Abstract
A 15-year-old boy, who had recently arrived back from a trip to Cambodia for a missionary camp, presented with several serpiginous thread-like skin lesions that began as small papules on the left upper extremities 2 weeks before his visit to Hospital. The skin lesions were pruritic and erythematous, and had migrated to the chest and abdomen. The histopathological findings showed only lymphocytic and eosinophilic infiltrations in the dermis of the biopsied skin lesion. The patient's serum reacted strongly to the Ancylostoma caninum antigen by an ELISA method. Therefore, he was diagnosed with cutaneous larva migrans by A. caninum. After the oral administration of albendazole and ivermectin, the skin lesions resolved without recurrence. This is the first reported case of a cutaneous larva migrans caused by Ancylostoma canimum diagnosed serologically using ELISA in Korea.
Keywords: Cutaneous larva migrans, Ancylostoma caninum, ELISA
INTRODUCTION
Cutaneous larva migrans (CLM) is a creeping eruption of the skin, which is caused by an accidental infection and migration of nematode larvae, usually the hookworm from dogs, cats and other mammals, through the epidermis (Davies et al., 1993). Ancylostoma braziliense is the most common cause of CLM (Davies et al., 1993). Other skin penetrating nematode larvae include Ancylostoma caninum, Uncinaria stenocephala, Bunostomum phlebotomum, and Strongyloides stercoralis (Davies et al., 1993; Lucchina and Wilson, 1999; Alonso, 2000). Sometimes other parasites including Gnathostoma spinigerum, Strongyloides procyonis, Dirofilaria repens, and some forms of myiasis can cause migratory skin lesions.
The third-stage larvae penetrate the human skin and migrate up to several centimeters a day, usually between the stratum germinativum and stratum corneum (Jang et al., 1995; Elder et al., 1997), which induces a localized eosinophilic inflammatory reaction in humans (Elder et al., 1997). Most larvae are unable to undergo further development or to invade deeper tissues and usually die within days or up to several months. The most common anatomic sites (usually 3 to 4 cm from the penetration site) include the feet, the buttocks, and the genitalia. The larval penetration and migration through the tissue produce a characteristic serpiginous skin eruption, which is usually erythematous, and is sometimes vesicular at the advancing end. Intense pruritus occurs frequently, and constant scratching may lead to secondary bacterial infections.
CLM is distributed worldwide, but is most commonly observed in many subtropical and tropical coastal areas, such as the southeastern US, Central and South America, Africa and other tropical areas. Occasionally, cases have been reported in non-endemic areas, but usually these occur in patients who have previously travelled to a tropical area. In Korea, more than 12 cases of CLM have been reported thus far, most of which had travelled to tropical areas such as Thailand, Vietnam and the Philippines (Jang et al., 1995; Lim et al., 1996; Chang et al., 1999; Kim et al., 1999; Suh et al., 2003). We report a case of CLM due to A. caninum that was believed to be the result of an infection obtained in Cambodia during a missionary camp and was diagnosed serologically using ELISA.
CASE RECORD
In July 2002, a 15-year-old boy visited the Seoul National University Hospital with a skin eruption on his left upper extremity, chest and abdomen, which had been presented for 2 weeks. The skin lesions were linear, serpiginous, elevated and erythematous (Fig. 1). It produced an intense itching sensation, and scaly plaque was observed on the surface of the lesions. He had returned from a trip to Cambodia for a missionary camp one week before the skin lesions erupted, but he denied any risk activities of cutaneous larva migrans such as walking barefoot on the ground or sun-bathing at the beach during his stay in Cambodia.
