Abstract
Background
Although clinical and experimental studies have demonstrated a correlation between obstructive jaundice and the development of sepsis, the mechanism has not been fully elucidated.
Purpose
The aim of this study was to investigate the influence of biliary obstruction on bacterial translocation as a possible source of infection in cases of obstructive jaundice.
Material and Methods
Two groups of 12 Wistar rats were examined: rats subjected to common bile duct (CBD) ligation (group A) and rats subjected to a sham operation (group B). After 7 days, blood samples were taken and liver, spleen, and mesenteric lymph nodes (MLNs) from the ileocecal area were removed, divided into small pieces, and cultured. Quantitative culture results were determined by the number of colony-forming units (CFU) per milliliter of homogenate. Bacterial translocation was defined as the presence of a positive culture of MLNs, blood, liver, and/or spleen. Samples for histopathological examination were taken from the mucosa of the ileum and the colon and evaluated for inflammatory and destructive changes.
Results
There was no evidence of bacterial translocation to MLNs, blood, spleen, or liver detected in any of the 12 sham-operated control rats. In contrast, bacterial translocation was demonstrated in 8 of the 12 CBD-ligated rats (P < 0.01). In all eight cases in which translocation occurred, Escherichia coli were cultured from the MLNs. There were no histological changes in the mucosal samples of the control animals. In the CBD-ligated rats, hyperemia, vacuolization, reduction of goblet cells, decreased mitotic activity, and infiltration by lymphocytes and polymorphonuclear leukocytes (PMNLs) were detected. Cases in which translocation occurred were significantly associated with decreased mitotic activity in the colon (r = −0.5, P < 0.01) and higher infiltration by PMNLs in the ileum (r = −0.62, P < 0.05).
Conclusion
Obstructive jaundice in a rat model predisposes to bacterial translocation. This suggests a mechanism whereby jaundiced patients are susceptible to septic complication.
Keywords: Bacterial translocation, Intestinal barrier, Obstructive jaundice
Introduction
Bacterial translocation (BT) is defined as the phenomenon by which live bacteria and/or their products cross the intestinal barrier to sterile body sites, such as the mesenteric lymph nodes (MLNs), spleen, liver, and bloodstream [1]. BT is suggested to be an important component in development of sepsis [1, 2].
Sepsis and multisystem organ failure (MOF) are major complications leading to increased postoperative morbidity and mortality in patients with obstructive jaundice [3]. Although clinical and experimental studies have demonstrated a correlation between obstructive jaundice and the development of sepsis, the mechanism has not been fully elucidated [4].
This study examined the influence of biliary obstruction on bacterial translocation in a rat model as a possible source of systemic infection in such cases.
Material and methods
Experimental design
This study was carried out at the animal lab of the National Liver Institute, Menoufeyia University. A total of 24 Wistar rats weighing 200–250 g were studied. Rats were caged at room temperature and exposed to a 12-h/12-h light/dark cycle. They were allowed free access to water and rat chow. The animals were randomized into two groups of 12 rats each: group A, obstructive jaundice group; and group B, sham operation. All animals were anesthetized with intramuscular injection of 50 mg/kg of ketamine hydrochloride. Midline laparotomies were performed under sterile conditions. In group A, the common bile duct was dissected, double-ligated by 6/0 silk and divided. For group B, the common bile duct was dissected but not tied. Laparotomy wound was closed in one layer by 4/0 silk. The rats were allowed to recover from anesthesia and given free access to food and water.
After 7 days, the animals were anesthetized as mentioned above, the abdomen and the chest were opened under sterile conditions, and the animals were killed by cardiac blood aspiration. Aspirated blood was used for culture and measurement of serum bilirubin. Liver, spleen, and MLNs from the ileocecal area were aseptically dissected, removed, and all organs were divided into small pieces.
Microbiological assays
The pieces of tissue were weighed separately, placed in a sterile grinding tube, homogenized in Ringer solution, and plated at multiple dilutions on McConkey agar and blood agar. Plates were cultured for 48 h under aerobic (McConkey and blood agar) and anaerobic (blood agar) conditions. Colonization is expressed as the number of colony-forming units (CFUs). Quantitative culture results were determined by the number of CFUs per milliliter of homogenate.
