Summary
Although the auditory system has limited information processing resources, the acoustic environment is infinitely variable. To properly encode the natural environment, the developing central auditory system becomes somewhat specialized through experience-dependent adaptive mechanisms that operate during a sensitive time window. Recent studies have demonstrated that cellular and synaptic plasticity occurs throughout the central auditory pathway. Acoustic-rearing experiments can lead to an over-representation of the exposed sound frequency, and this is associated with specific changes in frequency discrimination. These forms of cellular plasticity are manifest in brain regions, such as midbrain and cortex, that interact through feed-forward and feedback pathways. Hearing loss leads to a profound re-weighting of excitatory and inhibitory synaptic gain throughout the auditory CNS, and this is associated with an over-excitability that is observed in vivo. Further behavioral and computational analyses may provide insights into how theses cellular and systems plasticity effects underlie the development of cognitive functions such as speech perception.
Introduction
The evidence that auditory experience influences central nervous system maturation is both convincing and challenging. Our certainty stems from the many reports showing that early hearing loss or over-exposure to a narrow range of sound cues can profoundly disrupt the development of central coding properties and maps. However, some nettlesome issues remain. First, if we are to understand whether central properties are fashioned by an animal's acoustic environment, then it is necessary to know when they are labile and when they mature. Surprisingly, there are only a handful of studies that characterize how the central auditory system functions in young animals, and they all derive from anesthetized preparations. Therefore, it is important to explore the physiology of developing animals, and identify the critical periods during which functional properties are modifiable.
A second challenge for the study of plasticity is understanding how environmentally-induced changes to central auditory function correlate with changes to auditory perception. If not for psychophysical studies on developing humans, our understanding of perceptual development would be almost nonexistent. Therefore, behavioral studies in normal and manipulated animals are crucial to interpreting the influence of environment on central coding properties. Finally, the emergence of adult central coding properties can often be explained by the concurrent maturation of cochlear function; it is tricky to isolate and study the central mechanisms that are influenced by auditory experience, independent of the auditory periphery. Therefore, it is profitable to examine synaptic properties in brain slice preparations from normal and experimental animals. In this review, we evaluate recent advances in auditory plasticity during development, paying particular attention to each of these issues.
Auditory Development and Plasticity in Humans
The development of auditory perception in humans is a protracted affair that begins prenatally and continues through adolescence. It is well worth understanding the principles that have emerged from human studies because we are the only species for which comprehensive behavioral data exists. From 25 to 40 weeks post conception, the human fetus gradually becomes responsive to spectral and temporal features of sound, including speech, as measured by heart rate responses [1,2]. The age at which mature auditory performance is attained varies tremendously with the task, and may extend over a decade. Some percepts develop rapidly. Frequency resolution (tone detection in the presence of a second nearby tone), which is mature by 6 months. In contrast, frequency discrimination (hearing a difference between two sequential tones) is not adult-like until 4 years when assessed with tone of long duration. However, when brief tones are used, frequency discrimination continues to improve until 9 years, suggesting that attentional factors may contribute to the poorer performance of young children [3,4]. Sound localization matures over a somewhat longer interval; the minimum audible angle improves dramatically over the first five postnatal years, from ≈25° to ≈2°, although there is quite a bit of variability between subjects and development may continue for several years [3].
Percepts that take a long time to reach maturity include the detection of temporalcues, such as frequency and amplitude modulation. These cues contribute to human speech perception [5,6], making them of particular interest. Thus, the ability to detect amplitude modulations continues to improve beyond 7 years of age, and may not reach adult values until after 10 years [7,8]. Similarly, detection of frequency modulation continues to mature until about 8 years [9]. Using another measure of temporal resolution, the ability to detect a tone followed immediately by a noise (backward masking), it has been found that thresholds for 10 year-old children are still significantly poorer than those of adults [10]. This slow progression of perceptual development parallels the prolonged development of the human central auditory system. Sound-evoked cortical potentials reach a mature state during the first ten years of life, and this correlates with axonal maturation in supragranular cortical layers [11,12].
Together, these studies show that auditory behavior emerges by degrees, and suggest that acoustic experience can shape specific percepts during discrete intervals. Newborns can respond selectively to their mother's voice with an increased heart rate, presumably due to their acoustic experience in utero [13,14]. Influences of prenatal acoustic experience on sound perception have been further demonstrated in the ability of neonates to discriminate sentences of different languages or differing emotional expressions based on prosodic and rhythmic cues [15,16]. Recent studies also indicate advanced development of cortical response properties to speech sounds in preterm infants compared to control subjects of the same gestation ages, suggesting that maturation of the auditory system is driven by sensory experience [17]. Such precocious development may be related to accelerated brain anatomical development in preterm infants, including faster maturation of cerebral white matter [18]. Although auditory system development may be accelerated in preterm infants, longitudinal studies have generally revealed impaired auditory and speech processing in school-age children of premature birth, suggesting that the influence of experience is limited by intrinsic neurodevelopmental processes [19].
Prenatal adaptation to the prosodic structures of native speech may allow the neonatal auditory system to parse continuous streams of speech sounds into perceptually salient acoustic units, which may further shape representations of speech sounds at the phonetic level. Humans are born with language-universal phonetic representation and perception, which gradually becomes specialized for native speech by twelve months of age, losing perceptual sensitivity to some phonetic contrasts that are not used in the native language [20]. Such a process is likely due to active reorganization of the perceptual space rather than passive atrophy of foreign speech sound representations, because sensitivity to some foreign phonetic contrasts is preserved [20].
A central issue in speech perception research concerns the origin of categorical perception of speech sounds. Early studies reported evidence of categorical speech sound perception in two-month old infants [21], suggesting that it may be innate. More recent studies, however, have revealed profound within-category sensitivity and graded representations for consonants at ages of six months and older [22 *]. Parallel studies indicate that perceptual categories may be formed through passive exposure to speech sounds with bimodal, but not unimodal, distributions along the voice-onset time continuum [23 **,24]. Similar learning of the statistical structure of speech inputs presumably results in reorganized perceptual space [25], and facilitated perception of native speech sound [26]. While some studies suggest that simple acoustic exposure is sufficient to alter perceptual behaviors in infants [23,24], others argue that attention and social interaction are required [27,28].
The development of non-invasive brain imaging and recording methods has resulted in an explosive growth of research into human central auditory development. Despite these efforts, it remains unclear how experience shapes the cortical representation of speech sounds, and how this affects speech perception. For example, functional magnetic resonance imaging (fMRI) studies revealed weaker activation of the auditory areas by prototypical speech sounds as compared to less prototypical ones [29,30], suggesting that frequent experience of speech sounds refines their cortical representations. On the other hand, studies of mismatch negativity of event-related potentials showed more pronounced responses to native speech sounds than to non-native ones [31], suggesting that experience leads to more distinct representations of native speech sounds. More systematic investigations are needed to elucidate the neural mechanisms of experience-dependent sensory development in humans. Although the maturation of cortical sound representations has been extensively studied for the mechanisms underlying auditory perceptual development, the prolonged maturation of auditory brain stem responses to speech sound could also contribute to the progressive development of speech perception [32].
Learning to recognize speech sounds seems effortless for young children, yet it can be extremely difficult for adults, suggesting a sensitive period for the auditory system. This idea is supported by studies on children with hearing loss. Children identified on the basis of a speech or language delay demonstrated a correlation with the severity of hearing loss, including a significant effect of fluctuating conductive hearing loss [33,34]. An anatomical study suggests that congenital hearing loss leads to a significant reduction of white matter within Heschl's gyrus, perhaps reflecting a loss of myelination or axons [35]. A compelling example of a sensitive period is found in the studies of profoundly deaf children who receive an intracochlear prosthetic device that converts acoustic signals to patterned electrical stimulation. The auditory and language performance of prelingually deafened adults who received a cochlear implant generally remains well below that of postlingually deafened adults [36]. However, patients who are implanted at younger ages generally exhibit better language acquisition and may attain performance comparable to their normal hearing peers if implanted before 12 months [37-39]. For congenitally deaf children, cochlear implants also improved the abilty to fuse auditory and visual information when watching and listening to a speaker, and the benefit was more pronounced when children were implanted before 2.5 years [40]. Functional studies provide further support for a critical period. Cortical auditory evoked potentials were recorded in response to a synthesized speech syllable in children with normal hearing and those with a cochlear prosthesis. Children with the longest period of auditory deprivation (>7 years) before receiving the prosthesis had abnormally long cortical response latencies to the speech sound [41,42]. In contrast, the auditory brain stem evoked response does not display the same dependence on age of implantation [43].
