Abstract
Presenilin-1 (PS1) mutations cause many cases of early-onset inherited Alzheimer's disease, in part, by increasing the production of neurotoxic forms of amyloid β-peptide (A β). However, Aβ -independent effects of mutant PS1 on neuronal Ca2+ homeostasis and sensitivity to excitatory neurotransmitters have been reported. Here we show that cholinergic modulation of hippocampal synaptic plasticity is impaired in PS1 mutant knockin (PS1KI) mice. Whereas activation of muscarinic receptors enhances LTP at CA1 synapses of normal mice, it impairs LTP in PS1KI mice. Similarly, mutant PS1 impairs the ability of the cholinesterase inhibitor phenserine to enhance LTP. The NMDA current is decreased in CA1 neurons of PS1KI mice and is restored by intracellular Ca2+chelation. Similar alterations in acetylcholine and NMDA receptor-mediated components of synaptic plasticity are evident in 3×TgAD mice with PS1, amyloid precursor protein and tau mutations, suggesting that the adverse effects of mutant PS1 on synaptic plasticity can occur in the absence or presence of amyloid and tau pathologies.
Keywords: NMDA, PS1KI mice, LTP, Butyrycholinesterase, Alzheimer disease
1. Introduction
The deficits in short-term memory that typify the early stages of Alzheimer's disease (AD) are believed to result from impaired plasticity of synapses without overt structural damage to neurons (Selkoe, 2002; Coleman et al., 2004; Mattson, 2004). While the cause(s) of the most common late-onset form of AD is unknown, mutations in presenilin-1 (PS1) cause many cases of early-onset familial AD (FAD; Rademakers et al., 2003). PS1 is an integral membrane protein and the enzymatic component of the γ-secretase enzyme complex that generates Aβ; PS1 mutations increase production of the neurotoxic 42 amino acid form of Aβ (Hardy, 1997). However, FAD PS1 mutations have also been shown to perturb neuronal Ca2+ homeostasis by increasing the amount of Ca2+ in the endoplasmic reticulum resulting in enhanced Ca2+ release in response to activation of ryandodine and IP3 receptors (Guo et al., 1997; Chan et al., 2000). Wild-type PS1 functions as a Ca2+ leak channel and FAD mutations compromise this biological activity of PS1 (Tu et al., 2006). The consequences of perturbed neuronal Ca2+ homeostasis caused by PS1 mutations for synaptic plasticity are unknown.
An increase in synaptic strength called long-term potentiation (LTP) can be experimentally induced at hippocampal CA1 synapses by high frequency stimulation of afferent axons (Dineley et al., 2001). As with learning and memory, LTP involves activation of glutamate receptors of the AMPA and NMDA subtypes, and is modulated by acetylcholine acting at muscarinic receptors (Muller et al., 1988; Blitzer et al., 1990; Collingridge, 2003). LTP is mediated by Ca2+ influx though NMDA receptors and voltage-dependent Ca2+ channels and is enhanced by acetylcholine-induced Ca2+ release from IP3-sensitive ER stores (Nagase et al., 2003; Malenka and Baer, 2004; Shinoe et al., 2005; Li et al., 2007). Impaired cholinergic signaling is implicated in the early memory loss in AD and, indeed, acetylcholinesterase inhibitors improve cognition in some AD patients (Lleo et al., 2006). Interestingly, whereas LTP is impaired by Aβ oligomers in APP mutant transgenic mice (Chapman et al., 1999; Walsh et al., 2002; Klyubin et al., 2005). LTP is enhanced in PS1 mutant mice that lack amyloid pathology (Parent et al., 1999; Oddo et al., 2003), possibly as the result of enhanced Ca2+ release. Here we employ PS1 mutant knockin mice (PS1KI) and 3×TgAD mice to reveal an adverse effect of mutant PS1 on cholinergic modification of LTP, and suppression of NMDA currents which can be restored by intracellular Ca2+ chelation.
