Abstract
Although hepatocellular carcinoma (HCC) is the liver cancer that requires repeated treatments because of a high tendency for recurrence, few data have been available about whether repeated treatments, including those to reduce tumor mass, are effective in prolonging survival. We retrospectively analyzed the effectiveness of tumor-mass-reduction therapy for the prognosis of patients with recurrent HCC. To analyze the effectiveness of various modalities of therapies with a single criterion, we defined a tumor-mass-reduction grade (TMRG), which was retrospectively evaluated by dynamic CT or MRI. Grade A: no evident HCC remains untreated; Grade B1: more than 50% of lesions are treated; and Grade B2: less than 50% of lesions are treated. Subjects were stratified by Child-Pugh classification and the number of admissions for HCC treatment. In those classified as Child-Pugh A, a better survival rate was obtained, depending on the degree of TMRG from the first to the fifth admission (P < .01), suggesting that these patients are endurable for repeated therapies and benefit from the many sessions of treatment. In those classified as Child-Pugh B, on the second to the fifth admissions, survival rates showed statistical difference depending on the TMRG (P < .01), which may suggest that only a few sessions of treatment are meaningful. In those classified as Child-Pugh C, any number of mass-reduction treatment sessions did not improve the survival rate. In conclusion, repeated tumor-mass-reduction therapies for recurrent HCC are most beneficial in Child-Pugh A patients. Patients with Child-Pugh B who experience several recurrence episodes and any patients with Child-Pugh C may benefit more from modalities other than tumor-mass-reduction therapies.
Keywords: Hepatocellular carcinoma, Tumor-mass-reduction, Child-Pugh classification, Hospitalization
Introduction
Hepatocellular carcinoma (HCC) is the most common malignant tumor in the liver, with high recurrence rates, either as intrahepatic metastasis or multicentric carcinogenesis [1–3]. In Japan, more than 90% of HCCs occur from chronic liver diseases caused by hepatitis B or hepatitis C virus infection. Development of local ablation methods such as radiofrequency ablation [4] and surgical resection [5] has remarkably reduced the rates of local recurrence, mainly adjacent to treated lesions. However, despite these therapeutic developments, HCC recurs frequently, because of multicentric carcinogenesis arising from an already cirrhotic liver and also because of insufficient treatment, consequently requiring repeated therapy.
However, a more complete local ablation may not be enough: for better outcome, the residual hepatic function should be maintained in a better state, because it can be the other factor determining the prognosis [6, 7]. For example, CLIP score, a new scoring system proposed by the Cancer of the Liver Italian Program, shows that both the residual hepatic function as evaluated by Child-Pugh score and tumor factors, including tumor morphology, AFP levels, and portal vein thrombosis, determine the prognosis [8–10]. From this viewpoint, a dilemma exists; treatments performed for HCC may influence, or even damage, liver function to some extent, thus worsening the prognosis than that without any treatments.
In spite of the fact that majority of HCC patients need repeated treatments for recurrent diseases, the survival benefit of such treatments, especially medical interventions just to reduce tumor development, remains uncertain. Moreover, many of the studies reported so far are somewhat impractical because in the clinical management of HCC, we choose to combine various methods of treatments to obtain the best results, and most studies address the contribution of only a single modality or combined therapies of a few modalities for prognosis [4, 11–14]. This lack of data is partly due to the existence of a variety of therapeutic options in the treatment of HCC, which complicates analysis, and the ethical consideration when dealing with patients whose lives are in jeopardy on account of malignant diseases.
However, regardless of the difficulty of analysis, we need to obtain data to answer this unresolved question: Are repeated treatments for HCC effective for the improvement of prognosis? To answer this question, we retrospectively analyzed 878 HCC patients in total admitted to Tohoku University Hospital from 1989 to 2003. As discussed in this article, our results support the idea that to treat recurrent HCC, we need to consider the number of times the patients were hospitalized and residual liver function.
