Abstract
Raman scattering spectroscopy and elastic light scattering intensity (ESLI) were used to simultaneously measure levels of Ca-dipicolinic acid (CaDPA) and changes in spore morphology and refractive index during germination of individual B. subtilis spores with and without the two redundant enzymes (CLEs), CwlJ and SleB, that degrade spores’ peptidoglycan cortex. Conclusions from these measurements include: 1) CaDPA release from individual wild-type germinating spores was biphasic; in a first heterogeneous slow phase, Tlag, CaDPA levels decreased ∼15% and in the second phase ending at Trelease, remaining CaDPA was released rapidly; 2) in L-alanine germination of wild-type spores and spores lacking SleB: a) the ESLI rose ∼2-fold shortly before Tlag at T1; b) following Tlag, the ESLI again rose ∼2-fold at T2 when CaDPA levels had decreased ∼50%; and c) the ESLI reached its maximum value at ∼Trelease and then decreased; 3) in CaDPA germination of wild-type spores: a) Tlag increased and the first increase in ESLI occurred well before Tlag, consistent with different pathways for CaDPA and L-alanine germination; b) at Trelease the ESLI again reached its maximum value; 4) in L-alanine germination of spores lacking both CLEs and unable to degrade their cortex, the time ΔTrelease (Trelease–Tlag) for excretion of ≥75% of CaDPA was ∼15-fold higher than that for wild-type or sleB spores; and 5) spores lacking only CwlJ exhibited a similar, but not identical ESLI pattern during L-alanine germination to that seen with cwlJ sleB spores, and the high value for ΔTrelease.
INTRODUCTION
Optical trapping, also called optical tweezers, can capture and manipulate biological particles using low-power near-infrared laser beams and has allowed extensive study of single cells and molecules (1–5). Here we report use of simultaneous elastic and inelastic light scattering to monitor the germination of single bacterial spores in an optical trap following addition of various germinants
When a trapped cell is illuminated with the same or a second laser beam, it scatters light in all directions. The scattered light contains both elastic and inelastic components; the elastic scattered light has the same frequency as the illuminating laser beam and the inelastic scattered light has shifted frequencies. Elastic scattering light intensity (ESLI) can provide morphological information about a cell’s size, shape and refractive index (6–7). Analysis of the inelastic scattered light, or Raman spectroscopy, can provide information about cells’ molecular composition, since Raman scattering intensity is dependent on the amount of any particular molecular component (8–10). Consequently, simultaneous measurement of elastic and inelastic light scattering may provide valuable information on individual cells.
Spores of bacteria of Bacillus species are metabolically dormant, very resistant to a variety of harsh conditions, and can survive for many years (11–12). However, spores can rapidly return to active growth through germination followed by outgrowth (13). Germination is normally triggered by specific nutrients, although can also be initiated by some non-nutrient agents. Nutrient germinants bind to specific receptors located in the spore’s inner membrane, triggering the release of spore small molecules, notably the large depot (∼10% spore dry wt) of pyridine-2,6-dicarboxylic acid (dipicolinic acid (DPA)) (13–14). DPA is located exclusively in the spore’s central region or core as a 1:1 chelate with divalent cations, predominantly Ca2+ (CaDPA). Release of small molecules and their replacement by water comprise Stage I of germination, and Stage I events, in particular CaDPA release, trigger hydrolysis of the spore’s peptidoglycan (PG) cortex by either of two redundant cortex-lytic enzymes (CLEs), CwlJ and SleB, in B. subtilis spores. Cortex hydrolysis then allows swelling of the spore core and further water uptake, resulting in a core water content similar to that in growing cells. This full core rehydration completes Stage II of germination, and allows metabolism and macromolecular synthesis to begin (13).
The kinetics of germination of a spore population is usually monitored by measuring the optical density at 600 nm (OD600) of spore cultures, which falls ∼60% upon completion of Stage II of germination (13), or by measuring DPA release (15–18). However, the kinetics of germination of individual spores cannot be determined from these population measurements due to significant heterogeneity in the germination of individual spores (19–21). Kinetic germination would greatly change the amount CaDPA present in or release from the spores, which was recently used as a biomarker for the rapid detection of Bacillus spores by FAST coherent anti-Stokes Raman spectroscopy (22, 23).
The kinetics of germination of single bacterial spores has been monitored by phase contrast microscopy (21), as a phase-bright dormant spore becomes phase-dark upon germination due to the decrease in the core’s refractive index accompanying germination. However, the full decrease in the core’s refractive index is due to CaDPA release as well as to full core rehydration (13). The kinetics of CaDPA release during germination of single spores has also been followed by Raman (19) and surface enhanced Raman spectroscopy (24). In particular, laser tweezers Raman spectroscopy (LTRS) has demonstrated significant heterogeneity in the kinetics of CaDPA release during germination of individual spores (19). However, there have been no simultaneous measurements of both morphological changes in and CaDPA release from single germinating spores, which could give precise ordering of various events during germination.
In this work, we report measurements of the ESLI and Raman scattering from single B. subtilis spores with and without CLEs following addition of various germinants using the experimental setup shown in Fig. 1. The spore was held in an optical trap and analysis of its Raman scattering allowed determination of the kinetics of CaDPA release, while ESLI gave information about changes in spore morphology and refractive index. These analyses have provided new insight into the process of bacterial spore germination.