2. Materials and Methods
2.1. Animals
Male (9-12 month-old) presenilin-1 mutant (M146V) knockin mice (Guo et al., 1999) and 3×TgAD mice (Oddo et al., 2003), and wild-type mice of the same genetic background (C57BL/6) were used in this study. Mice were housed 4 per cage with continuous access to food and water, and were maintained on a 12 h light/12 h dark cycle. All procedures were approved by the National Institute on Aging Animal Care and Use Committee.
2.2. Slice preparation and electrophysiology
Hippocampal slices were prepared using procedures described previously (Wang et al., 1998, 2004b). Briefly, transverse slices of whole brain were cut at a thickness of 350 μm, and were allowed to recover for at least 1 h in a holding chamber in artificial cerebral spinal fluid (ACSF), bubbled with 95/5% (O2/CO2) at room temperature up to 6 h. Field potentials were recorded from CA1 stratum radiatum using pipettes (1-3 MΩ) filled with bubbled ACSF, placed in stratum radiatum in response to stimulation of Schaffer collateral/commissural afferents. The stimuli (30 μs duration at 0.033 Hz) were delivered through fine bipolar tungsten electrodes; a stimulation intensity was used that evoked a response that was approximately 30-40% of the maximum fEPSP, and LTP was induced by high frequency stimulation (HFS, 100Hz 1s). Whole-cell excitatory postsynaptic currents (EPSC) were recorded from CA1 pyramidal neurons. The neurons were visualized by differential interference contrast microscopy using a 40× water immersion lens. Series resistance was 10 to 15 MΩ. The patch electrode (3-5 MΩ) contained (in mM): potassium gluconate, 130; KCl, 10; EGTA, 10; CaCl2, 1; MgCl2, 3; HEPES, 20; MgATP, 5; Na-GTP, 0.5; QX 314 10; pH 7.2 (in some studies 20 mM BAPTA was included in the intracellular solution); the osmolality was 280 mmol/kg. AMPA and NMDAR currents were generated simultaneously by series voltage steps (20 mV and150 ms) from −60 mV to +60 mV. To avoid capacitance transients generated by the step effect on the current, there was a delay of 50 ms between the start of the step and pre-synaptic stimulation. Data were collected using an Axopatch 200B (EPSCs) or an Axopatch 1D (fEPSPs, Molecular Devices, Sunnyvale, CA) amplifier. Signals were filtered at 2 kHz, digitized at 10 kHz, and analyzed using pCLAMP 8 software (Molecular Devices). All recording solutions for LTP studies contained 50 μM picrotoxin to block GABAA activity. All slices were pre-selected; only those with a steep input-output curve were included in the study. During recording, slices were maintained at 30-32°C.
2.3. Reagents
(-) Phenserine(+) tartrate (PT) was synthesized as described previously (Yu et al., 1997), and prepared as a concentrated stock in ethanol:Tween 80 (1:3) which was then diluted to the final concentration in ACSF. The vehicle had no significant effect on the fEPSP or EPSC. Carbachol was prepared as a 200 mM stock in water and was diluted in ACSF before perfusion. All other reagents were obtained from Sigma Chemical Co.
3. Results
3.1. Cholinergic agonist-induced enhancement of LTP is reversed in PS1 mutant knockin mice
The M1 muscarinic agonist arbachol (50 μM) was bath applied to slices from wild-type and PS1KI mice while recording basal synaptic transmission. Carbachol induced a similar response in slices from WT and PS1KI mice characterized by a rapid depression of baseline fEPSP which was maintained for at least 30 minutes (Fig. 1A). The fEPSP amplitude rapidly returned to the baseline level upon washout of the carbachol. These findings are in agreement with previous data showing that although muscarinic agonists suppress basal synaptic transmission or induce LTD through a presynaptic M1 receptor, they do not affect LTP induction (McCutchen et al., 2006). It is possible that an elevated postsynaptic intracellular Ca2+ in response to carbachol may partially compensate for the presynaptic depression.
