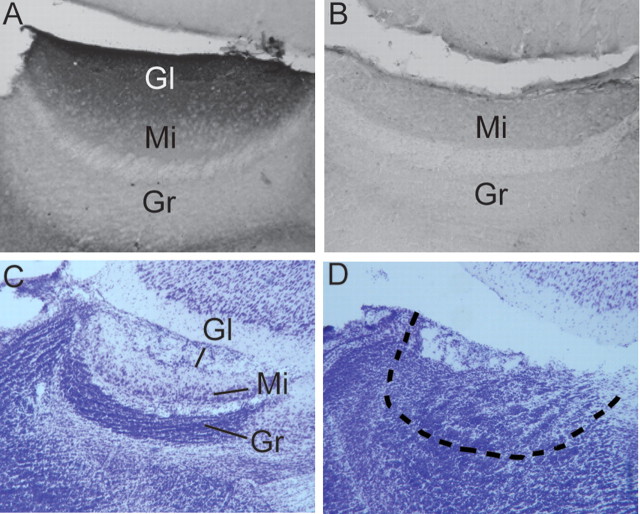
In experiments 1 and 3, olfactory bulb sections were cut sagittally at 30 μm on a freezing sledge microtome (Leica Microsystems). Every other section was immersed free-floating in 0.1 m PBS, and remaining sections were stored in antifreeze at −20°C. Free-floating sections were later processed for soybean agglutinin conjugated with horseradish peroxidase (SBA-HRP) staining to ensure that the VNO was successfully removed (Wysocki and Wysocki, 1995). SBA selectively binds to the glomeruli of the AOB in the mouse (Key and Giorgi, 1986), thus the absence of any staining in the AOB indicates that VNO axonal inputs are no longer present. Sections were mounted onto gelatin-coated slides and coverslipped with Permount. Only VNOx subjects in which SBA staining was completely absent in both hemispheres were included in the study (Fig. 1 A,B). One of 12 animals in experiment 1 and 5 of 14 animals in experiment 3 were excluded from the lesion groups based on SBA histology.
Figure 1.
A–D, Representative photomicrographs showing histological confirmation of successful surgical removal of the VNO or of electrolytic lesions of the AOB in female mice. All sections are shown in the sagittal plane. A, SBA staining is present in the glomerular layer of the AOB of a subject that received a sham lesion of the VNO. B, SBA staining is totally absent in the AOB of a mouse from which the ipsilateral VNO was successfully removed several months earlier. C, The layers of the AOB are shown by cresyl violet staining in a female mouse that previously received a sham lesion of the AOB. D, The dotted line outlines the boundary of what would have been the AOB granule cell layer in a mouse that previously received an electrolytic lesion of the AOB. Gl, Glomerular cell layer; Mi, mitral cell layer; Gr, granule cell layer.
Snouts were cut coronally at 10 μm on a cryostat (Leica Microsystems) maintained at −25°C. One section every 150 μm was transferred directly onto positively charged Superfrost Plus glass slides and dried overnight. Sections were rinsed and stained with H&E (supplemental Fig. S1A,B, available at www.jneurosci.org as supplemental material) to assess the presence of blood clots in the nasal sinuses. One VNOi and two VNOx subjects in experiment 3 and one VNOx subject in experiment 1 showed evidence of small blood clots partially blocking one side of the nasal cavity (supplemental Fig. S1C,D, available at www.jneurosci.org as supplemental material). We verified that these subjects were able to discriminate male and female urinary volatiles in habituation/dishabituation tests, indicating that the animals were not anosmic. Therefore, all behavioral data from these subjects were retained in our experiments. All other subjects were free of any sinus blockage at the time of killing.
In experiment 2, olfactory bulb sections were cut sagittally at 50 μm on a freezing sledge microtome, immersed in 0.1 m PBS, and all sections were immediately mounted onto gelatin-coated slides in sequence (lateral–medial). Slides were dried overnight, rinsed with distilled water, stained with Cresyl Violet to differentiate cell layers (Fig. 1 C,D), and finally coverslipped with Permount. An independent observer, blind to the lesion status of the subjects, examined the slides to determine the extent of the lesions. Four AOBx subjects were excluded from the study because >25% of the AOB remained intact. The extent of the AOB lesion damage in the remaining 8 subjects is shown in supplemental Figure S2 (available at www.jneurosci.org as supplemental material).
Sequence of endocrine manipulations and behavioral tests
A timeline showing the sequence of treatments and behavioral tests in each experiment is given in supplemental Figure S3 (available at www.jneurosci.org as supplemental material).
Experiment 1.
Male-typical sexual behavior was first assessed in ovary-intact, VNOx, and VNOi female subjects as well as testes-intact males using the testing paradigm in which male and female stimulus animals were simultaneously introduced into each subject's home cage. Subjects were then ovariectomized and given subcutaneous E2 implants and subsequently retested under E2 as well as E2+P treatment, at which time testes-intact males were also retested. Tests for male-typical sexual behavior were conducted on two occasions under each endocrine condition, and data were averaged according to lesion status and endocrine state. Data obtained from testes-intact males in each of these tests were averaged. Habituation/dishabituation tests of odor discrimination were then conducted in E2-primed subjects. All subjects were given E2+P before lordosis tests, and group-housed mounting data were collected immediately after these lordosis tests on days 3 and 4 of the series. The E2 capsules were then removed; all subjects were rehoused individually after 7 d of TP treatment and tested again on two occasions, using the initial mating paradigm in which stimulus animals were simultaneously introduced into each subject's home cage.
Experiment 2.