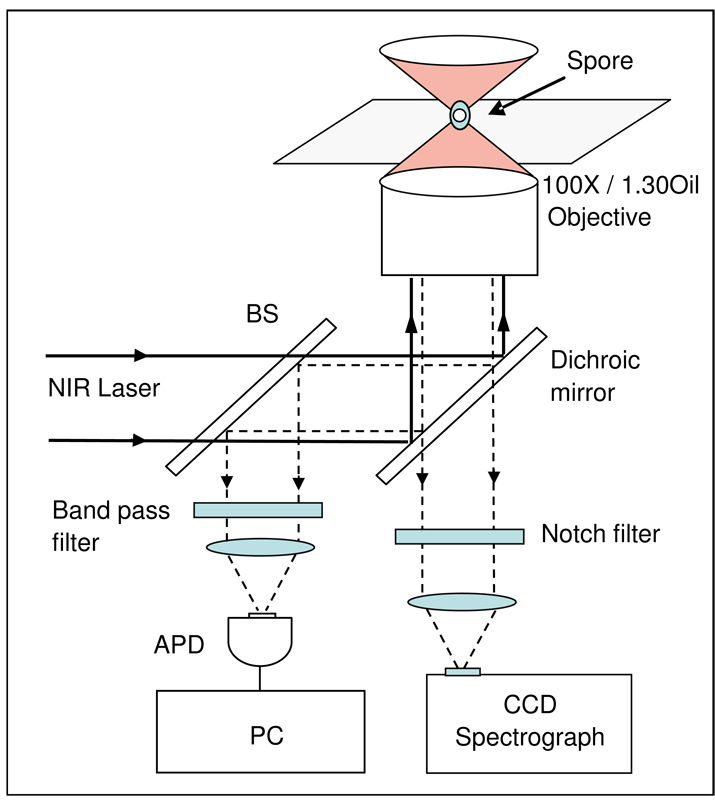
Figure 1.
Experimental setup for analysis of elastic and inelastic light scattering from a single optically trapped spore. A near-infrared (NIR) laser beam at 785 nm is introduced in an inverted microscope (Nikon TE2000 DIC) equipped with an objective by a dichroic mirror to form a single-beam optical trap. A germinating spore in liquid is trapped in the focus of the laser beam about 10 µm above the coverslip.
MATERIALS AND METHODS
Optical trap, Raman spectroscopy and ESLI
The optical trap and Raman spectroscopy setup was as described (10, 19). As shown in Fig. 1, a germinating spore in liquid is trapped in the focus of the laser beam about 10 µm above the coverslip. The backwards inelastic Raman scattering light from the trapped spore passes through the dichroic mirror and a notch filter, and is focused onto the entrance slit of a spectrograph and detected by a charge-coupled detector (Symphony CCD, Jobin Yvon). Raman spectra were recorded from 500 to 1800 cm−1 with a spectral resolution of ∼6 cm−1. The backwards elastic scattering light from the trapped spore is reflected by a beam splitter (BS), passes through a band-pass filter, and is detected by an avalanche photodiode (APD) (Hamamatsu S2383) and recorded with a personal computer (PC) equipped with a data acquisition card (National Instruments, Inc. PCI-6024E). The sensitive area of the APD was much larger than the image of trapped spore so that the total ESLI was detected. A video camera was used to record the time-lapse images of the trapped spore (not shown in Fig. 1).
Strains, spore preparation and storage
B. subtilis strains used in this work were isogenic and were: PS832, a prototrophic laboratory derivative of strain 168; PS533 (25) carrying plasmid pUB110 that encodes resistance to kanamycin (10 µg/ml); strain FB113 (26) lacking CwlJ and SleB; and strains FB111 and FB112 (26) lacking CwlJ or SleB, respectively. Spores of B. subtilis strains were prepared at 37°C on 2xSG medium agar plates and purified and stored as described (27). All spore preparations used in this work were free (>98%) of growing or sporulating cells and germinated spores as determined by phase contrast microscopy.
Spore germination
Prior to germination with L-alanine, B. subtilis spores in water were heat activated for 30 min at 70°C and then cooled on ice; heat activation was not needed for CaDPA germination. B. subtilis spores were germinated in a microscope sample holder kept either at 37°C with 10 mM L-alanine in 25 mM Tris-HCl buffer (pH 7.4), or at 25°C with 60 mM CaDPA (1:1 mixture of 120 mM CaCl2 and 120 mM DPA, made pH 7.5 with Tris base).
Monitoring germination dynamics of individual spores
After adding spores to pre-heated buffer plus germinants, a single spore was trapped at the focus of the near-infrared laser beam with a power of 3 mW. Raman spectra of the trapped spore were acquired with a CCD acquisition time of 45 s until spore germination as monitored by DPA release was complete or for 60 min. The intensities and bright-field images of elastic scattering light from the spore were recorded by the APD with a resolution time of 15 ms and a video camera, respectively. This procedure was repeated for another individual spore following loading of a new spore sample. The CaDPA level in individual spores was determined from intensities of the Raman spectral band at 1017 cm−1 (10, 19), normalized to the CaDPA level at time zero, and plotted as a function of the germination time. The effect of the low-power optical trap (∼3 mW) on spore germination was minimal due to the low absorption in the near-IR region (19).