Materials and methods
Study population
We enrolled 386 HCC patients who were admitted to Tohoku University Hospital from December 1989 to December 2003. Initially 320 patients were admitted to this hospital for their first treatment of HCC, and then 66 patients were newly referred to this hospital for the treatment of recurrent HCC. Most of patients experienced more than one hospitalization (median: 2.0; range: 1–14) owing to recurrence, so the total number of subjects who were admitted to the hospital for the treatment of HCC were 878. The diagnosis of HCC was performed by the combination of dynamic CT (or dynamic MRI), and tumor markers (AFP and DCP). Majority of patients who were diagnosed or suspected as having HCC were rendered to hepatic subtraction angiography (DSA), often to angio-CT (CTHA and CTAP [15, 16] for a definite diagnosis. If a lesion was difficult to be diagnosed as HCC by methods described above, it was further evaluated histologically (16 cases). Any subjects whose hepatic tumors are diagnosed as other than HCC or who have extrahepatic metastases at the entry were excluded from this study. The modalities of treatment include transcatheter arterial embolization (TAE) [14, 17–19], transcatheter arterial infusion (TAI) chemotherapy [20–24], percutaneus ethanol injection (PEI) [25], percutaneus microwave coagulation therapy (PMCT) [11], radiofrequency ablation (RFA) [26–28], radiation therapy (RT) [29–32], and hepatic resection [5]. No antiviral drugs were given as an adjuvant therapy to any patient in this study. Our principle in HCC treatment was to obtain an as sufficient control of HCC lesions (represented as necrosis or shrinkage of lesions) as possible and limiting the liver damage accompanying treatments as much as possible. To satisfy this principle, we chose either of these treatments or several modalities in combination. If we could not achieve complete necrosis because of the therapeutically difficult location, multiple distribution, poor liver function, or a poor general condition, we performed tumor-mass-reduction therapies as suboptimum treatments to reduce tumor growth, assuming that even such palliative treatments could prolong survival. The endpoint of a series of combination therapy in one hospitalization was determined when all lesions were regarded to be completely treated, when further curative treatments were difficult to perform owing to technical difficulty, poor liver function, distant metastasis, any serious complications, or poor performance status. Thereafter, these subjects were discharged from the hospital, and took a medical examination including dynamic CT, dynamic MRI, abdominal ultrasonography, or a blood test at least every 3 months as an outpatient at the hospital. If new lesions emerged or insufficiently controlled lesions developed during the follow-up periods, these subjects were hospitalized again for the detailed medical examination described above and received repeated treatments with the same principle.
Study design
In general, evaluation was made retrospectively according to the dynamic CT or dynamic MRI performed at least 1 month later from the last treatment in the previous hospitalization. However, if possible to follow up, we referred to any dynamic CT or MRI performed at any subsequent points of time to detect local recurrence after an interval of several months. Also, if available, we referred to an angio-CT performed during subsequent hospitalization for confirmation of suspected lesions. With those concepts, we defined a set of criteria for grading tumor-mass-reduction as follows:
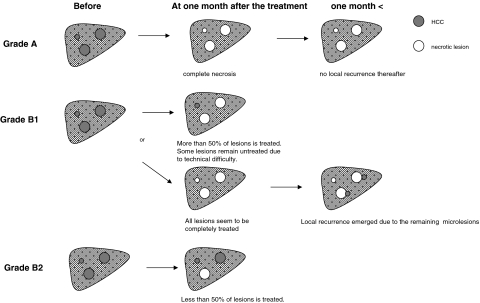
Grade A: No evident cancerous lesion remains untreated after a series of treatments in one admission. In other words, complete necrosis of all HCC lesions is regarded to be obtained in this group, by evaluating all cross sections in dynamic CT or MRI.
Grade B: Cancerous lesions remain untreated after a series of treatments in one admission. This grade is categorized further into two subgroups, according to the percentage of the treated volume compared to the pretreated volume. However, if appropriate, the largest cross section of a lesion is preferably evaluated, because in many cases the largest cross section could be regarded to represent the whole lesion.
Grade B1: More than 50% of the lesions are estimated to be treated.
Grade B2: Less than 50% of the lesions are estimated to be treated.
If more than one HCC lesion exists, this categorization is made by calculating the sum of all lesions. The schematic demonstration of TMRG is illustrated in Fig. 1. For instance, when a lesion was treated without an evident viable lesion and no recurrent lesion appeared by any retrospective evaluation, it was categorized as Grade A (the best result in our classification). However, if a patient with HCC was seemingly treated completely at discharge despite microlesions being actually left untreated failing detection, they were categorized as Grade B1 if these unchecked lesions adjacent to the treated lesions became manifest later. Thus only lesions that emerged adjacent to the treated lesions were evaluated to be the local recurrence due to insufficient therapy. Moreover, when a lesion could not be completely treated owing to some reasons, but residual viable lesion could be evaluated as less than 50% of pretreated lesions, it was also categorized as Grade B1 (not completely, but relatively well treated). When more than 50% of pretreated lesions could not be treated, it was categorized as Grade B2 (the worst result in our classification).
Fig. 1.
Schema of the concept of tumor-mass-reduction-grade used in the current study
Statistical analysis
For the analysis of baseline characteristics, the Kolmogorov–Smirnov normality test was performed, and continuous variables that show normal distribution were expressed as mean ± SD, then compared using the Student t-test. Continuous variables that do not show normal distribution were expressed as median, then compared by the Mann–Whitney U test. Categorical variables were compared with the use of the χ2 test. For these analyses, P values <0.05 were considered statistically significant. The survival of each tumor-mass-reduction grade (TMRG) was calculated by the Kaplan–Meier method and the differences between the curves were evaluated with the log-rank test. P values <0.01 were used for statistical significance. All statistical analyses were performed using StatView ver5.0.