Male-typical sexual behavior was first assessed in ovariectomized female AOBi and AOBx subjects without hormone replacement as well as in testes-intact males, again on two occasions, using the testing paradigm in which both male and female stimulus animals were simultaneously introduced into each subject's home cage. Data for testes-intact males were averaged, as were data for female subjects with respect to lesion status. Subjects were then given subcutaneous E2 implants, and habituation/dishabituation tests of odor discrimination were conducted. All subjects were then given E2+P for lordosis tests, and group-housed mounting data were collected immediately after lordosis testing on days 3 and 4 of the series. The E2 capsules were then removed; all subjects were rehoused individually after 7 d of TP treatment and tested on two occasions for male-typical sexual behavior (data were averaged according to lesion status) using the initial paradigm in which stimulus animals were simultaneously introduced into each subject's home cage.
Experiment 3.
After VNOx or VNOi surgery, ovariectomized subjects were given subcutaneous E2 implants whereupon they were given habituation/dishabituation tests of odor discrimination. These tests were followed by the urinary odor preference tests. Subjects then underwent a series of tests to evaluate male-typical sexual behavior in which male or female stimulus animals were individually introduced into each subject's home cage in separate single tests given while subjects received E2, E2+P, and finally TP. Additional single tests were given with each type of stimulus, and mount durations were recorded while subjects continued to receive TP.
Results
Experiment 1
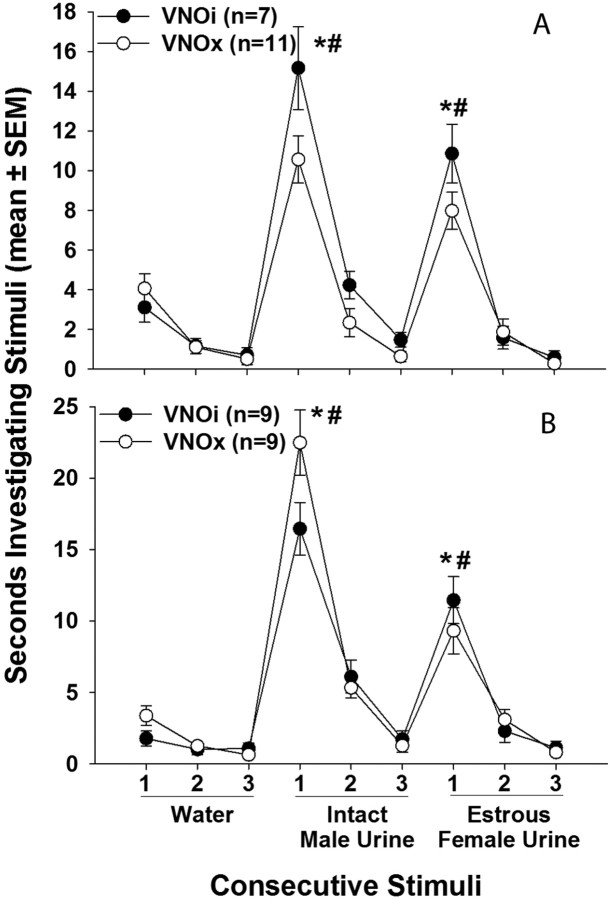
There were no significant differences in the proportion of VNOi versus VNOx subjects that mounted either the male or female stimulus animals in tests given while subjects were ovary-intact, or ovariectomized and treated with E2, E2+P, or TP (Fig. 2 A). In fact, no subjects displayed mounting or pelvic-thrusting behaviors toward either stimulus when tested while ovary-intact. After ovariectomy and treatment with TP, 4 of 7 VNOi versus 1 of 11 VNOx females displayed mounting and pelvic-thrusting behavior toward the female stimulus, and 2 of 7 VNOi versus 1 of 11 VNOx females displayed these behaviors toward the male stimulus. Supplemental Video 1 (available at www.jneurosci.org as supplemental material) illustrates mounting and pelvic thrusting shown by a representative VNOi female first toward a castrated, urine-swabbed male and then toward an estrous female mouse. An example of these same behaviors displayed by a testes-intact male mouse is provided in supplemental Video 2 (available at www.jneurosci.org as supplemental material). When given TP, VNOi subjects showed a significantly higher number of mounts (p = 0.022) than VNOx subjects toward the estrous female (Fig. 2 B) during the 30 min mating tests, and a similar nonsignificant trend was observed for the number of pelvic thrusts displayed by the two groups (Fig. 2 C). Both VNOi and VNOx subjects showed similar numbers of anogenital investigations (data not shown). No significant effects of endocrine treatment were revealed by the within-groups comparisons. Finally, the percentage of females showing mounts, the number of mounts, and the number of pelvic thrusts toward either type of stimulus animal were all much lower than those observed in testes-intact males. As reported by Pankevich et al. (2004), testes-intact males (with intact VNOs) mounted both types of stimulus mouse, although more mounts were directed toward estrous females than toward castrated males.
Figure 2.
