Abstract
The delivery of active molecules into cells requires the efficient translocation of the plasma membrane barrier. Penetratin is a promising cell penetrating peptide is which crosses the cell membrane by a receptor and metabolic energy-independent mechanism. In previous work, we have shown that basic peptides induce membrane invaginations (i.e., tubes formation by induction of negative curvature of membranes) suggesting a new mechanism for cellular uptake of cell penetrating peptides: “physical endocytosis”. These effects on membrane curvature are favored in pure liquid disordered but not in pure liquid ordered (raft-like) membrane domains. Herein, we present experiments in heterogeneous membranes composed of mixed domains. The results show that Penetratin is able to induce invaginations in membranes in which liquid ordered and liquid disordered membranes coexist. We suggest that Penetratin is able to recruit specific lipids locally forming fluid membrane patches dispersed inside a liquid ordered membrane zone resulting in the invagination of tubes composed of heterogeneous membrane domains.
Key words: membrane invagination, membrane domains, penetratin, physical endocytosis
Penetratin, a peptide derived from the Antennapedia homeoprotein binding domain1 is one of the first cell penetrating peptides (CPP) used to introduce active molecules into cells.2 However, the mechanisms of CPPs membrane translocation are still in debate. Several models were proposed to explain the cellular uptake of CCPs: direct membrane penetration,3 inverted micelle formation,4 penetration by endocytosis5 or macropinocytosis6 and the translocation thanks to the neutralization of arginin residues by complexation with the phosphate groups of phospholipids.7 The high diversity of molecular hypothesis for the role of energy-independent steps in cell uptake results in controversial data.8 In experiments with Giant unilamellar vesicles (GUV) composed of phosphatidylcholine (PC) and phosphatidylglycerol (PG) (9/1), different basic peptides were shown to invaginate membranes in the form of narrow tubular structures.9–11 This metabolic energy-independent “physical endocytosis” was suggested to be a new mechanism underlying the cellular uptake of basic cell penetrating peptides. This tubulation was observed with GUV in the fluid liquid disordered sate (PC or PC/PG) but not in GUV with a pure rigid liquid ordered raft-like membrane (sphingomyelin (SM) and cholesterol (Chol).12 However, the addition of 10% PG in the ordered membranes (SM/Chol/PG) induces peptide aggregation at the membrane surface and an accumulation of vesicles (grapes). We have interpreted the occurrence of these grape-like vesicular structures as the result of the peptide inefficiency to support the “normal” elongation of the tubes on a quite rigid ordered membrane.
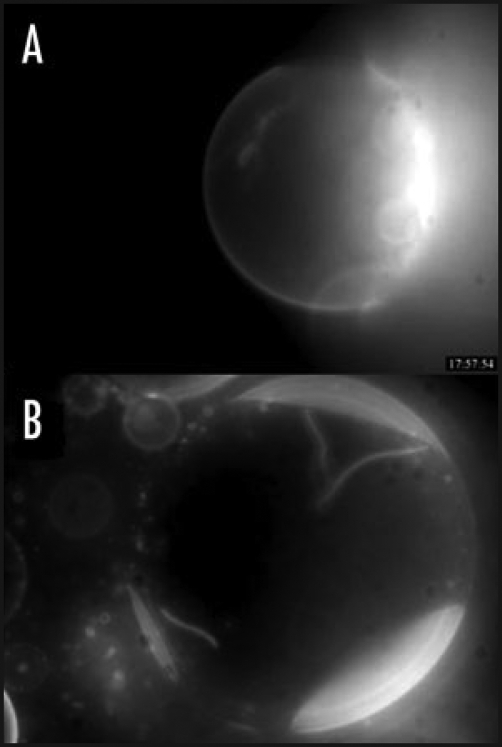
Herein, we investigated the action of Penetratin on heterogeneous membranes in which both; liquid ordered and liquid disordered domains coexist. Figure 1A shows a vesicle containing three different lipids: PC a lipid that favors the liquid disordered state of the membrane bilayer, SM and cholesterol that associate to form liquid ordered lamellar phases (raft domains). The fluorescent liquid disordered marker Di-Q (4-(p-(dihexadecylamino)styryl)-N-methylquinolinium iodide) (Ex/Em: 562/600+) purchased from Molecular Probes was added (1%) to distinguish the different lamellar phases. Thus the ordered zone is dark and the disordered membrane is fluorescent. In these GUV Penetratin was able to induce the formation of fluorescent tubes (liquid disordered membranes) in agreement with a precedent work.12 In this case the presence of small amounts of SM or cholesterol inside the PC domain cannot be excluded. Figure 1B shows a vesicle of the same composition (without Di-Q) containing the liquid ordered domain lipid marker GM1 (1%). To observe the different domains, we added a rhodamine-labelled cholera toxin which is a GM1 ligand. In this case the ordered domain is fluorescent and the disordered membrane dark. As shown, Penetratin induces the formation of tubes containing the cholera-toxin associated GM1 lipid.