Results
Baseline characteristics of subjects
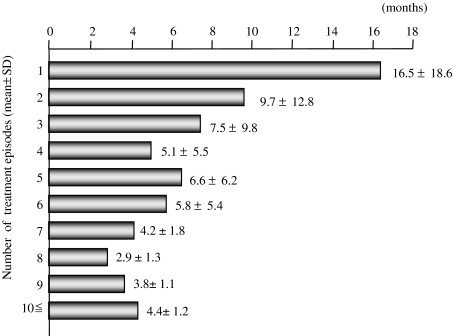
A total of 320 subjects received therapy for the initial occurrence of HCC (Table 1a). The average age at the initial therapy was 63.3 ± 9.3 (range, 28–81). Overall, 386 patients were enrolled from December 1989 to December 2003, and the total number of subjects we analyzed in this study was 878 (M:F = 594:284; mean age: 64.6 ± 8.8) (Table 1b). Among 386 patients, 198 patients died up to December 2003 owing to the development of HCC (n = 135), hepatic failure (n = 35), variceal bleeding (n = 11), insufficiency of other organs (n = 16), or unknown causes (n = 10). The etiology of background liver disease was 69.5% of HCV infection, 17.0% of HBV infection, 5.2% of HCV and HBV superinfection, 3.2% of alcoholic liver injury, and 5.1% of other causes (Table 1b). The distribution of residual liver function was Child-Pugh A 68.6%, Child-Pugh B 29.5%, and Child-Pugh C 1.9% (Table 1b). Median value for AFP was 61.6 ng/dl (range, 0–106), and for DCP was 26.0 AU/l (range, 0–193,000) (Table 1b). Median tumor size was 25.0 mm (range, 5–190). Forty-four percent of patients had solitary tumor, 30.3% two or three tumors, 7.4% four or five tumors, and 18.4% more than five tumors (Table 1). Between subjects at the initial treatment and the total subjects during the whole course of treatment, age, tumor size, and survival showed statistical significance, although other parameters were statistically insignificant (Table 1a, b). Therapeutic modalities we selected for recurrent HCC included 27 patterns (Table 2). The intervals of period between hospitalizations were almost in inverse proportion to the number of times for hospitalization (Fig. 2), indicating that the speed for recurrence was gradually accelerated.
Table 1.
Baseline characteristics of subjects
| (a) n = 320 | (b) n = 878 | P value | ||
|---|---|---|---|---|
| Age (mean ± SD) | 63.3 ± 9.3 | 64.6 ± 8.8 | 0.025* | |
| Gender | M/F | 211/109 | 594/284 | 0.952 |
| Etiology (%) | HCV | 71.9 | 69.5 | 0.101 |
| HBV | 19.0 | 17.0 | ||
| HBV + HCV | 4.5 | 5.2 | ||
| Alcohol | 2.9 | 3.2 | ||
| Others | 1.6 | 5.1 | ||
| Number of treatments (median) | 2.0 (1–14) | |||
| Tumor size (mm) (median) | 30.0 (8–190) | 25.0 (5–190) | 0.001* | |
| Number of tumors (%) | 1 | 59.1 | 44.0 | 0.061 |
| 2, 3 | 29.2 | 30.3 | ||
| 4, 5 | 5.0 | 7.4 | ||
| > 5 | 6.6 | 18.4 | ||
| Vascular invasion | Yes/No | 26/294 | 74/804 | 0.983 |
| Child-Pugh class (%) | A | 69.7 | 68.6 | 0.875 |
| B | 28.8 | 29.5 | ||
| C | 1.6 | 1.9 | ||
| Median survival time (months) | 56.8 (2.5–157.1) | 37.4 (1.0–157.1) | <0.0001* | |
| Alpha-fetoprotein (ng/dl) (median) | 46.6 (0–106) | 61.6 (0–106) | 0.059 | |
| DCP (AU/L) (median) | 19.0 (0–193,000) | 26.0 (0–193,000) | 0.140 | |
| Total bilirubin (mg/dl) | 1.3 ± 0.6 | 1.3 ± 1.1 | 0.187 | |
| Serum albumin (mg/dl) | 3.6 ± 0.6 | 3.6 ± 0.6 | 0.922 | |
| Prothrombin activity (%) | 79.1 ± 16.5 | 79.6 ± 30.9 | 0.757 | |
| ICG R15 (%) | 26.1 ± 14.5 | 27.4 ± 15.3 | 0.194 | |
| Aspartate aminotransaminase (IU/dl) | 79.7 ± 45.5 | 76.8 ± 45.0 | 0.331 | |
| Alanine aminotransferase (IU/dl) | 71.8 ± 46.2 | 66.4 ± 45.8 | 0.070 | |
| Platelet count (104/mm3) | 11.2 ± 5.9 | 10.5 ± 5.8 | 0.155 | |
| Alkaline phosphatase (IU/dl) | 291 ± 177 | 307 ± 172 | 0.147 | |
Note: (a) Subjects who received the initial treatments for the first occurrence of HCC. (b) Total subjects. Values are mean ± standard deviation
Abbreviations: DCP: des-gamma carboxy prothrombin; ICG R15: retention of indocyanine green at 15 min
*P < 0.05
Table 2.