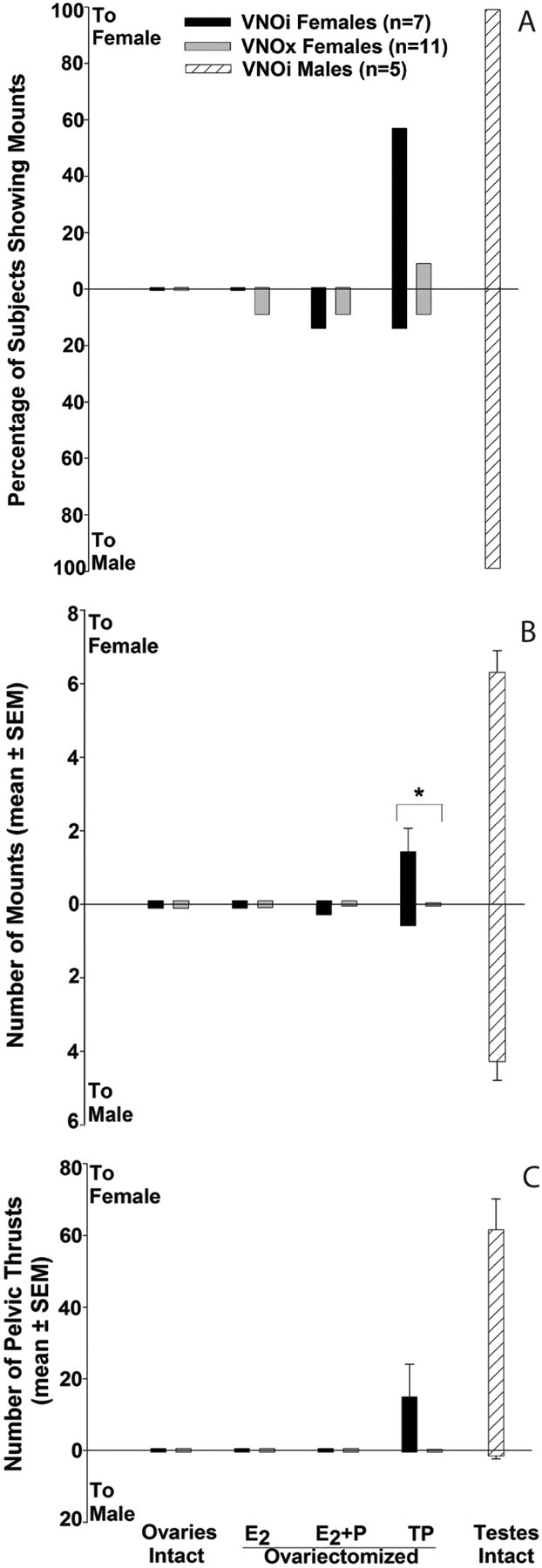
A–C, Effect of bilateral VNO removal (VNOx) or sham operation (VNOi) on the percentage of female mice showing mounting (A), the number of mounts (B), and the number of pelvic thrusts (C) shown toward an estrous female (to female) versus a castrated, urine-swabbed male (to male) when presented simultaneously in each subject's home cage in experiment 1. Endocrine treatments for each set of tests (2 per treatment) are listed across the x-axis. Comparative data for testes-intact males in which the VNO was also intact (VNOi) are depicted by . *Between-groups differences in mean mounting frequency were assessed using Mann–Whitney U tests.
. *Between-groups differences in mean mounting frequency were assessed using Mann–Whitney U tests.
Both VNOi and VNOx female subjects successfully dishabituated from the third presentation of water to the first presentation of intact male urine (VNOi, p = 0.016; VNOx, p < 0.001), as well as from the third presentation of intact male urine to the first presentation of estrous female urine (VNOi, p = 0.016; VNOx, p < 0.001) presented outside of the home cage (Fig. 3 A). Subsequent tests using testes-intact male versus estrous female urine inside of the home cage, as well as testes-intact male versus castrated male urine both outside and inside the home cage, yielded similar results in which subjects successfully dishabituated to the first presentation of each stimulus regardless of lesion status (data not shown). This indicates that subjects in both groups could distinguish between the olfactory stimuli presented and thus were not anosmic. Additionally, VNOx subjects showed a significantly reduced lordosis quotient compared with VNOi subjects (VNOi, 23 ± 11; VNOx, 6 ± 3; t (16) = 2.57, p = 0.011), indicating that female-typical sexual behavior was impaired in the absence of a functional VNO. In the group-housed mounting tests, a significantly higher proportion (p = 0.025) of VNOi subjects (5 of 7) versus VNOx subjects (1 of 11) displayed mounting behavior toward one or more estrous female cage mates. Overall, a significantly higher proportion (p = 0.005) of ovariectomized VNOi subjects (7 of 7) versus ovariectomized VNOx subjects (2 of 11) showed mounting behavior in either the group-housed mounting tests when given E2+P treatment or in mating tests when ovariectomized and treated with TP.
Figure 3.
A, B, Effect of bilateral VNO removal (VNOx) or sham operation (VNOi) on the ability of female mice to discriminate between testes-intact male and estrous female volatile urinary odors presented outside of the home cage in experiment 1 (A) and experiment 3 (B). Each stimulus was presented three consecutive times. *,# p < 0.05, two-tailed Wilcoxon test comparisons with the third presentation of the previous stimulus for the respective groups.
Experiment 2
Ovariectomized AOBi and AOBx subjects showed no mounting behavior when first tested after ovariectomy without hormone replacement. Both of these groups displayed appreciable mounting and pelvic-thrusting behavior when treated with TP (Fig. 4); however, no between-groups differences were seen in the number of mounts or pelvic thrusts displayed toward either stimulus. AOBi subjects showed a greater number of mounts (Fig. 4 B) (p = 0.031) and pelvic thrusts (Fig. 4 C) (p = 0.031) toward the female stimulus animal when ovariectomized and given TP compared with no hormone treatment. AOBx subjects had a similar though nonsignificant trend to show increased mounting and pelvic thrusting toward the female stimulus animal when given TP. TP treatment did not significantly stimulate mounting or pelvic thrusting behavior toward the male stimulus animal in either AOBi or AOBx females. Again, the percentage of females showing mounts, the number of mounts, and the number of pelvic thrusts displayed toward either stimulus were much lower than those observed in testes-intact males. All male subjects mounted both types of stimulus mouse; however as in experiment 1, males displayed more mounting and pelvic thrusting behavior directed toward the estrous female than toward the castrated stimulus male.