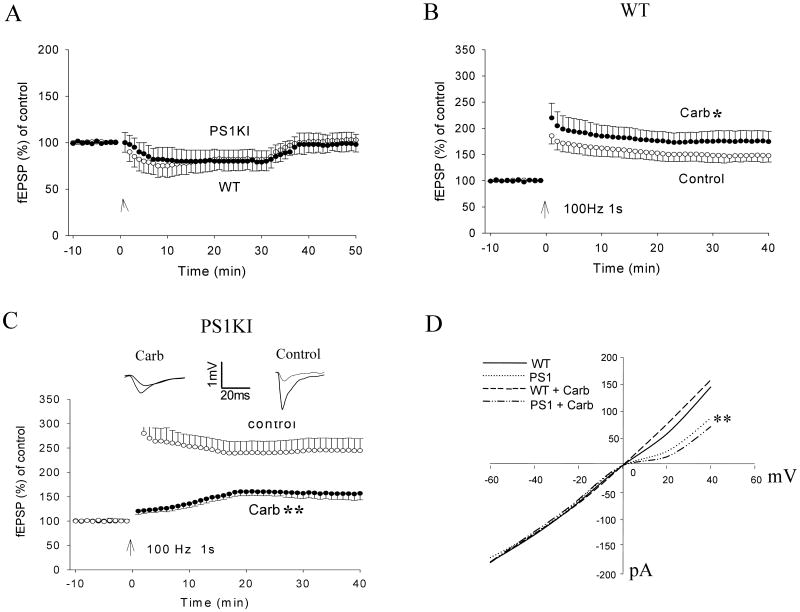
Figure 1.
Cholinergic agonist-induced enhancement of LTP is blocked and reversed in PS1 mutant knockin mice. A. Slices were perfused for 30 min with 50 μM carbachol (Carb) and wash out for 20 min. B and C. Plots of the normalized initial EPSP slope. Following a 10 minute period of baseline recording, high frequency stimulation (HFS; 100 Hz, 1 s) was applied at CA1 stratum radiatum. Slices were incubated for 60 minutes and perfused with 50 μM carbachol or vehicle (control) during the recording. In slices from wild-type (WT) mice, HFS induced a normal LTP and the LTP was significantly enhanced by carbachol. In slices from PS1KI mice, LTP was reduced in the presence of carbachol. Values are the mean and SEM (6-10 slices from 5 or 6 mice). *p< 0.05, **p< 0.01, compared to the control value. D. I-V curves of AMPA and NMDA currents generated from whole-cell patch clamp recordings in slices from WT and PS1KI mice treated with either vehicle or carbachol. Currents were generated by a series voltage steps from −60 mV to +60 mV. The NMDA current (induced at positive holding potentials) was decreased in neurons from PS1KI mice compared to WT mice, **p< 0.01.
As expected from previous studies (Blitzer et al., 1990), the amplitude of LTP at CA1 synapses in hippocampal slices from wild-type mice was increased by treatment with the M1 muscarinic agonist carbachol (Fig. 1B). The normalized field excitatory postsynaptic potential (fEPSP) slope was 175 ± 29% of baseline in carbachol-treated slices compared to 151 ± 17% in vehicle-treated control slices (P < 0.05). The amplitude of LTP was significantly greater in slices from PS1KI mice compared to wild-type mice (Fig. 1B, C) (245 ± 11%), consistent with previous reports (Parent et al., 1999; Oddo et al., 2003). However, in contrast to wild-type slices carbachol treatment caused a large and highly significant suppression of LTP in slices from PS1KI mice (Fig. 1C) (157 ± 12%) (P < 0.01).
Because NMDA and AMPA receptors are critically involved in the LTP process, we recorded NMDA and AMPA currents simultaneously using whole-cell patch clamp methods. In slices from wild-type mice, carbachol treatment enhanced the NMDA current (159 ± 16%) compared to vehicle (146 ± 17%) (Fig. 1D). In contrast, carbachol treatment decreased the NMDA current in slices from PS1KI mice (72 ± 10%) compared to vehicle (88 ± 13%). Carbachol had no significant effects on the AMPA current in slices from wild-type mice (carbachol, 179 ± 18%; vehicle, 178 ± 17%) or PS1KI mice (carbachol, 178 ± 19%; vehicle, 172 ± 16%).
