Abstract
Introduction
Lung transplant recipients with documented gastroesophageal reflux disease (GERD) are at increased risk for graft dysfunction. Here, we present the first large-animal model of gastric aspiration following allogeneic lung transplantation and some preliminary data demonstrating the effect of chronic aspiration on the direct and indirect pathways of allorecognition.
Methods
Left orthotopic lung transplants (n=3) were performed in miniature swine across an MHC class I disparity, followed by 12 days of high-dose cyclosporine (CyA). At the time of transplantation, a trans-tracheal catheter was placed at the carina, above the bronchial anastomosis. A gastrostomy tube was placed for daily aspiration of gastric contents. Subsequently, graft lungs were instilled with gastric aspirate daily (3cc/hr × 8hr/day) for 50 days. Recipients were followed with daily CBC, scheduled chest radiographs, and biopsies. In vitro studies, including cell-mediated lympholysis (CML), mixed lymphocyte reactions (MLR), and peptide proliferation assays (PPA), were performed. Results from these three recipients were compared to those of historical controls (n=6) who were treated identically, except for the tracheal cannulation and simulated gastric aspiration.
Results
Two of the experimental animals were sacrificed with non-viable lungs soon after the POD 50 biopsy. In both cases the native lung was normal. The third animal survived to 180 days without evidence of chronic rejection. After immunosuppressive treatment, all animals demonstrated donor-specific hyporesponsiveness by assays of direct alloresponse (CML, MLR). A significant response to synthetic donor-derived class I peptide, however, was seen in all animals. A more pronounced and diffuse response was seen in the animals rejecting their grafts. The historical controls showed medium-term graft survival with evidence of chronic rejection in the majority of animals, as previously reported.
Conclusion
In a model of GERD after lung transplantation, a spectrum of clinical outcomes was observed. The in vitro data suggest that acid reflux enhances the indirect alloresponse to processed donor class I antigen, giving mechanistic insight into the manner in which GERD may be deleterious to the transplanted lung.
Keywords: Lung transplantation, Gastroesophageal reflux disease (GERD), Tolerance
Introduction
Gastroesophageal reflux disease (GERD) is highly prevalent among patients with severe pulmonary disease awaiting lung transplantation (1–4). Proximal reflux has been implicated in allograft dysfunction following transplantation, suggesting a role for anti-reflux surgery in lung transplant recipients with GERD (5–8). Several centers have demonstrated that laparoscopic fundoplication is safe and effective in patients awaiting lung transplantation (5,8,9). Furthermore, recent studies suggest that lung transplant recipients with GERD demonstrate improved pulmonary allograft function following anti-reflux surgery (7,9).
Despite these clinical observations, the mechanism by which gastroesophageal reflux exerts a deleterious effect on the transplanted lung remains unknown. Theoretically, repetitive aspiration could simply lead to graft injury by direct toxicity. There exists, however, an association between aspiration and obliterative bronchiolitis (OB), a specific histopathologic finding of chronic rejection (10,11). This suggests that GERD potentially induces allograft injury through immune-mediated processes.
After transplantation, allorecognition may occur via the direct or indirect pathways. In direct allorecognition, host T cells recognize intact donor major histocompatibility complex (MHC) antigens presented by donor antigen presenting cells (APCs). Indirect allorecognition involves recipient recognition of donor derived MHC peptide after host APC processing. It has been hypothesized that the persistent indirect alloresponse is primarily responsible for chronic allograft rejection (12).
Our group has previously reported that a short course of high-dose cyclosporine (CyA) following class I-mismatched (class II-matched) lung transplantation in miniature swine reproducibly leads to chronic rejection (12). Here, we present the first large-animal model of repetitive gastric aspiration following allogeneic lung transplantation. Specifically, we sought to evaluate the effects of chronic aspiration on direct and indirect allorecognition in a clinically relevant, large-animal lung transplantation model.
