Abstract
Negative mood increases smoking reinforcement and risk of relapse. We explored associations of gene variants in the dopamine, opioid, and serotonin pathways with smoking reward (“liking”) and reinforcement (latency to first puff, total puffs) as a function of negative mood and expected vs. actual nicotine content of the cigarette. Smokers of European ancestry (n=72) were randomized to one of four groups in a 2 × 2 balanced-placebo design, corresponding to manipulation of actual (0.6 mg vs. 0.05 mg) and expected (told nicotine, told denicotinized) nicotine “dose” in cigarettes during each of two sessions (negative vs. positive mood induction). Following mood induction and expectancy instructions, they sampled and rated the assigned cigarette, and then smoked additional cigarettes ad lib during continued mood induction. The increase in smoking amount due to negative mood was associated with: DRD2 C957T (CC>TT or CT), SLC6A3 (presence of 9 repeat > absence of 9), and among those given a nicotine cigarette, DRD4 (presence of 7 repeat > absence of 7) and DRD2/ANKK1 TaqIA (TT or CT > CC). SLC6A3 and DRD2/ANKK1 TaqIA were also associated with smoking reward and smoking latency. OPRM1 (AA > AG or GG) was associated with smoking reward, but SLC6A4 VNTR was unrelated to any of these measures. These results warrant replication but provide the first evidence for genetic associations with the acute increase in smoking reward and reinforcement due to negative mood.
Keywords: smoking reward, reinforcement, mood, genetics, dopamine
INTRODUCTION
Acute situations or other contexts can modulate smoking reinforcement, supporting the notion that those situations may strengthen dependence and promote relapse in smokers trying to quit. For example, inducing negative mood in the laboratory consistently increases smoking behavior (Rose et al. 1983; Pomerleau and Pomerleau, 1987; Gilbert 1997; Conklin and Perkins 2005) and craving for cigarettes (Perkins and Grobe 1992; Willner and Jones, 1996). Negative mood also increases relapse risk in clinical studies (Lerman et al. 2003; Baker et al. 2004; Shiffman and Waters 2004).
Less studied, however, is the possibility of individual differences in vulnerability to these situational influences, especially those contributing to negative mood effects. Some have proposed that certain personality traits may increase sensitivity to smoking’s influence on mood regulation in particular situations (e.g., Gilbert 1997; Netter et al. 1998). As a specific example, smokers (and even nonsmokers) high in trait hostility experience greater alleviation of anger from nicotine intake via patch (Jamner et al. 1999; Fallon et al. 2004). Few other individual difference characteristics have been examined to understand why some smokers may be more vulnerable to smoking reinforcement during negative mood. Notably, no study, to our knowledge, has explored genetic moderation of the link between negative mood and acute smoking reinforcement or reward. Greater understanding of such genetic influences may provide direction for identifying smokers most vulnerable to relapse in response to negative mood, as well as for clarifying the mechanisms of smoking’s reinforcing effects during negative mood, a surprisingly murky area of research (Parrott 1998; Kassel et al. 2003).
Although no research has directly examined genetic factors on smoking reinforcement during acute negative mood, prior research suggests that the dopamine reward pathway and opioid and serotonergic systems may be important. Genetic variants in the TaqIA variant in the dopamine D2 receptor (DRD2/ANKK1), dopamine D4 receptor (DRD4), and dopamine transporter (SLC6A3) genes have been associated with smoking status, smoking intensity, and smoking cessation in some, but not all, studies (Munafo et al. 2004; Schnoll et al. , 2007). Additional studies suggest that genes in the dopamine pathway may interact with negative mood symptoms in associations with smoking behavior (Lerman et al. 1998; Audrain-McGovern et al. 2004). Genetic variation in the mu opioid receptor (OPRM1) gene also may be associated with smoking behavior and cessation (Lerman et al. 2004; Zhang et al. 2006; Munafo et al. 2007), as well as with negative mood effects of smoking abstinence (Lerman et al. 2004). Although most studies have not found significant associations of a common variant in the serotonin transporter (SLC6A4) gene with smoking behavior and cessation (Munafo et al. 2006; Trummer et al. 2006; David et al. 2007a), there is evidence that smoking behavior is related to a gene by neuroticism interaction (Hu et al. 2000; Lerman et al. 2000).
Aside from negative mood, other influences on smoking reward and reinforcement include the actual, as well as the expected, nicotine content (or “dose”) of cigarettes (Netter et al. 1998; Juliano and Brandon 2002; Perkins et al. 2003; Perkins et al. 2008). Regarding genetic influences on the reinforcing effects of actual nicotine content, one study showed an association between preference for nicotine versus denicotinized cigarettes with the mu opioid receptor polymorphism (OPRM1), at least in women (Ray et al. 2006). No research has examined genetic moderators of the influence of expected nicotine content (i.e. nicotine expectancy) on acute smoking reinforcement and reward.
In this study, we examined the association of specific genetic polymorphisms with the increase in smoking reward and reinforcement due to negative mood, taking into consideration the effects of actual and expected nicotine dose. Candidate genes and SNPs were selected based on the following criteria: (1) genes in pathways implicated in the neurobiology of nicotine, including the dopamine, serotonin and endogenous opioid pathway; (2) within those pathways, genes that have been linked to nicotine dependence phenotypes in prior research (Lerman et al. , 2007); and (3) within these genes, polymorphisms with documented functional effects (in vitro or in vivo) and minor allele frequencies >0.10). Based on these criteria, we selected the following polymorphisms: dopamine D4 receptor (DRD4 VNTR), dopamine D2 receptor (DRD2 C957T SNP and DRD2/ANKK1 TaqIA SNP (referred to here as ANKK1), the dopamine transporter (SLC6A3 VNTR), the mu opioid receptor exon 1 SNP (OPRM1 A118G), and the serotonin transporter promoter variant (SLC6A4 5HTTLPR VNTR).