Figure 1.
Penetratin induced tubulation in heterogeneous GUVs. (A) PC/SM/Chol/Di-Q (60/20/20/1) GUV showing the fluorescent liquid disordered domain (fluorescent Di-Q is a marker of liquid disordered domains), and a dark non fluorescent liquid ordered domain in the top of the GUV. The fluorescent tubes inside indicate that Penetratin is able to induce invaginations of membrane disordered domains. (B) PC/SM/Chol/GM1 (60/20/20/1) GUV showing the dark non fluorescent liquid disordered domain, and three fluorescent liquid ordered domains. The fluorescence from rhodamine-labelled cholera toxin binds specifically to the liquid ordered lipid marker GM1. The fluorescent tubes inside indicate that Penetratin is able to induce invaginations containing cholera toxin associated membrane ordered domains.
This result seems in contradiction with the fact that in pure liquid ordered membranes the peptide was unable to induce tubes.12 The explanation of this apparent paradox resides in the following assumption. If tubulation was due to the peptide capacity to induce negative curvature in fluid membranes, then this fluorescent tube must contain a mixture of lipids that reduces the rigidity of the ordered domain. In other words, these data suggest the formation of lipid-peptide complexes containing PC creating small fluid membrane zones coexisting inside a raft-like domain. This small local perturbation of the ordered domain would facilitate the observed membrane deformation. Fretz has shown that cholesterol depletion with methyl-beta-cyclodextrin of cell plasma membrane increases polyarginine (R8) uptake13 possibly due to the increase in the amount of the liquid disordered membrane domains proportion. Our data suggest that for the cell penetration of Penetratin, the preferential cellular membrane target would be the non-raft fluid plasma membrane domains. However, in membranes in which coexist fluid disordered domains with rigid ordered domains (the typical situation in biological membranes), a small fraction of lipids that favor the disordered domains render the membrane competent for tubulation. The remaining interesting question is whether the small disordered zones pre-exist inside an ordered domain, or whether the peptide recruits lipids that increase the fluidity inside the ordered domain. In conclusion, this mechanism could explain in part the capacity of Penetratin and other CPPs to be internalized by a metabolic energy independent pathway in the different cellular membrane domains.
Acknowledgements
This work was supported by CNRS and an ANR-PCV grant.