Methods
Animals
Transplant donors (n=3) and recipients (n=3) were selected from our herd of partially inbred miniature swine at 5–10 months of age. As previously described, these animals have been bred to homozygosity at the MHC class I and class II loci (13). In this study, transplants were performed using donor-recipient pairs that were class II matched (class I and minor antigen-disparate). These data were compared to a group of historical control recipients (n=6) that underwent the same protocol without the transtracheal instillation of gastric juice (see Table 1) (12). All animal care and procedures were in compliance with “Principles of Animal Care” formulated by the National Society for Medical Research and the “Guide for the Care and Use of Laboratory Animals” prepared by the Institute of Laboratory Animal Resources and published by the National Institutes of Health.
Table 1.
Comparison of lung allograft outcomes in control and experimental animals.
| Animal | Group | POD of first appearance of ACR (ISHLT grade) |
POD of first appearance of OB |
Graft survival (days) |
|---|---|---|---|---|
| 13515 | Control | 62 (2/4) | 238 | 249 |
| 13678 | Control | 28 (3/4) | N/A | 67 |
| 14123 | Control | 108 (1/4) | 184 | 184 |
| 14459 | Control | 91 (2/4) | 235 | 316 |
| 14780 | Control | 42 (4/4) | N/A | 69 |
| 14830 | Control | 27 (3/4) | 119 | 605 |
| 17237 | Experimental | 51 (3/4) | N/A | 51 |
| 17239 | Experimental | 28 (2/4) | 48 | 61 |
| 17256 | Experimental | 32 (3/4) | N/A | 187 |
Experimental design
Prior to transplantation, in vitro assays were performed to confirm an alloresponse between the transplant pairs. On POD 0, animals underwent left orthotopic lung transplantation, gastrostomy tube placement, and transtracheal catheter placement (detailed below). High-dose cyclosporine (generously provided by Novartis [East Hanover, New Jersey]), was initiated on POD 0 and continued for 12 days. Trough levels were monitored daily during this time, and the dose was adjusted for a target level of 400–800 ng/mL. Beginning on POD 1, animals received 24 cc of coarse-filtered gastric aspirate daily (3cc/hr × 8hr /day) for 50 days, administered through the transtracheal catheter by an infusion pump (Genie Plus, Kent Scientific; Torrington, CT). Animals were followed by daily physical examination and complete blood count. Serial chest radiography and open lung biopsies via limited thoracotomy were scheduled monthly, or more frequently if indicated by the animals’ clinical condition. Animals were sacrificed, if there was evidence of graft loss due to rejection or overwhelming infection.
Surgical procedures
Under general anesthesia two indwelling silastic catheters were placed in the external jugular veins. One catheter was utilized for the administration of CyA and other medications; the second catheter was used to obtain blood both for daily clinical monitoring as well as in vitro studies. An additional silastic catheter was tunneled subcutaneously and inserted trans-tracheally for administration of gastric aspirate at the level of the carina. A laparotomy was performed and a gastric tube was placed in the standard fashion. A left thoracotomy was performed and the recipient hilar structures isolated. Heparin was administered (300U/kg) and a pneumonectomy was performed. The donor lung was approached via median sternotomy. Heparin was administered (300U/kg) and the heart and lung cooled with iced saline. The left lung was flushed in situ with 4 L of cold Euro-Collins solution (Fresenius Medical Care AG, Bad Homburg, Germany) containing prostaglandin E1 (500 ug/L). The donor lung was prepared surgically and immediately transplanted. The bronchial anastomosis was performed using interrupted 4–0 polyglactin sutures (Vicryl; Ethicon, Inc. Somerville, NJ) and vascular anastomoses were performed using running 6–0 polypropylene (Prolene; Ethicon Inc.). A thoracostomy tube was placed to evacuate the pleural space; this was removed after recovery from anesthesia.
Isolation of PBMC
Heparinized whole blood was diluted with HBSS (Life Technologies, Grand Island, NY). The mononuclear cells were extracted by gradient centrifugation with lymphocyte separation media (Organon, Teknika, Durham, NC). The mononuclear cells were then washed with HBSS, and any contaminating red blood cells were lysed with ACK buffer (B&B Research Laboratories, Fiskeville, RI). The mononuclear cells were washed with HBSS after lysing and resuspended in tissue culture media appropriate for the in vitro assays to be performed.