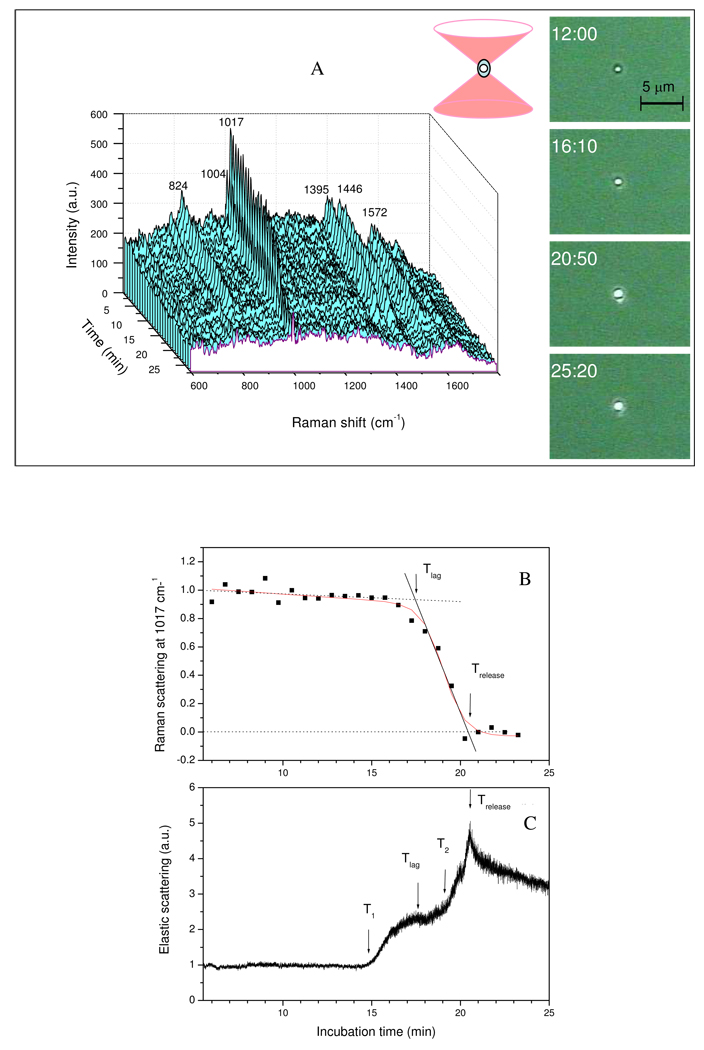
Spore germination parameters determined from these data (Fig. 2) were: a) Tlag - the time between addition of a germinant and initiation of rapid Ca-DPA release; as shown in Fig. 2B, the rate of the CaDPA release during L-alanine germination was initially slow, but became rapid as remaining CaDPA was released; the intersection of the slow release slope with the rapid release slope gives Tlag (19). The percentage of initial CaDPA remaining in the spore at Tlag was determined by the measured amount of CaDPA at the time point nearest to Tlag, with a precision of the acquisition time of 45 s; b) T1 – the time of initiation of the first rapid rise in ESLI; c) T2 – the time of initiation of the second rapid rise in ESLI; d) Trelease – the time when release of CaDPA is complete, determined by the intersection of the rapid release slope with the zero level of CaDPA; and e) ΔTrelease = (Trelease-Tlag).
Figure 2.
Figure 2A–C. A) Raman spectra and images, B) intensities of the CaDPA-specific Raman spectral band, and C) ESLI from a B. subtilis wild-type spore germinating in L-alanine in an optical trap. The Raman spectra, images and ESLI and the intensity of the CaDPA-specific 1017-cm−1 Raman band from a B. subtilis PS533 (wild-type) spore germinating in L-alanine were determined as described in Methods. The red solid line in Fig. 2B was a mathematical fit with a modified sigmoidal curve. The Raman band intensity and ESLI at each time point were normalized to values at the first time of measurement. For all measurements, λex = 785 nm, Pexc = 3 mW, and the acquisition time was 45 s for Raman scattering light and 15 ms for ESLI. The dark ring in the image at 12 min in A) gives the size of the trapped spore.
RESULTS
Germination of B. subtilis spores in L-alanine
Fig. 2A shows Raman spectra and images of elastic scattering light from a single wild-type B. subtilis spore germinating with L-alanine. These data indicate that: 1) peak heights of CaDPA specific bands at 824, 1017, 1395, and 1572 cm−1 fell slightly during Tlag (∼17 min) following addition of L-alanine (and see below), and then decreased rapidly and essentially to zero by Trelease (∼20 min) as seen previously (19); however, intensities of Raman spectral bands from other spore components such as phenylalanine (1004 cm−1) and the protein amide bond (1655 cm−1) remained unchanged after CaDPA release; 2) images of elastic scattering light from the spore prior to 15 min (∼ 2 min before Tlag) did not change; as seen in the 12 min image, the brightness of the elastic scattering pattern was relatively weak with only the spore core efficiently generating scattering light (note that the spore’s total size can be determined from the dark edge in the 12 min image); 3) the brightness and size of the spore’s strong elastic scattering pattern increased abruptly just prior to Tlag (Fig. 2A, shown at 16:10; and see below), and increased again at about Trelease (Fig. 2A, shown at 20:50); 4) after Trelease, the brightness of the elastic scattering pattern gradually decreased, although the overall size of the elastic scattering pattern was nearly unchanged. This behavior was seen in analyses of multiple individual spores, although values of Tlag and Trelease varied considerably (see below).
Fig. 2B shows the intensity of the CaDPA-specific 1017-cm−1 band of this same single germinating spore normalized to its value at the first time of measurement; Following L-alanine addition, CaDPA release began slowly but became more rapid at Tlag. While most CaDPA was released between Tlag and Trelease, ∼20% was released prior to Tlag. Fig. 2C shows the total ESLI from this same spore following L-alanine addition. As indicated above, the ESLI remained low until just prior to Tlag, but ∼2 min earlier (∼15 min) the ESLI began to increase rapidly, rising ∼2-fold by Tlag and remaining nearly constant prior to T2 (∼ 19.3 min), when the ESLI increased again to a maximum at Trelease (∼20.7 min) and then gradually decreased. The increases in the ESLI prior to, during and after the release of spore’s CaDPA indicated that significant changes in either the state or the morphology of the spore are taking place at these times.