Modalities of treatments performed for HCC
| n (%) | |
|---|---|
| Surgical resection | 36 (4.1) |
| PEI | 113 (12.9) |
| PEI, PMCT | 16 (1.8) |
| PEI, PMCT, RFA | 2 (0.2) |
| PEI, PMCT, TAE | 1 (0.1) |
| PEI, RFA | 19 (2.2) |
| PEI, RFA, RT | 1 (0.1) |
| PEI, RFA, TAE | 9 (1.0) |
| PEI, RT | 1 (0.1) |
| PEI, TAE | 134 (15.3) |
| PEI, TAE, RT | 1 (0.1) |
| PEI, TAI | 6 (0.7) |
| PMCT | 6 (0.7) |
| PMCT, RFA | 1 (0.1) |
| PMCT, RFA, TAE | 1 (0.1) |
| PMCT, TAE | 8 (0.9) |
| PMCT, TAI | 1 (0.1) |
| RFA | 111 (12.6) |
| RFA, TAE | 24 (2.7) |
| RFA, TAI | 2 (0.2) |
| RT | 5 (0.6) |
| RT, TAE | 9 (1.0) |
| RT, TAE, TAI | 4 (0.5) |
| RT, TAI | 10 (1.1) |
| TAE | 294 (33.5) |
| TAE, TAI | 5 (0.6) |
| TAI | 58 (6.6) |
| Total | 878 (100) |
Abbreviations: PEI, percutaneous ethanol injection; PMCT, percutaneous microwave coagulation therapy; RFA, radiofrequency ablation; TAE, transcatheter arterial embolization; TAI, transcatheter arterial infusion; RT, radiation therapy
Fig. 2.
Mean periods of time until the next hospitalization for treatments of HCC
The evaluation of prognosis of initial treatments for the first occurrence of HCC
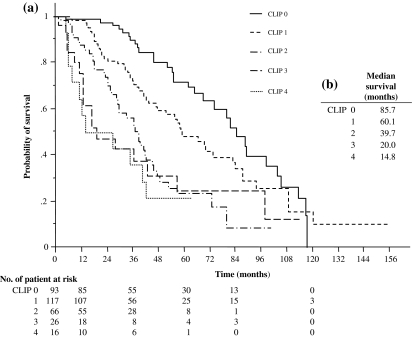
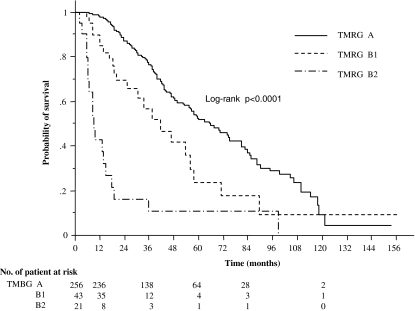
When subjects who received the initial treatment for the first occurrence of HCC were stratified using the CLIP score (Fig. 3a, b), each group of score showed better survival with the previous report in Japan or Italy [8–10], suggesting that our strategy for HCC treatments was up to the standard. Furthermore, when these patients were stratified with the mass-reduction grade, each mass-reduction group was discriminated well from each other with statistical significance (P < 0.0001) (Fig. 4). The 1-year survival rates of Grade A, B1, and B2 patients were 98.4, 90.0, and 42.6%, respectively; the 3-year survival rates of Grades A, B1, and B2 were 76.6, 56.6, and 16.0%, respectively; and the 5-year survival rates of Grades A, B1, and B2 were 52.7, 23.7, and 10.0%, respectively (Fig. 3). These analyses of the initial treatment show that this grading system can function for evaluating the relationship between the prognosis and the grade of mass reduction.
Fig. 3.
(a) Survival curves for different CLIP scores in patients who have undergone initial treatments for the first occurrence of HCC. Data for two were excluded because of missing values of AFP. (b) Median survival of each CLIP score
Fig. 4.
Survival curves for three different grades of tumor-mass-reduction in patients who received initial treatments for HCC (n = 320; log-rank test, P values <0.01 were considered statistically significant)
The relationship between prognosis and tumor-mass-reduction therapy for recurrent HCC
On the basis of the evaluation performed above, we used this system for the analysis of recurrent HCC. The number of hospitalizations for treatment of HCC ranged from 1 to 14. We separated the subjects into three groups using Child-Pugh classifications A, B, and C, and then analyzed each category of patients according to the number of hospitalizations to receive treatment for HCC. Since the number of subjects who were categorized into strata of more than the eighth admission was too small to analyze, we analyzed the strata of admission from the first to the eighth admission. As Table 3 shows, in Child-Pugh A, different grades of mass reduction brought significant differences in survival (P < 0.01) from the first to the fifth admission, suggesting that patients with Child-Pugh A are endurable for repeated therapies and benefited from many sessions of treatments without reducing their prognosis. In Child-Pugh B, our analysis showed somewhat confusing data, that is, biphasic statistical significance at the second and fifth hospitalizations (P < 0.01). In Child-Pugh C, no HCC treatment, regardless of curative or just tumor-mass-reduction treatments, brought any statistical differences in improving survival at any point of time, indicating that reduction therapy is consistently meaningless in such patients.
