Abstract
The modes of action of antibiotics are mainly characterized by their effects on their targets. Previously,1,2 and in a recent paper,3 we have reported our discovery of a new mechanism for the action of some antibiotics. Rather than directly interfering with a vital bacterial pathway, these antibiotics act by triggering the bacterial toxin-antitoxin chromosomal module mazEF, thereby causing the bacteria to commit suicide. We also showed that antibiotics that inhibit transcription and/or translation cause mazEF-mediated cell death by forming Reactive Oxygen Species (ROS).3 Moreover, we found that after treatment by such antibiotics, the mazEF system cannot be activated, and thus ROS cannot be formed, without the presence of communication signaling peptide called the Extracellular Death Factor (EDF). Our results challenge the classical division between bacteriostatic and bactericidal antibiotics. Our study further provides evidence that mode of action of antibiotics may also be determined by the ability of the bacteria to communicate through the signaling peptide EDF. In this Addendum article we present a model of how the presence of some antibiotics may result in this novel downstream pathway.
Key words: antibiotics, toxin-antitoxin, mazEF, quorum sensing, bacterial communication, ROS, EDF
As multi-drug resistance among bacterial pathogens is becoming more and more widespread, the scientific and medical communities are desperately seeking new antibiotics.4,5
Currently, the most common approach is to create a second generation of these antibiotics in which the activities of the existing antibiotics are improved.4 However, this approach is also limiting. Probably, the only solution to the lack of new antibiotics will be through a deeper understanding of the events occurring downstream to the antibiotics-target interaction in the bacterial cell.
Traditionally, antibiotics have been divided into two separate groups: (i) bacteriostatic antibiotics inhibit cellular functions and thus lead to the arrest of cell growth; (ii) bactericidal antibiotics cause cell death.6 The mechanisms of antibiotic action have mainly been studied in relation to their primary target interactions. Three main kinds of mechanisms have been distinguished: Inhibitors of protein synthesis, DNA damage-causing agents and inhibitors of cell wall biosynthesis.7 Only more recently, the mechanisms of the action of antibiotics has been studied in relation to common downstream mechanisms, like ROS-formation, that lead to cell death.3,8 We have reported on one such ROS-formation pathway triggered by antibiotics in E. coli.3 In this pathway, the outcome of the action of the antibiotics appears to be unique: instead of preventing one or more vital bacterial cellular pathway, the antibiotic activates a built-in bacterial death pathway that we have called the E. coli mazEF programmed cell death system.3 mazEF is a toxin-antitoxin (TA) module found on the chromosomes of many bacteria including pathogens.9–13 E. coli mazF specifies for the stable toxin MazF, and mazE specifies for the labile antitoxin, MazE.9 Since MazE is a labile protein, preventing MazF-mediated lethal action requires the continuous production of MazE. Thus, any stressful condition that prevents the expression of the chromosomally borne mazEF module will lead to the reduction of MazE in the cell, permitting the toxin MazF to act freely.9,14,15 