The other information given by the ESLI from a single spore during L-alanine germination was in the intensity fluctuation of the scattered light as reflected in the amplitude of the intensity curve (Fig. 2C). While the intensity fluctuation was low before T1, the fluctuation increased shortly after T1, and increased further after Trelease. The increased fluctuation in ESLI may be due to increases in the Brownian motion of scattering centers in or on the spore, since the detected ESLI is the coherent superposition of the scattering light fields from the spore’s various scattering centers.
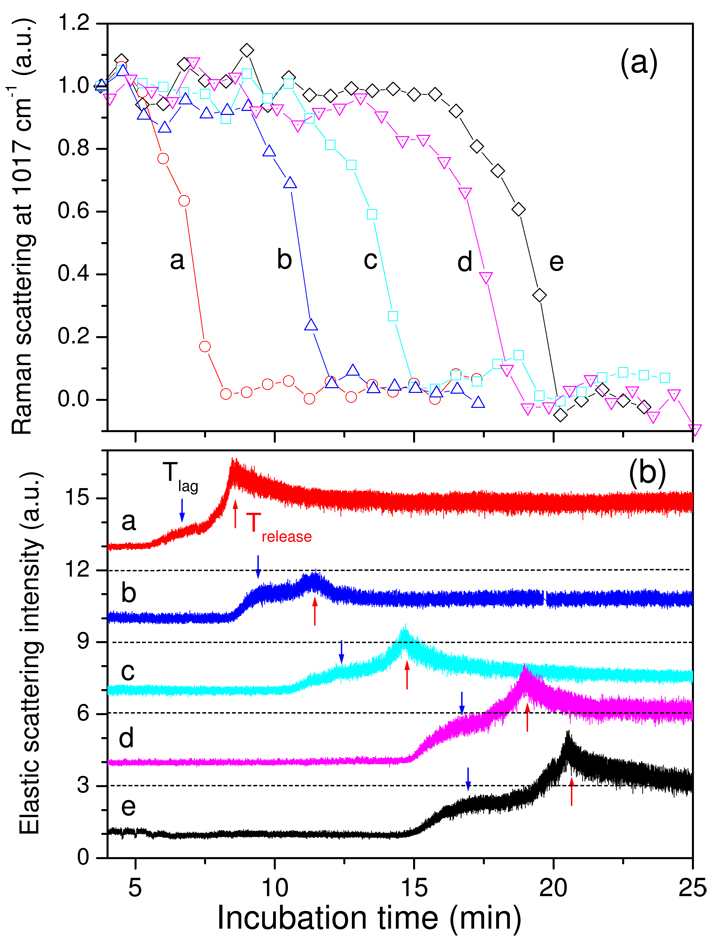
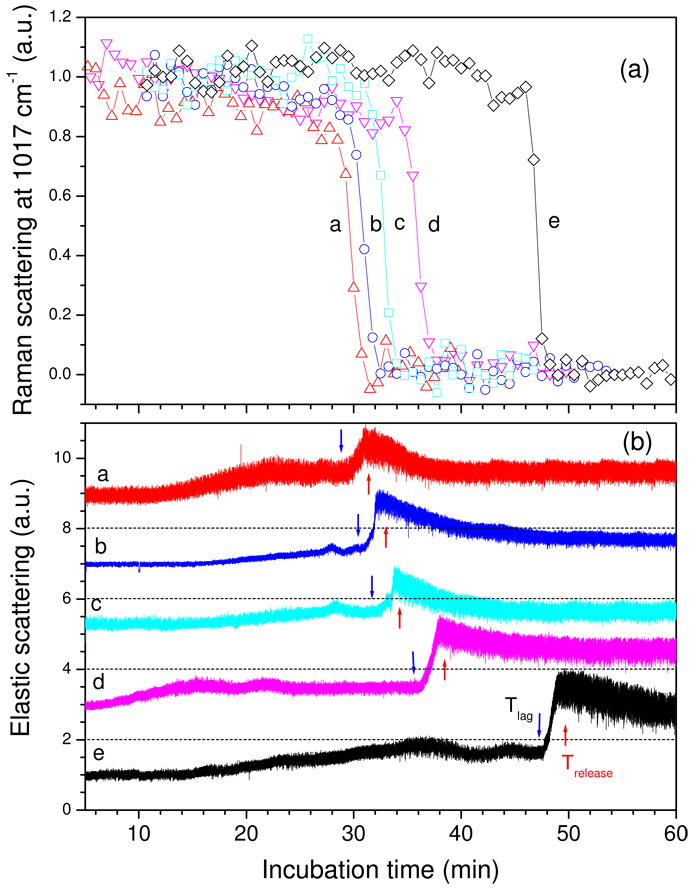
Analysis of multiple individual wild-type spores germinating with L-alanine (Fig. 3a,b) revealed the same general features described above. Thus 10–20% of total CaDPA was released in Tlag, and the biggest variation in the kinetic parameters for CaDPA release between different spores was in values for Tlag (Table 1), as seen with germinating B. thuringiensis spores (19). However, while Tlag values varied considerably, the timing of Tlag, Trelease, T1 and T2 relative to one another were identical, and the time for release of ≥75% of spore CaDPA (ΔTrelease) was always 2–3 min (Fig. 3a; Table 1).
Figure 3.
Figure 3a,b. CaDPA release and ESLI from five wild-type B. subtilis spores during L-alanine germination. a) The relative intensities of the 1017-cm−1 CaDPA Raman band from five individual B. subtilis PS533 (wild-type) spores (a-e) germinating with L-alanine were determined as described in Methods and values are given relative to those at the beginning of the experiment. b) The relative ESLI from the five spores described in a) were determined as described in Methods, and were normalized to the first values measured. Normalized ESLI for spores a-d were shifted upwards by 3 on the y-axis for display purposes. The dashed lines at 3, 6, 9, and 12 scattering intensities are the levels of zero ESLI for spores d, c, b and a, respectively, and the blue and red arrows indicate the Tlag and Trelease times, respectively, for each spore.