Abbreviations
- Chol
cholester
- CPP
cell penetrating peptide
- GUV
giant unilamellar vesicle
- PC
phosphatidylcholine
- PG
phosphatidylglycerol
- SM
sphingomyelin
Footnotes
Previously published online as a Communicative & Integrative Biology E-publication: http://www.landesbioscience.com/journals/cib/article/8073
References
- 1.Joliot A, Pernelle C, Deagostini-Bazin H, Prochiantz A. Antennapedia homeobox peptide regulates neural morphogenesis. Proc Natl Acad Sci USA. 1991;88:1864–1868. doi: 10.1073/pnas.88.5.1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perez F, Lledo PM, Karagogeos D, Vincent JD, Prochiantz A, Ayala J. Rab3A and Rab3B carboxy-terminal peptides are both potent and specific inhibitors of prolactin release by rat cultured anterior pituitary cells. Mol Endocrinol. 1994;8:1278–1287. doi: 10.1210/mend.8.9.7838160. [DOI] [PubMed] [Google Scholar]
- 3.Su Y, Mani R, Hong M. Asymmetric insertion of membrane proteins in lipid bilayers by solid-state NMR paramagnetic relaxation enhancement: A cell-penetrating peptide example. J Am Chem Soc. 2008;130:8856–8864. doi: 10.1021/ja802383t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Derossi D, Calvet S, Trembleau A, Brunissen A, Chassaing G, Prochiantz A. Cell internalization of the third helix of the Antennapedia homeodomain is receptor-independent. J Biol Chem. 1996;271:18188–18193. doi: 10.1074/jbc.271.30.18188. [DOI] [PubMed] [Google Scholar]
- 5.Drin G, Cottin S, Blanc E, Rees AR, Temsamani J. Studies on the internalization mechanism of cationic cell-penetrating peptides. J Biol Chem. 2003;278:31192–31201. doi: 10.1074/jbc.M303938200. [DOI] [PubMed] [Google Scholar]
- 6.Khalil IA, Kogure K, Futaki S, Harashima H. High density of octaarginine stimulates macropinocytosis leading to efficient intracellular trafficking for gene expression. J Biol Chem. 2006;281:3544–3551. doi: 10.1074/jbc.M503202200. [DOI] [PubMed] [Google Scholar]
- 7.Rothbard JB, Jessop TC, Lewis RS, Murray BA, Wender PA. Role of membrane potential and hydrogen bonding in the mechanism of translocation of guanidinium-rich peptides into cells. J Am Chem Soc. 2004;126:9506–9507. doi: 10.1021/ja0482536. [DOI] [PubMed] [Google Scholar]
- 8.Vives E. Present and future of cell-penetrating peptide mediated delivery systems: “is the Trojan horse too wild to go only to Troy?“. J Control Release. 2005;109:77–85. doi: 10.1016/j.jconrel.2005.09.032. [DOI] [PubMed] [Google Scholar]
- 9.Menger FM, Seredyuk VA, Kitaeva MV, Yaroslavov AA, Melik-Nubarov NS. Migration of poly-L-lysine through a lipid bilayer. J Am Chem Soc. 2003;125:2846–2847. doi: 10.1021/ja021337z. [DOI] [PubMed] [Google Scholar]
- 10.Lamaziere A, Chassaing G, Trugnan G, Ayala-Sanmartin J. Transduction peptides: structural-functional analyses in model membranes. J Soc Biol. 2006;200:229–233. doi: 10.1051/jbio:2006026. [DOI] [PubMed] [Google Scholar]
- 11.Lamazière A, Burlina F, Wolf C, Chassaing G, Trugnan G, Ayala-Sanmartin J. Non-metabolic membrane tubulation and permeability induced by bioactive peptides. PLoS ONE. 2007;2:201. doi: 10.1371/journal.pone.0000201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lamazière A, Wolf C, Lambert O, Chassaing G, Trugnan G, Ayala-Sanmartin J. The homeodomain derived peptide Penetratin induces curvature of fluid membrane domains. PLoS ONE. 2008;3:1938. doi: 10.1371/journal.pone.0001938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fretz MM, Penning NA, Al-Taei S, Futaki S, Takeuchi T, Nakase I, et al. Temperature-, concentration- and cholesterol-dependent translocation of L- and D-octa-arginine across the plasma and nuclear membrane of CD34+ leukaemia cells. Biochem J. 2007;403:335–342. doi: 10.1042/BJ20061808. [DOI] [PMC free article] [PubMed] [Google Scholar]