The development of non-phonetic processing of language is beyond the scope of this review. It is worth noting, however, that speech sound perception in infancy is positively correlated with later development in word comprehension [44]. The finding is consistent with the notion that different language components develop hierarchically—i.e., prenatal adaptation to prosodic structures of native language improves subsequent phonetic and phonological learning, which in turn facilitates lexical development. These developmental stages occur in different, but possibly overlapping, sensitive periods [20].
Developmental Plasticity of Auditory Cortex
Many studies of development and plasticity in animal models have focused on the primary auditory cortex (AI). Early reports suggested that the frequency map within rat AI was poorly organized shortly after the onset of sound-evoked responses [45,46]. However, recent studies demonstrate that AI displays a precise tonotopic map shortly after hearing onset, with frequency tuning actually becoming somewhat broader during early development [47-50]. Because frequency tuning at the cochlear nucleus gradually becomes sharper during development [51], the observed increase in tuning bandwidth in AI neurons probably reflects central auditory development. In addition to frequency tuning and cortical maps, cortical responses to rapidly repeating or frequency-modulated sounds also improve during early development [52,53].
The development of acoustic representations is profoundly influenced by the acoustic environment [46]. For example, repeated exposure to a tone in developing rats and mice results in enlarged cortical representations of that tone [46,49,52,54,55]. The absence of experience with rapidly pulsed sounds, or over-exposure to slow-rate sounds, leads to reduced entrainment of cortical responses to tones of high repetition rate [45,56]. Temporal features of the acoustic input also influence spectral plasticity—the auditory cortical neurons tend to over-represent sounds repeated at the temporal rates of species specific vocalizations [57]. These principles extend to the selectivity for frequency-modulated sounds. When juvenile bats are devocalized, such that they do not hear the frequency modulated signals that they emit to navigate through space, cortical neurons are subsequently found to be less selective for sweep direction and rate [53]. The converse is also true: exposure of juvenile rats to frequency-modulated sweeps results in more auditory cortical neurons selective for the direction and rate of the exposure sound [50].
In most studies of developmental plasticity, animals are either deprived of structured sensory inputs or repeatedly exposed to a single stimulus. Those studies represent the first steps in understanding auditory developmental plasticity, and may provide insights to hearing loss-induced brain alterations. However, the neural mechanisms associated with developmental plasticity may not be properly engaged under acoustically impoverished conditions. Indeed, exposing juvenile rats to tone sequences results in complex reorganization of the AI map [58]. In cats exposed to a continuous stream of tones delivered in a random fashion, cortical neuron responses to the exposed frequencies are suppressed, rather than enhanced, suggesting that complex spectral interactions shape cortical representations [59]. More systematic investigations of these interactions using statistically structured acoustic inputs may reveal cortical mechanisms of statistical learning.
Acoustic representations in the primary auditory cortex are susceptible to modulation by sensory exposure during defined epochs of early development, often referred to as the critical period. For example, the cortical representations of a tone near threshold may be enlarged by repeated exposure to the tone during a narrow time window from postnatal day eleven to day thirteen, when cortical responses are just emerging [49]. However, this critical period is not fixed in time, but rather depends on the temporal structure of sensory inputs. Masking temporal information with continuous tones or noise retards development of spectral tuning, and delays the critical period for frequency map plasticity [45]. Interestingly, when temporal information is masked for a narrow band of frequencies, only the neurons representing those frequencies remain immature and plastic, suggesting that experience-dependent maturation of the auditory cortex is a local rather than global process [60 *,61]. A recent study indicated that multiple sensitive periods exist for specific sound features, such as the characteristic frequency, tuning bandwidth, and frequency modulation [50]. Sensitive periods for more complex sound features tend to occur later, with each sensitive period approximately coincidental with the emergence or maturation of the cortical representation of the corresponding feature [50].
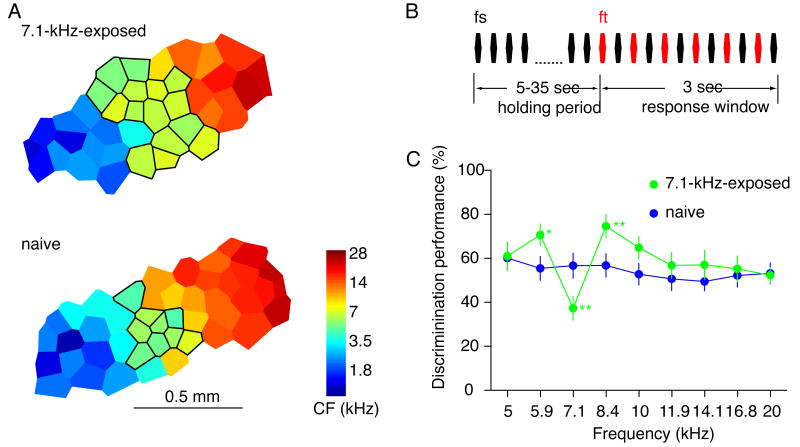
It has long been assumed that cortical plasticity serves to improve sensory perception. Improving one perceptual behavior may, however, come at the expense of impairing another. Categorical perception of sounds, for instance, is defined in part by impaired discrimination ability near the category prototypes. A recent study reported that early exposure of rat pups to a single frequency tone enlarged cortical representations of that tone, but impaired discrimination of nearby frequencies (Figure 1) [54 **]. The discrimination of the neighboring frequencies was improved. Drawing the parallel between these findings and those on speech perception in human infants, the authors argue that experience-induced increases in sensory representation may be a mechanism for categorical perception [54,62 *]. Further perceptual testing will be necessary to establish whether tone-reared rats exhibit true categorical perception. Alternatively, enlarged representations may serve to improved stimulus detection and stronger stimulus salience.
Figure 1. Repeated exposure to a tone influences cortical tonotopic map and frequency discrimination.
A. Representative cortical tonotopic maps from a naïve rat and a rat that had been exposed to 7.1-kHz tone pips from postnatal day 9 to day 30. Each polygon corresponds to a recording site, and the color codes for the characteristic frequency of the recorded neurons. The outlined areas had characteristic frequencies in a range of 7.1 kHz ± 0.2 octaves. Note the enlarged representation of frequencies near 7.1 kHz in the tone-exposed animal. B. Schematic of the frequency discrimination task. After a tone of the standard frequency was played for a variable period, the standard tone and a target tone was played alternatively. The animal was trained to detect the difference between the standard and the target frequencies and make a nose poke within three second after the target onset. C. Animal performances in discriminating a 0.3-octave frequency difference. The 7.1-kHz-exposed animals showed impaired performance near the exposure frequency, and improved performance at neighboring frequencies. * indicates p < 0.05, and ** p < 0.01. (Modified from [54]).
While developmental plasticity can be induced by simple sensory exposure, the adult nervous system is largely refractory to such treatment. However, sensory exposure may cause robust plasticity effects when it is paired with activation of neuromodulatory systems [63-65]. In a way, the neuromodulatory actions may be regarded as reactivating developmental plasticity in adulthood. Studies on the visual pathway provide the clearest understanding of reactivation of plasticity.
Developmental Plasticity of the Auditory Brain Stem
A unique characteristic of the central auditory pathway is that information is processed by many brain stem nuclei before ascending to the thalamus and cortex. Therefore, it is possible that early experience effects some or all of these structures. In fact, the original studies to demonstrate an environmental influence on auditory coding properties were carried out in a midbrain nucleus called the inferior colliculus (IC). Foremost among these were experiments that asked whether binaural coding properties that support sound localization were influenced by experience.