Table 1.
Values of T1, Tlag, T2, and Trelease and CaDPA remaining in spores during germination of 5 wild-type spores with L-alanine. Values are from Fig. 3a,b, and values in parentheses are the percentage of initial CaDPA remaining in the spore at these times.
| Spore # | T1 - min | Tlag - min | T2 - min | Trelease - min |
|---|---|---|---|---|
| a | 5.4 (90) | 6.4 (71) | 7.6 (17) | 8.5 (0.8) |
| b | 8.3 (94) | 9.3 (90) | 10.7 (60) | 11.6 (13) |
| c | 10.5 (99) | 11.3 (82) | 13.5 (60) | 14.8 (11) |
| d | 14.7 (87) | 16.5 (69) | 17.5 (60) | 19.1 (0.1) |
| e | 14.8 (99) | 17.0 (80) | 19.3 (40) | 20.7 (0.1) |
| Average | 10.7 (94) | 12.0 (78) | 13.4 (48) | 14.8 (5) |
Germination of B. subtilis spores lacking CLEs
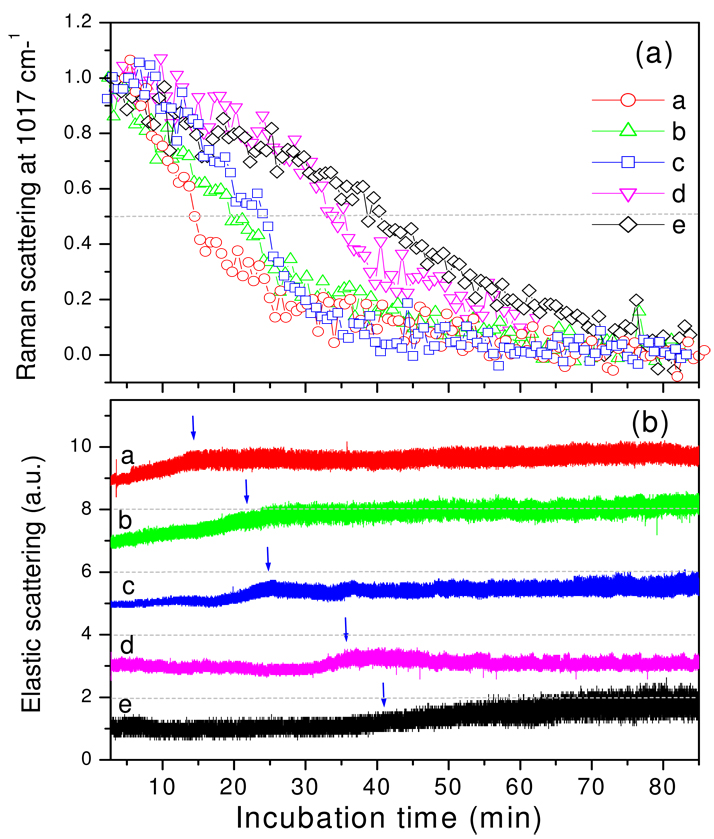
The changes in the ESLI during wild-type spore germination with L-alanine described above could be due to morphological changes accompanying cortex PG hydrolysis, to changes in the hydration of the spore core accompanying CaDPA release, or to both processes. To determine which of the latter are most important, we germinated cwlJ sleB spores that cannot degrade cortex PG during spore germination with L-alanine (26). The results (Fig. 4a,b) were striking as: i) the ΔTrelease for CaDPA increased to 30–60 min; ii) most of the changes seen in ESLI with germinating wild-type spores were not seen, as the most notable change was a moderately slow (5–10 min) increase of ∼0.5–2-fold in scattering intensity that began when ∼25% of CaDPA had been released, and ended when ∼75% had been released; after this point there were no further notable changes; and iii) the amplitude of the fluctuation in ESLI did, however, increase notably after the increase in intensity noted above.
Figure 4.
Figure 4a,b. CaDPA release and ESLI from B. subtilis spores that cannot degrade their cortex during L-alanine germination. a) The relative intensities of the 1017-cm−1 CaDPA band from five B. subtilis FB113 (cwlJ sleB) spores that do not degrade their cortex during germination with L-alanine were determined as described in Methods. b) The ESLI from the five spores germinating in a) were determined as described in Methods. These latter values were normalized to values obtained in the first measurement, and normalized values were shifted by 2 on the y-axis for display purposes. The dashed lines at 2, 4, 6 and 8 are the levels of zero ESLI for spores d, c, b and a, respectively, and the vertical blue arrows indicate the time at which the DPA level of individual spores was reduced by 50%.
To determine which CLE, CwlJ or SleB was responsible for increasing ΔTrelease during L-alanine germination, we examined CaDPA release and ESLI from individual spores lacking only CwlJ or SleB (Table 2). While values of Tlag varied considerably between different spore preparations for reasons that remain unclear, ΔTrelease values were 2–4 min for spores that contained both CwlJ and SleB or only CwlJ. In contrast, spores that lacked CwlJ, either alone or along with SleB, had ΔTrelease values of ∼42 min. Thus only CwlJ plays a role in accelerating rates of CaDPA release from B. subtilis spores germinating in L-alanine.
Table 2.