Heart blood samples were cultured both aerobically and anaerobically and monitored for 7 days. Positive blood cultures were plated out on appropriate media and species identified by standard bacteriological techniques.
Bacterial translocation was defined as the presence of a positive culture of MLNs, blood, liver, and/or spleen.
Processing of histological samples
Samples from the central part of the ileum and the colon were taken and fixed with neutral formalin 10%. Paraffin sections were stained with hematoxylin and eosin, acridine orange, and a modified Gram stain. Several inflammatory and destructive changes, such as hyperemia, hemorrhages, vacuolization, necroses, goblet cell counts, mitotic activity, lymphocyte infiltration, infiltration by polymorphonuclear leukocytes (PMNLs), and the presence of microbes within crypts were evaluated.
Statistical analysis
Data were expressed as mean. Statistical significance was assessed using Student t test and Mann–Whitney U test for bacterial translocation and Fisher test for comparison of changes between groups.
Results
Serum bilirubin
Significantly raised bilirubin levels were present in rats 1 week after bile duct ligation compared with those in sham-operated controls (12.8 vs 0.8 mg/dL; P < 0.0001, Mann–Whitney U test).
Bacterial translocation
There was no evidence of bacterial translocation to MLN, blood, spleen, or liver detected in any of the 12 sham-operated control rats. In contrast, bacterial translocation was demonstrated in 8 of the 12 CBD-ligated rats (P < 0.01, Fisher exact test). In all eight cases in which translocation occurred, Escherichia coli were cultured from the MLNs. E. coli was the sole organism isolated in 5 of 10 MLN cultures. In addition, one animal also had Gram-positive bacilli cultured from the MLNs. The least frequently translocated bacteria were enterococci, staphylococci, streptococci, propionibacteria, and Gram-negative nonfermenters. Bacterial yield was variable, ranging from 5 to more than 108 CFUs per milliliter of homogenate (Table 1 and Fig. 1). There was no evidence of colonization with anaerobic bacteria.
Table 1.
Incidence of bacterial translocation in blood and tissue specimens
| Organ | Sham-operated (n = 12) | Common bile duct-ligated (n = 12) | P value | ||
|---|---|---|---|---|---|
| Incidence (%) | CFU/ml | Incidence | CFU/ml | ||
| MLNs | 0.0 | – | 8/12 (67%) | 822 ± 345 (850) | <0.01 |
| Spleen | 0.0 | – | 6/12 (50%) | 463 ± 196 (458) | <0.01 |
| Liver | 0.0 | – | 4/12 (33%) | 362 ± 223 (213) | <0.01 |
| Blood | 0.0 | – | 4/12 (33%) | 262 ± 123 (113) | <0.01 |
CFU/ml: Mean ± SD of colony-forming units per homogenate of tissue, with median values in parentheses
Fig. 1.
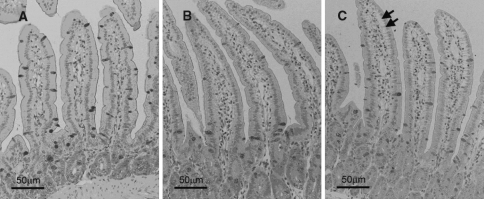
Histology samples obtained from the small intestinal mucosa: (A) control group; (B) and (C) common bile duct ligated group. Arrows indicate vacuolization at the basal area of intestinal villus epithelium
When the counts in individual animals were correlated, a strong positive association was found between the counts of E. coli in blood and in other organs (P < 0.001). Correlation coefficients (r) were as follows: MLNs, r = 0.79; liver, r = 0.85; spleen, r = 0.83 (Table 2).
Table 2.
Correlation coefficient (r) between the E coli counts in blood and other organs
| MLNs | Spleen | Liver | P value | |
|---|---|---|---|---|
| Count of E coli in blood | 0.79 | 0.83 | 0.85 | <0.001 |
Histological studies of intestinal mucosa
There were no histological changes in the mucosal samples of the control animals except for a mild reduction in mitotic activity in one rat. In the CBD-ligated rats, hyperemia, vacuolization, reduction of goblet cells, decreased mitotic activity, and infiltration by lymphocytes and PMNLs were detected. However, the expression of these characteristics in individual animals was highly variable (Fig. 1 and Table 3).
Table 3.