METHODS
Participants
Participants were 72 young adult smokers of European ancestry (34m, 38f) who smoked at least 10 cigarettes per day and had been smoking for at least 1 year. They were participants in a study of the effects of mood on smoking behavior as a function of actual and expected nicotine dose (Perkins et al. 2008). Mean ± SE sample characteristics for these 72 participants are as follows: age of 28.1 ± 1.3 yrs, nicotine yield of preferred brand of 1.01 ± 0.02 mg, daily smoking rate of 17.6 ± 0.6 cigarettes/day, and Fagerstrom Test of Nicotine Dependence (FTND; Heatherton et al. 1991) score of 4.4 ± 0.2.
Independent Variables
Negative versus positive mood was manipulated within-subjects, during two experimental sessions on separate days. (Note that “mood” is the context for smoking, while “affect” is the self-reported experience produced by mood.) Actual nicotine content (given nicotine, given denicotinized, or “denic”) and expected nicotine content (told nicotine, told denic) of cigarettes were manipulated between-subjects, to form the 2 × 2 balanced-placebo design. The same actual and expected nicotine conditions were administered across days. Genotype was another between-subjects factor and is described later.
Negative and Positive Mood Induction Procedure
The mood induction procedure produces robust differences in self-reported affect and has been described in detail elsewhere (Conklin and Perkins 2005). This procedure involves the combination of pictorial slides from the International Affective Picture System (IAPS; Lang et al. 1988) and mood congruent classical music. Briefly, slides are presented for 12 sec each as musical selections play (negatively-valenced slides and music for negative mood induction, positively-valenced slides and music for positive mood induction), to foster sustained negative mood or positive mood, depending on the assigned mood condition for that session.
Cigarettes (actual nicotine “dose”)
Half of the sample was randomly assigned to the “given nicotine” cigarette group and half to the “given denic” group. The “nicotine” brand was QuestR 1 (yield of 0.6 mg nicotine, 9 mg tar), and the denicotinized (“denic”) brand was QuestR 3 (yield < 0.05 mg nicotine, 9 mg tar), both sold commercially by Vector Group, Ltd. (Miami, FL). Menthol smokers received menthol Quest; non-menthol smokers received non-menthol Quest. All cigarettes had identifiable markings covered.
Expectancy for nicotine
Half of each “given” group was randomly assigned to two expectancy subgroups, “told nicotine” and “told denic”. When presented with the cigarette to be smoked that session, they were given the following instructions, based on those of Juliano and Brandon (2002), via computer screen to manipulate their expectancy for nicotine:
Told nicotine: “You will be smoking a regular cigarette. This is a normal cigarette with a normal amount of nicotine.”
Told denic: “You will be smoking a placebo cigarette. This is a normal cigarette except it contains no nicotine.”
Dependent Measures
Smoking reward (“liking”) and reinforcement (latency to first puff, amount of puffs) were the primary dependent measures. We also assessed affect to verify manipulation of mood.
Smoking Reward
The “reward” value of smoking was assessed using the single “liking” item from the Rose Sensory Questionnaire (see Westman et al. 1996), which was rated on a 0 to 100 visual analog scale (VAS) anchored by “not at all” and “extremely”, respectively.
Smoking Reinforcement
Smoking reinforcement during the 14-min ad lib smoking period (see Procedure, below) was determined by: 1) the latency to first puff, and 2) the total number of puffs taken. Latency was viewed as an index of smoking’s incentive salience (Berridge 2007), and number of puffs was seen as a measure of overall drug consumption (Everitt and Robbins 2005). These measures were obtained by behavioral observation of smoking behavior from videotapes of the sessions (see Perkins et al. 1997; Conklin and Perkins 2005). Inter-rater reliability of these measures exceeds 99% (Perkins et al. 1997).
Affect
Self-reported affect was assessed with the Diener and Emmons (1984) Mood Scale to verify successful manipulation of affect via the mood induction procedure. The Mood Scale consists of 9 VAS items rated from 0 (“not at all”) to 100 (“very much”) that yield a positive affect (PA) and negative affect (NA) score. PA scale items are “happy”, “joyful”, “pleased”, and “enjoyment/fun”, while NA scale items are “depressed/blue”, “unhappy”, “frustrated”, “worried/anxious”, and “angry/hostile”.
Procedures
Participants engaged in two 90-min sessions, each after overnight (>12 hrs) abstinence from smoking, verified by expired-air carbon monoxide (CO). Mean±SE CO was 6.5 ± 0.3 ppm upon arrival, confirming overnight abstinence. Sessions differed only by the valence of the mood induction condition, positive or negative (in counter-balanced order). Participants were randomly assigned to one of four groups, varying in each combination of actual (given nicotine, 0.6 versus given denic, 0.05 mg) and expected (told nicotine versus told denic) nicotine “dose” in the cigarettes provided; “given” and “told” conditions remained the same between sessions.
For each session, subjects first rested quietly and completed the affect measure. They repeated this measure following 5 mins of initial mood induction (negative, positive). They were then given their expectancy instructions (i.e. told condition) and the assigned cigarette under single blind conditions. As the music from the mood induction procedure continued, participants took 4 puffs to “sample” the cigarette (smoking exposure period), using computer-presented puffing instructions to standardize intake, as described previously (Perkins et al. 2004; Perkins et al. 2006). They rated the cigarette for reward (“liking”) and completed a check of the expectancy manipulation (see below). After resumption of the mood induction procedure for 3 mins, they were allowed to smoke ad lib more cigarettes of that same type as the mood induction continued for 14 mins. Latency to the first puff and the total number of puffs consumed were assessed during this ad lib smoking period.
Because effects of expectancy rely on successful manipulation of expectancy (Hull and Bond 1986; Martin and Sayette 1993), we verified the success of this manipulation after the smoking exposure period, before the ad lib smoking period. Subjects were asked to circle one of two options indicating how much nicotine they believed was in the cigarette they were given, either “normal amount of nicotine” or “no nicotine”. Analyses were limited to the 72 (out of 120 total subjects of European ancestry) who believed their dose instruction on both days (i.e. under both mood induction conditions).
All subjects provided informed consent after the nature and consequences of participation were explained. This research was approved by the Institutional Review Board of the University of Pittsburgh Medical Center.