Table 3.
Association between TMRG and survival stratified by Child-Pugh classification and the frequency of treatments
| Child-Pugh A | Child-Pugh B | Child-Pugh C | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. | TMRG | n | MS | P value | n | MS | P value | n | MS | P value |
| 1 | A | 177 | 75.1 | <0.0001* | 76 | 57.7 | 0.065 | 3 | 37.3 | 0.1159 |
| B1 | 30 | 46.5 | 12 | 25.5 | 1 | 20.0 | ||||
| B2 | 16 | 8.6 | 4 | 36.1 | 1 | 13.9 | ||||
| 2 | A | 112 | 54.4 | 0.0020* | 43 | 34.4 | <0.0001* | 2 | 19.2 | 0.1573 |
| B1 | 26 | 24.7 | 11 | 20.2 | 1 | 10.7 | ||||
| B2 | 3 | 9.6 | 3 | 3.0 | ||||||
| 3 | A | 62 | 42.9 | <0.0001* | 25 | 30.1 | 0.0737 | 2 | 2.2 | 0.1387 |
| B1 | 18 | 26.1 | 9 | 25.5 | 1 | 9.9 | ||||
| B2 | 7 | 6.2 | 4 | 7.8 | 3 | 4.9 | ||||
| 4 | A | 36 | 42.6 | 0.0020* | 13 | 34.2 | 0.0772 | 0 | 0.8084 | |
| B1 | 14 | 14.1 | 10 | 12.9 | 2 | 4.9 | ||||
| B2 | 2 | 9.5 | 1 | 12.0 | 1 | 6.4 | ||||
| 5 | A | 21 | 39.6 | <0.0001* | 11 | 23.7 | <0.0001* | |||
| B1 | 11 | 31.8 | 8 | 13.8 | ||||||
| B2 | 6 | 7.4 | 1 | 5.5 | ||||||
| 6 | A | 14 | 39.6 | 0.1795 | 3 | 15.6 | 0.0125 | |||
| B1 | 6 | 33.2 | 7 | 14.2 | ||||||
| B2 | 2 | 6.7 | 2 | 5.8 | ||||||
| 7 | A | 8 | 26.1 | 0.8072 | 0 | 0.9189 | ||||
| B1 | 8 | 19.6 | 6 | 9.2 | ||||||
| B2 | 2 | 9.5 | 1 | 13.0 | ||||||
| 8 | A | 3 | 32.6 | 0.7820 | 0 | 0.3173 | ||||
| B1 | 3 | 18.2 | 1 | 4.7 | ||||||
| B2 | 2 | 18.2 | 1 | 4.2 | ||||||
Abbreviations: No., number of hospitalization; TMRG, tumor-mass-reduction grade; MS, median survival (months)
Discussion
Preceding this study, our clinical observation in treatments of HCC had given us an impression that treatments for recurrent HCC might not be always effective in view of prognosis. Although some data were available regarding the effectiveness of the initial therapy for HCC, effectiveness of a single therapy or therapies in combination with a limited number of modalities [4, 11–13, 26, 33], little data have been available regarding if repeated treatments for recurrent HCC and combined treatments for the better control of HCC are effective in improving prognosis. Furthermore, we observed that mere numbers and distribution of HCC or residual liver function as evaluated by Child-Pugh score etc. might not determine the outcome of HCC therapy. We speculated that “repeated sessions of HCC treatments” per se might worsen prognosis or induce recurrence, although the accurate mechanism for the aggravation of prognosis by this “repeating” was nebulous. Actually, in our data, the intervals between recurrences got shorter and shorter, while the events of recurrence increased (Fig. 2). One possible mechanism could be that during each session of therapy not only were the HCC lesions necrotized, but the surrounding liver tissues were also injured, cumulatively damaging liver functions. Although this possibility may be applicable in some cases, it does not always seem to be true, because our analysis showed that although in some cases Child-Pugh classification changed, indicating deterioration, in many cases, the scores did not change during a course of repeated treatments. Only 16.5% of subjects underwent changes in their grading. For example, among 223 patients who were graded as Child-Pugh A at the first treatment, 44 subjects underwent deterioration to Grade B or C, at the following therapy. Eight among 90 Child-Pugh B patients at the first treatment underwent deterioration to Grade C at the next occasion (data not shown). Another possibility is that the therapeutic stimuli may induce transformation of HCC, making it more malignant and more resistant to therapies. Although this speculation may not be always true, it may explain some cases, because a study suggested that anoxia caused by TAE induced Bcl-2 expression, which changed HCC cells more tolerant to apoptosis [34]. The other possibility is that during HCC treatments, multiple microlesions or precancerous lesions of HCC may be left untreated failing detection that later develop into intractable lesions [1–3, 20]. In such cases, the probability of multicentric carcinogenesis may be increased if the period from the initial onset of HCC becomes longer; the probability of intrahepatic metastasis of HCC with poorer differentiation may be increased if each session of treatment leaves viable cancer cells undetectable by medical examination. Whatever mechanism is true, the data of repeated treatments for HCC is urgently needed.