The central auditory system can extract information about the location of a sound source by comparing the level of sound, or the time of its arrival, at the two ears. In most vertebrates, these interaural level (ILD) and time differences (ITD) are first computed in brain stem nuclei, and are manifest throughout the ascending pathway to the cortex. A disruption of these cues during development leads to dramatic alterations in binaural coding properties in a broad range of vertebrates [66].
When developing barn owls are reared with a plug inserted into one ear, they compensate for the abnormal cues, and are eventually able to localize sounds accurately. Much of this behavioral compensation is due to a visually-guided mechanism operating within the superior colliculus whereby binaural properties are adjusted to bring an auditory space map back into alignment with the primary visual space map [67]. However, ILD coding can be influenced by experience even when owls are deprived of vision. Modified ILD coding properties are detected in the brain stem nucleus that initially computes this percept (called VLVp), and changes to ILD coding become quite profound in midbrain neurons [68,69]. Similarly, rearing gerbils in omnidirectional noise, a stimulus that limits experience with interaural signals, leads to a disruption of ITD coding in a brain stem nucleus called DNLL; ITD coding was normal in adults exposed to the same stimulus [70 *]. One recent study has shown that binaural properties within the guinea pig cochlear nucleus, the first structure to receive afferents from the cochlea, are altered within one day of unilateral hearing loss, suggesting that ILD coding remains extremely dynamic, even in adult animals [71].
If the auditory brain stem is a primary site of developmental plasticity, does experience also influence frequency processing, as described above for cortex? In fact, early studies demonstrated similar effects in rodents. When mice are reared in repetitive clicks, a stimulus that co-activates afferents across a broad frequency range, single IC neurons become more broadly tuned to frequency [72]. Similarly, when rats are reared with a single tone, the IC becomes more responsive to that frequency [73]. A particularly clever experiment showing the influence of activity on tonotopic development made use of an electrode array that was inserted into the cochleae of deafened cats. Animals were reared with repetitive electrical pulses, delivered either to one position along the cochlea (i.e., one frequency region), or alternating between two different positions. When one position was activated, that region of the cochlea was subsequently able to drive a larger fraction of the IC tonotopic axis, as compared to unstimulated animals. In contrast, when two positions were activated alternately, the expansion was prevented [74,75]. Each of these experiments support the general idea that co-active synapses establish stronger connections during IC development which may increase the representation of a frequency. Furthermore, there is some evidence to suggest behavioral consequences of early acoustic stimulation. In mice with genetically determined progressive hearing loss, exposure to pulses of broadband noise can partially rescue the response of IC neurons to high frequencies that would ordinarily be lost during development, and improve the behavioral response to these same frequencies [76]. In somewhat older animals with noise-induced hearing loss, alterations in the cortical frequency map can be ameliorated by exposing the animals with a multi-tone complex [77].
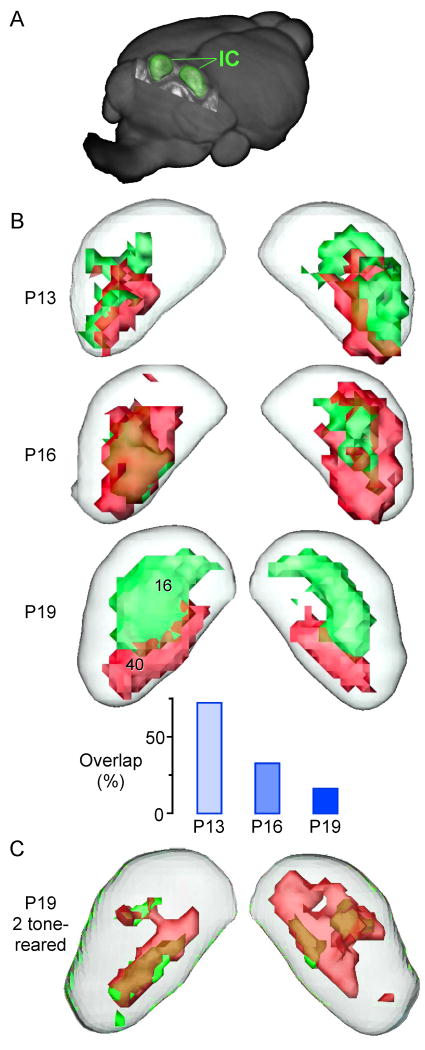
The influence of rearing environment can lead to a large-scale reorganization of the IC tonotopic axis, as demonstrated with an imaging technique called manganese enhanced MRI (MEMRI). Pure tone stimulation causes Mn+2 ions to become localized at the site of neural activity, presumably via influx through calcium channels. MRI is then used to identify this site, providing a 3-dimensional map of frequency-specific responses. As shown in Figure 2, a much larger percentage of IC responded to each test frequency at the onset of hearing, but these patterns became quite restricted by 3 weeks postnatal. When animals were reared with two tones presented simultaneously, a significant volume of the IC became responsive to both rearing frequencies [78 *]. The mechanistic basis for this plasticity is somewhat mysterious because the broad frequency responses observed at hearing onset originate primarily in the cochlea, and brain stem afferent projections are topographically organized at this age, although they do undergo a period of anatomical refinement [79-82]. One possibility is that local axonal projections establish and strengthen contacts across the IC tonotopic laminae during development [83]. Alternatively, it is possible that the IC inherits a modified tonotopy from the auditory cortex via descending projections [84]. Inactivation of the auditory cortex while rearing animals with tones or noise could help to resolve this issue.
Figure 2. The inferior colliculus tonotopic map is reorganized when mice are reared in a two-tone environment.
A. A volumetric representation of the whole mouse brain was reconstructed from 100 μm images using manganese-enhanced magnetic resonance imaging (MEMRI). The two lobes of the inferior colliculus (IC) are shown in green. B. Three-dimensional maps of 16kHz (green) and 40kHz (red) activity are shown for the IC at 3 postnatal ages. The bar graph summarizes the decrease in overlap volume between these two frequency regions. C. When animals are exposed to a synchronous two-tone stimulus (16+40kHz) from P9-17, a significant volume of IC becomes responsive to both rearing frequencies. (Modified from [78])
Some of these functional effects may be due to activity-dependent remodeling of axonal connections within the auditory brain stem, while others may be due to changes in synaptic strength (below). The elimination of cochlear activity in postnatal animals can prevent the normal refinement of both excitatory and inhibitory connections. The cat auditory nerve afferents to the cochlear nucleus (CN) remain topographically organized following neonatal deafening, but when normalized for the smaller CN, the projections are >30% broader than in control animals [85 *]. Similar findings have been obtained for excitatory afferents from the CN to superior olivary nuclei or to the IC [66]. Inhibitory projections are relatively common in the auditory brain stem, and the anatomical specificity of these afferents are also influenced by cochlear activity [86-89]. Thus, many of the functional changes that occur following environmental manipulations may reflect alterations of inhibitory synapse specificity or function.
Synaptic Mechanisms
Studies of developmental plasticity must separate the contribution of cochlear maturation from those in the CNS. This is particularly challenging in the auditory system because descending projections from cortex influence subcortical centers and, ultimately, the cochlea [84]. Furthermore, there are significant changes to the pinna, ear canal, middle ear, and cochlea during normal development, and each of them can influence central coding properties. For example, many of the developmental changes displayed by ferret cortex neurons in response to spatial cues are determined by maturation of the pinna [90,91]. One way to circumvent this problem is to measure synapse function in brain slices obtained from normal or environmentally manipulated animals.
Dramatic changes in synaptic strength are induced by developmental hearing loss throughout the auditory CNS. For the most part, bilateral hearing loss leads to enhanced neuron excitability brought about by homeostatic changes in synaptic gain. Depending on the region, increased excitability can be governed by quite distinct mechanisms. In a genetically deaf mouse, for example, excitatory synaptic currents become larger in the cochlear nucleus (CN), but are unaffected in the MNTB; instead, MNTB neurons become more excitable due to the down-regulation of a potassium channel [92,93]. Functional changes in the CN of deaf mice are accompanied by dramatic morphological changes, including a loss of synaptic vesicles, and an increase in postsynaptic density area [94].