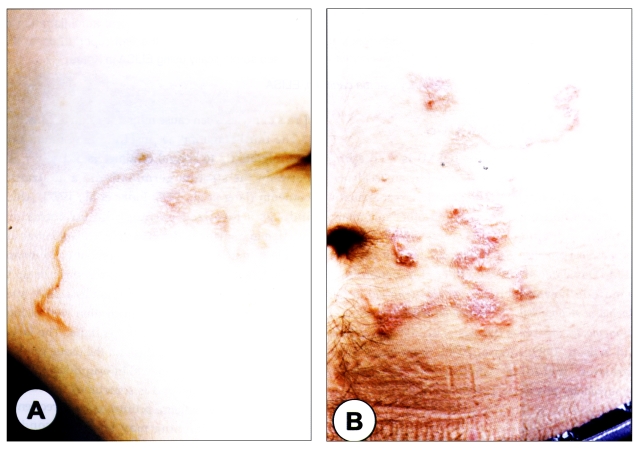
Fig. 1.
Erythematous linear elevated serpiginous plaques on the left upper extremities (A) and the abdomen (B).
The laboratory findings revealed an increased number of WBC (19,060/mm3) with a normal eosinophil count and normal serum IgE level. A stool examination showed no eggs of the parasites and the chest X-ray was within the normal limits. A pathological examination on the biopsied specimen showed only dermal patchy infiltrations of lymphocytes and eosinophils, and neither the worm section nor evidence of tunnel formation by the migrating larvae were found (Fig. 2). The patient s serum was serologically negative by multiple-dot ELISA using 12 parasite antigens: they were Dirofilaria immitis, Toxocara canis, Ascaris suum, Anisakis simplex, Gnathostoma doloresi, Strongyloides ratti, Paragonimus westermani, Paragonimus miyazakii, Fasciola hepatica, Clonorchis sinensis, Spirometra erinacei, and Cysticercus cellulosae. However, by microplate ELISA using antigens of G. doloresi and A. caninum, the patient's serum showed positive reaction against the antigen of A. caninum, but did not against Gnathostoma antigen (Table 1). Therefore, he was serologically diagnosed with cutaneous larva migrans caused by a larval infection of A. caninum. After an oral treatment with 400 mg/day of albendazole for three days and ivermectin at a single dose of 150 µg/kg, the skin lesions resolved without recurrence after a follow up period of 6 months.
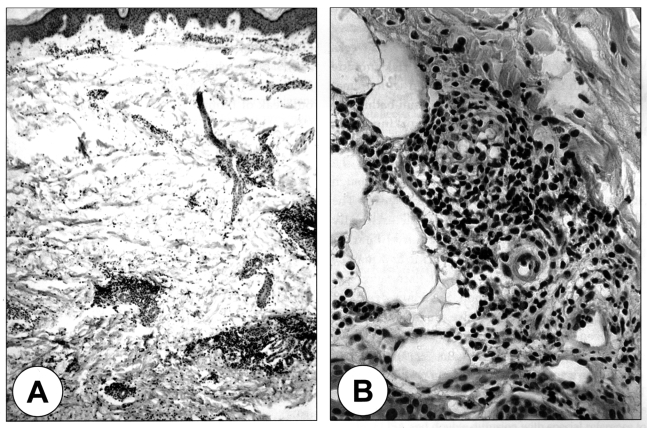
Fig. 2.
A. Patchy inflammatory cellular infiltrations in the dermis of the lesion. H & E stain, × 40. B. Magnification of the inflammatory lesion, which is composed of mostly lymphocytes and eosinophils. × 200.
Table 1.
Absorbances of the patient's sera against the Gnathostoma dorolesi and Ancylostoma caninum antigens by ELISAa)
a)The absorbance was measured at 490 nm in an ELISA reader, and an absorbance > 0.30 was considered serologically positive.
DISCUSSION
Human are usually an aberrant, dead-end host, and acquire the parasite by skin contact with infective larvae of animal hookworms in the soil. The infective larvae may remain viable in the soil for several weeks (Jones, 1993). Activities that pose a risk include contact with sand or soil contaminated with animal feces, such as playing in a sandbox, sun-bathing or walking barefoot on a beach, and working in the crawl spaces under houses (Pardo and Kerdel, 1992).