3.2. Cholinesterase inhibitor impairs LTP in PS1KI mice
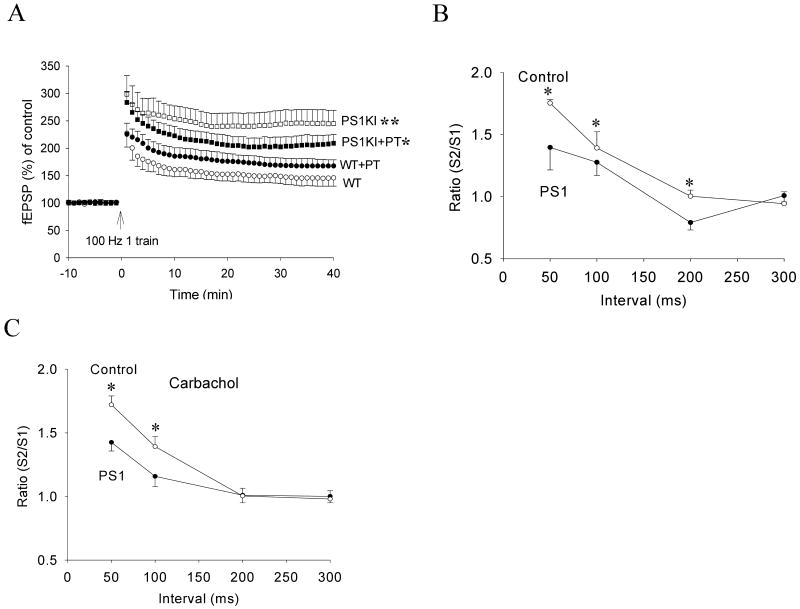
To determine whether mutant PS1 affects the modulation of hippocampal synaptic plasticity by endogenous acetylcholine, we evaluated the effects of the cholinesterase inhibitor phenserine tartrate (PT) on LTP in slices from wild-type and PS1KI mice. In slices from wild-type mice, PT treatment significantly increased the normalized fEPSP slope compared to vehicle-treated slices. In slices from PS1KI mice, the magnitude of LTP was significantly decreased in response to PT treatment (Fig. 2A). We also measured paired pulse facilitation (PPF), an indicator of release of neurotransmitter from presynaptic terminals, in order to elucidate whether mutant PS1 alters the presynaptic component of LTP. The S2/S1 ratio (with a 50 ms interpulse interval) was significantly lower at CA1 synapses in PS1KI mice compared wild-type mice (Fig. 2B, 1.4 ± 0.18 vs. 1.75 ± 0.03, n = 6 and 8), suggesting that the PS1 mutation results in a decrease in neurotransmitter release. In the presence of carbachol (50 μM), the PPF ratio was not altered in slices from either wild-type or PS1KI mice (Fig. 2C, 1.42 ± 0.07 vs. 1.72 ± 0.07, n = 6 and 12), suggesting that the effects of carbachol on synaptic plasticity impairments are likely mediated by postsynaptic mechanisms.
Figure 2.
The cholinesterase inhibitor phenserine tartrate (PT) enhances LTP in wild-type mice, but inhibits LTP in PS1KI mice. A. The normalized EPSP slopes in slices from wild-type (WT) and PS1KI mice that had been treated with 5 μM PT or vehicle. Values are the mean and SEM (6-8 slices from 5-6 mice). *p< 0.05 compared to the PS1KI value, **p< 0.01 compared to the WT value). B. Paired pulse facilitation (PPF) analysis showing the S1/S2 ratios for increasing stimulation interpulse intervals in slices from WT and PS1KI mice (8-10 slices from 5-6 mice). *p< 0.05 compared to the PS1KI value. C. PPF analysis in the presence of 50 μM carbachol.