However, to address this subject, we confronted a challenging situation. To begin with, we found that randomized controlled trials (RCTs) were quite difficult to conduct in our setting owing to ethical reasons. Those patients whose life expectancy is limited on account of malignant diseases do not dare to risk reducing their chances of survival by participating in such studies, because medical care is affordable for almost all patients in Japan. Therefore, although RCTs would have been the most reliable way to assess this subject, we had to find a way to analyze the effectiveness of HCC treatments in the absence of controls.
Second, most studies that have attempted to analyze treatments for HCC have been limited to the analysis of only a single modality [11–13, 33]. However, in practice, we treat HCC with modalities prudently combined because it can usually better control HCC than does a single modality. Thus, in a situation where data for survival are lacking, our tentative goal has been to control HCC lesions as completely as possible using available modalities, with the expectation that such treatments improve survival.
Finally, because HCC is a cancer that frequently tends to recur and needs to be treated repeatedly, our practical interest was not only the influence of the initial treatment upon prognosis but also the total influence of repeated therapy. Most previous reports, however, deal with the prognosis when a modality is selected as an initial treatment. Since actually an identical modality is seldom selected consistently for one patient and various therapies are selected for recurrent lesions, analysis becomes too complicated.
Considering these situations, we took a step for the analysis with the following concepts: (1) We analyzed not only a single modality, but also treatments sequentially combined to obtain the best control, performed during one hospitalization. Such treatments include seven kinds of monotherapy and 21 kinds of combination therapy (Table 2). (2) We analyzed not only an initial treatment but every repeated treatment performed at each hospitalization. (3) We defined our original classification for evaluation of HCC treatments in order to apply it commonly for all single or combined treatments. To be evaluated as “a sufficient therapeutic effect,” enough safety margin will be usually necessary for PEI, RFA, etc.; sufficient deposit of lipiodol completely covering the margin of tumors for TAE; and complete disappearance of tumors for chemotherapy. However, when aiming to evaluate a variety of therapeutic patterns, it was not feasible to apply these criteria individually. Therefore, by making the best use of retrospective analysis, we simply judged the effectiveness of therapies by mainly focusing on recurrent lesions retrospectively using our original evaluation system described above.
In this study, we showed that both Child-Pugh classification and number of times of hospitalization for HCC therapy determine the effectiveness of mass reduction therapy on the prognosis of HCC. This observation is also true in view of tumor markers because regardless of different Child-Pugh grades, majority of patients underwent decreases of AFP and DCP levels. Furthermore, additional decrease of AFP and DCP levels tend to be acquired by the better TMRG (data not shown). In Child-Pugh A, up to the fifth hospitalization, better prognosis was achieved in proportion to grades of mass reduction, but in subsequent hospital admissions, attempts for mass reduction did not make any difference in the prognosis. In Child-Pugh B, rather confused results were obtained; only at the second and fifth admission, TMRGs showed statistical significance in prognosis. The reason that there was no statistical difference on the first admission of Child-Pugh B patients may be that a good survival rate was achieved even by the B2 grade of treatment (Table 2). In the majority of these patients, Child-Pugh grade was maintained at the same level without worsening into C after therapy. This observation may indicate, that good survival can be achieved by therapy that does not damage liver function even if the TMRG is not so satisfactory. On the other hand, although the precise reason why Child-Pugh B patients on the fifth admission showed statistical significance is unclear, it may be explained simply by the lack of analyzed subject.
We emphasize that the number of times for hospitalization indicated in this study is neither universal nor absolute, so some differences will exist from institute to institute. Also we admit that our study fundamentally has limits in terms of analytical methods. One limit is that this study is a nonrandomized retrospective analysis. The other limit is that we do not have any data of natural history. Without the data of no treatment, we cannot exactly decide, which grade of mass reduction can improve prognosis compared to that of no treatment or just a symptomatic treatment. Regardless of these limits, however, an unchangeable advantage of this analytical method is, that if there is no statistical difference between the various grades, it can be demonstrated that any attempts for mass reduction will fail to improve prognosis.
Despite these relations or limits, our analysis seems to be presenting important suggestions. In Child-Pugh A, many occasions of hospitalization for HCC therapy seem to be endurable (in our institute, they were up to five) and can be of benefit for prognostic improvement by more curative treatments; in Child-Pugh B, only initial few occasions of hospitalization for HCC therapy may be beneficial; in Child-Pugh C, any therapeutic attempts are useless. For the better prognosis of HCC patients with Child-Pugh B, it may be crucial to administer new modalities including liver transplantation as early as possible, while the same strategy is also relevant for any HCC patients with Child-Pugh C. Comparing with our data, which addressed repeated treatments by various modalities in combination, further studies are warranted, including the analysis of survival benefit due to liver transplantation.