The incubation period is usually 1 to 6 days. The eruption usually lasts between 2 to 8 weeks, although it has been reported to last for up to 2 years. The lesions, single or multiple, are characteristically erythematous, raised and vesicular, linear or serpentine. They are intensely pruritic and may be painful (Lucchina and Wilson, 1999). Excoriation and impetiginization are common. Systemic signs and symptoms such as wheezing, dry cough, and urticaria have been reported in patients with an extensive infection (Lucchina and Wilson, 1999). The only common systemic sign is a moderate peripheral blood eosinophilia (Alonso, 2000).
The confirmatory diagnosis of CLM requires isolation of the larvae from the lesion. However, the organisms are rarely if ever recovered by a biopsy, therefore, a diagnosis of CLM is based on the characteristic skin lesions as well as the exposure history (Jones, 1993; Jang et al., 1995; Lim et al., 1996; Chang et al., 1999). In this case, the serpiginous skin lesions were characteristic of CLM, but the patient denied being exposed to larval infection while he stayed in Cambodia. Moreover, there is no elevation in the eosinophil count, and we could not find the characteristic pathological findings of CLM. Therefore, ELISA was performed on the patient's serum, which reacted with A. caninum antigens. As far as the authors are aware, this is the first case of a CLM that was diagnosed serologically by ELISA in Korea.
In the present case, the possibility of CLM due to Gnathostoma larvae was excluded, because the patient denied eating raw freshwater fish both in Cambodia and Korea, and his serum did not react strongly with the antigen of G. doloresi. Although the sensitivity or specificity was lower than when using homologous antigens, the crude extract of adult G. doloresi worms has been shown to be useful for the diagnosis of gnathostomiasis due to G. hispidum and G. spinigerum (Tada et al., 1987). The diagnostic usefulness of G. doloresi antigen has been verified in 300 cases of CLM in Mexico (Diaz Camacho et al., 1998) and in an outbreak of human gnathostomiasis in Miyanmar (Chai et al., 2003).
CLM is a self-limited process, which means that therapy is optional. However, the unpredictable duration and severity generally leads to intervention. The suggested treatment options include local cryosurgery, topical or oral thiabendazole, oral albendazole, or oral ivermectin (Stone and Mullins, 1965; Bouchaud et al., 2000; Blackwell and Vega-Lopez, 2001). Physical modalities for CLM such as cryotherapy and surgical excision are often ineffective, as the larva is usually 1-2 cm ahead of the visible tract and can easily be missed, even though these modalities may be appropriate in pregnancy (Blackwell and Vega-Lopez, 2001). Oral thiabendazole has been reported to have a very high efficacy and is usually given at a dose of 25 mg/kg twice daily for 3 days (Stone and Mullins, 1965). The pruritus subsides by the first day, and the lesions clear within 1 to 2 weeks. However, there is a relatively high incidence of side-effects including nausea, anorexia, headache and gastrointestinal disturbances (Stone and Mullins, 1965). Oral albendazole and topical thiabendazole as a 15% cream are also effective, particularly in the early phase. More recently, ivermectin has been used to treat CLM in a single dose of 150-200 µg/kg (Bouchaud et al., 2000). Both thiabendazole and albendazole have been shown to be teratogenic in animals and are therefore contraindicated in pregnancy (Blackwell and Vega-Lopez, 2001).
As the international travel becomes more common, people are at a higher risk of contracting infectious diseases during their travel. In Korea, CLM is an imported infectious disease rather than an indigenous one. Therefore, clinicians should pay more attention to patients presenting a creeping skin eruption, who had recently travelled abroad, particularly to tropical or subtropical areas.