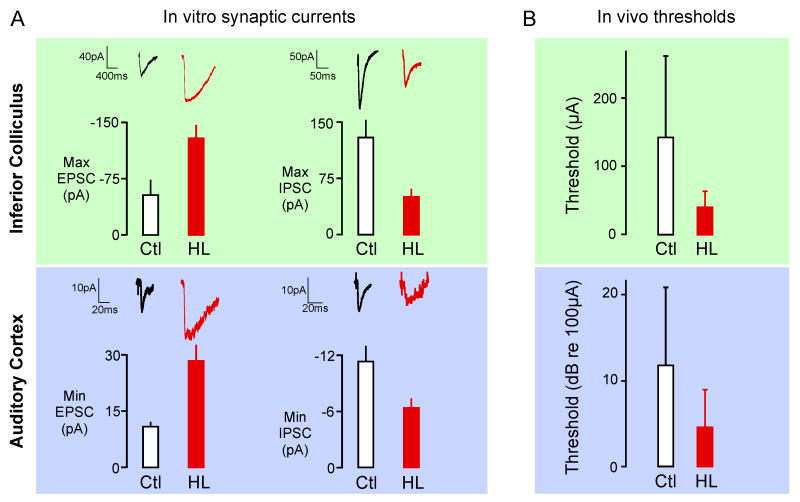
Studies in the auditory midbrain and cortex suggest that excitatory and inhibitory synaptic alterations go hand-in-hand (Figure 3A). In the IC, maximum evoked excitatory currents are larger following bilateral cochlear ablation, whereas maximum inhibitory currents decline. The decreased inhibitory strength occurs, in part, because the chloride equilibrium potential is not maintained [95,96]. In the auditory cortex, putative monosynaptic thalamus-evoked excitatory currents are twice as large following hearing loss, while monosynaptic intracortically-evoked inhibitory currents are ≈50% of normal. Here, inhibitory strength declines, in part, because of decreased GABAA receptor trafficking to the postsynaptic membrane [97-99]. Even a moderate form of hearing loss can induce synaptic alterations in auditory cortex. In animals reared with a conductive loss, that raises hearing thresholds but does not injure the cochlea, thalamic afferents displayed greater excitatory synaptic depression during repetitive stimulation [100]. Together, synaptic modifications of this sort may help to explain why in vivo thresholds are actually lower (i.e, better) in deafened animals when the cochlea is activated electrically (Figure 3) [74,101].
Figure 3. Hearing loss increases excitability in the central auditory pathway.
A. Synaptic currents were measured in two central loci following bilateral hearing loss at P10. In the inferior colliculus (top, green background), maximum evoked excitatory synaptic currents were larger, and maximum evoked inhibitory currents were smaller (solid red bars), as compared to those recorded in control neurons (open black bars). In the auditory cortex (bottom, blue background), minimum evoked excitatory currents were larger, and minimum evoked inhibitory currents were smaller (solid red bars), as compared to those recorded in control neurons (open black bars). All differences between control (Ctl) and hearing loss (HL) are significant. (Modified from [95,97,98,113]) B. Following hearing loss in developing cats, stimulating electrodes were inserted into the cochlea, and electrically-evoked responses were measured in two brain areas. In both the inferior colliculus (top, green background), and the auditory cortex (bottom, blue background), the threshold stimulus was lower (i.e., better) following a period of hearing loss, as compared to acutely implanted animals. Differences between control (Ctl) and hearing loss (HL) are significant. (Modified from [74,101]). Thus, the balance between excitatory and inhibitory synaptic currents was shifted to favor excitation, and this may help to explain the lower in vivo thresholds.
Environmentally-induced changes in auditory coding properties, discussed above, are thought to be mediated by activity-dependent changes in synaptic strength. One putative mechanism has been characterized in the lateral superior olive (LSO), a brain stem nucleus that responds selectively to ILD cues by integrating excitatory inputs driven by the ipsilateral ear with inhibitory inputs from the medial nucleus of the trapezoid body (MNTB) which are driven by the contralateral ear. Developing inhibitory MNTB terminals undergo synapse refinement and elimination in two phases: a decrease in functional connections occurs before hearing onset, and an anatomical elimination of terminal boutons occurs after [79,80,102]. The latter process depends on an intact cochlea, and during this time MNTB terminals display use-dependent long-term depression (LTD), suggesting a role in synaptic remodeling [103]. Inhibitory synaptic LTD is mediated by GABA signaling. MNTB terminals release GABA during a transient period of postnatal development, and LTD can be induced by focal application of GABA, or blocked with a GABAB receptor antagonist [104]. Thus, activity-dependent pruning of MNTB arbors may depend on LTD after the onset of hearing, but may rely on a distinct mechanism beforehand [105].
Recently, it has been suggested that experience is required for the normal maturation of synaptic plasticity mechanisms. Excitatory synaptic long-term potentiation (LTP) becomes more prominent during auditory cortex development. However, LTP is compromised following hearing loss while LTD persists [106 *]. Similarly, hearing loss leads to a down-regulation of two molecules that are implicated in LTP (BDNF and CREB), and expression can be restored with electrical stimulation of cochlea [107 *]. In contrast, rearing young animals (but not adults) in white noise leads to increased excitatory LTP and decreased LTD [108]. This suggests that the system remains in a more labile state, consistent with earlier findings [104].
Conclusions
The developing auditory CNS is remarkably labile, at least in response to flagrant environmental manipulations. Despite some shortcomings, we would argue that these experiments do hint at principles that apply to normal development, and they certainly suggest several promising research directions. First, it is likely that much, if not all, of the central auditory system is initially malleable in response to environmental manipulations. Hearing loss can elicit structural and functional changes from cochlear nucleus through auditory cortex. Even exposure to a continuous auditory stimulus tends to produce a functional reorganization that is as large at the inferior colliculus as in auditory cortex. However, the auditory pathway is a loop of ascending and descending projections [84]. We do not yet understand whether each auditory locus responds to the environment autonomously, or whether corticofugal projections provide descending control over brain stem plasticity.
Second, profound changes in the strength of central auditory synapses are associated with auditory deprivation. There is a growing appreciation that the behavioral deficits associated with early hearing loss require a consideration of both cochlear and brain function. We presume that alterations in synaptic gain are also associated with environmental manipulations in which animals are over-exposed to a specific sound cue. In principle, brain slice experiments can be used to assess synapse function after passive exposure or training in vivo [109], but it has yet to be applied to the developing auditory system.
Third, it appears that (human) auditory perceptual skills and (non-human) auditory coding properties do not develop in unison. Furthermore, they are each vulnerable to environmental manipulations during different periods of development. To date, the acoustic manipulations that lead to this conclusion are relatively crude, and we must begin to use stimuli with meaningful (to the nervous system) statistical structure. Perhaps most crucial will be our ability to identify and control behavioral context. For example, mallard ducklings can imprint on a chicken call, but only when reared with contact; tactile isolates do not develop this preference despite an identical acoustic rearing environment [110]. Active participation in an auditory task during development, and the increased attention that it leverages, must also be considered [111]. When juvenile zebra finches are engaged in a task that provides them with exposure to a tutor song, it is found that relatively few stimuli are optimal for learning; song learning actually decline when too many stimuli are provided [112].
Finally, in the few instances where behavioral performance has been examined following an environmental manipulation, the outcome displays a compelling correlate to functional modifications within the auditory CNS [54,67,76]. While these results are encouraging, we would hasten to point out that we know almost nothing about the normal development of perception (in other than humans), and our understanding of functional maturation is based solely on anesthetized animals and brain slices. Behavioral studies on immature non-human animals, and associated physiology studies on awake animals during the period of normal maturation will better permit us to assign underlying neural mechanisms, and will be quite helpful for interpreting the results from environmental manipulations.
Acknowledgments
This work was supported by DC006864 (DHS), DC009259 (SB), and American Tinnitus Association (SB).
Footnotes
Conflicts of Interest: The authors have no conflicts of interest with the publication of this article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Dan H. Sanes, Center for Neural Science, New York University, 4 Washington Place, New York, NY 10003, Email: sanes@cns.nyu.edu
Shaowen Bao, Helen Wills Neuroscience Institute, 210X Barker Hall, University of California, Berkeley, Berkeley, California 94720, Email: sbao@berkeley.edu.