Genotyping
We performed the genotyping of the SNPs using the GoldenGate™ Assay on the Illumina platform (Illumina, San Diego, CA) at the Genomics Core Facility at USC/Norris Comprehensive Cancer Center (Director, David Van Den Berg). The assay utilizes a multiplexed oligonucleotide ligation assay (OLA) on genomic DNA and PCR amplification with universal primers. For additional details see http://www.illumina.com/products/prod_snp.ilmn. Data from the assay array is read by the BeadArray Reader (Illumina) and genotype calling is performed in BeadStudio software (Illumina, Inc.) using the genotyping module. The system includes automated protocols for the entire genotyping process utilizing robotics, barcoding, and extensive data and process tracking to ensure high call rates and accuracy. Additionally, genotyping quality control was further monitored by the inclusion of replicates and CEPH trios. VNTR genotyping was as described elsewhere (George et al. , 1993; Lesch et al. , 1996; Vandenbergh et al. , 2002). Results were analyzed using GeneMarker® v1.5 (SoftGenetics).
The coding of genotypes for analysis, based on prior literature, was as follows: 1) DRD2 C957T (CC vs. CT vs. TT); 2) 5HTTLPR (or SLC6A4, presence or abstinence of the short allele); 3) DRD4 VNTR (presence or absence of the 7-repeat allele); 4) SLC6A3 (presence or absence of the 9-repeat allele); 5) OPRM1 A118G (presence or absence of the G allele); and 6) DRD2/ANKK1 (TT or CT vs. CC; note: T is the less common TaqIA A1 allele).
Analyses
The overall effects on smoking reward and reinforcement due to mood and actual or expected nicotine dose in the full sample are reported elsewhere (Perkins et al. 2008). Of primary interest in the current analysis were interactions involving gene X mood (positive, negative) on smoking reward and reinforcement. We first determined any influence of the order of mood induction conditions across sessions on responses, but found no such order effects, allowing us to collapse across order. Differences in affective responses (NA, PA) to mood induction as a function of each gene were then analyzed separately using analysis of variance (ANOVA), with mood induction (negative, positive) as the within-subjects factor.
The primary analyses examined the influence of genes on smoking reward and reinforcement in response to the nicotine, expectancy, and mood manipulations, using ANOVA. Gene, nicotine (nicotine versus denic cigarette), and expectancy (i.e. told nicotine versus told denic) were between-subjects factors, and mood was the within-subjects factor. Because we found virtually no interactions that differed by sex, sex was not included as a between-subjects factor in primary analyses in order to retain power for the other effects of interest. To further minimize chances that significant interactions were due to very small n’s in certain cells, we focused only on interactions involving gene X mood, which would suggest differential sensitivity to mood effects on smoking associated with gene variants. We also examined the possible moderating influences of actual or expected nicotine indicated in the triple interactions involving gene X mood X nicotine or gene X mood X dose expectancy. Significant interactions were followed up with paired comparisons within the relevant subgroups of differences in smoking reward or reinforcement due to negative versus positive mood induction using Fisher’s LSD t-tests (Huitema 1980).
RESULTS
Genotype distributions
The distribution of genotypes, by sex, is presented in Table 1. Because no significant effects were observed for 5HTTLPR (SLC6A4), this gene will not be discussed further.
Table 1.
Frequencies of alleles for each gene, by sex.
| Males | Females | Total | ||
|---|---|---|---|---|
| DRD2 C957T | TT | 11 (32.4%) | 10 (27.8%) | 21 |
| TC | 14 (41.2%) | 20 (55.6%) | 34 | |
| CC | 9 (26.5%) | 6 (16.7%) | 15 | |
| 5HTTLPR | Absence of Short | 11 (32.4%) | 12 (32.4%) | 23 |
| Presence of Short | 23 (67.6%) | 25 (67.6%) | 48 | |
| DRD4 | Absence of 7 | 25 (75.8%) | 25 (75.8%) | 50 |
| Presence of 7 | 8 (24.2%) | 8 (24.2%) | 16 | |
| SLC6A3 | Absence of 9 | 21 (61.8%) | 19 (51.4%) | 40 |
| Presence of 9 | 13 (38.2%) | 18 (48.6%) | 31 | |
| OPRM1 | AA | 25 (73.5%) | 26 (70.3%) | 51 |
| AG or GG | 9 (26.5%) | 11 (29.7%) | 20 | |
| ANKK1 TaqIA | TT or TC | 18 (52.9%) | 16 (47.1%) | 34 |
| CC | 16 (50%) | 16 (50%) | 32 |
Mood manipulation check
The differences in negative and positive affect due to the mood induction were highly significant, F (1,70)’s = 21.86 and 70.77, respectively, both p<.001, verifying that mood was successfully manipulated. Negative affect increased from a mean (SE) of 21.8 + 2.0 at baseline to 29.2 + 2.3 during negative mood induction, and decreased from 20.5 + 1.9 to 17.9 + 1.9 during positive mood induction. Similarly, positive affect decreased from 44.5 + 2.2 at baseline to 23.6 + 2.2 during negative mood induction, and was maintained from 44.1 + 2.3 to 43.6 + 2.6, respectively, during positive mood induction. There were no other significant effects, including interactions of mood X sex. In analyses of genetic influences on negative and positive affect responses to mood induction, we saw no significant effects at all, indicating that the affective responses to mood induction were not associated with the genes of interest.