Figure 4.
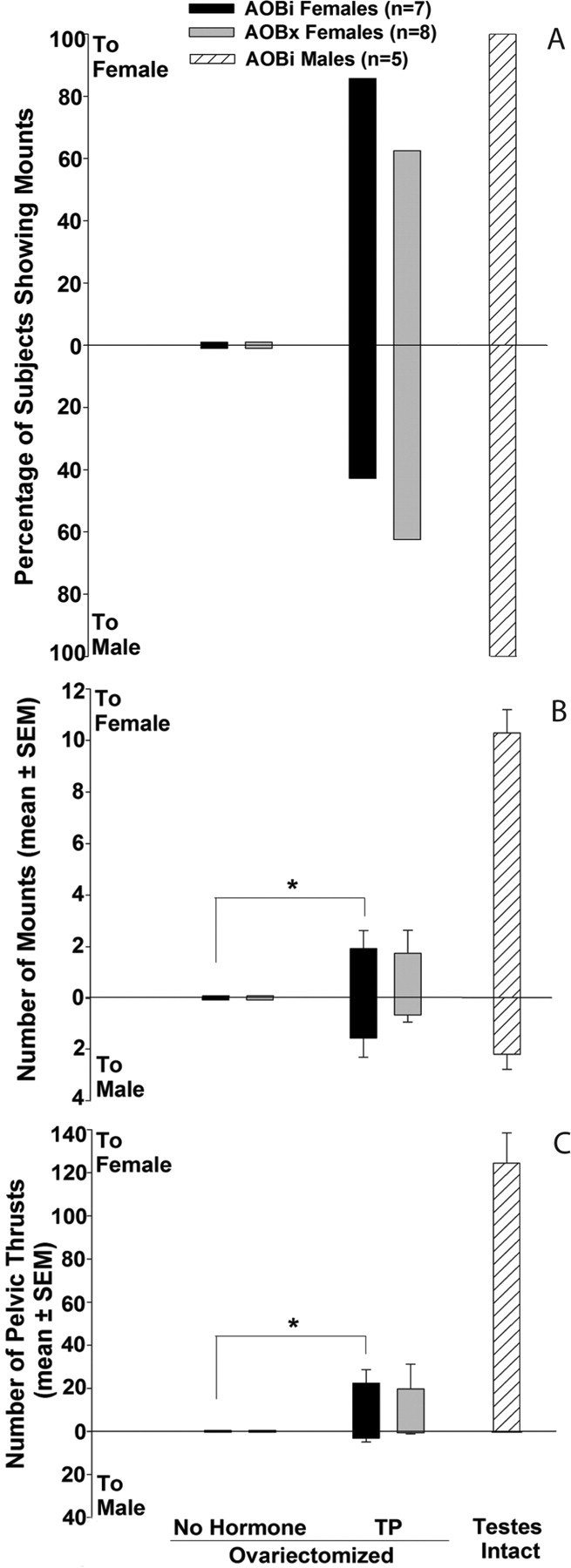
A–C, Effect of bilateral accessory olfactory bulb lesions (AOBx) or sham operation (AOBi) on the percentage of female mice showing mounting (A), the number of mounts (B), and the number of pelvic thrusts (C) shown toward an estrous female (to female) versus a castrated, urine-swabbed male (to male) when presented simultaneously in each subject's home cage in experiment 2. Endocrine treatments for each set of tests (2 tests per treatment) are listed across the x-axis. Comparative data for testes-intact males in which the AOB was intact (AOBi) are depicted by  . *Within-group differences in mounting and pelvic thrusting frequencies (mean ± SEM) were assessed using two-tailed Wilcoxon tests.
. *Within-group differences in mounting and pelvic thrusting frequencies (mean ± SEM) were assessed using two-tailed Wilcoxon tests.
Both AOBi and AOBx subjects successfully dishabituated from the third presentation of water to the first presentation of testes-intact male urine (AOBi, p = 0.016; AOBx, p = 0.008), as well as from the third presentation of testes-intact male urine to the first presentation of estrous female urine (AOBi, p = 0.016; AOBx, p = 0.008) presented outside of the home cage (data not shown). Subsequent tests using testes-intact male versus estrous female urine inside the home cage, as well as testes-intact male versus castrated male urine both outside and inside the home cage, yielded similar results in which subjects successfully dishabituated to the first presentation of each stimulus regardless of lesion status (data not shown). Thus, a functional AOB is not required for females to discriminate urinary odors from mice of different sexes or endocrine states. Additionally, AOBx subjects showed a significantly reduced lordosis quotient compared with AOBi subjects (AOBi, 22 ± 9; AOBx, 6 ± 4; t (13) = 2.733, p = 0.009), indicating that female-typical sexual behavior was impaired in the absence of a functional AOB. In the group-housed mounting tests, no significant differences in the proportion of AOBi (two of seven) versus AOBx (zero of eight) subjects displaying mounting behavior toward one or more estrous female cage mates were observed. Overall, similar proportions of AOBi (six of seven) and AOBx (six of eight) subjects showed mounting behavior in either the group-housed mounting tests when given E2+P treatment or in mating tests when ovariectomized and treated with TP.