References
- 1.Shahidullah S, Hepper PG. Frequency Discrimination by the Fetus. Early Hum Dev. 1994;36:13–26. doi: 10.1016/0378-3782(94)90029-9. [DOI] [PubMed] [Google Scholar]
- 2.Groome LJ, Mooney DM, Holland SB, Smith YD, Atterbury JL, Dykman RA. Temporal pattern and spectral complexity as stimulus parameters for eliciting a cardiac orienting reflex in human fetuses. Percept Psychophys. 2000;62:313–320. doi: 10.3758/bf03205551. [DOI] [PubMed] [Google Scholar]
- 3.Saffran JR, Werker JF, Werner LA. The infant's auditory world: hearing, speech, and the beginnings of language. In: Kuhn D, Siegler RS, editors. Cognition, perception, and language. 6th. John Wiley & Sons Inc.; 2006. pp. 58–108. [Google Scholar]; Damon W, Lerner RM, editors. Handbook of child psychology. Vol. 2 Series Editor. [Google Scholar]
- 4.Moore DR, Ferguson MA, Halliday LF, Riley A. Frequency discrimination in children: perception, learning and attention. Hear Res. 2008;238:147–154. doi: 10.1016/j.heares.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 5.Rosen S. Temporal information in speech: acoustic, auditory and linguistic aspects. Philos Trans R Soc Lond B Biol Sci. 1992;336:367–373. doi: 10.1098/rstb.1992.0070. [DOI] [PubMed] [Google Scholar]
- 6.Shannon RV, Zeng FG, Kamath V, Wygonski J, Ekelid M. Speech recognition with primarily temporal cues. Science. 1995;270:303–304. doi: 10.1126/science.270.5234.303. [DOI] [PubMed] [Google Scholar]
- 7.Hall JW, 3rd, Grose JH. Development of temporal resolution in children as measured by the temporal modulation transfer function. J Acoust Soc Am. 1994;96:150–154. doi: 10.1121/1.410474. [DOI] [PubMed] [Google Scholar]
- 8.Banai K, Sabin AT, Kraus N, Wright BA. The development of sensitivity to amplitude and frequency modulation follow distinct time courses. ARO Abstract. 2007;30:928. [Google Scholar]
- 9.Dawes P, Bishop DV. Maturation of visual and auditory temporal processing in school-aged children. J Speech Lang Hear Res. 2008;51:1002–1015. doi: 10.1044/1092-4388(2008/073). [DOI] [PubMed] [Google Scholar]
- 10.Wright BA, Zecker SG. Learning problems, delayed development, and puberty. Proc Natl Acad Sci U S A. 2004;101:9942–9946. doi: 10.1073/pnas.0401825101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ceponiene R, Rinne T, Naatanen R. Maturation of cortical sound processing as indexed by event-related potentials. Clin Neurophysiol. 2002;113:870–882. doi: 10.1016/s1388-2457(02)00078-0. [DOI] [PubMed] [Google Scholar]
- 12.Moore JK, Guan YL. Cytoarchitectural and axonal maturation in human auditory cortex. J Assoc Res Otolaryngol. 2001;2:297–311. doi: 10.1007/s101620010052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kisilevsky BS, Hains SMJ, Lee K, Xie X, Huang HF, Ye HH, Zhang K, Wang ZP. Effects of experience on fetal voice recognition. Psychol Sci. 2003;14:220–224. doi: 10.1111/1467-9280.02435. [DOI] [PubMed] [Google Scholar]
- 14.DeCasper AJ, Fifer WP. Of human bonding: newborns prefer their mothers' voices. Science. 1980;208:1174–1176. doi: 10.1126/science.7375928. [DOI] [PubMed] [Google Scholar]
- 15.Nazzi T, Bertoncini J, Mehler J. Language discrimination by newborns: toward an understanding of the role of rhythm. J Exp Psychol Hum Percept Perform. 1998;24:756–766. doi: 10.1037//0096-1523.24.3.756. [DOI] [PubMed] [Google Scholar]
- 16.Mastropieri D, Turkewitz G. Prenatal experience and neonatal responsiveness to vocal expressions of emotion. Dev Psychobiol. 1999;35:204–214. doi: 10.1002/(sici)1098-2302(199911)35:3<204::aid-dev5>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 17.Nishida T, Kusaka T, Isobe K, Ijichi S, Okubo K, Iwase T, Kawada K, Namba M, Imai T, Itoh S. Extrauterine environment affects the cortical responses to verbal stimulation in preterm infants. Neurosci Lett. 2008;443:23–26. doi: 10.1016/j.neulet.2008.07.035. [DOI] [PubMed] [Google Scholar]
- 18.Gimenez M, Miranda MJ, Born AP, Nagy Z, Rostrup E, Jernigan TL. Accelerated cerebral white matter development in preterm infants: a voxel-based morphometry study with diffusion tensor MR imaging. Neuroimage. 2008;41:728–734. doi: 10.1016/j.neuroimage.2008.02.029. [DOI] [PubMed] [Google Scholar]
- 19.Foster-Cohen S, Edgin JO, Champion PR, Woodward LJ. Early delayed language development in very preterm infants: evidence from the MacArthur-Bates CDI. J Child Lang. 2007;34:655–675. doi: 10.1017/s0305000907008070. [DOI] [PubMed] [Google Scholar]
- 20.Werker JF, Tees RC. Speech perception as a window for understanding plasticity and commitment in language systems of the brain. Dev Psychobiol. 2005;46:233–251. doi: 10.1002/dev.20060. [DOI] [PubMed] [Google Scholar]
- 21.Eimas PD, Siqueland ER, Jusczyk P, Vigorito J. Speech perception in infants. Science. 1971;171:303–306. doi: 10.1126/science.171.3968.303. [DOI] [PubMed] [Google Scholar]
- 22.McMurray B, Aslin RN. Infants are sensitive to within-category variation in speech perception. Cognition. 2005;95:B15–26. doi: 10.1016/j.cognition.2004.07.005. [DOI] [PubMed] [Google Scholar]; * This study shows that infant are sensitive to within-category variations in stop consonants. The authors argue that such a representation is adaptive--it preserves plasticity until more information about native speech input is available.
- 23.Maye J, Werker JF, Gerken L. Infant sensitivity to distributional information can affect phonetic discrimination. Cognition. 2002;82:B101–111. doi: 10.1016/s0010-0277(01)00157-3. [DOI] [PubMed] [Google Scholar]; ** This study show that perceptual categories can be established in infants by exposure to sounds bimodallym, but not unimodally, distributed along a phonetic continuum.