Such stressful conditions can be caused by (i) antibiotics, like rifampicin, that inhibit transcription and/or translation,1 and (ii) those, like nalidixic acid, that cause DNA damage.15–18 We found that antibiotics of both groups cause: (a) mazEF-mediated cell death; and (b) the production of ROS through MazF action.3 In addition, we found that antibiotics in the first group cause mazEF-mediated cell death that is ROS-dependent.3 Moreover, we have recently discovered that E. coli mazEF-mediated cell death is a population phenomenon, which occurs in a dense culture but not in a diluted culture.19 This because for mazEF activation a quorum sensing communication molecule called Extracellular Death Factor (EDF) is also required. EDF is the linear penta-peptide NNWNN.19
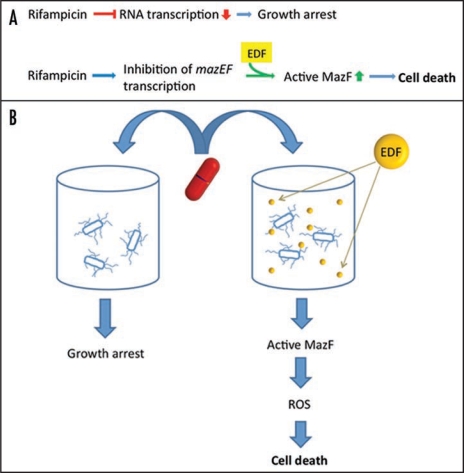
Perhaps our most striking discovery is that adding synthetic EDF to an E. coli mazEF+ strain that is defective in the production of EDF causes a switch in the anti-bacterial action of the transcriptional inhibitor rifampicin (Figs. 1A and 5 in ref. 3). Activation of the EDF-mazEF system causes rifampicin, that is normally bacteriostatic, only causing growth arrest, to become bactericidal, causing cell death. (Fig. 1A and B). We further confirmed the fundamental role of the communication factor EDF in the bactericidal action of rifampicin by showing that adding the inhibitor of EDF (iEDF) along with EDF neutralized the action of EDF so that under those conditions, rifampicin remains bacteriostatic (Fig. 5E in ref. 3).
Figure 1.
Traditionally, rifampicin was thought to arrest the growth of bacterial cultures by generally inhibiting cellular transcription by interfering with the activity of its primary target, RNA polymerase. Previously,1,2 and in our recent paper,3 we showed that rifampicin prevents the expression of the chromosomally borne mazEF module and leads to the reduction of MazE in the cell, permitting toxin MazF to act freely. The activation of MazF requires the quorum sensing factor Extracellular Death Factor (EDF) (in yellow). (B) Graphic illustration of (A). Following treatment by rifampicin, EDF, the communication signaling peptide, activates the mazEF system, resulting in ROS formation and subsequently in cell death.
In summary, we deal with two novel aspects of the modes of action of antibiotics: (a) The results of our study3 challenge the classical division between bacteriostatic and bactericidal antibiotics: under some conditions, we found that bacteriostatic antibiotics could become bactericidal; (b) We reported,3 for the first time, that the mode of action of antibiotics can be determined by the ability of the bacteria to communicate through a bacterial communication signaling molecule (Fig. 1B). Thus, we have introduced novel conceptual dimensions to our understanding of the modes of action of various antibiotics. Moreover, more practically, we offer a method that may become the basis for designing new, much needed, antibiotic drugs.
Acknowledgements
We thank F.R. Warshaw-Dadon (Jerusalem, Israel) for her critical reading of the manuscript. This research was supported by grant No. 177/07 from the Israel Science Foundation (ISF) administrated by the Israel Academy of Science and Humanities, and by grant No. 2005029 from United State-Israel Bi-national Science Foundation (BSF), and by Grant Number GM069509 of the National Institute of Health (NIH-USA).