Experiment 3
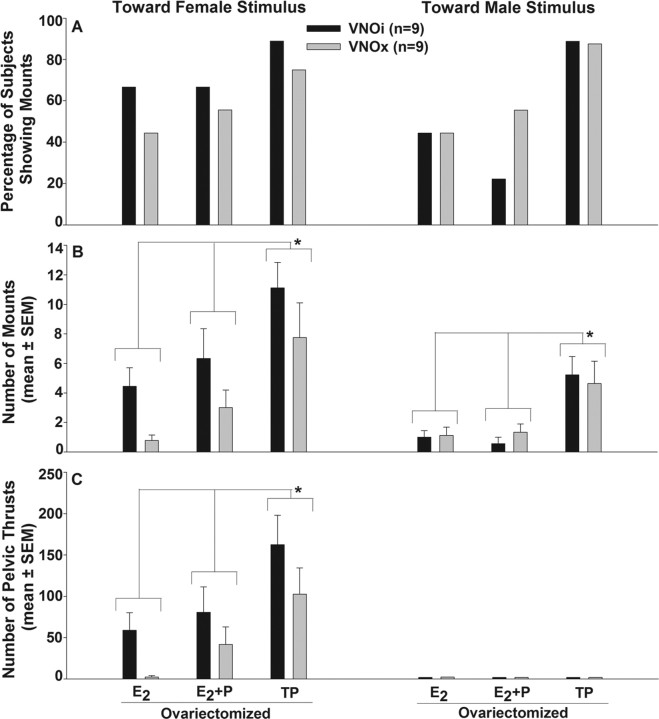
A similar, maximal percentage of VNOi and VNOx subjects showed mounting behavior toward both a stimulus female and a stimulus male after ovariectomy and treatment with TP (Fig. 5 A). Two-way ANOVAs with repeated measures and Student–Newman–Keuls post hoc tests revealed an effect of TP treatment on the number of mounts (Fig. 5 B) toward the female stimulus (F (2,50) = 17.2; p < 0.001), mounts toward the male stimulus (F (2,50) = 14.9; p < 0.001), and pelvic thrusts (Fig. 5 C) toward the female stimulus (F (2,50) = 16.2; p < 0.001). No females displayed pelvic-thrusting behavior toward the male stimulus. In the final set of mating tests in which ovariectomized subjects were further tested while receiving TP, a similar high percentage of VNOi and VNOx females displayed mounting behavior. In addition, VNOi and VNOx females showed equivalent frequencies and durations of mounting toward male or female stimulus animals (supplemental Fig. S4, available at www.jneurosci.org as supplemental material).
Figure 5.
A–C, Effect of bilateral VNO removal (VNOx) or sham operation (VNOi) on the percentage of female mice showing mounting (A), the number of mounts (B), and the number of pelvic thrusts (C) shown toward an estrous female versus a castrated, urine-swabbed male when presented individually in separate tests in each subject's home cage in experiment 3. Endocrine treatments for each test are given across the x-axis. *Two-way ANOVAs followed by Student–Newman–Keuls post hoc tests revealed an effect of TP treatment on both mounting and pelvic thrusting frequency that was equivalent in both VNOi and VNOx females.
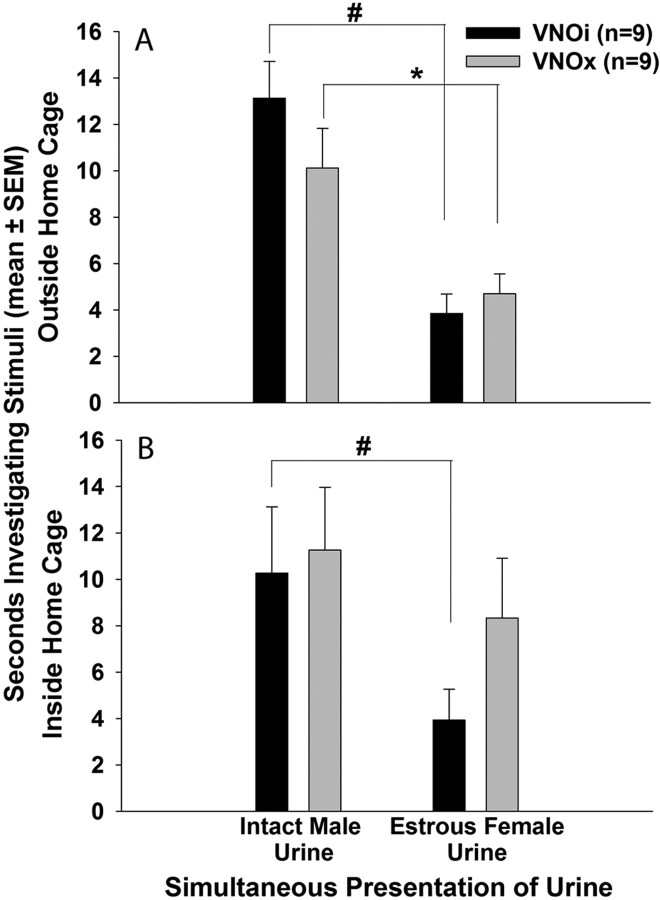
Both VNOi and VNOx subjects successfully dishabituated from the third presentation of water to the first presentation of testes-intact male urine (VNOi, p = 0.004; VNOx, p = 0.004), as well as from the third presentation of testes-intact male urine to the first presentation of estrous female urine (VNOi, p = 0.004; VNOx, p = 0.008) presented outside of the home cage (Fig. 3 B). Again, this indicates that VNOx subjects could discriminate urinary odors of male versus female conspecifics and thus were not anosmic. In the simultaneous odor choice tests, subjects in both groups preferred to investigate testes-intact male urine over estrous female urine (VNOi, t (16) = 5.207, p < 0.001; VNOx, t (16) = 2.826, p = 0.006) when the stimuli were placed outside of the home cage (Fig. 6 A). When the stimuli were moved inside of the home cage, thereby providing direct nasal access to both volatile and nonvolatile components of urinary odors, VNOi subjects maintained their preference for male urine (t (16) = 2.013, p = 0.031), whereas VNOx subjects spent an equivalent amount of time investigating each stimulus (Fig. 6 B).