- 24.Maye J, Weiss DJ, Aslin RN. Statistical phonetic learning in infants: facilitation and feature generalization. Dev Sci. 2008;11:122–134. doi: 10.1111/j.1467-7687.2007.00653.x. [DOI] [PubMed] [Google Scholar]
- 25.Kuhl PK. Human adults and human infants show a “perceptual magnet effect” for the prototypes of speech categories, monkeys do not. Percept Psychophys. 1991;50:93–107. doi: 10.3758/bf03212211. [DOI] [PubMed] [Google Scholar]
- 26.Kuhl PK, Stevens E, Hayashi A, Deguchi T, Kiritani S, Iverson P. Infants show a facilitation effect for native language phonetic perception between 6 and 12 months. Dev Sci. 2006;9:F13–F21. doi: 10.1111/j.1467-7687.2006.00468.x. [DOI] [PubMed] [Google Scholar]
- 27.Toro JM, Sinnett S, Soto-Faraco S. Speech segmentation by statistical learning depends on attention. Cognition. 2005;97:B25–34. doi: 10.1016/j.cognition.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 28.Kuhl PK. Is speech learning ‘gated’ by the social brain? Dev Sci. 2007;10:110–120. doi: 10.1111/j.1467-7687.2007.00572.x. [DOI] [PubMed] [Google Scholar]
- 29.Guenther FH, Nieto-Castanon A, Ghosh SS, Tourville JA. Representation of sound categories in auditory cortical maps. J Speech Lang Hear Res. 2004;47:46–57. doi: 10.1044/1092-4388(2004/005). [DOI] [PubMed] [Google Scholar]
- 30.Myers EB. Dissociable effects of phonetic competition and category typicality in a phonetic categorization task: an fMRI investigation. Neuropsychologia. 2007;45:1463–1473. doi: 10.1016/j.neuropsychologia.2006.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Naatanen R, Lehtokoski A, Lennes M, Cheour M, Huotilainen M, Iivonen A, Vainio M, Alku P, Ilmoniemi RJ, Luuk A, et al. Language-specific phoneme representations revealed by electric and magnetic brain responses. Nature. 1997;385:432–434. doi: 10.1038/385432a0. [DOI] [PubMed] [Google Scholar]
- 32.Johnson KL, Nicol T, Zecker SG, Kraus N. Developmental plasticity in the human auditory brainstem. J Neurosci. 2008;28:4000–4007. doi: 10.1523/JNEUROSCI.0012-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schonweiler R, Ptok M, Radu HJ. A cross-sectional study of speech- and language-abilities of children with normal hearing, mild fluctuating conductive hearing loss, or moderate to profound sensoneurinal hearing loss. Int J Pediatr Otorhinolaryngol. 1998;44:251–258. doi: 10.1016/s0165-5876(98)00075-5. [DOI] [PubMed] [Google Scholar]
- 34.Psillas G, Psifidis A, Antoniadou-Hitoglou M, Kouloulas A. Hearing assessment in pre-school children with speech delay. Auris Nasus Larynx. 2006;33:259–263. doi: 10.1016/j.anl.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 35.Emmorey K, Allen JS, Bruss J, Schenker N, Damasio H. A morphometric analysis of auditory brain regions in congenitally deaf adults. Proc Natl Acad Sci U S A. 2003;100:10049–10054. doi: 10.1073/pnas.1730169100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fallon JB, Irvine DR, Shepherd RK. Cochlear implants and brain plasticity. Hear Res. 2008;238:110–117. doi: 10.1016/j.heares.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Svirsky MA, Teoh SW, Neuburger H. Development of language and speech perception in congenitally, profoundly deaf children as a function of age at cochlear implantation. Audiol Neurootol. 2004;9:224–233. doi: 10.1159/000078392. [DOI] [PubMed] [Google Scholar]
- 38.Miyamoto RT, Hay-McCutcheon MJ, Kirk KI, Houston DM, Bergeson-Dana T. Language skills of profoundly deaf children who received cochlear implants under 12 months of age: a preliminary study. Acta Otolaryngol. 2008;128:373–377. doi: 10.1080/00016480701785012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dettman SJ, Pinder D, Briggs RJ, Dowell RC, Leigh JR. Communication development in children who receive the cochlear implant younger than 12 months: risks versus benefits. Ear Hear. 2007;28:11S–18S. doi: 10.1097/AUD.0b013e31803153f8. [DOI] [PubMed] [Google Scholar]
- 40.Schorr EA, Fox NA, van Wassenhove V, Knudsen EI. Auditory-visual fusion in speech perception in children with cochlear implants. Proc Natl Acad Sci U S A. 2005;102:18748–18750. doi: 10.1073/pnas.0508862102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ponton CW, Eggermont JJ. Of kittens and kids: altered cortical maturation following profound deafness and cochlear implant use. Audiol Neurootol. 2001;6:363–380. doi: 10.1159/000046846. [DOI] [PubMed] [Google Scholar]
- 42.Sharma A, Dorman MF, Spahr AJ. A sensitive period for the development of the central auditory system in children with cochlear implants: implications for age of implantation. Ear Hear. 2002;23:532–539. doi: 10.1097/00003446-200212000-00004. [DOI] [PubMed] [Google Scholar]
- 43.Gordon KA, Papsin BC, Harrison RV. Activity-dependent developmental plasticity of the auditory brain stem in children who use cochlear implants. Ear Hear. 2003;24:485–500. doi: 10.1097/01.AUD.0000100203.65990.D4. [DOI] [PubMed] [Google Scholar]
- 44.Tsao FM, Liu HM, Kuhl PK. Speech perception in infancy predicts language development in the second year of life: a longitudinal study. Child Dev. 2004;75:1067–1084. doi: 10.1111/j.1467-8624.2004.00726.x. [DOI] [PubMed] [Google Scholar]
- 45.Chang EF, Merzenich MM. Environmental noise retards auditory cortical development. Science. 2003;300:498–502. doi: 10.1126/science.1082163. [DOI] [PubMed] [Google Scholar]
- 46.Zhang LI, Bao S, Merzenich MM. Persistent and specific influences of early acoustic environments on primary auditory cortex. Nat Neurosci. 2001;4:1123–1130. doi: 10.1038/nn745. [DOI] [PubMed] [Google Scholar]
- 47.Bonham BH, Cheung SW, Godey B, Schreiner CE. Spatial organization of frequency response areas and rate/level functions in the developing AI. J Neurophysiol. 2004;91:841–854. doi: 10.1152/jn.00017.2003. [DOI] [PubMed] [Google Scholar]
- 48.Pienkowski M, Harrison RV. Tone frequency maps and receptive fields in the developing chinchilla auditory cortex. J Neurophysiol. 2005;93:454–466. doi: 10.1152/jn.00569.2004. [DOI] [PubMed] [Google Scholar]
- 49.de Villers-Sidani E, Chang EF, Bao S, Merzenich MM. Critical period window for spectral tuning defined in the primary auditory cortex (A1) in the rat. J Neurosci. 2007;27:180–189. doi: 10.1523/JNEUROSCI.3227-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Insanally MN, Kover H, Kim H, Bao S. Feature-dependent sensitive periods in the development of complex sound representation. J Neurosci. 2009;29:5456–5462. doi: 10.1523/JNEUROSCI.5311-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Saunders JC, Dolgin KG, Lowry LD. The maturation of frequency selectivity in C57BL/6J mice studied with auditory evoked response tuning curves. Brain Res. 1980;187:69–79. doi: 10.1016/0006-8993(80)90495-3. [DOI] [PubMed] [Google Scholar]
- 52.Chang EF, Bao S, Imaizumi K, Schreiner CE, Merzenich MM. Development of spectral and temporal response selectivity in the auditory cortex. Proc Natl Acad Sci U S A. 2005;102:16460–16465. doi: 10.1073/pnas.0508239102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Razak KA, Richardson MD, Fuzessery ZM. Experience is required for the maintenance and refinement of FM sweep selectivity in the developing auditory cortex. Proc Natl Acad Sci U S A. 2008;105:4465–4470. doi: 10.1073/pnas.0709504105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Han YK, Kover H, Insanally MN, Semerdjian JH, Bao S. Early experience impairs perceptual discrimination. Nat Neurosci. 2007;10:1191–1197. doi: 10.1038/nn1941. [DOI] [PubMed] [Google Scholar]; ** This study shows that early experience of a sound leads to reduced discrimination performance near that sound. The effects can be accounted for by the observed expansion of representation of the experienced sound.
- 55.Takahashi K, Hishida R, Kubota Y, Kudoh M, Takahashi S, Shibuki K. Transcranial fluorescence imaging of auditory cortical plasticity regulated by acoustic environments in mice. Eur J Neurosci. 2006;23:1365–1376. doi: 10.1111/j.1460-9568.2006.04662.x. [DOI] [PubMed] [Google Scholar]
- 56.Zhou X, Merzenich MM. Enduring effects of early structured noise exposure on temporal modulation in the primary auditory cortex. Proc Natl Acad Sci U S A. 2008;105:4423–4428. doi: 10.1073/pnas.0800009105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim H, Bao S. Selective increase in representations of sounds repeated at an ethological rate. J Neurosci. 2009;29:5163–5169. doi: 10.1523/JNEUROSCI.0365-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nakahara H, Zhang LI, Merzenich MM. Specialization of primary auditory cortex processing by sound exposure in the “critical period”. Proc Natl Acad Sci U S A. 2004;101:7170–7174. doi: 10.1073/pnas.0401196101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Norena AJ, Gourevitch B, Aizawa N, Eggermont JJ. Spectrally enhanced acoustic environment disrupts frequency representation in cat auditory cortex. Nat Neurosci. 2006;9:932–939. doi: 10.1038/nn1720. [DOI] [PubMed] [Google Scholar]
- 60.de Villers-Sidani E, Simpson KL, Lu YF, Lin RC, Merzenich MM. Manipulating critical period closure across different sectors of the primary auditory cortex. Nat Neurosci. 2008;11:957–965. doi: 10.1038/nn.2144. [DOI] [PMC free article] [PubMed] [Google Scholar]; * The study shows that exposure to band-limited noises delays the maturation and closure of the critical period in a section of the primary auditory cortex that represents frequencies of the noise band.