Values of Tlag, ΔTrelease, T1, and T2 for individual spores germinating in L-alanine or CaDPA. 10 spores of strains PS832, FB111, FB112, and FB113 were germinated with L-alanine and 5 spores of PS533 were germinated with L-alanine or CaDPA as described in Fig. 3–5, and values for Tlag, ΔTrelease, T1, and T2 were determined as described in Methods. Values in parentheses are the percentage of initial Ca-DPA remaining in spores at Tlag.
| Strain | Tlag – min | ΔTrelease – min | T1 - min | T2 - min |
|---|---|---|---|---|
| PS533 (wt) in L-alanine | 12.0 ± 4.5 (78.0) | 2.7 ± 0.6 | 10.7 ± 4.1 | 13.4 ± 4.8 |
| PS832 (wt) in L-alanine | 6.3 ± 1.7 (82.3) | 3.7 ± 0.8 | 4.6 ± 2.6 | 8.0 ± 2.3 |
| PS533 (wt) in CaDPA | 33.9 ± 7.0 (87.8) | 2.3 ± 0.2 | 12.7 ± 4.4 | 35.1 ± 7.4 |
| FB113 (cwlJ sleB) in L-alanine | 9.5 ± 3.2 (89.5) | 42.9 ± 13.3 | 21.0 ± 13.0 | Not observed |
| FB111 (cwlJ) in L-alanine | 33.5 ± 5.8 (78.9) | 40.7 ± 8.6 | 24.1 ± 2.4 | Not observed |
| FB112 (sleB) in L-alanine | 36.7 ± 9.8 (68.1) | 3.6 ± 2.1 | 34.4 ± 8.9 | 38.2 ± 9.9 |
The parameters for changes in ESLI during L-alanine germination of spores lacking only SleB were also similar to those obtained with wild-type spores, when the longer Tlag value of the sleB spores was ignored (Table 2). However, during L-alanine germination of spores lacking only CwlJ there was only a single rapid increase in ESLI, as seen with germinating cwlJ sleB spores, although the increase in ESLI with cwlJ spores took place well before Tlag, while it was well after Tlag with germinating cwlJ sleB spores (Table 2).
Germination of B. subtilis spores in exogenous CaDPA
In addition to nutrient germinants, spores can also germinate with a number of non-nutrient agents, one of which is CaDPA itself, and the pathway for spore germination with CaDPA is different than that with nutrient germinants (13). Raman spectra from individual wild-type B. subtilis spores germinating with CaDPA showed that the peak heights of CaDPA specific bands at 824, 1017, 1395, and 1572 cm−1 slowly decreased prior to Tlag and then rapidly decreased to zero by Trelease after subtraction of the signal due to external CaDPA (Fig. 5a; and data not shown). As with L-alanine germination, absolute values for Tlag and Trelease in CaDPA germination were heterogeneous and intensities of Raman peaks from other spore components did not change even after full CaDPA release (Fig. 5a; Table 2; and data not shown).
Figure 5.
Figure 5a,b. CaDPA release and ESLI from five B. subtilis spores germinating with CaDPA. a) The intensities of the CaDPA-specific 1017-cm−1 Raman band from five B. subtilis PS533 (wild-type) spores (a-e) germinating with CaDPA were determined as described in Methods; the signal intensity due to the CaDPA added to trigger germination has been subtracted. b) The ESLI normalized to the values of the first measurement from the five spores germinating with CaDPA in a) were determined as described in Methods. The normalized ESLI values were shifted by 2 on the y-axis for display purposes. The dashed dark lines at 2, 4, 6, and 8 are the zero levels of ESLI for spores d, c, b and a, respectively, and the blue and red vertical arrows indicate the Tlag and Trelease times, respectively, for the individual spores.
In contrast to L-alanine germination in which T1, the time the ESLI first began to increase, was only slightly prior to Tlag, with CaDPA germination T1 was well before Tlag (Fig. 5b). Following T1 the ESLI increased slowly until leveling off at T2, again well before Trelease (Table 2). Between Tlag and T2, the majority of spore’s CaDPA was still in the spore’s core (Fig. 5a), but the ESLI increased sharply ∼2-fold beginning at T2, reaching a maximum level at Trelease (Fig. 5b). After Trelease, the ESLI gradually decreased, and as seen with L-alanine germination, the intensity fluctuation increased when the ESLI increased following T2, and the fluctuation increased further after Trelease.
DISCUSSION
Two parameters have been measured during the germination of individual B. subtilis spores with the nutrient germinant L-alanine, CaDPA levels and ESLI. These analyses confirm features of CaDPA release seen during wild-type B. thuringiensis spore germination with L-alanine (19) including: i) a significant Tlag between addition of a germinant and rapid CaDPA release; ii) a marked heterogeneity in Tlag between individual spores; and iii) release of the great majority of CaDPA in only a few min (ΔTrelease) as the spore’s CaDPA level falls to zero at Trelease. These features of CaDPA release were also seen in germination by exogenous CaDPA, which triggers germination by activating CwlJ (26). Notably, Tlag values were heterogeneous for CaDPA germination while ΔTrelease times were comparable to those for L-alanine germination.
One novel finding in this work is that during nutrient and CaDPA germination of B. subtilis spores there was slow release of 15–20% of the spores’ CaDPA in Tlag that preceded rapid release of the majority of CaDPA during ΔTrelease. Reexamination of data on CaDPA release from B. thuringiensis spores during nutrient germination (19) has also indicated that 10–20% of total CaDPA is released in Tlag during the germination of these spores (data not shown). The significance of this slow CaDPA release in Tlag is not known, but it is tempting to speculate that this is important in germination, perhaps by slowly elevating the core water content. A second novel finding was that values for ΔTrelease were accelerated greatly by the CLE CwlJ, while SleB had no effect. A decrease in the rate of CaDPA release during nutrient germination of B. subtilis cwlJ spore populations has been reported (28). However, current work indicates that the effect of CwlJ is on ΔTrelease not on Tlag and this effect of CwlJ has also been seen during nutrient germination of individual B. megaterium spores (29). Thus CwlJ action somehow accelerates CaDPA efflux across the spore’s inner membrane during germination.