Cigarette liking
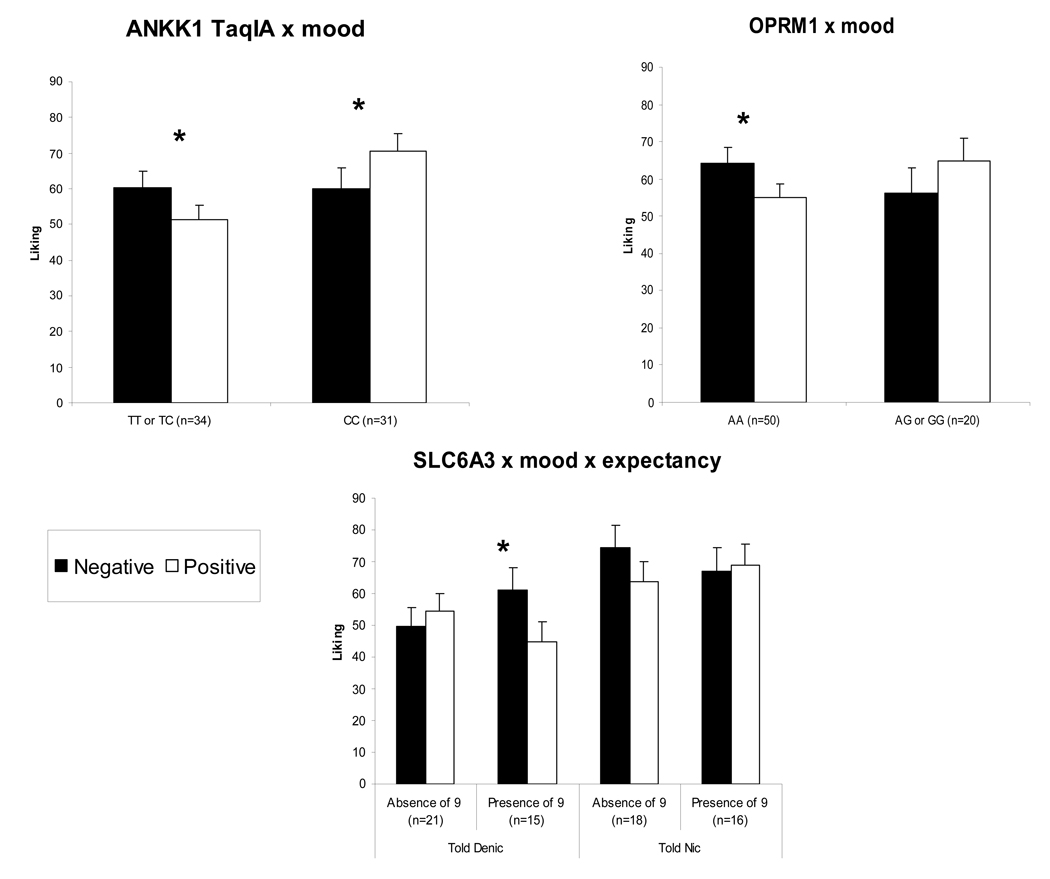
In ANOVAs of cigarette liking, significant interactions were observed for DRD2/ANKK1 TaqIA X mood, F(1,49)=6.56, p<.05, and OPRM1 X mood, F(1,54)=5.20, p<.05. As shown in Figure 1, liking was higher during negative versus positive mood induction for DRD2/ANKK1 TaqIA TT or CT genotypes but higher during positive versus negative mood for the CC genotype. Liking was also higher during negative versus positive mood induction for the OPRM1 AA genotype but not the AG or GG genotypes. Also found was a significant interaction of SLC6A3 X mood X expectancy, F(1,54)=5.73, p<.05. Among those told and believing the cigarette was a denic, liking was higher during negative versus positive mood induction for those with the SLC6A3–9 allele but not among those without the 9 allele. There was no influence of genotype among those told and believing the cigarette had nicotine.
Figure 1.
Mean±SE “liking” (reward) for the assigned cigarette after initial exposure (4 puffs) during negative versus positive mood induction, by genotype and, where relevant, expected (“told”) nicotine dose. * p<.05 for differences between mood induction conditions within subgroups.
Smoking behavior
Latency
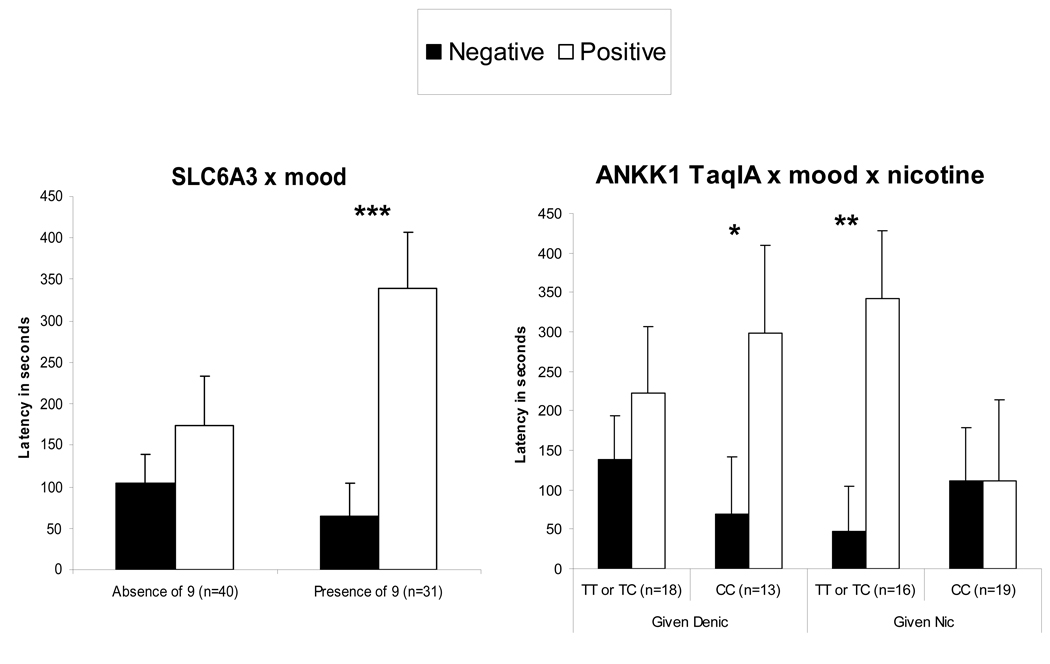
For latency to first puff during the ad lib smoking period, the interactions of SLC6A3 X mood, F(1,55)=4.76, p<.05, and of DRD2/ANKK1 TaqIA X mood X nicotine, F(1,50)=5.15, p<.05, were significant. As shown in Figure 2, latency was faster during negative versus positive mood for those with the SLC6A3–9 allele but not among those without the 9 allele. Among those given the nicotine cigarette, latency was faster during negative versus positive mood in those with the DRD2/ANKK1 TaqIA TT or CT genotypes but not CC genotype. Yet, opposite results were seen among those given the denic cigarette, as latency was faster during negative mood for those with the ANKK1 TaqIA CC genotype but not TT or CT genotypes.