Abbreviations
- ROS
reactive oxygen species
- EDF
extracellular death factor
Footnotes
Previously published online as a Communicative & Integrative Biology E-publication: http://www.landesbioscience.com/journals/cib/article/7876
References
- 1.Sat B, Hazan R, Fisher T, Khaner H, Glaser G, Engelberg-Kulka H. Programmed cell death in Escherichia coli: some antibiotics can trigger mazEF lethality. J Bacteriol. 2001;183:2041–2045. doi: 10.1128/JB.183.6.2041-2045.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Engelberg-Kulka H, Sat B, Reches M, Amitai S, Hazan R. Bacterial programmed cell death as a target for antibiotics. Trends Microbiol. 2004;12:66–71. doi: 10.1016/j.tim.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 3.Kolodkin-Gal I, Sat B, Keshet A, Engelberg- Kulka H. Communication factor EDF and the toxin-antitoxin mazEF determine the mode of action of antibiotics. PLoS Biology. 2008;6:319. doi: 10.1371/journal.pbio.0060319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taubes G. The bacteria fight back. Science. 2008;321:356–361. doi: 10.1126/science.321.5887.356. [DOI] [PubMed] [Google Scholar]
- 5.Payne DJ. Desperately seeking new antibiotics. Science. 2008;321:1644–1645. doi: 10.1126/science.1164586. [DOI] [PubMed] [Google Scholar]
- 6.Pankey GA, Sabath LD. Clinical relevance of bacteriostatic versus bactericidal mechanisms of action in the treatment of Gram-positive bacterial infection. Clin Infect Dis. 2004;38:864–870. doi: 10.1086/381972. [DOI] [PubMed] [Google Scholar]
- 7.Walsh C. Molecular mechanisms that confer antibacterial drug resistance. Nature. 2000;406:775–781. doi: 10.1038/35021219. [DOI] [PubMed] [Google Scholar]
- 8.Kohanski MA, Dwyer DJ, Hayate B, Lawrence CA, Collins JJ. A common mechanism of cellular death induced by bacterial antibiotics. Cell. 2007;130:797–810. doi: 10.1016/j.cell.2007.06.049. [DOI] [PubMed] [Google Scholar]
- 9.Aizenman E, Engelberg-Kulka H, Glaser G. An Escherichia coli chromosomal “addiction module” regulated by guanosine 3′,5′-bispyrophosphate: a model for programmed bacterial cell death. Proc Natl Acad Sci USA. 1996;93:6059–6063. doi: 10.1073/pnas.93.12.6059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mittenhuber G. Occurrence of mazEF-like antitoxin/toxin systems in bacteria. J Mol Microbiol Biotechnol. 1999;1:295–302. [PubMed] [Google Scholar]
- 11.Engelberg-Kulka H, Sat B, Hazan R. Bacterial programmed cell death and antibiotics. ASM News. 2002;67:617–625. [Google Scholar]
- 12.Pandey DP, Gerdes K. Toxin-antitoxin loci are highly abandan in free-living but lost from host-associated prokaryotes. Nucl Acids Res. 2005;33:966–976. doi: 10.1093/nar/gki201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hayes F. Toxins-antitoxins: plasmid maintenance, programmed cell death and cell cycle arrest. Science. 2003;301:1496–1499. doi: 10.1126/science.1088157. [DOI] [PubMed] [Google Scholar]
- 14.Sat B, Reches M, Engelberg-Kulka H. The Escherichia coli mazEF suicide module mediates thymineless death. J Bacteriol. 2003;185:1803–1807. doi: 10.1128/JB.185.6.1803-1807.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hazan R, Sat B, Engelberg-Kulka H. Escherichia coli mazEF-mediated cell death is triggered by various stressful conditions. J Bacteriol. 2004;186:3663–3669. doi: 10.1128/JB.186.11.3663-3669.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Engelberg-Kulka H, Amitai S, Kolodkin-Gal I, Hazan R. Programmed cell death and multicellular behavior in bacteria. PLoS Genet. 2006;2:1518–1526. doi: 10.1371/journal.pgen.0020135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Godoy VG, Jarosz DF, Walker FL, Siommons LA, Walker GC. Y-family DNA polymerase respond to DNA damage-independent inhibition of replication fork progression. EMBO J. 2006;25:868–879. doi: 10.1038/sj.emboj.7600986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kolodkin-Gal I, Engelberg-Kulka H. Induction of Escherichia coli chromosomal mazEF by stressful conditions causes an irreversible loss of viability. J Bacteriol. 2006;188:3420–3423. doi: 10.1128/JB.188.9.3420-3423.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kolodkin-Gal I, Hazan R, Gaathon A, Carmeli S, Engelberg-Kulka H. A linear penta-peptide is a quorum sensing factor required for mazEF-mediated cell death in Escherichia coli. Science. 2007;318:652–655. doi: 10.1126/science.1147248. [DOI] [PubMed] [Google Scholar]