Assays of direct alloresponse
Cell-mediated lympholysis (CML) assays were performed as previously described (14). Media consisted of RPMI 1640 (Life Technologies) supplemented with 6% FCS (Sigma), 100U/mL penicillin (GIBCO), 135ug/mL streptomycin (GIBCO), 50ug/mL gentamicin (GIBCO), 10mM HEPES (Cellgro), 2mM L-glutamine (Life Technologies), 1mM sodium pyruvate (BioWhittaker), nonessential amino acids (BioWhittaker), and 5×10−5 Mβ2 ME (Sigma). Mixed lymphocyte cultures contained 4×106 responder PBMCs and 4×106 irradiated (2500 rad) stimulator PBMCs and were incubated in 2mL of CML media in 24 well plates (Costar) for 6 days at 37°C and 6% CO2. On day 6, effector cells were harvested and tested for cytolytic activity against 51Cr-labeled PHA targets in a 5.5 hour 51CR release assay. Supernatants were harvested using the Skatron collection system (Skatron, Sterling, VA) and 51Cr-release was determined on a gamma counter (Micromedics, Huntsville, AL). Results were expressed as the percent specific lysis, calculated as % specific lysis = [(experimental release-spontaneous release) / (maximum release-spontaneous release)] × 100.
Mixed lymphocyte reaction (MLR) assays were performed as previously described (14). Culture media was identical to CML media except that 6% FPS was substituted instead of FCS. Cultures containing 4×106 responder and 4×106 irradiated (2500 rad) stimulator PBMCs were incubated in 200uL of MLR media in 96 well flat bottomed plates (Costar) for 5 days at 37°C and 6% CO2. After the 5 day incubation, one µCi of [3H]thymidine was added to each well, followed by an additional 5 hour incubation under the same conditions. [3H]-incorporation was determined in triplicate samples by β-scintillation counting. Results were expressed as stimulation indices (SI), calculated as SI = average count per million (cpm) for a responder-stimulator pair / cpm of the same responder stimulated by an autologous stimulator.
Synthetic SLA Class I Peptides
There are two known SLA class I loci in the pig (PC1 and PC14), where “C” refers to the SLA class I homozygous haplotype of the donor swine used in this study. Most of the polymorphisms are located within the hypervariable regions of the α1 and α2 domains of the heavy chain (15). Four peptides spanning the full length of the hypervariable region of the PC1 α1 helix were synthesized and labeled PC1–1 (amino acid (aa) 3–27), PC1–2 (aa 35–52), PC1–3 (aa 53–73) and PC1–4 (aa 71–90). Three peptides spanning the full length of the hypervariable region of the PC14 α1 helix were synthesized and labeled PC14–1 (aa 3–27), PC14–2 (aa 45–59) and PC14–3 (aa 60–85).
Peptide proliferation assay
Peptide proliferation assays (PPA) were performed as previously described (16,17). Culture media was identical to the MLR assay media. 8×105 responder PBMCs and 10ug of synthetic peptide, or 5uL of 1:20 CFA as a positive control, were incubated in 200uL of media in 96 well flat bottomed plates (Costar) for 5 days at 37°C and 6% CO2. After the 5 day incubation, one µCi of [3H]thymidine was added to each well, and incubated for an additional 5 hours to allow for incorporation. [3H]-incorporation was determined in triplicate samples by β-scintillation counting. Results were expressed as stimulation indices (SI), calculated as SI = average count per million (cpm) for a responder-peptide pair / cpm of the same responder stimulated by media alone.
Histology
Biopsies were placed in 10% formalin. Tissue was then imbedded in paraffin and stained with hematoxylin and eosin (H&E) and Verhoeff (elastin) stains. A pathologist (SLH) evaluated all specimens in a blinded fashion. The lung rejection scoring was based on the International Society for Heart and Lung Transplantation criteria.