Figure 2.
Mean±SE latency (secs) to first puff during the ad lib smoking period under negative versus positive mood induction, by genotype and, where relevant, actual (“given”) nicotine dose. Smaller values indicate faster latency to first puff. * p<.05, ** p<.01, *** p<.001 for differences between mood induction conditions within subgroups.
Amount
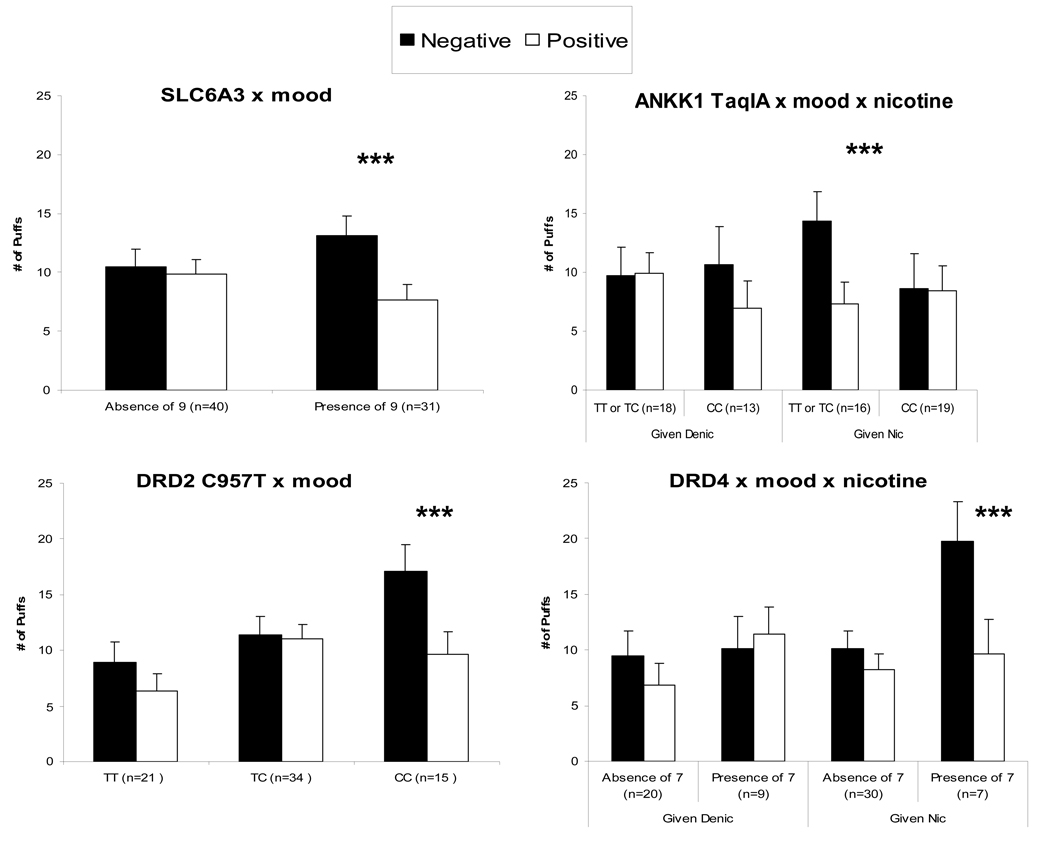
For number of puffs, interactions of SLC6A3 X mood, F(1,55)=6.31, p<.05, and of DRD2/ANKK1 X mood X nicotine, F(1,50)=6.12, p<.05, were significant, similar to findings for latency to first puff (above). Also significant were interactions of DRD2 C957T X mood, F(2,47)=3.51, p<.05, and DRD4 X mood X nicotine, F(1,50)=8.17, p<.01. As shown in Figure 3, puffs were greater during negative versus positive mood for those with the SLC6A3–9 allele, and for those with the DRD2 C957T CC genotype. For DRD2/ANKK1 TaqIA and DRD4, genotype was associated with puff number only among those given a nicotine cigarette. In these subjects, puffs were greater during negative versus positive mood for those with the DRD2/ANKK1 TaqIA TT or CT genotype, and for those with the DRD4–7 allele.
Figure 3.
Mean±SE total number of puffs during the 14-min ad lib smoking period under negative versus positive mood induction, by genotype and, where relevant, actual (“given”) nicotine dose. Asterisks as in Figure 2.
DISCUSSION
To our knowledge, this is the first study to examine genetic associations with differences in smoking reward and reinforcement due to acute mood manipulation. Past research has shown that negative mood acutely increases ad lib smoking behavior, indicating that smoking is more reinforcing during negative mood. The novel finding from this study is that this influence of mood is associated with genes in the dopamine reward pathway and endogenous opioid pathway.
Among the significant findings, two genes believed related to the dopamine D2 receptor, the DRD2/ANKK1 TaqIA and the DRD2 C957T, were associated with sensitivity to mood effects on smoking reward and reinforcement. Smokers carrying the minor “T” allele of DRD2/ANKK1 TaqIA reported increased cigarette liking during negative versus positive mood, while those heterozygous for the common variant liked the cigarette more during positive versus negative mood induction. The minor allele group also had an increased number of puffs and shorter latency to smoking during negative mood, if they were smoking a nicotine cigarette. These findings are consistent with evidence for an increased risk of cigarette consumption among carriers of the DRD2/ANKK1 TaqIA minor allele (Munafo et al. 2004). However, this finding may not fit as well with prior evidence that this subgroup of smokers is less responsive to treatment with the antidepressant bupropion for smoking cessation (David et al. 2007b). Smokers homozygous for the DRD2 C957T allele (CC) also took more cigarette puffs during negative versus positive mood, perhaps consistent with prior evidence for their increased risk for relapse while on placebo and their enhanced therapeutic response to bupropion (Lerman et al. 2006).
Two other genes thought to be involved in the dopamine pathway were associated with increased smoking reinforcement due to negative mood. The increase in smoking amount due to negative mood among those given nicotine (but not denic) cigarettes was greater in smokers carrying the DRD4 7 repeat allele, which has been associated with increased risk of smoking relapse (Shields et al. 1998; David et al. 2008). We also found that carriers of the 9-repeat allele of the dopamine transporter (SLC6A3) polymorphism had a shorter latency to smoking and increased number of puffs during negative mood induction, consistent with associations of this allele with stress-induced cigarette craving (Erblich et al. 2004).