- 61.Zhou X, Nagarajan N, Mossop BJ, Merzenich MM. Influences of un-modulated acoustic inputs on functional maturation and critical-period plasticity of the primary auditory cortex. Neuroscience. 2008;154:390–396. doi: 10.1016/j.neuroscience.2008.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim H, Bao S. Distributed representation of perceptual categories in the auditory cortex. J Comput Neurosci. 2008;24:277–290. doi: 10.1007/s10827-007-0055-5. [DOI] [PubMed] [Google Scholar]; * This paper investigates, with a computational model of the auditory cortex, the potential consequences of experience-induced cortical plasticity. The authors argue that cortical plasticity in the form of enlarged representation potentially contributes to categorical perception.
- 63.Bakin JS, Weinberger NM. Induction of a physiological memory in the cerebral cortex by stimulation of the nucleus basalis. Proc Natl Acad Sci U S A. 1996;93:11219–11224. doi: 10.1073/pnas.93.20.11219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bao S, Chan VT, Merzenich MM. Cortical remodelling induced by activity of ventral tegmental dopamine neurons. Nature. 2001;412:79–83. doi: 10.1038/35083586. [DOI] [PubMed] [Google Scholar]
- 65.Kilgard MP, Merzenich MM. Cortical map reorganization enabled by nucleus basalis activity. Science. 1998;279:1714–1718. doi: 10.1126/science.279.5357.1714. [DOI] [PubMed] [Google Scholar]
- 66.Moore DR, King AJ. Plasticity of binaural systems. In: Parks TN, Rubel EW, Fay RR, Popper AN, editors. Plasticity of the Auditory System. Springer; 2004. pp. 96–172. [Google Scholar]
- 67.Knudsen EI. Mechanisms of experience-dependent plasticity in the auditory localization pathway of the barn owl. J Comp Physiol [A] 1999;185:305–321. doi: 10.1007/s003590050391. [DOI] [PubMed] [Google Scholar]
- 68.Knudsen EI, Mogdans J. Vision-independent adjustment of unit tuning to sound localization cues in response to monaural occlusion in developing owl optic tectum. J Neurosci. 1992;12:3485–3493. doi: 10.1523/JNEUROSCI.12-09-03485.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mogdans J, Knudsen EI. Site of auditory plasticity in the brain stem (VLVp) of the owl revealed by early monaural occlusion. J Neurophysiol. 1994;72:2875–2891. doi: 10.1152/jn.1994.72.6.2875. [DOI] [PubMed] [Google Scholar]
- 70.Seidl AH, Grothe B. Development of sound localization mechanisms in the mongolian gerbil is shaped by early acoustic experience. J Neurophysiol. 2005;94:1028–1036. doi: 10.1152/jn.01143.2004. [DOI] [PubMed] [Google Scholar]; * Together with two other reports (Kapfer et al., 2002; Werthat et al., 2008), it has been found that inhibitory terminals become spatially restricted on brain stem neurons that compute interaural time differences (ITD), a response property that supports azimuthal sound localization. Importantly, when animals are deprived of ITD cues during development by rearing them in omnidirectional noise, the refinement of inhibitory terminals is prevented and ITD coding properties do not develop normally.
- 71.Sumner CJ, Tucci DL, Shore SE. Responses of ventral cochlear nucleus neurons to contralateral sound after conductive hearing loss. J Neurophysiol. 2005;94:4234–4243. doi: 10.1152/jn.00401.2005. [DOI] [PubMed] [Google Scholar]
- 72.Sanes DH, Constantine-Paton M. The sharpening of frequency tuning curves requires patterned activity during development in the mouse, Mus musculus. J Neurosci. 1985;5:1152–1166. doi: 10.1523/JNEUROSCI.05-05-01152.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Poon PW, Chen X. Postnatal exposure to tones alters the tuning characteristics of inferior collicular neurons in the rat. Brain Res. 1992;585:391–394. doi: 10.1016/0006-8993(92)91243-8. [DOI] [PubMed] [Google Scholar]
- 74.Snyder RL, Rebscher SJ, Cao KL, Leake PA, Kelly K. Chronic intracochlear electrical stimulation in the neonatally deafened cat I: Expansion of central representation. Hear Res. 1990;50:7–33. doi: 10.1016/0378-5955(90)90030-s. [DOI] [PubMed] [Google Scholar]
- 75.Leake PA, Snyder RL, Rebscher SJ, Moore CM, Vollmer M. Plasticity in central representations in the inferior colliculus induced by chronic single- vs two-channel electrical stimulation by a cochlear implant after neonatal deafness. Hear Res. 2000;147:221–241. doi: 10.1016/s0378-5955(00)00133-7. [DOI] [PubMed] [Google Scholar]
- 76.Willott JF, Turner JG. Neural plasticity in the mouse inferior colliculus: relationship to hearing loss, augmented acoustic stimulation, and prepulse inhibition. Hear Res. 2000;147:275–281. doi: 10.1016/s0378-5955(00)00137-4. [DOI] [PubMed] [Google Scholar]
- 77.Norena AJ, Eggermont JJ. Enriched acoustic environment after noise trauma reduces hearing loss and prevents cortical map reorganization. J Neurosci. 2005;25:699–705. doi: 10.1523/JNEUROSCI.2226-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yu X, Sanes DH, Aristizabal O, Wadghiri YZ, Turnbull DH. Large-scale reorganization of the tonotopic map in mouse auditory midbrain revealed by MRI. Proc Natl Acad Sci U S A. 2007;104:12193–12198. doi: 10.1073/pnas.0700960104. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Manganese-enhanced magnetic resonance imaging (MEMRI) was used to acquire suprathreshold tone-evoked activity patterns in the developing mouse inferior colliculus (IC). The activity patterns elicited by each of 2 widely spaced frequencies were overlapping at first, but become quite specific at P19. However, the 3D tonotopic map was reorganized in mice reared with the two tones presented simultaneously. The results highlight the importance of analyzing subcortical mammalian brain regions in plasticity experiments.
- 79.Kim G, Kandler K. Elimination and strengthening of glycinergic/GABAergic connections during tonotopic map formation. Nat Neurosci. 2003;6:282–290. doi: 10.1038/nn1015. [DOI] [PubMed] [Google Scholar]
- 80.Sanes DH, Siverls V. Development and specificity of inhibitory terminal arborizations in the central nervous system. J Neurobiol. 1991;22:837–854. doi: 10.1002/neu.480220805. [DOI] [PubMed] [Google Scholar]
- 81.Leake PA, Snyder RL, Hradek GT. Postnatal refinement of auditory nerve projections to the cochlear nucleus in cats. J Comp Neurol. 2002;448:6–27. doi: 10.1002/cne.10176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Henkel CK, Keiger CJ, Franklin SR, Brunso-Bechtold JK. Development of banded afferent compartments in the inferior colliculus before onset of hearing in ferrets. Neuroscience. 2007;146:225–235. doi: 10.1016/j.neuroscience.2007.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Oliver DL, Kuwada S, Yin TC, Haberly LB, Henkel CK. Dendritic and axonal morphology of HRP-injected neurons in the inferior colliculus of the cat. J Comp Neurol. 1991;303:75–100. doi: 10.1002/cne.903030108. [DOI] [PubMed] [Google Scholar]
- 84.Suga N. Role of corticofugal feedback in hearing. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2008;194:169–183. doi: 10.1007/s00359-007-0274-2. [DOI] [PubMed] [Google Scholar]
- 85.Leake PA, Hradek GT, Chair L, Snyder RL. Neonatal deafness results in degraded topographic specificity of auditory nerve projections to the cochlear nucleus in cats. J Comp Neurol. 2006;497:13–31. doi: 10.1002/cne.20968. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Together with an earlier report (Leake et al., 2002), this study demonstrates that topographic specificity at the first central auditory synapse is quite precise before the onset of sound driven responses, but is disrupted significantly by hearing loss.