3.3. PS1 mutation suppresses NMDA current in an intracellular Ca2+-dependent manner
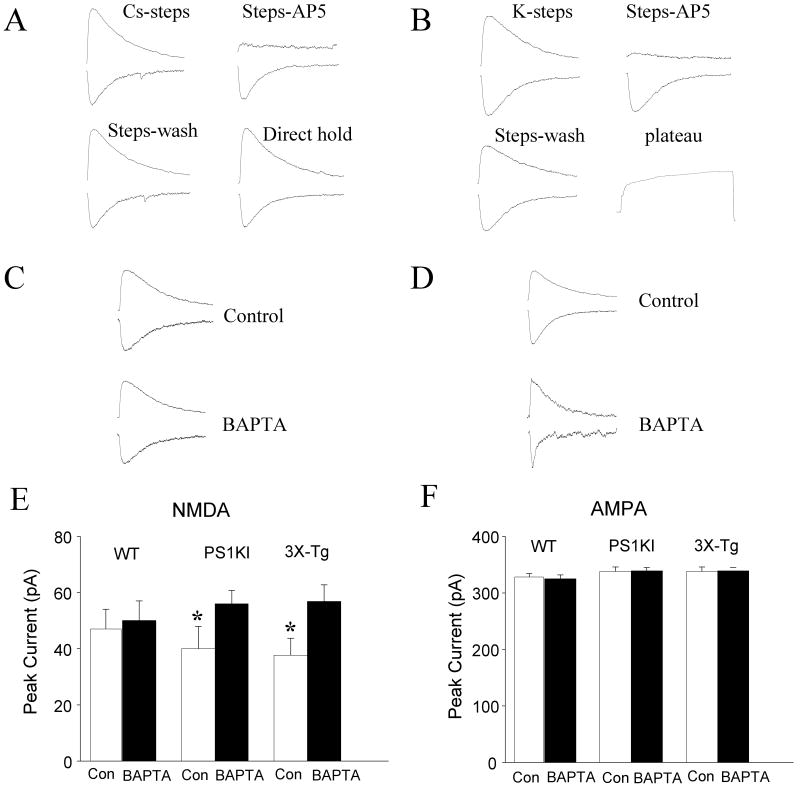
We next recorded whole-cell NMDA and AMPA currents from wild-type and PS1KI mice (Fig. 3A, B). In wild-type CA1 neurons the NMDA receptor current amplitude (measured at +40 mV using 60% of the maximum stimulation intensity) was 154 pA ± 7 pA. The AMPA receptor current amplitude (recorded at -60 mV) was 328 ± 6 pA. Perfusion of wild-type neurons with the intracellular Ca2+ chelator BAPTA had no significant effects on NMDA or AMPA current amplitudes (Fig. 3C). The NMDA current amplitude was significantly lower in neurons from PS1KI mice compared to wild-type mice. Intracellular Ca2+ chelation increased the NMDA current in neurons expressing mutant PS1 to a level as great, or greater than, that of wild-type mice. In contrast, AMPA currents were unaffected by mutant PS1 or BAPTA (Fig. 3D). Because PS1KI mice do not exhibit amyloid or tau pathologies (Guo et al., 1999), our data suggested that the abnormalities of NMDA currents and muscarinic modulation of LTP were independent of Aβ and tau. To confirm the latter conclusion we therefore measured NMDA and AMPA currents in slices from 3×TgAD mice which exhibit Aβ and tau pathology in CA1 neurons and impaired cognitive function (Oddo et al., 2003; Billings et al., 2005; Halagappa et al., 2007). Similar to neurons in PS1KI mice, neurons in 3×TgAD mice exhibited a significantly lower NMDA current compared to wild-type neurons (Fig. 3E). The AMPA current in CA1 neurons from 3×TgAD mice was not different than the AMPA current in CA1 neurons from wild-type or PS1KI mice. As in neurons of PS1KI mice, perfusion of neurons from 3×TgAD mice with BAPTA restored the NMDA current to a level similar to that of wild-type neurons (Fig. 3F).
Figure 3.