Abbreviations
- AFP
Alpha-fetoprotein
- CT
Computed tomography
- CTAP
CT during arterioportography
- CTHA
CT during hepatic arteriography
- DCP
des-gamma carboxy prothrombin
- HCC
Hepatocellular carcinoma
- MRI
Magnet resonance imaging
References
- 1.Miyagawa S, Kawasaki S, Makuuchi M. Comparison of the characteristics of hepatocellular carcinoma between hepatitis B and C viral infection: tumor multicentricity in cirrhotic liver with hepatitis C. Hepatology 1996;24(2):307–10. [DOI] [PubMed]
- 2.Sugimoto R, Okuda K, Tanaka M, Aoyagi S, Kojiro M. Metachronous multicentric occurrence of hepatocellular carcinoma after surgical treatment – clinicopathological comparison with recurrence due to metastasis. Oncol Rep 1999;6(6):1303–8. [DOI] [PubMed]
- 3.Yamamoto T, Kajino K, Kudo M, Sasaki Y, Arakawa Y, Hino O. Determination of the clonal origin of multiple human hepatocellular carcinomas by cloning and polymerase chain reaction of the integrated hepatitis B virus DNA. Hepatology 1999;29(5):1446–52. [DOI] [PubMed]
- 4.Lencioni R, Cioni D, Crocetti L, Bartolozzi C. Percutaneous ablation of hepatocellular carcinoma: state-of-the-art. Liver Transpl 2004;10(2 Suppl 1):S91–7. [DOI] [PubMed]
- 5.Makuuchi M, Kosuge T, Takayama T, Yamazaki S, Kakazu T, Miyagawa S, et al. Surgery for small liver cancers. Semin Surg Oncol 1993;9(4):298–304. [DOI] [PubMed]
- 6.Llovet JM, Bru C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis 1999;19(3):329–38. [DOI] [PubMed]
- 7.Levy I, Sherman M. Staging of hepatocellular carcinoma: assessment of the CLIP, Okuda, and Child-Pugh staging systems in a cohort of 257 patients in Toronto. Gut 2002;50(6):881–5. [DOI] [PMC free article] [PubMed]
- 8.The Cancer of the Liver Italian Program (CLIP) Investigators. A new prognostic system for hepatocellular carcinoma: a retrospective study of 435 patients. Hepatology 1998;28(3):751–5. [DOI] [PubMed]
- 9.Ueno S, Tanabe G, Sako K, Hiwaki T, Hokotate H, Fukukura Y, et al. Discrimination value of the new western prognostic system (CLIP score) for hepatocellular carcinoma in 662 Japanese patients. Cancer of the Liver Italian Program. Hepatology 2001;34(3):529–34. [DOI] [PubMed]
- 10.The Cancer of the Liver Italian Program (CLIP) Investigators. Prospective validation of the CLIP score: a new prognostic system for patients with cirrhosis and hepatocellular carcinoma. Hepatology 2000;31(4):840–5. [DOI] [PubMed]
- 11.Seki T, Wakabayashi M, Nakagawa T, Imamura M, Tamai T, Nishimura A, et al. Percutaneous microwave coagulation therapy for patients with small hepatocellular carcinoma: comparison with percutaneous ethanol injection therapy. Cancer 1999;85(8):1694–702. [DOI] [PubMed]
- 12.Llovet JM, Real MI, Montana X, Planas R, Coll S, Aponte J, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet 2002;359(9319):1734–9. [DOI] [PubMed]
- 13.Lo CM, Ngan H, Tso WK, Liu CL, Lam CM, Poon RT et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology 2002;35(5):1164–71. [DOI] [PubMed]
- 14.Okano H, Shiraki K, Inoue H, Ito T, Yamanaka T, Deguchi M et al. Combining transcatheter arterial chemoembolization with percutaneous ethanol injection therapy for small size hepatocellular carcinoma. Int J Oncol 2001;19(5):909–12. [DOI] [PubMed]
- 15.Hayashi M, Matsui O, Ueda K, Kawamori Y, Gabata T, Kadoya M. Progression to hypervascular hepatocellular carcinoma: correlation with intranodular blood supply evaluated with CT during intraarterial injection of contrast material. Radiology 2002;225(1):143–9. [DOI] [PubMed]
- 16.Matsui O, Kadoya M, Kameyama T, Yoshikawa J, Takashima T, Nakanuma Y, et al. Benign and malignant nodules in cirrhotic livers: distinction based on blood supply. Radiology 1991;178(2):493–7. [DOI] [PubMed]
- 17.Hsu HC, Wei TC, Tsang YM, Wu MZ, Lin YH, Chuang SM. Histologic assessment of resected hepatocellular carcinoma after transcatheter hepatic arterial embolization. Cancer 1986;57(6):1184–91. [DOI] [PubMed]