Statistical analysis
Statistical significance of in vitro assay results was calculated as previously described. In vitro assays were performed using PBMC isolated from donor MHC-identical and recipient MHC-identical animals. The mean stimulation index for the synthetic peptides was calculated from this panel of naïve controls. Experimental values exceeding three standard deviations above the mean control values for each peptide were considered significant.
Results
Survival
Three experimental animals underwent orthotopic left lung transplants as described above. Gastric aspirate was administered via tracheal catheter for the first 50 postoperative days. Two animals were sacrificed with non-viable, rejected grafts at POD 51 and POD 61. A third animal was electively sacrificed on POD 187. When compared to historical controls (12), there was no significant difference in survival by Kaplan-Meier analysis (Table 1).
Histopathology
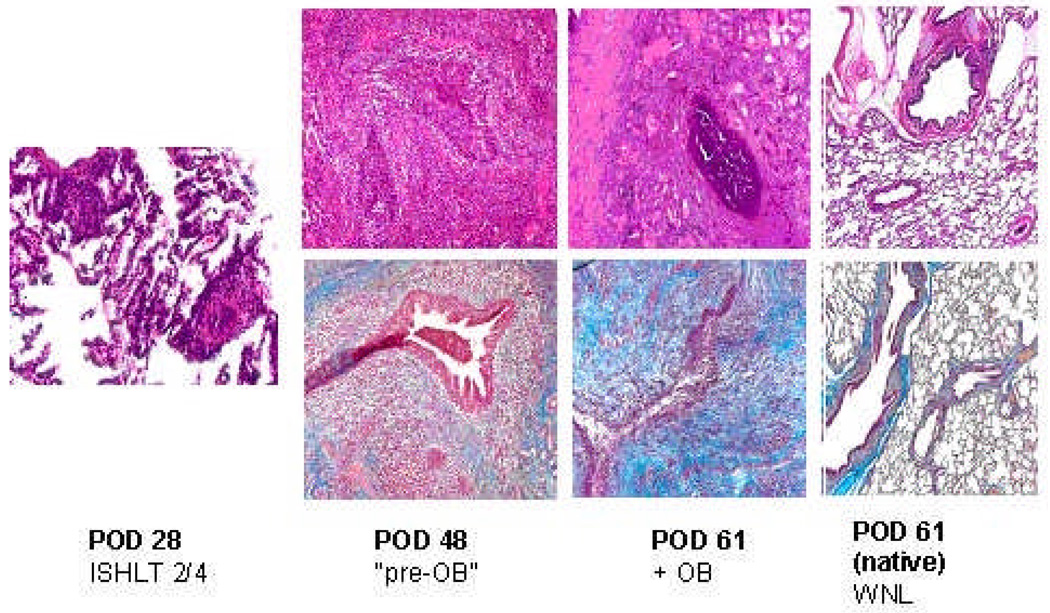
In two experimental animals, simulated gastric aspiration induced a severe fibrotic reaction in the graft. In one of these animals, the pathologic changes of obliterative bronchiolitis were seen significantly earlier than in any of the untreated controls (Figure 1). A second animal developed diffuse fibrosis in the graft. The third animal had no evidence of fibrosis and survived long-term, although necropsy revealed bilateral pneumonia and pulmonary abscesses. Although two (out of six) untreated control animals died of acute cellular rejection between PODs 67 and 69, the severe fibrotic changes observed in the experimental animals were not seen (Table 1).
Figure 1.
On the histopathology of animal 17237, there was evidence of low-grade acute cellular rejection on POD 28. By POD 48, lesions of OB were seen. At the time of sacrifice, there was diffuse fibrosis in the graft. The native lung remained normal.