- 86.Sanes DH, Takacs C. Activity-dependent refinement of inhibitory connections. Eur J Neurosci. 1993;5:570–574. doi: 10.1111/j.1460-9568.1993.tb00522.x. [DOI] [PubMed] [Google Scholar]
- 87.Kapfer C, Seidl AH, Schweizer H, Grothe B. Experience-dependent refinement of inhibitory inputs to auditory coincidence-detector neurons. Nat Neurosci. 2002;5:247–253. doi: 10.1038/nn810. [DOI] [PubMed] [Google Scholar]
- 88.Werthat F, Alexandrova O, Grothe B, Koch U. Experience-dependent refinement of the inhibitory axons projecting to the medial superior olive. Dev Neurobiol. 2008;68:1454–1462. doi: 10.1002/dneu.20660. [DOI] [PubMed] [Google Scholar]
- 89.Franklin SR, Brunso-Bechtold JK, Henkel CK. Bilateral cochlear ablation in postnatal rat disrupts development of banded pattern of projections from the dorsal nucleus of the lateral lemniscus to the inferior colliculus. Neuroscience. 2008;154:346–354. doi: 10.1016/j.neuroscience.2008.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mrsic-Flogel TD, Schnupp JW, King AJ. Acoustic factors govern developmental sharpening of spatial tuning in the auditory cortex. Nat Neurosci. 2003;6:981–988. doi: 10.1038/nn1108. [DOI] [PubMed] [Google Scholar]
- 91.Campbell RA, King AJ, Nodal FR, Schnupp JW, Carlile S, Doubell TP. Virtual adult ears reveal the roles of acoustical factors and experience in auditory space map development. J Neurosci. 2008;28:11557–11570. doi: 10.1523/JNEUROSCI.0545-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Oleskevich S, Walmsley B. Synaptic transmission in the auditory brainstem of normal and congenitally deaf mice. J Physiol. 2002;540:447–455. doi: 10.1113/jphysiol.2001.013821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Leao RN, Berntson A, Forsythe ID, Walmsley B. Reduced low-voltage activated K+ conductances and enhanced central excitability in a congenitally deaf (dn/dn) mouse. J Physiol. 2004;559:25–33. doi: 10.1113/jphysiol.2004.067421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lee DJ, Cahill HB, Ryugo DK. Effects of congenital deafness in the cochlear nuclei of Shaker-2 mice: an ultrastructural analysis of synapse morphology in the endbulbs of Held. J Neurocytol. 2003;32:229–243. doi: 10.1023/B:NEUR.0000010082.99874.14. [DOI] [PubMed] [Google Scholar]
- 95.Vale C, Sanes DH. The effect of bilateral deafness on excitatory and inhibitory synaptic strength in the inferior colliculus. Eur J Neurosci. 2002;16:2394–2404. doi: 10.1046/j.1460-9568.2002.02302.x. [DOI] [PubMed] [Google Scholar]
- 96.Vale C, Schoorlemmer J, Sanes DH. Deafness disrupts chloride transporter function and inhibitory synaptic transmission. J Neurosci. 2003;23:7516–7524. doi: 10.1523/JNEUROSCI.23-20-07516.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kotak VC, Fujisawa S, Lee FA, Karthikeyan O, Aoki C, Sanes DH. Hearing loss raises excitability in the auditory cortex. J Neurosci. 2005;25:3908–3918. doi: 10.1523/JNEUROSCI.5169-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kotak VC, Takesian AE, Sanes DH. Hearing loss prevents the maturation of GABAergic transmission in the auditory cortex. Cereb Cortex. 2008;18:2098–2108. doi: 10.1093/cercor/bhm233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sarro EC, Kotak VC, Sanes DH, Aoki C. Hearing loss alters the subcellular distribution of presynaptic GAD and postsynaptic GABAA receptors in the auditory cortex. Cereb Cortex. 2008;18:2855–2867. doi: 10.1093/cercor/bhn044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Xu H, Kotak VC, Sanes DH. Conductive hearing loss disrupts synaptic and spike adaptation in developing auditory cortex. J Neurosci. 2007;27:9417–9426. doi: 10.1523/JNEUROSCI.1992-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Raggio MW, Schreiner CE. Neuronal responses in cat primary auditory cortex to electrical cochlear stimulation.III. Activation patterns in short- and long-term deafness. J Neurophysiol. 1999;82:3506–3526. doi: 10.1152/jn.1999.82.6.3506. [DOI] [PubMed] [Google Scholar]
- 102.Sanes DH. The development of synaptic function and integration in the central auditory system. J Neurosci. 1993;13:2627–2637. doi: 10.1523/JNEUROSCI.13-06-02627.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kotak VC, Sanes DH. Long-lasting inhibitory synaptic depression is age- and calcium-dependent. J Neurosci. 2000;20:5820–5826. doi: 10.1523/JNEUROSCI.20-15-05820.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chang EH, Kotak VC, Sanes DH. Long-term depression of synaptic inhibition is expressed postsynaptically in the developing auditory system. J Neurophysiol. 2003;90:1479–1488. doi: 10.1152/jn.00386.2003. [DOI] [PubMed] [Google Scholar]
- 105.Kandler K, Gillespie DC. Developmental refinement of inhibitory sound-localization circuits. Trends Neurosci. 2005;28:290–296. doi: 10.1016/j.tins.2005.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kotak VC, Breithaupt AD, Sanes DH. Developmental hearing loss eliminates long-term potentiation in the auditory cortex. Proc Natl Acad Sci U S A. 2007;104:3550–3555. doi: 10.1073/pnas.0607177104. [DOI] [PMC free article] [PubMed] [Google Scholar]; * This study shows that excitatory synaptic long-term potentiation (LTP) emerges at the time of hearing onset in auditory cortex layer 5 neurons, and this particular form of plasticity is eliminated by the loss of hearing. Thus, normal auditory experience may be essential for the maturation of synaptic plasticity mechanisms.
- 107.Tan J, Widjaja S, Xu J, Shepherd RK. Cochlear implants stimulate activity-dependent CREB pathway in the deaf auditory cortex: implications for molecular plasticity induced by neural prosthetic devices. Cereb Cortex. 2008;18:1799–1813. doi: 10.1093/cercor/bhm206. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Two molecular components implicated in synaptic plasticity (Brain-derived neurotrophic factor and cAMP response element binding protein) are down-regulated in auditory cortex following early hearing loss. However, electrical stimulation of the cochlea restores their expression.
- 108.Speechley WJ, Hogsden JL, Dringenberg HC. Continuous white noise exposure during and after auditory critical period differentially alters bidirectional thalamocortical plasticity in rat auditory cortex in vivo. Eur J Neurosci. 2007;26:2576–2584. doi: 10.1111/j.1460-9568.2007.05857.x. [DOI] [PubMed] [Google Scholar]
- 109.Rioult-Pedotti MS, Friedman D, Donoghue JP. Learning-induced LTP in neocortex. Science. 2000;290:533–536. doi: 10.1126/science.290.5491.533. [DOI] [PubMed] [Google Scholar]
- 110.Gottlieb G. Social Induction of Malleability in Ducklings - Sensory Basis and Psychological Mechanism. Anim Behav. 1993;45:707–719. [Google Scholar]
- 111.Fritz JB, Elhilali M, David SV, Shamma SA. Auditory attention--focusing the searchlight on sound. Curr Opin Neurobiol. 2007;17:437–455. doi: 10.1016/j.conb.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 112.Tchernichovski O, Lints T, Mitra PP, Nottebohm F. Vocal imitation in zebra finches is inversely related to model abundance. Proc Natl Acad Sci U S A. 1999;96:12901–12904. doi: 10.1073/pnas.96.22.12901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Vale C, Sanes DH. Afferent regulation of inhibitory synaptic transmission in the developing auditory midbrain. J Neurosci. 2000;20:1912–1921. doi: 10.1523/JNEUROSCI.20-05-01912.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]