References
- 1.Alonso FF. Cutaneous larva migrans. In: Harper J, Oranje A, Neil P, editors. Textbook of pediatric dermatology. Oxford, UK: Blackwell Science; 2000. pp. 527–530. [Google Scholar]
- 2.Blackwell V, Vega-Lopez F. Cutaneous larva migrans: clinical features and management of 44 cases presenting in the returning traveller. Br J Dermatol. 2001;145:434–437. doi: 10.1046/j.1365-2133.2001.04406.x. [DOI] [PubMed] [Google Scholar]
- 3.Bouchaud O, Houze S, Schiemann R, et al. Cutaneous larva migrans in travelers: a prospective study, with assessment of therapy with ivermectin. Clin Infect Dis. 2000;31:493–498. doi: 10.1086/313942. [DOI] [PubMed] [Google Scholar]
- 4.Chai JY, Han ET, Shin EH, et al. An outbreak of gnathostomiasis among Korean emigrants in Miyanmar. Am J Trop Med Hyg. 2003;69:67–73. [PubMed] [Google Scholar]
- 5.Chang SE, Seo CW, Choi JH, Sung KJ, Moon KC, Koh JK. A case of cutaneous larva migrans showing a larva on biopsy. Korean J Dermatol. 1999;37:547–549. [Google Scholar]
- 6.Davies HD, Sakuls P, Keystone JS. Creeping eruption. A review of clinical presentation and management of 60 cases presenting to a tropical disease unit. Arch Dermatol. 1993;129:588–591. doi: 10.1001/archderm.129.5.588. [DOI] [PubMed] [Google Scholar]
- 7.Diaz Camacho SP, Zazueta Ramos M, Ponce Torrecillas E, et al. Clinical manifestations and immunodiagnosis of gnathostomiasis in Culiacan, Mexico. Am J Trop Med Hyg. 1998;59:908–915. doi: 10.4269/ajtmh.1998.59.908. [DOI] [PubMed] [Google Scholar]
- 8.Elder D, Elenitsas R, Jaworsky C, Johnson BJ. Lever's histopathology of the skin. 8th ed. Philadelphia, UK: Lippincott-Raven; 1997. pp. 560–561. [Google Scholar]
- 9.Jang IK, Lee DW, Cho BK. A case of cutaneous larva migrans. Korean J Dermatol. 1995;33:396–400. [Google Scholar]
- 10.Jones WB., 2nd Cutaneous larva migrans. South Med J. 1993;86:1311–1313. doi: 10.1097/00007611-199311000-00032. [DOI] [PubMed] [Google Scholar]
- 11.Kim JW, Kim DJ, Kim IH, Song HJ, Oh CH. A case of cutaneous larva migrans. Korean J Dermatol. 1999;37:423–426. [Google Scholar]
- 12.Lim MH, Lee SC, Won YH, Chun IK. A case of creeping eruption. Korean J Dermatol. 1996;34:485–488. [Google Scholar]
- 13.Lucchina LC, Wilson ME. Cysticercosis and other helminthic infections. In: Freedberg IM, Eisen AZ, Wolff K, Austen KF, Goldsmith LA, Katz SI, editors. Dermatology in general medicine. New York, USA: McGraw-Hill; 1999. pp. 87–100. [Google Scholar]
- 14.Pardo RJ, Kerdel FA. Parasites, arthropod, and hazardous animals of dermatologic significance. In: Moshella SL, Hurley HJ, editors. Dermatology. Philadelphia, USA: W.B. Saunders Company; 1992. pp. 2005–2009. [Google Scholar]
- 15.Stone OJ, Mullins JF. Thiabendazole effectiveness in creeping eruption. Arch Dermatol. 1965;91:427–429. doi: 10.1001/archderm.1965.01600110013004. [DOI] [PubMed] [Google Scholar]
- 16.Suh HS, Jee MS, Kim HH, et al. Two cases of cutaneous larva migrans. Korean J Dermatol. 2003;41:92–95. [Google Scholar]
- 17.Tada I, Araki T, Matsuda H, Araki K, Akahane H, Mimori T. A study on immunodiagnosis of gnathostomiasis by ELISA and double diffusion with special reference to the antigenicity of Gnathostoma doloresi. Southeast Asian J Trop Med Public Health. 1987;18:444–448. [PubMed] [Google Scholar]