Direct alloresponse
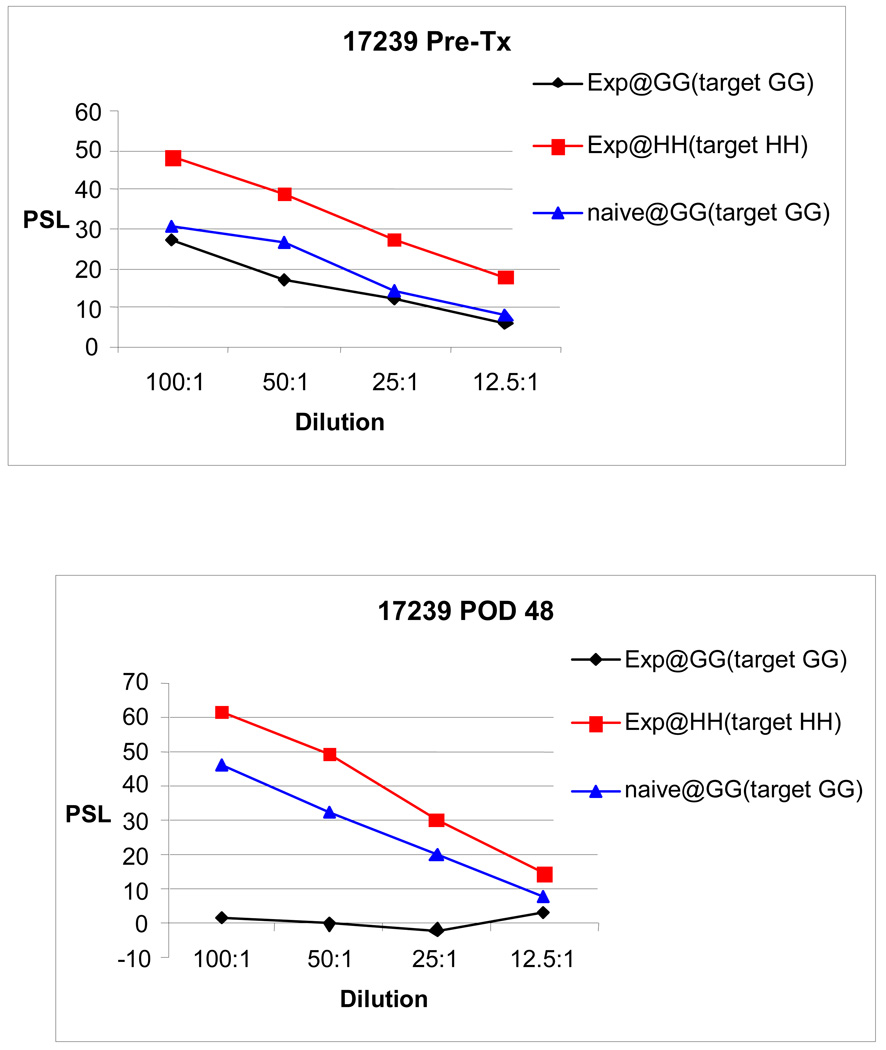
All pulmonary allograft recipients demonstrated an in vitro response to donor prior to transplantation by both CML and MLR. Reflecting the class I disparity, a more vigorous response was detected by CML. Assays were repeated at scheduled biopsies. All experimental animals demonstrated donor-specific hyporesponsiveness by CML and MLR following their course of immunosuppression (Figure 2). This hyporesponsiveness was maintained throughout the animals’ lifespan.
Figure 2.
Representative CML data demonstrate donor-specific hyporesponsiveness after class I-mismatched (class II-matched) lung transplantation, following a short course of high-dose CyA.