- 18.Matsui O, Kadoya M, Yoshikawa J, Gabata T, Arai K, Demachi H, et al. Small hepatocellular carcinoma: treatment with subsegmental transcatheter arterial embolization. Radiology 1993;188(1):79–83. [DOI] [PubMed]
- 19.Sato Y, Fujiwara K, Ogata I, Ohta Y, Hayashi S, Oka Y, et al. Transcatheter arterial embolization for hepatocellular carcinoma. Benefits and limitations for unresectable cases with liver cirrhosis evaluated by comparison with other conservative treatments. Cancer 1985;55(12):2822–5. [DOI] [PubMed]
- 20.Tanaka K, Shimada H, Togo S, Takahashi T, Endo I, Sekido H, et al. Use of transcatheter arterial infusion of anticancer agents with lipiodol to prevent recurrence of hepatocellular carcinoma after hepatic resection. Hepatogastroenterology 1999;46(26):1083–8. [PubMed]
- 21.Yamasaki T, Kurokawa F, Takami T, Omori K, Kawaguchi K, Tsuchiya M, et al. Arterial infusion chemotherapy using cisplatin, 5-fluorouracil, and isovorin for patients with advanced hepatocellular carcinoma, pilot study: is a high dose of the biochemical modulator effective? Hepatol Res 2003;27(1):36–44. [DOI] [PubMed]
- 22.Lai YC, Shih CY, Jeng CM, Yang SS, Hu JT, Sung YC, et al. Hepatic arterial infusion chemotherapy for hepatocellular carcinoma with portal vein tumor thrombosis. World J Gastroenterol 2003;9(12):2666–70. [DOI] [PMC free article] [PubMed]
- 23.Tanioka H, Tsuji A, Morita S, Horimi T, Takamatsu M, Shirasaka T, et al. Combination chemotherapy with continuous 5-fluorouracil and low-dose cisplatin infusion for advanced hepatocellular carcinoma. Anticancer Res 2003;23(2C):1891–7. [PubMed]
- 24.Ando E, Tanaka M, Yamashita F, Kuromatsu R, Yutani S, Fukumori K, et al. Hepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma with portal vein tumor thrombosis: analysis of 48 cases. Cancer 2002;95(3):588–95. [DOI] [PubMed]
- 25.Shiina S, Tagawa K, Niwa Y, Unuma T, Komatsu Y, Yoshiura K, et al. Percutaneous ethanol injection therapy for hepatocellular carcinoma: results in 146 patients. Am J Roentgenol 1993;160(5):1023–8. [DOI] [PubMed]
- 26.Bloomston M, Binitie O, Fraiji E, Murr M, Zervos E, Goldin S, et al. Transcatheter arterial chemoembolization with or without radiofrequency ablation in the management of patients with advanced hepatic malignancy. Am J Surg 2002;68(9):827–31. [PubMed]
- 27.Wood TF, Rose DM, Chung M, Allegra DP, Foshag LJ, Bilchik AJ. Radiofrequency ablation of 231 unresectable hepatic tumors: indications, limitations, and complications. Ann Surg Oncol 2000;7(8):593–600. [DOI] [PubMed]
- 28.Yamasaki T, Kurokawa F, Shirahashi H, Kusano N, Hironaka K, Okita K. Percutaneous radiofrequency ablation therapy for patients with hepatocellular carcinoma during occlusion of hepatic blood flow. Comparison with standard percutaneous radiofrequency ablation therapy. Cancer 2002;95(11):2353–60. [DOI] [PubMed]
- 29.Uno T, Itami J, Shiina T, Toita T, Mikuriya S, Hatano K, et al. Radiation therapy in patients with unresectable hepatocellular carcinoma. Cancer Chemother Pharmacol 1992;31(Suppl):S106–10. [DOI] [PubMed]
- 30.Matsuura M, Nakajima N, Arai K, Ito K. The usefulness of radiation therapy for hepatocellular carcinoma. Hepatogastroenterology 1998;45(21):791–6. [PubMed]
- 31.Tazawa J, Maeda M, Sakai Y, Yamane M, Ohbayashi H, Kakinuma S, et al. Radiation therapy in combination with transcatheter arterial chemoembolization for hepatocellular carcinoma with extensive portal vein involvement. J Gastroenterol Hepatol 2001;16(6):660–5. [DOI] [PubMed]
- 32.Chia-Hsien Cheng J, Chuang VP, Cheng SH, Lin YM, Cheng TI, Yang PS, et al. Unresectable hepatocellular carcinoma treated with radiotherapy and/or chemoembolization. Int J Cancer 2001;96(4):243–52. [DOI] [PubMed]
- 33.Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology 2003;37(2):429–42. [DOI] [PubMed]
- 34.Kobayashi N, Ishii M, Ueno Y, Kisara N, Chida N, Iwasaki T, et al. Co-expression of Bcl-2 protein and vascular endothelial growth factor in hepatocellular carcinomas treated by chemoembolization. Liver 1999;19(1):25–31. [DOI] [PubMed]