The ESLI analysis revealed some unexpected features of spore germination, as there were two large, rapid increases in ESLI during L-alanine germination of wild-type spores, one at T1 just prior to Tlag and a second at T2 during ΔTrelease; these two increases were then followed by a slow decline. The two changes in ESLI were also seen during CaDPA germination, but there was only one increase during cwlJ spore germination. Changes in ELSI could be due to changes in spore size, shape or refractive index (6, 7). Overall spore shape, including that of the spore core, does not change noticeably during the complete process of spore germination (30), so changes in this parameter seem unlikely to change the ESLI observed in the current work. Similarly, although the volume encompassed by the spore core increases 2- to 3-fold upon completion of cortex lysis and core swelling, the size of the spore does not change, and the ESLI was obtained from the entire B. subtilis spore. This leaves changes in refractive index as the likely cause of changes in ESLI during spore germination. The physical origin of increases in ESLI could be an increase in the inhomogeneity of refractive index inside the spore or on the spore’s surface. This effect generates more scattering centers so that more incident photons are scattered from cellular compartments that have a higher refractive index than their surroundings, even though the overall refractive index of the spore is not increased. Prior to the addition of nutrient or non-nutrient germinants, the refractive index is relatively uniformly distributed in the spore, except for the high value in the core due to its extremely high level of CaDPA. Thus, the observed ESLI is relatively weak from a dormant spore (Fig. 2A). However, dramatic changes take place in the refractive index of the spore core during germination, first as CaDPA is released and replaced by water, and then as cortex lysis and core swelling proceed to completion. The key question is what causes the changes in ESLI seen during spore germination in L-alanine. There appear to be a number of major possibilities: 1) abrupt mixing of water in the spore core resulting in solubilization of some of the core’s CaDPA; 2) full release of CaDPA resulting in a drastic fall in core refractive index; and 3) full core hydration due to cortex lysis, core swelling and water uptake. Surprisingly, the first rapid increase in ESLI generally took place before Tlag, when only small amounts of CaDPA had been released, while the second increase took place midway in ΔTrelease, when ∼ 50% of CaDPA had been released. These same increases in ESLI were also seen in CaDPA germination, but while the increase at T2 was at the same time relative to Tlag as in L-alanine germination, T1 was well before Tlag, and well before release of much CaDPA. In contrast to the two increases in ESLI seen during germination of wild-type spores or those lacking only SleB, spores lacking CwlJ or CwlJ and SleB exhibited only one increase. This increase was after Tlag with cwlJ sleB spores, but before Tlag with cwlJ spores.
As noted above, changes in ELSI due to alterations in refractive index could be due to abrupt changes in the heterogeneity of the refractive index in some spore compartment – most likely the core. Thus the first change could be due to the initiation of mixing of water in the spore core beginning the process of solublizing the core’s CaDPA that likely exists primarily in an insoluble form. The second abrupt change in ELSI could then be due to a second rapid decrease in core refractive index accompanying the swelling of the spore core due to cortex hydrolysis. CwlJ and SleB are located primarily on the outer and inner edges of the cortex, respectively (13), and thus we presume that CwlJ hydrolyses cortical PG from the outside in, while SleB works from the inside out. This might result in an abrupt jump in core volume when the two waves of cortex hydrolysis meet. However, when SleB is the only CLE present, the core may swell slowly and smoothly. The slow decrease in ESLI seen after its final rise could then be due to a slow decrease in the heterogeneity of the core’s refractive index due to complete mixing of core components following full core expansion. Unfortunately, we do not yet know precisely when cortex hydrolysis actually takes place relative to CaDPA release during nutrient germination, whether CwlJ and SleB do indeed work only from the outside in and the inside out, respectively, and how rapidly the core expands when the cortex is hydrolyzed, since this expansion requires remodeling of the germ cell wall to accommodate the core’s increased volume. We also do not know the rate of diffusion of various core components in various stages of spore germination, although a small, soluble protein is essentially immobile in dormant and Stage I germinated spores after CaDPA has been released, and becomes mobile upon completion of germination (31). We also do not know the meaning of the increase in fluctuation of the ESLI as germination proceeds. However, most of this increase took place only after the first rise in ESLI. Further complicating analysis of events in spore germination is that recent work has indicated that muropeptides (MP) produced by PG degradation, perhaps even by hydrolysis of the spore cortex, can also trigger germination, and by a pathway distinct from the nutrient germinant receptor pathway (32). Thus triggering cortex hydrolysis via the germinant receptor pathway in an individual spore could result in further stimulation of germination of that spore via the MP germination pathway. However, the details of the MP germination pathway have not been determined.
In conclusion, Raman scattering spectroscopy and ESLI have been used to simultaneously measure levels of CaDPA and changes in spore morphology and refractive index during germination of individual Bacillus spores. These analyses have provided new insight into the process of bacterial spore germination.
Acknowledgements
This work was supported by a grant from the Army Research Office (YQL/PS), by a Research Development Award from East Carolina University (YQL), and by a grant from the National Institutes of Health (GM19698) (PS).