Histological changes in the intestinal mucosa
| Histological changes | Sham-operated (n = 12) | Common bile duct-ligation (n = 12) |
|---|---|---|
| Hyperemia in ileum | NS | 7 |
| Hemorrhage in ileum | NS | 8 |
| Necrosis in ileum | NS | 8 |
| Microbes in crypts of ileum | NS | 7 |
| Microbes in crypts of colon | NS | 9 |
| Infiltration with PMNLs in ileum | NS | 6 |
| Vacuolization | NS | 7 |
| Reduction of goblet cells | NS | 6 |
NS: Not significant
Correlation between bacterial translocation and histological changes is demonstrated in Table 4.
Table 4.
Correlations between bacterial counts and histological changes in CBD ligation group
| Histological changes | r | P value |
|---|---|---|
| Hyperemia in ileum | 0.47 | <0.05 |
| Hemorrhage in ileum | 0.53 | <0.05 |
| Necrosis in ileum | 0.54 | <0.01 |
| Microbes in crypts of ileum | 0.47 | <0.05 |
| Microbes in crypts of colon | 0.62 | <0.01 |
| Infiltration with PMNLs in ileum | 0.43 | <0.05 |
| Total score of ileum | 0.68 | <0.01 |
| Total score of colon | 0.60 | <0.05 |
r: correlation coefficient
Correlating translocation and the histology of mucosae of all infected animals, it was found that higher incidences of translocation cases were significantly associated with decreased mitotic activity in the colon (r = −0.5, P < 0.01) and higher infiltration by PMNLs in the ileum (r = −0.62, P < 0.05).
Discussion
Sepsis is a major complication leading to increased postoperative morbidity and mortality in patients with obstructive jaundice. Several studies have suggested that the source of systemic bacterial infection in obstructive jaundice originate from the gastrointestinal tract.
The normal intestinal flora, especially the anaerobes, act to resist overgrowth or colonization by other endogenous or extraneous bacteria [3]. Under normal circumstances, the intestinal mucosa has a complex barrier system that prevents the penetration of gut microflora. Certain pathological conditions can induce the passage of viable bacteria from the lumen of the gastrointestinal tract to other systemic organs, a process termed bacterial translocation [5].
In this study, we tried to investigate the influence of bile duct ligation on bacterial translocation in an experimental animal model. Our experimental model is simple and easily reproducible. While there was no evidence of bacterial translocation to MLN, blood, spleen, or liver detected in any of the 12 sham-operated control rats, bacterial translocation was demonstrated in 8 of the 12 CBD-ligated rats (P < 0.01). In all eight cases in which translocation occurred, Escherichia coli were cultured from the MLNs. E. coli was the sole organism isolated in 5 of 10 MLN cultures. Correlating translocation and the histology of the gut mucosa, it was found that higher incidences of cases with bacterial translocation were significantly associated with decreased mitotic activity in the colon (r = −0.5, P < 0.01) and higher infiltration by PMNLs in the ileum (r = −0.62, P < 0.05). This indicates that there is a significant correlation between bacterial translocation and histological evidence of mucosal injury, a mechanism whereby viable bacteria from the lumen of the gastrointestinal tract may pass to the bloodstream and other organs, causing systemic infection. These findings support the hypothesis that the source of bacterial infection in obstructive jaundice may originate from the gastrointestinal tract.
Bacterial overgrowth following experimental biliary obstruction has been shown in [6] and [7], which also describe histological evidence of mucosal injury, with subepithelial edema involving the ileal villi and lifting of the epithelium from the lamina propria. Morphological evidence of ileal mucosal injury with reduction in villus height and total mucosal thickness in jaundiced rats has been demonstrated in other studies [8]. Furthermore, impaired mononuclear cell function has been demonstrated in jaundiced rats as well [9].