PS1 mutation suppresses NMDA current, in an intracellular Ca2+-dependent manner. A and B. Representative recordings of NMDA (A) and AMPA (B) currents in wild-type neurons in the absences or presence of the NMDA receptor antagonist AP5. C and D. Representative recordings of NMDA (C) and AMPA (D) currents in neurons perfused with a patch pipette solution lacking (Control) or containing the Ca2+ chelator BAPTA (20 mM). E and F. Graphs showing summary data for peak NMDA (E) and AMPA (F) currents, recorded without (Con) or with 20 mM BAPTA in the patch pipette in neurons from WT, PS1KI and 3×TgAD mice. Values are the mean and SEM (6-10 neurons in 5-8 slices). *p< 0.05 compared to the corresponding control value for WT mice and to the BAPTA value for mice of the same genotype.
3.4. Cholinesterase inhibitor decreases LTP at hippocampal synapses in 3×TgAD mice
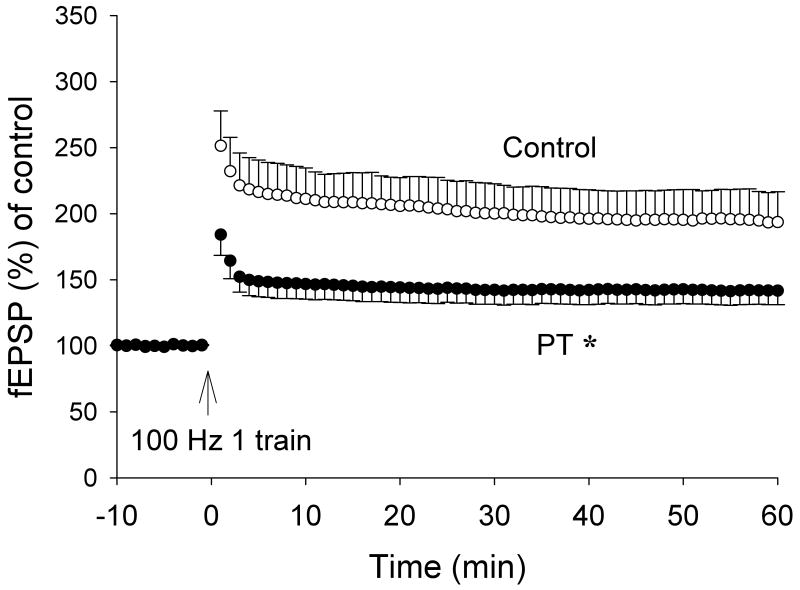
We also found that LTP was inhibited when slices from 3×TgAD mice were treated with the cholinesterase inhibitor PT (Fig. 4), an abnormality similar to that found in PS1KI mice. The amplitude of LTP was reduced by more than 50% at CA1 synapses in slices from 3×TgAD mice compared to non-transgenic control mice. The latter findings are consistent with previous data showing that neurons from PS1KI mice and 3×TgAD mice exhibit late-onset elevated input resistance and exagerated ER Ca2+ signals (IP3- and spike-evoked) relative to wild type mice (Guo et al., 1997; Chan et al., 2000; Stutzmann et al., 2006).
Figure 4.
Cholinesterase inhibition results in a decreased LTP amplitude at CA1 synapses in hippocampal slices from 3×TgAD mice. The normalized field EPSP slopes are shown for slices from 3×TgAD mice that had been treated with 5 μM PT or vehicle. Values are the mean and SEM (6-8 slices from 5-6 mice). *p< 0.01 compared to the control value.