References
- 1.Ashkin A, Dziedzic JM, Bjorkholm JE, Chu S. Opt. Lett. 1986;11:288–290. doi: 10.1364/ol.11.000288. [DOI] [PubMed] [Google Scholar]
- 2.Neuman KC, Block SM. Rev Sci Instr. 2004;75:2787–2809. doi: 10.1063/1.1785844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ashkin A, Dziedzic JM, Yamane T. Nature. 1987;330:769–771. doi: 10.1038/330769a0. [DOI] [PubMed] [Google Scholar]
- 4.Mehta AD, Rief M, Spudich JA, Smith DA, Simmons RM. Science. 1999;283:1689–1695. doi: 10.1126/science.283.5408.1689. [DOI] [PubMed] [Google Scholar]
- 5.Visscher K, Schnitzer MJ, Block SM. Nature. 1999;400:184–189. doi: 10.1038/22146. [DOI] [PubMed] [Google Scholar]
- 6.Doornbos RMP, Schaeffer M, Hoekstra AG, Sloot PMA, de Grooth BG, Greve J. Appl. Opt. 1996;35:729–734. doi: 10.1364/AO.35.000729. [DOI] [PubMed] [Google Scholar]
- 7.Watson D, Hagen N, Diver J, Marchand P, Chachisvillis M. Biophys. J. 2004;87:1298–1306. doi: 10.1529/biophysj.104.042135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Puppels GJ, de Mul FFM, Otto C, Greve J, Robert-Nicoud M, Arndt-Jovin DJ, Jovin TM. Nature. 1990;347:301–303. doi: 10.1038/347301a0. [DOI] [PubMed] [Google Scholar]
- 9.Xie CA, Dinno MA, Li YQ. Opt. Lett. 2002;27:249–251. doi: 10.1364/ol.27.000249. [DOI] [PubMed] [Google Scholar]
- 10.Huang SS, Chen D, Pelczar PL, Vepachedu VR, Setlow P, Li YQ. J. Bacteriol. 2007;189:4681–4687. doi: 10.1128/JB.00282-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Piggot PJ, Hilbert DW. Curr. Opinion. Microbiol. 2004;7:579–586. doi: 10.1016/j.mib.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 12.Setlow P. J. Appl. Microbiol. 2006;101:514–525. doi: 10.1111/j.1365-2672.2005.02736.x. [DOI] [PubMed] [Google Scholar]
- 13.Setlow P. Curr. Opinion. Microbiol. 2003;6:550–556. doi: 10.1016/j.mib.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 14.Setlow B, Wahome PG, Setlow P. J. Bacteriol. 2008;190:4759–4763. doi: 10.1128/JB.00399-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hindle AA, Hall EAH. Analyst. 1999;124:1599–1604. doi: 10.1039/a906846e. [DOI] [PubMed] [Google Scholar]
- 16.Scott LR, Ellar DJ. J. Bacteriol. 1978;135:133–137. doi: 10.1128/jb.135.1.133-137.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shafaat HS, Ponce A. Appl. Environ. Microbiol. 2006;72:6808–6814. doi: 10.1128/AEM.00255-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheung HY, Cui J, Sun SQ. Microbiology. 1999;145:1043–1048. doi: 10.1099/13500872-145-5-1043. [DOI] [PubMed] [Google Scholar]
- 19.Chen D, Huang SS, Li YQ. Anal. Chem. 2006;78:6936–6941. doi: 10.1021/ac061090e. [DOI] [PubMed] [Google Scholar]
- 20.Stringer SC, Webb MD, George SM, Pin C, Peck MW. Appl. Environ. Microbiol. 2005;71:4998–5003. doi: 10.1128/AEM.71.9.4998-5003.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hashimoto T, Frieden WR, Conti SF. J Bacteriol. 1969;98:1011–1020. doi: 10.1128/jb.98.3.1011-1020.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pestov DM, Zhi M, Sariyanni Z, Kalugin NG, Kolomenskii AA, Murawski R, Paulus GG, Sautenkov VA, Schuessler H, Sokolov AV, Welch GR, Rostovtsev YV, Siebert T, Akimov DA, Graefe S, Kiefer W, Scully MO. Proc. Natl. Acad. Sci. 2005;102:14976–14981. doi: 10.1073/pnas.0506529102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scully MO, Kattawar GW, Lucht RP, Opatrny T, Pilloff H, Rebane A, Sokolov AV, Zubairy MS. Proc. Natl. Acad. Sci. 2002;99:10994–11001. doi: 10.1073/pnas.172290899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Evanoff DD, Jr, Heckel J, Caldwell TP, Christensen KA, Chumanov G. J. Amer. Chem. Soc. 2006;128:12618–12619. doi: 10.1021/ja0642717. [DOI] [PubMed] [Google Scholar]
- 25.Setlow B, Setlow P. J. Bacteriol. 1996;178:3486–3495. doi: 10.1128/jb.178.12.3486-3495.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paidhungat M, Ragkousi K, Setlow P. J. Bacteriol. 2001;183:4886–4893. doi: 10.1128/JB.183.16.4886-4893.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nicholson WL, Setlow P. In: Molecular Biological Methods for Bacillus. Harwood CR, Cutting SM, editors. Chichester, UK: John Wiley and Sons; 1990. pp. 391–450. [Google Scholar]
- 28.Ishikawa S, Yamane Y, Sekiguchi J. J. Bacteriol. 1998;180:1375–1380. doi: 10.1128/jb.180.6.1375-1380.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Setlow B, Peng L, Loshon CA, Li YQ, Christie G, Setlow P. J. Appl. Microbiol. 2009 doi: 10.1111/j.1365-2672.2009.04210.x. In press. DOI: 10.1111/j.1365-2672.2009.04210.x. [DOI] [PubMed] [Google Scholar]
- 30.Gould GW. In: The Bacterial Spore. Gould GW, Hurst A, editors. New York: Academic Press; 1969. pp. 397–444. [Google Scholar]
- 31.Cowan AE, Koppel DE, Setlow B, Setlow P. Proc. Natl. Acad. Sci. 2003;100:4209–4214. doi: 10.1073/pnas.0636762100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shah IM, Laaberki MH, Popham DL, Dworkin J. Cell. 2008;135:486–496. doi: 10.1016/j.cell.2008.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]