The mechanism by which jaundice promotes bacterial translocation is unknown [2]. The component of bile that influences bacterial translocation is unknown; both bile salts and secretory immunoglobulin A may be important. Direct mucosal injury, bacterial overgrowth and gut immune function are relevant to models of bacterial translocation [10]. Irrespective of the underlying mechanism, once bacterial translocation of E. coli is initiated, a self-perpetuating cycle of translocation may be sustained, because endotoxin is itself a potent promoter of translocation [9]. It was reported that bile salts inhibit the growth of intestinal bacteria and contribute to the regulation of gut microflora [10]. Therefore, removal of intraluminal bile salts by ligation of the bile duct may cause a change in the endogenous bacterial flora, loss of mucosal integrity, and increased bacterial translocation to the MLNs and liver [7]. Returning bile to the gastrointestinal lumen has been assumed to be associated with reduced postoperative endotoxemia, renal impairment, and mortality in animal models [11]. Internal biliary drainage has also been considered to be important in the recovery of mononuclear phagocyte function [12]. Construction of choledochoduodenostomy in jaundiced rats is associated with reduced bacterial translocation when compared with rats subjected to external biliary drainage, implying an improvement in intestinal-barrier function with internal biliary drainage [7]. The same authors reported that internal biliary drainage reduced endotoxemia, restored immune competence, and increased survival following a septic challenge in an experimental model. In a large study of patients undergoing surgery for pancreatic cancer, patients who underwent internal stenting before surgery had a reduced incidence of infection. Furthermore, external biliary drainage is associated with a higher incidence of infection than internal drainage, which may relate to its failure to affect bacterial translocation [13].
In conclusion, this study supports the hypothesis that obstructive jaundice is associated with significant bacterial translocation. Further studies would be needed to explore the possible mechanisms by which bacteria translocate across the intestinal barrier and to find out measures to reduce this phenomenon aiming at reducing septic complications in cases of obstructive jaundice.
References
- 1.Deriy LV, Beno DW, Uhing MR, Jiyamapa-Serna VA, Kimura RE. Splenectomy ablates endotoxin-induced IFN-[gamma] response in rats. Shock 2002;17:312–5 [DOI] [PubMed]
- 2.Lu L, Walker WA. Pathologic and physiologic interactions of bacteria with the gastrointestinal epithelium. Am J Clin Nutr 2001;73:1124S–30S [DOI] [PubMed]
- 3.Seki S, Habu Y, Kawamura T, Takeda K, Dobashi H, Ohkawa T, Hiraide H. The liver as a crucial organ in the first line of host defense: the roles of Kupffer cells, natural killer (NK) cells and NK1.1 Ag+ T cells in T helper 1 immune responses. Immunol Rev 2000;174:35–46 [DOI] [PubMed]
- 4.Qiu G, Wang C, Smith R, Harrison K, Yin K. Role of IFN-[gamma] in bacterial containment in a model of intra-abdominal sepsis. Shock 2001;16:425–9 [DOI] [PubMed]
- 5.Rigato O, Salomao R. Impaired production of interferon-[gamma] and tumor necrosis factor-[alpha] but not of interleukin 10 in whole blood of patients with sepsis. Shock 2003;19:113–6 [DOI] [PubMed]
- 6.Deitch EA, Rutan R, Waymack JP. Bacterial translocation studies of mice and men. Am J Gastroenterol 1998;93:277–8 [DOI] [PubMed]
- 7.Ding P, Smidt I, Tamme K. Translocation of indigenous microflora in an experimental model of sepsis. J Med Microbiol 2000;49:431–9 [DOI] [PubMed]
- 8.Godshall CJ, Scott MJ, Burch PT, Peyton JC, Cheadle WG. Natural killer cells participate in bacterial clearance during septic peritonitis through interactions with macrophages. Shock 2003;19:144–9 [DOI] [PubMed]
- 9.Lichtman SM. Bacterial translocation in humans. J Pediatr Gastroenterol Nutr 2001;33:1–10 [DOI] [PubMed]
- 10.Nussler NC, Stange B, Nussler AK, Settmacher U, Langrehr JM, Neuhaus P, Hoffman RA. Upregulation of intraepithelial lymphocyte (IEL) function in the small intestinal mucosa in sepsis. Shock 2001;16:454–8 [DOI] [PubMed]
- 11.Moore FA. The role of the gastrointestinal tract in post injury multiple organ failure. Am J Surg 1999;178:449–53 [DOI] [PubMed]
- 12.Trede M, Schwall G. The complications of pancreatectomy. Ann Surg 1998;207:39–47 [DOI] [PMC free article] [PubMed]
- 13.Hart AL, Stagg AJ, Frame M, Graffner H, Glise H, Falk P, Kamm MA. The role of the gut flora in health and disease, and its modification as therapy. Aliment Pharmacol Ther 2002;16:1383–93 [DOI] [PubMed]