Indirect alloresponse
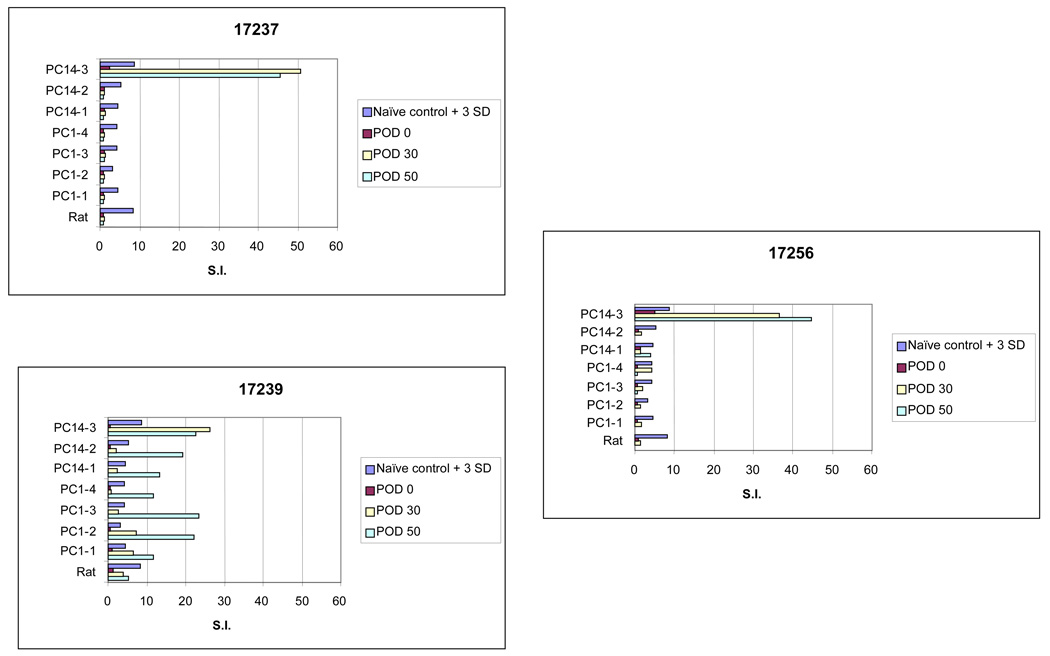
Peptide proliferation assays were performed to measure the indirect alloresponse to donor peptide. Prior to transplantation, the recipient response to synthetic donor class I peptide was indistinguishable from naïve controls. Following transplantation, all animals developed and maintained a response to at least 1 donor peptide (Figure 3). In general, the most pronounced response was seen to a single peptide, designated PC 14–3. Although these findings are consistent with our prior results, the stimulation indices measured in our GERD transplant model were significantly higher than those seen in animals spontaneously rejecting their allografts, either acutely or chronically (12). In fact, the only other circumstance under which we have observed such high stimulation indices has been when we intentionally immunized swine donor-derived peptide by subcutaneous administration with complete Freund’s adjuvant (12).
Figure 3.
The responses to donor class I-derived peptides in all GERD animals are shown. During the 50-day period of daily aspiration, continued shedding of alloantigen resulted in augmented in vitro responses to donor-derived peptides.
Discussion
Numerous clinical reports have linked repetitive aspiration of gastric contents secondary to GERD to pulmonary allograft dysfunction (5–8). At several centers, lung transplant candidates with GERD are now offered anti-reflux surgery prior to transplantation (5,8,9). Despite this body of clinical evidence, the mechanism by which gastric aspiration damages the allograft remains unknown. To date, only one rodent model has been employed to evaluate the effects of GERD on the pulmonary allograft (11). Here, we present the first large-animal model of simulated GERD after lung transplantation, in an effort to further elucidate the mechanism of GERD-mediated graft injury. Specifically, we sought to determine the effects of simulated chronic gastric aspiration on the direct and indirect alloresponse.
We have previously demonstrated that miniature swine recipients of class I-mismatched lung transplants treated with 12 days of high-dose cyclosporine have a mean graft survival of 248 days (12). At the time of rejection, animals are sensitized to donor-derived class I peptides. This phenomenon is observed despite donor specific hyporesponsiveness by CML, a measure of direct alloreactivity. We concluded, therefore, that chronic rejection in this swine model of lung transplantation is primarily mediated via the indirect allorecognition pathway. This contention has been substantiated by other pre-clinical research and human studies (16,18–20).
Furthermore, we have reported that immunization with donor class I peptides leads to accelerated rejection of pulmonary allografts and a more pronounced in vitro response to donor class I (12). This suggests that immunization with donor-derived peptides promotes the development of T-cell clones that are alloreactive through the indirect pathway.