Figure 6.
A, B, Effect of bilateral VNO removal (VNOx) or sham operation (VNOi) on the preference of ovariectomized, estradiol-primed female mice for urinary odors from testes-intact male versus estrous female mice presented simultaneously. Subjects' preference for volatile urinary odors presented outside the home cage is shown in A, and subjects' preference for volatile plus nonvolatile urinary odors presented inside the home cage is shown in B. #, *Within- group differences were assessed using one-tailed Student's t tests.
Discussion
In contrast to Kimchi et al. (2007), disabling vomeronasal signaling either by surgical VNO removal (experiments 1 and 3) or by lesioning the AOB (experiment 2) did not significantly increase mounting or pelvic-thrusting behaviors displayed by female mice toward either an estrous female or a castrated, urine-swabbed male. In experiment 1, these male-typical sexual behaviors were completely absent in ovary-intact female mice (as used in Kimchi et al., 2007) and were only minimally expressed in ovariectomized E2-treated and E2+P-treated subjects. Considerably more mounting and pelvic-thrusting behaviors, more often directed toward the stimulus female than the male, were displayed when ovariectomized VNOi females were treated with TP. In experiment 2, AOBi and AOBx subjects showed equivalent increases in male-typical sex behavior when ovariectomized and given TP, and a similar effect of TP was seen in both VNOi and VNOx females studied in experiment 3. Previous studies (Herrada and Dulac, 1997; Halem et al., 2001) showed that aspects of VNO sensory neuron function are sexually differentiated in rodents. Together, however, our results provide no indication that female-typical VNO signaling inhibits the expression of male-typical sex behavior in female mice.
In experiments 1 and 2, the numbers of mounts and pelvic thrusts displayed by ovariectomized female subjects with male-typical circulating levels of testosterone were much lower than in testes-intact male mice. Thus, whereas female mice possess the neural circuitry controlling the display of male-typical sexual behavior, a sex difference exists in the response of this circuitry to the activational effect of testosterone in adulthood. This sex difference is presumably the result of organizational effects of perinatal exposure to testosterone in the male (Phoenix et al., 1959) and, as shown in experiments 1 and 2, has nothing to do with an inhibitory effect of tonic VNO signaling in the female.
Previous studies in rats (Ball, 1940; Beach, 1942; Pfaff, 1970; Södersten, 1972; Emery and Sachs, 1975; Fang and Clemens, 1999) and mice (Edwards and Burge, 1971) showed that adult administration of E2 or testosterone activated male-typical mounting behavior in ovariectomized females that were tested with an estrous female. Likewise, in experiment 3 maximal, equivalent levels of male-typical sex behavior were seen in VNOi and VNOx female mice when they were given TP after ovariectomy and tested separately with an estrous female. Neonatal administration of TP further augmented the ability of female mice to display male-typical mating behavior in later life (Manning and McGill, 1974), a finding that is, again, consistent with the traditional view that male-typical brain and behavioral sexual differentiation results from the perinatal action of testosterone in the male nervous system. Several more recent studies suggest that a degree of male-typical, perinatal sex steroid signaling also contributes to the ability of normal female mice to display appreciable mounting and pelvic-thrusting behavior in adulthood. Thus, female mice in which E2 signaling was reduced because of a null mutation of the estrogen receptor-α (Wersinger et al., 1997) or the aromatase (Bakker et al., 2002) genes showed significantly less male-typical mounting behavior than wild-type females after adult ovariectomy and testosterone or E2 treatment. Conversely, female α-fetoprotein knock-out mice, whose brains are exposed fetally to increased E2, showed increased mounting behavior toward estrous stimulus females after adult ovariectomy and E2 treatment (Bakker et al., 2006). Finally, ovariectomized female mice with a null mutation of the androgen receptor gene showed significantly less mounting behavior than wild-type control females after adult ovariectomy and treatment with E2 (Sato et al., 2004). Our results reaffirm the ability of adult testosterone to stimulate appreciable male-typical sexual behavior in female mice and provide no support for the suggestion (Kimchi et al., 2007) that VNO signaling inhibits activity of the circuitry controlling this behavior in females.
Habituation/dishabituation urinary odor discrimination tests in experiments 1–3 yielded two important findings. First, females with or without a functional VNO or AOB were able to distinguish between volatile male and female urinary odors as well as urinary odors from males in different endocrine states. Similar results were previously obtained in VNOi and VNOx male mice (Pankevich et al., 2004). These results argue against the previous suggestion (Stowers et al., 2002; Kimchi et al., 2007) that a functional VNO is necessary for sex discrimination in both male and female mice. Second, previous studies (Pankevich et al., 2004; Keller et al., 2006) were disregarded by Kimchi et al. (2007) because, they argued, blood clots resulting from VNO removal surgery likely occluded the nasal sinuses so as to render subjects anosmic. Clearly, the fact that lesioned and sham-operated subjects in all three of the present experiments successfully discriminated between different volatile urinary odors indicates that they were not anosmic. Additionally, histological analysis of the snouts of VNOx and VNOi animals from experiments 1 and 3 revealed that even when small blood clots were present (in 4 of 36 females given VNOx or VNOi surgery), investigation times in the habituation/dishabituation tests were not attenuated compared with subjects that were completely free of sinus blood clots at the completion of the study.