Regarding the two non-dopamine related gene variants examined here, the serotonin transporter promoter variant was unrelated to any of the effects examined, while the functional OPRM1 A118G variant was associated with smoking reward under negative versus positive mood induction. Specifically, smokers carrying the common AA genotype liked the cigarette more in the negative mood condition, while ratings of liking did not differ significantly as a function of mood condition in those with the minor “G” allele. The minor G allele is thought to be a reduced activity allele for the mu opioid receptor, as it relates to reduced mRNA and protein expression (Zhang et al. 2005). These in vitro data are consistent with evidence that smokers carrying the common AA OPRM1 genotype are more likely than those carrying the “reduced activity” G allele to relapse, and those with the AA genotype also report higher levels of abstinence-induced negative affect (Lerman et al. 2004). Further, female smokers with the OPRM1 AA genotype are more likely to choose nicotine over denicotinized cigarettes than female smokers carrying the G allele (Ray et al. 2006). The present findings, therefore, suggest that the AA genotype of OPRM1 may predispose to smoking relapse, in part, via effects of negative mood on smoking reward.
In addition to the novelty of the study focus, genetic associations with increased smoking reward and reinforcement due to negative mood, strengths of this study include some of the methods used. The mood induction procedure was successful in manipulating mood as intended, as we have previously demonstrated (Conklin and Perkins 2005). Moreover, the magnitude of negative and positive affect changes due to the mood induction procedures did not vary by genotypes, ruling out the notion that these genetic associations with increased smoking reward and reinforcement due to negative mood resulted from some genotypes experiencing greater intensity of negative affect. Second, the within-subject manipulation of mood reduced error variance in the statistical comparisons, all of which involved mood as a factor, as well as genotype. Third, inclusion in analyses of only those smokers who believed their dose instructions resulted in participants whose expectancies for nicotine were successfully manipulated, allowing for a test of genetic association with the influence of expectancies on smoking behavior during negative mood, if present.
The study contained several limitations as well, primarily the small numbers of subjects in some of the genotype subgroups varying in actual nicotine or expectancy for nicotine (e.g., those with the DRD4–7 allele). Consequently, despite the within-subject manipulation of mood, we may not have had adequate power to detect other genetic influences on smoking reward and reinforcement due to negative mood. This problem reflects the practical conflict between studying a limited amount of data from a large sample versus extensive phenotyping of a smaller sample (e.g., assessing prospective responses to different mood induction conditions while varying actual and expected nicotine, as done in this study). A second limitation is that some of the significant effects may have resulted from chance, with 6 genes and 3 gene X mood effects of interest (gene X mood, gene X mood X nicotine, gene X mood X expectancy) for each of the 3 main dependent measures. Yet, the fact that interactions involving SLC6A3 X mood and DRD2/ANKK1 TaqIA X mood were significant for each dependent measure suggests a consistency in associations that is not likely due to chance. Given the complete lack of prior research on genetic associations with smoking reward and reinforcement due to mood, this study was exploratory in nature and the findings were intended to be heuristic and not conclusive. This study should be replicated with larger samples to verify the findings. Third, the ad lib smoking period during mood induction was only 14 mins, and more extended duration of mood may reveal greater or smaller changes in smoking behavior due to genotypes.
In summary, these findings suggest that some dopamine and opioid genes are associated with the degree to which negative mood increases acute smoking reward and reinforcement. Future research should examine associations of these genes with smoking reward and reinforcement under other mood conditions or other acute behavioral manipulations (e.g. stressful challenge, co-administration of alcohol). The associations of genes in other neurobiological pathways with smoking reward and reinforcement during negative mood should also be examined. Results may help identify possible mechanisms to explain increased risk of dependence onset and persistence (i.e. relapse) in those with certain genotypes.
Acknowledgments
The authors thank Robert Ferrell, Nancy Petro, David Conti, Denise Nishita, Yungang He, John Scott, Michael Sayette, and Lacey Neagle for their assistance.
This research was supported by NIDA Grants DA16483 (KAP) and DA19478 (KAP), NCI and NIDA Grant P50 CA/DA84718 (CL), and NIDA grant U01 DA20830 (NLB).