Although additional experimental animals will be needed to further define the impact of GERD on the transplanted lung, the present study suggests the enhancement of indirect allorecognition is one mechanism by which chronic aspiration harms the pulmonary allograft. Chronic aspiration can be seen as an ongoing, in vivo, immunization that leads to continuous exposure and recognition of donor antigen. Although a short course of high-dose CyA blunts the direct immune response, as demonstrated by donor-specific hyporesponsiveness in CML assays, GERD-mediated inflammation leads to augmented presentation of donor class I antigen and the development of a robust indirect alloresponse. In the present study, the in vitro proliferative responses to donor-derived class I allopeptides, measured as stimulation indices, were well above those seen in controls. Prior to this study, vigorous responses such as these have only been observed in the setting of intentional immunization of recipient animals with donor peptide prior to transplantation (12).
Although there was significant reactivity to donor class I peptide in all three animals, the broadest response was seen in animal 17239, which developed significant in vitro responses to all seven donor derived class I peptides after simulated reflux was initiated. This animal developed lesions consistent with OB and was sacrificed with evidence of severe acute rejection and chronic rejection on POD 61. The other 2 animals developed profound and persistent responses to the synthetic class I peptide designated PC 14–3, previously established as the most antigenic of the seven synthetic class I peptides. Response to the other class I peptides was variable, but also significantly higher than naïve control animals.
Indirect allorecognition represents a significant clinical dilemma because donor allopeptides are continuously shed from the allograft and current immunosuppressive agents fail to prevent indirect allorecognition. Although further investigation is warranted, the present study using a novel large-animal model of GERD suggests that the inflammatory effects of GERD promote the continued shedding of alloantigens, enhancing the ongoing indirect alloresponse to the lung graft.
Acknowledgments
Supported by NIH R01HL67110, and fellowships from the International Society for Heart and Lung Transplantation (Weiss) and the Thoracic Surgery Foundation for Research and Education (Sahara).
References
- 1.Sweet MP, Herbella FA, Leard L, et al. The prevalence of distal and proximal gastroesophageal reflux in patients awaiting lung transplantation. Ann Surg. 2006;244:491–497. doi: 10.1097/01.sla.0000237757.49687.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.D'Ovidio F, Singer LG, Hadjiliadis D, et al. Prevalence of gastroesophageal reflux in end-stage lung disease candidates for lung transplant. Ann Thorac Surg. 2005;80:1254–1260. doi: 10.1016/j.athoracsur.2005.03.106. [DOI] [PubMed] [Google Scholar]
- 3.Sweet MP, Patti MG, Leard LE, et al. Gastroesophageal reflux in patients with idiopathic pulmonary fibrosis referred for lung transplantation. J Thorac Cardiovasc Surg. 2007;133:1078–1084. doi: 10.1016/j.jtcvs.2006.09.085. [DOI] [PubMed] [Google Scholar]
- 4.Button BM, Roberts S, Kotsimbos TC, et al. Gastroesophageal reflux (symptomatic and silent): a potentially significant problem in patients with cystic fibrosis before and after lung transplantation. J Heart Lung Transplant. 2005;24:1522–1529. doi: 10.1016/j.healun.2004.11.312. [DOI] [PubMed] [Google Scholar]