When male and female stimulus animals were introduced individually in separate tests (experiment 3), we observed more mounting and pelvic thrusting toward the female stimulus animals in both VNOi and VNOx subjects than was seen in experiments 1 and 2 when social stimuli were presented simultaneously. Interestingly, Fang and Clemens (1999) reported that ovariectomized E2+P-treated female rats showed robust female-oriented mounting behavior, which was dramatically reduced in the presence of a male rat. Little pelvic-thrusting behavior was displayed by female subjects toward castrated male stimulus animals in any of our studies. This likely reflects the resistance to female mounts shown by outbred Swiss Webster male mice, even after castration. As shown in supplemental Figure S4 (available at www.jneurosci.org as supplemental material), while VNOi and VNOx subjects mounted both male and female stimulus animals a similar number of times, the total time spent mounting the female (∼12.7% and ∼12.5% of the total time, respectively) versus the male (∼0.3% and ∼0.6%, respectively) stimulus animal was dramatically different, indicating that subjects typically were not able to sustain male mounts long enough to show pelvic-thrusting behavior.
It is unclear why robust male-typical sexual behavior was observed in female mice lacking a functional VNO, whereas little mounting or pelvic thrusting behavior was observed in control females studied by Kimchi et al. (2007). It is standard practice to monitor sexually dimorphic behaviors in gonadally intact subjects as well as gonadectomized subjects given adult sex hormones (Becker et al., 2005); however, Kimchi et al. (2007) conducted their experiments only in ovary-intact females. It is noteworthy that the TRPC2−/− females studied by Kimchi et al. (2007) had significantly elevated plasma levels of free testosterone compared with heterozygous controls. Although these levels were much lower than those typically observed in testes-intact male mice, the possibility remains that TRPC2−/− females (and perhaps VNOx females because well) were more responsive to testosterone than other groups of females. When we clamped circulating testosterone in our ovariectomized VNOx and VNOi subjects as well as in our ovariectomized AOBx and AOBi subjects, no enhancing effect of disrupted VNO signaling on the expression of male-typical sexual behavior was seen. Thus, the presence of circulating testosterone as opposed to the absence of VNO signaling was the main factor controlling the expression of male-typical sex behavior in our mice.
Kimchi et al. (2007) conducted their experiments using either TRPC2−/− mice bred on a mixed C57BL/6J × 129/Sv genetic background or VNOx mice from either this mixed background or a pure C57BL/6J inbred strain, whereas our experiments were performed using Swiss Webster outbred mice. Our results show that VNO-mediated inhibition of neural circuits controlling male-typical sex behavior is not a general characteristic of female mice, regardless of strain.
Although we observed no effect of disrupted VNO signaling on male-typical mating behavior, we did observe several other behavioral effects that corroborate previous reports of the effects of disrupting VNO function in mice of both sexes. In experiment 1, we corroborated the report of Keller et al. (2006) that VNOx female mice showed significantly reduced lordosis quotients compared with VNOi subjects after treatment with ovarian hormones. A similar reduction in lordosis behavior was also seen in AOBx female mice (experiment 2), suggesting that VNO signaling plays an essential role in regulating the neural circuits that control female-typical mating behavior. In experiment 3, both VNOi and VNOx subjects preferred to investigate volatile male versus estrous female urinary odors, presumably after their detection by the main olfactory system. However, when given direct nasal access to the stimuli, only VNOi subjects showed this preference. These observations support our previous conclusion (Pankevich et al., 2004; Keller et al., 2006) that VNO signaling motivates mice of both sexes to prolong their contact with opposite-sex nonvolatile pheromones so as to facilitate reproductive success.
Footnotes
This work was supported by National Institutes of Health Grant HD044897. We thank Dr. Geralyn Messerlian of Brown University for providing the serum testosterone radioimmunoassay measurements for this study and Drs. James Cherry, Julie Bakker, and Ningdong Kang for their assistance.
References
- Bakker J, Honda S, Harada N, Balthazart J. The aromatase knock-out mouse provides new evidence that estrogens are required for the development of the female brain. J Neurosci. 2002;22:9104–9112. doi: 10.1523/JNEUROSCI.22-20-09104.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker J, De Mees C, Douhard Q, Balthazart J, Gabant P, Szpirer J, Szpirer C. α-Fetoprotein protects the developing female mouse brain from masculinization and defeminization by estrogens. Nat Neurosci. 2006;9:220–226. doi: 10.1038/nn1624. [DOI] [PubMed] [Google Scholar]
- Ball J. The effect of testosterone on the sex behavior of female rats. J Comp Psychol. 1940;29:151–165. [Google Scholar]
- Baum MJ, Keverne EB. Sex difference in attraction thresholds for volatile odors from male and estrous female mouse urine. Horm Behav. 2002;41:213–219. doi: 10.1006/hbeh.2001.1749. [DOI] [PubMed] [Google Scholar]