References
- Audrain-McGovern J, Lerman C, Wileyto EP, Rodriguez D, Shields PG. Interacting effects of genetic predisposition and depression on adolescent smoking progression. Amer J Psychiatr. 2004;161:1224–1230. doi: 10.1176/appi.ajp.161.7.1224. [DOI] [PubMed] [Google Scholar]
- Baker TB, Piper ME, McCarthy D, Majeskie MR, Fiore MC. Addiction motivation reformulated: an affective processing model of negative reinforcement. Psychol Rev. 2004;111:33–51. doi: 10.1037/0033-295X.111.1.33. [DOI] [PubMed] [Google Scholar]
- Berridge KC. The debate over dopamine’s role in reward: the case for incentive salience. Psychopharmacol. 2007;191:391–431. doi: 10.1007/s00213-006-0578-x. [DOI] [PubMed] [Google Scholar]
- Conklin CA, Perkins KA. Subjective and reinforcing effects of smoking during negative mood induction. J Abnormal Psychol. 2005;114:153–164. doi: 10.1037/0021-843X.114.1.153. [DOI] [PubMed] [Google Scholar]
- David SP, Munafo MR, Murphy MF, Proctor M, Walton RT, Johnstone EC. Genetic variation in the dopamine D4 receptor (DRD4) gene and smoking cessation: follow-up of a randomised clinical trial of transdermal nicotine patch. Pharmacogenomics J. 2008;8:122–128. doi: 10.1038/sj.tpj.6500447. Epub 2007 Mar 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David SP, Munafò MR, Murphy MF, Walton RT, Johnstone EC. The serotonin transporter 5-HTTLPR polymorphism and treatment response to nicotine patch: follow-up of a randomized controlled trial. Nic Tobacco Res. 2007a;9:225–231. doi: 10.1080/14622200601078566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David SP, Strong DR, Munafò MR, Brown RA, Lloyd-Richardson EE, Wileyto PE, et al. Bupropion efficacy for smoking cessation is influenced by the DRD2 TaqIA polymorphism: analysis of pooled data from two clinical trials. Nic Tobacco Res. 2007b;9:1251–1257. doi: 10.1080/14622200701705027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diener E, Emmons RA. The independence of positive and negative affect. J Pers Soc Psychol. 1984;47:1105–1117. doi: 10.1037//0022-3514.47.5.1105. [DOI] [PubMed] [Google Scholar]
- Erblich J, Lerman C, Self DW, Diaz GA, Bovbjerg DH. Stress-induced cigarette craving: effects of the DRD2 TaqI RFLP and SLC6A3 VNTR polymorphisms. Pharmacogenomics J. 2004;4:102–109. doi: 10.1038/sj.tpj.6500227. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Fallon JH, Keator DB, Mbogori J, Turner J, Potkin SG. Hostility differentiates the brain metabolic effects of nicotine. Cog Brain Res. 2004;18:142–148. doi: 10.1016/j.cogbrainres.2003.10.003. [DOI] [PubMed] [Google Scholar]
- George SR, Cheng R, Nguyen T, Israel Y, O'Dowd BF. Polymorphisms of the D4 dopamine receptor alleles in chronic alcoholism. Biochem Biophys Res Commun. 1993;196:107–114. doi: 10.1006/bbrc.1993.2222. [DOI] [PubMed] [Google Scholar]
- Gilbert DG. The situation x trait adaptive response (STAR) model of drug use, effects, and craving. Human Psychopharmacol. 1997;12:S89–S102. [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom K-O. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hu S, Brody CL, Fisher C, Gunzerath L, Nelson ML, Sabol SZ, et al. Interaction between the serotonin transporter gene and neuroticism in cigarette smoking behavior. Mol Psychiatr. 2000;5:181–188. doi: 10.1038/sj.mp.4000690. [DOI] [PubMed] [Google Scholar]
- Huitema BE. Analysis of covariance and alternatives. New York: John Wiley & Sons; 1980. [Google Scholar]
- Hull JG, Bond CF. Social and behavioral consequences of alcohol consumption and expectancy: a meta-analysis. Psychol Bull. 1986;99:347–360. [PubMed] [Google Scholar]
- Jamner LD, Jarvik ME, Shapiro D. Nicotine reduces the frequency of anger reports in high but not low hostile smokers and nonsmokers: an ambulatory study. Exper Clin Psychopharmacol. 1999;7:454–463. doi: 10.1037//1064-1297.7.4.454. [DOI] [PubMed] [Google Scholar]
- Juliano LM, Brandon TH. Effects of nicotine dose, instructional set, and outcome expectancies on the subjective effects of smoking in the presence of a stressor. J Abnormal Psychol. 2002;111:88–97. doi: 10.1037//0021-843x.111.1.88. [DOI] [PubMed] [Google Scholar]
- Kassel JD, Stroud LR, Paronis CA. Smoking, stress, and negative affect: correlation, causation, and context across stages of smoking. Psychol Bull. 2003;129:270–304. doi: 10.1037/0033-2909.129.2.270. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Ohman A, Vaitl D. The International Affective Picture System [Photographic Slides] Gainesville, FL: The Center for Research in Psychophysiology, University of Florida; 1988. [Google Scholar]
- Lerman C, Caporaso NE, Audrain J, Main D, Boyd NR, Shields PG. Interacting effects of the serotonin transporter gene and neuroticism in smoking practices and nicotine dependence. Mol Psychiatr. 2000;5:189–192. doi: 10.1038/sj.mp.4000672. [DOI] [PubMed] [Google Scholar]
- Lerman C, Caporaso N, Main D, Audrain J, Boyd NR, Bowman ED, Shields PG. Depression and self-medication with nicotine: the modifying influence of the dopamine D4 receptor gene. Health Psychol. 1998;17:56–62. doi: 10.1037//0278-6133.17.1.56. [DOI] [PubMed] [Google Scholar]
- Lerman C, Jepson C, Wileyto EP, Epstein LH, Rukstalis M, Patterson F, et al. Role of functional genetic variation in the dopamine D2 receptor (DRD2) in response to bupropion and nicotine replacement therapy for tobacco dependence: results of two randomized clinical trials. Neuropsychopharmacol. 2006;31:231–242. doi: 10.1038/sj.npp.1300861. [DOI] [PubMed] [Google Scholar]
- Lerman CE, Schnoll RA, Munafò MR. Genetics and smoking cessation: Improving outcomes in smokers at risk. Am J Prev Med. 2007;33(6S):S398–S405. doi: 10.1016/j.amepre.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerman C, Shields PG, Wileyto EP, Audrain J, Hawk LH, Jr, Pinto A, et al. Effects of dopamine transporter and receptor polymorphisms on smoking cessation in a bupropion clinical trial. Health Psychol. 2003;22:541–548. doi: 10.1037/0278-6133.22.5.541. [DOI] [PubMed] [Google Scholar]