- 5.Linden PA, Gilbert RJ, Yeap BY, et al. Laparoscopic fundoplication in patients with end-stage lung disease awaiting transplantation. J Thorac Cardiovasc Surg. 2006;131:438–446. doi: 10.1016/j.jtcvs.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 6.Hartwig MG, Appel JZ, Davis RD. Antireflux surgery in the setting of lung transplantation: strategies for treating gastroesophageal reflux disease in a high-risk population. Thorac Surg Clin. 2005;15:417–427. doi: 10.1016/j.thorsurg.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 7.Davis RD, Jr, Lau CL, Eubanks S, et al. Improved lung allograft function after fundoplication in patients with gastroesophageal reflux disease undergoing lung transplantation. J Thorac Cardiovasc Surg. 2003;125:533–542. doi: 10.1067/mtc.2003.166. [DOI] [PubMed] [Google Scholar]
- 8.Gasper WJ, Sweet MP, Hoopes C, et al. Antireflux surgery for patients with end-stage lung disease before and after lung transplantation. Surg Endosc. 2007 doi: 10.1007/s00464-007-9494-3. [DOI] [PubMed] [Google Scholar]
- 9.Cantu E, III, Appel JZ, III, Hartwig MG, et al. J. Maxwell Chamberlain Memorial Paper. Early fundoplication prevents chronic allograft dysfunction in patients with gastroesophageal reflux disease. Ann Thorac Surg. 2004;78:1142–1151. doi: 10.1016/j.athoracsur.2004.04.044. [DOI] [PubMed] [Google Scholar]
- 10.Palmer SM, Miralles AP, Howell DN, Brazer SR, Tapson VF, Davis RD. Gastroesophageal reflux as a reversible cause of allograft dysfunction after lung transplantation. Chest. 2000;118:1214–1217. doi: 10.1378/chest.118.4.1214. [DOI] [PubMed] [Google Scholar]
- 11.Hartwig MG, Appel JZ, Li B, et al. Chronic aspiration of gastric fluid accelerates pulmonary allograft dysfunction in a rat model of lung transplantation. J Thorac Cardiovasc Surg. 2006;131:209–217. doi: 10.1016/j.jtcvs.2005.06.054. [DOI] [PubMed] [Google Scholar]
- 12.Shoji T, Wain JC, Houser SL, et al. Indirect recognition of MHC class I allopeptides accelerates lung allograft rejection in miniature swine. Am J Transplant. 2005;5:1626–1634. doi: 10.1111/j.1600-6143.2005.00925.x. [DOI] [PubMed] [Google Scholar]
- 13.Sachs DH, Leight G, Cone J, Schwartz S, Stuart L, Rosenberg S. Transplantation in miniature swine. I. Fixation of the major histocompatibility complex. Transplantation. 1976;22:559–567. doi: 10.1097/00007890-197612000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Leight GS, Sachs DH, Rosenberg SA. Transplantation in miniature swine. II. In vitro parameters of histocompatibility in MSLA homozygous minipigs. Transplantation. 1977;23:271–276. [PubMed] [Google Scholar]
- 15.Sullivan JA, Oettinger HF, Sachs DH, Edge AS. Analysis of polymorphism in porcine MHC class I genes: alterations in signals recognized by human cytotoxic lymphocytes. J Immunol. 1997;159:2318–2326. [PubMed] [Google Scholar]
- 16.Lee RS, Yamada K, Houser SL, et al. Indirect recognition of allopeptides promotes the development of cardiac allograft vasculopathy. Proc Natl Acad Sci U S A. 2001;98:3276–3281. doi: 10.1073/pnas.051584498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shoji T, Johnston DR, Hoerbelt R, et al. Indirect allorecognition of MHC class I peptides accelerates pulmonary alllograft rejection in miniature swine. Am J Transpl. 2004;4:S316. doi: 10.1111/j.1600-6143.2005.00925.x. [DOI] [PubMed] [Google Scholar]
- 18.SivaSai KS, Smith MA, Poindexter NJ, et al. Indirect recognition of donor HLA class I peptides in lung transplant recipients with bronchiolitis obliterans syndrome. Transplantation. 1999;67:1094–1098. doi: 10.1097/00007890-199904270-00002. [DOI] [PubMed] [Google Scholar]
- 19.Baker RJ, Hernandez-Fuentes MP, Brookes PA, Chaudhry AN, Cook HT, Lechler RI. Loss of direct and maintenance of indirect alloresponses in renal allograft recipients: implications for the pathogenesis of chronic allograft nephropathy. J Immunol. 2001;167:7199–7206. doi: 10.4049/jimmunol.167.12.7199. [DOI] [PubMed] [Google Scholar]
- 20.Molajoni ER, Cinti P, Orlandini A, et al. Mechanism of liver allograft rejection: the indirect recognition pathway. Hum Immunol. 1997;53:57–63. doi: 10.1016/S0198-8859(97)00029-3. [DOI] [PubMed] [Google Scholar]