- Baum MJ, Brown JJ, Kica E, Rubin BS, Johnson RS, Papaioannou VE. Effect of a null mutation of the c-fos proto-oncogene on sexual behavior of male mice. Biol Reprod. 1994;50:1040–1048. doi: 10.1095/biolreprod50.5.1040. [DOI] [PubMed] [Google Scholar]
- Beach FA. Execution of the complete masculine copulatory pattern by sexually receptive female rats. J Genet Psychol. 1942;60:137–142. [Google Scholar]
- Becker JB, Arnold AP, Berkley KJ, Blaustein JD, Eckel LA, Hampson E, Herman JP, Marts S, Sadee W, Steiner M, Taylor J, Young E. Strategies and methods for research on sex differences in brain and behavior. Endocrinology. 2005;146:1650–1673. doi: 10.1210/en.2004-1142. [DOI] [PubMed] [Google Scholar]
- Bruce HM. An exteroceptive block to pregnancy in the mouse. Nature. 1959;184:105. doi: 10.1038/184105a0. [DOI] [PubMed] [Google Scholar]
- Edwards DA, Burge KG. Early androgen treatment and male and female sexual behavior in mice. Horm Behav. 1971;2:49–58. [Google Scholar]
- Emery DE, Sachs BD. Ejaculatory pattern in female rats without androgen treatment. Science. 1975;190:484–486. doi: 10.1126/science.1174387. [DOI] [PubMed] [Google Scholar]
- Fang J, Clemens LG. Contextual determinants of female-female mounting in laboratory rats. Anim Behav. 1999;57:545–555. doi: 10.1006/anbe.1998.1025. [DOI] [PubMed] [Google Scholar]
- Halem HA, Baum MJ, Cherry JA. Sex difference and steroid modulation of pheromone-induced immediate early genes in the two zones of the mouse accessory olfactory system. J Neurosci. 2001;21:2474–2480. doi: 10.1523/JNEUROSCI.21-07-02474.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrada G, Dulac C. A novel family of putative pheromone receptors in mammals with a topographically organized and sexually dimorphic distribution. Cell. 1997;90:763–773. doi: 10.1016/s0092-8674(00)80536-x. [DOI] [PubMed] [Google Scholar]
- Keller M, Pierman S, Douhard Q, Baum MJ, Bakker J. The vomeronasal organ is required for the expression of lordosis behaviour, but not sex discrimination in female mice. Eur J Neurosci. 2006;23:521–530. doi: 10.1111/j.1460-9568.2005.04589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelliher KR, Spehr M, Li XH, Zufall F, Leinders-Zufall T. Pheromonal recognition memory induced by TRPC2-independent vomeronasal sensing. Eur J Neurosci. 2006;23:3385–3390. doi: 10.1111/j.1460-9568.2006.04866.x. [DOI] [PubMed] [Google Scholar]
- Key B, Giorgi PP. Soybean agglutinin binding to the olfactory systems of the rat and mouse. Neurosci Lett. 1986;69:131–136. doi: 10.1016/0304-3940(86)90591-4. [DOI] [PubMed] [Google Scholar]
- Kimchi T, Xu J, Dulac C. A functional circuit underlying male sexual behaviour in the female mouse brain. Nature. 2007;448:1009–1014. doi: 10.1038/nature06089. [DOI] [PubMed] [Google Scholar]
- Manning A, McGill TE. Neonatal androgen and sexual behavior in female house mice. Horm Behav. 1974;5:19–31. doi: 10.1016/0018-506x(74)90004-x. [DOI] [PubMed] [Google Scholar]
- Pankevich DE, Baum MJ, Cherry JA. Olfactory sex discrimination persists, whereas the preference for urinary odorants from estrous females disappears in male mice after vomeronasal organ removal. J Neurosci. 2004;24:9451–9457. doi: 10.1523/JNEUROSCI.2376-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaff D. Nature of sex hormone effects on rat sex behavior: specificity of effects and individual patterns of response. J Comp Physiol Psychol. 1970;3:349–358. doi: 10.1037/h0030242. [DOI] [PubMed] [Google Scholar]
- Phoenix CH, Goy RW, Gerall AA, Young WC. Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology. 1959;65:369–382. doi: 10.1210/endo-65-3-369. [DOI] [PubMed] [Google Scholar]
- Sato T, Matsumoto T, Kawano H, Watanabe T, Uematsu Y, Sekine K, Fukuda T, Aihara K, Krust A, Yamada T, Nakamichi Y, Yamamoto Y, Nakamura T, Yoshimura K, Yoshizawa T, Metzger D, Chambon P, Kato S. Brain masculinization requires androgen receptor function. Proc Natl Acad Sci U S A. 2004;101:1673–1678. doi: 10.1073/pnas.0305303101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah NM, Breedlove SM. Females can also be from Mars. Nature. 2007;448:999–1000. doi: 10.1038/nature05892. [DOI] [PubMed] [Google Scholar]
- Södersten P. Mounting behavior in the female rat during the estrous cycle, after ovariectomy, and after estrogen or testosterone administration. Horm Behav. 1972;3:307–320. [Google Scholar]
- Spors H, Sobel N. Male behavior by knockout. Neuron. 2007;55:689–693. doi: 10.1016/j.neuron.2007.08.016. [DOI] [PubMed] [Google Scholar]
- Stowers L, Holy TE, Meister M, Dulac C, Koentges G. Loss of sex discrimination and male-male aggression in mice deficient for TRPC2. Science. 2002;295:1493–1500. doi: 10.1126/science.1069259. [DOI] [PubMed] [Google Scholar]
- Vandenbergh JG. Acceleration and inhibition of puberty in female mice by pheromones. J Reprod Fertil Suppl. 1973;19:411–419. [PubMed] [Google Scholar]
- Wersinger SR, Sannen K, Villalba C, Lubahn DB, Rissman EF, De Vries GJ. Masculine sexual behavior is disrupted in male and female mice lacking a functional estrogen receptor α gene. Horm Behav. 1997;32:176–183. doi: 10.1006/hbeh.1997.1419. [DOI] [PubMed] [Google Scholar]
- Whitten WK. Occurrence of anoestrus in mice caged in groups. J Endocrinol. 1959;18:102–107. doi: 10.1677/joe.0.0180102. [DOI] [PubMed] [Google Scholar]
- Wysocki CJ, Wysocki LM. Surgical removal of the mammalian vomeronasal organ and its verification. In: Speilman AI, Brand JG, editors. Experimental cell biology of taste and olfaction. New York: CRC; 1995. pp. 49–57. [Google Scholar]