- Lerman C, Wileyto EP, Patterson F, Rukstalis M, Audrain-McGovern J, Restine S, et al. The functional mu opioid receptor (OPRM1) Asn40Asp variant predicts short-term response to nicotine replacement therapy in a clinical trial. Pharmacogenomics J. 2004;4:184–192. doi: 10.1038/sj.tpj.6500238. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, et al. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- Martin CS, Sayette MA. Experimental design in alcohol administration research: Limitations and alternatives in the manipulation of dosage-set. J Stud Alc. 1993;54:750–761. doi: 10.15288/jsa.1993.54.750. [DOI] [PubMed] [Google Scholar]
- Munafo MR, Clark TG, Johnstone EC, Murphy MFG, Walton RT. The genetic basis for smoking behavior: a systematic review and meta-analysis. Nic Tobacco Res. 2004;6:583–597. doi: 10.1080/14622200410001734030. [DOI] [PubMed] [Google Scholar]
- Munafò MR, Elliot KM, Murphy MF, Walton RT, Johnstone EC. Association of the mu-opioid receptor gene with smoking cessation. Pharmacogenomics J. 2007;7:353–361. doi: 10.1038/sj.tpj.6500432. [DOI] [PubMed] [Google Scholar]
- Munafò MR, Johnstone EC, Wileyto EP, Shields PG, Elliot KM, Lerman C. Lack of association of 5-HTTLPR genotype with smoking cessation in a nicotine replacement therapy randomized trial. Cancer Epidemiol Biomarkers Prev. 2006;15:398–400. doi: 10.1158/1055-9965.EPI-05-0648. [DOI] [PubMed] [Google Scholar]
- Netter P, Hennig J, Huwe S, Olbrich R. Personality related effects of nicotine, mode of application, and expectancies on performance, emotional states, and desire for smoking. Psychopharmacol. 1998;135:52–62. doi: 10.1007/s002130050485. [DOI] [PubMed] [Google Scholar]
- Parrott AC. Nesbitt’s Paradox resolved? Stress and arousal modulation during cigarette smoking. Addiction. 1998;93:27–39. doi: 10.1046/j.1360-0443.1998.931274.x. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Doyle T, Ciccocioppo M, Conklin C, Sayette M, Caggiula AR. Sex differences in the influence of nicotine and dose instructions on subjective and reinforcing effects of smoking. Psychopharmacol. 2006;184:600–607. doi: 10.1007/s00213-005-0103-7. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Grobe JE. Increased desire to smoke during acute stress. Br J Addict. 1992;87:1037–1040. doi: 10.1111/j.1360-0443.1992.tb03121.x. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Grobe JE, Caggiula AC. Acute reinforcing effects of low-dose nicotine nasal spray in humans. Pharmacol Biochem Behav. 1997;56:235–241. doi: 10.1016/s0091-3057(96)00216-x. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Jacobs L, Ciccocioppo M, Conklin CA, Sayette M, Caggiula A. The influence of instructions and nicotine dose on the subjective and reinforcing effects of smoking. Exper Clin Psychopharmacol. 2004;12:91–101. doi: 10.1037/1064-1297.12.2.91. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Ciccocioppo M, Conklin C, Milanak M, Grottenthaler A, Sayette M. Mood influences on acute smoking responses are independent of nicotine intake and dose expectancy. J Abnormal Psychol. 2008;117:79–93. doi: 10.1037/0021-843X.117.1.79. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Sayette MA, Conklin CA, Caggiula AR. Placebo effects of tobacco smoking and other nicotine intake. Nic Tobacco Res. 2003;5:695–709. doi: 10.1080/1462220031000158636. [DOI] [PubMed] [Google Scholar]
- Pomerleau CS, Pomerleau OF. The effects of a psychological stressor on cigarette smoking and subsequent behavioral and physiological responses. Psychophysiol. 1987;24:278–285. doi: 10.1111/j.1469-8986.1987.tb00295.x. [DOI] [PubMed] [Google Scholar]
- Ray R, Jepson C, Patterson F, Strasser AA, Rukstalis M, Perkins K, et al. Association of OPRM1 Asn40Asp variant with the relative reinforcing value of nicotine in female smokers. Psychopharmacol. 2006;188:355–363. doi: 10.1007/s00213-006-0504-2. [DOI] [PubMed] [Google Scholar]
- Rose JE, Ananda S, Jarvik ME. Cigarette smoking during anxiety-provoking and monotonous tasks. Addict Behav. 1983;8:353–359. doi: 10.1016/0306-4603(83)90035-7. [DOI] [PubMed] [Google Scholar]
- Schnoll RA, Johnson TA, Lerman C. Genetics and smoking behavior. Curr Psychiatr Rep. 2007;9:349–357. doi: 10.1007/s11920-007-0045-3. [DOI] [PubMed] [Google Scholar]
- Shields P, Lerman C, Audrain J, Bowman ED, Main D, Boyd NR, Caporaso NE. Dopamine D4 receptors and the risk of cigarette smoking in African-Americans and Caucasians. Cancer Epidemiol Biomarkers Prev. 1998;7:453–458. [PubMed] [Google Scholar]
- Shiffman S, Waters AJ. Negative affect and smoking lapses: a prospective analysis. J Consult Clin Psychol. 2004;72:192–201. doi: 10.1037/0022-006X.72.2.192. [DOI] [PubMed] [Google Scholar]
- Trummer O, Köppel H, Wascher TC, Grünbacher G, Gutjahr M, Stanger O, et al. The serotonin transporter gene polymorphism is not associated with smoking behavior. Pharmacogenomics J. 2006;6:397–400. doi: 10.1038/sj.tpj.6500389. [DOI] [PubMed] [Google Scholar]
- Vandenbergh DJ, Bennett CJ, Grant MD, Strasser AA, O'Connor R, Stauffer RL, et al. Smoking status and the human dopamine transporter variable number of tandem repeats (VNTR) polymorphism: failure to replicate and finding that never-smokers may be different.[see comment] Nic Tobacco Res. 2002;4:333–340. doi: 10.1080/14622200210142689. [DOI] [PubMed] [Google Scholar]
- Westman EC, Behm FM, Rose JE. Dissociating the nicotine and airway sensory effects of smoking. Pharmacol Biochem Behav. 1996;53:309–315. doi: 10.1016/0091-3057(95)02027-6. [DOI] [PubMed] [Google Scholar]
- Willner P, Jones C. Effects of mood manipulation on subjective and behavioural measures of cigarette craving. Behav Pharmacol. 1996;7:355–363. doi: 10.1097/00008877-199608000-00007. [DOI] [PubMed] [Google Scholar]
- Zhang L, Kendler KS, Chen X. The mu-opioid receptor gene and smoking initiation and nicotine dependence. Behav Brain Funct. 2006;2:28. doi: 10.1186/1744-9081-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Wang D, Johnson AD, Papp AC, Sadée W. Allelic expression imbalance of human mu opioid receptor (OPRM1) caused by variant A118G. J Biol Chem. 2005;280:32618–32624. doi: 10.1074/jbc.M504942200. Erratum in: J Biol Chem. 2005 280:38888. [DOI] [PubMed] [Google Scholar]