4. Discussion
Our findings identify novel adverse effects on hippocampal synaptic plasticity of a PS1 mutation that causes early-onset FAD. Treatment of slices from wild-type mice with the muscarinic agonist carbachol or the cholinesterase inhibitor PT resulted in enhanced LTP. In contrast, carbachol and PT impaired LTP at CA1 synapses in slices from PS1KI mice. In addition, the NMDA current amplitude was significantly lower in CA1 neurons of PS1KI mice compared to wild-type mice. There is no evidence of degeneration of hippocampal neurons or basal forebrain cholinergic neurons or synapses in mice expressing PS1 mutations alone or in combination with an APP mutation (Guo et al., 1999; Oddo et al., 2003), making it unlikely that a major structural abnormality is responsible for the perturbed synaptic function caused by mutant PS1. Instead, the abnormalities in hippocampal synaptic plasticity are likely the result of the perturbed endoplasmic reticulum Ca2+ regulation previously documented in studies of cultured neurons expressing FAD PS1 mutations. Those studies showed that PS1 mutations cause Ca2+ to accumulate at higher levels in the endoplasmic reticulum resulting in an enhanced release of Ca2+ in response to muscarinic agonists (Guo et al., 1997; 1999; Begley et al., 1999; Leissring et al., 2001), as well as enhanced Ca2+-induced Ca2+ release through ryanodine receptors (Chan et al., 2000; Pack-Chung et al., 2000). Apparently, wild-type PS1 functions as a Ca2+ leak channel in the endoplasmic reticulum membrane, and FAD-linked PS1 mutations impair this Ca2+ leak function (Tu et al., 2006). Consistent with a loss-of-function mechanism, cells lacking PS1 exhibit impaired Ca2+ responses to glutamate (Yang and Cook, 2004). Considerable evidence suggests that there is an optimal level of postsynaptic Ca2+ elevation required for LTP and memory, and that a further increase in the Ca2+ level can impair LTP and memory ((Jouvenceau et al., 1999; Price et al., 1999; Tonkikh et al., 2006). Excessive Ca2+ release in response to muscarinic receptor activation could explain the impairment of LTP caused by carbachol and PT in neurons expressing mutant PS1. Excessive elevation of intracellular Ca2+ could also be responsible for reduced NMDA currents which are known to be inactivated by Ca2+ (Legendre et al., 1993; Furukawa et al., 1997). Indeed, we found that the intracellular Ca2+ chelator BAPTA restored NMDA currents in CA1 neurons of PS1KI mice.
Increasing evidence suggests that FAD PS1 mutations exert adverse effects on neuronal function that are independent of, or in addition to, altered γ-secretase activity and increased Aβ production (Guo et al., 1997, 1999; Herms et al., 2003; Shen and Kelleher, 2007). Alterations in neuronal Ca2+ regulation similar to those in PS1KI mice have been documented in neurons of 3×TgAD mice (Smith et al., 2005). We found that, as in PS1KI mice, CA1 neurons in 3×TgAD mice exhibited reductions in NMDA current that were reversed by intracellular Ca2+ chelation, as well as LTP impairment in response to cholinergic stimulation. Because CA1 neurons in 3×TgAD mice exhibit accumulation of Aβ oligomers and hyper-phosphorylated tau, whereas CA1 neurons in PS1KI mice do not (Guo et al., 1999; Oddo et al., 2003, 2006; Billings et al., 2005), our findings suggest that the adverse effects of PS1 mutations on cholinergic and NMDA-mediated synaptic plasticity are independent of Aβ and tau pathologies. Indeed, families have recently been identified in which PS1 mutations cause frontotemporal dementia [48] (Zekanowski et al., 2006) or cardiomyopathy and heart failure (Li et al., 2006), disorders that lack amyloid pathology. The mechanisms identified in the present study may explain the increased Ca2+ responses and increased afterhyperpolarization of hippocampal neurons (Barrow et al., 2000) and the defective associative learning (Wang et al., 2004a) previously documented in studies of presenilin mutant mice.
It has previously been reported that neurons expressing mutant presenilin-1 exhibit enhanced Ca2+ release from ryanodine- and IP3-sensitive ER stores, apparently as the result of an increase in the ER Ca2+ content (Guo et al., 1997; Chan et al., 2000). While some data suggest that Ca2+ release from ER can enhance LTP (Raymond and Redmond, 2002), other findings suggest the opposite. For example, mice lacking the type 3 ryanodine receptor exhibit enhanced LTP at hippocampal CA1 synapses and improved spatial learning ability (Futatsugi et al., 1999). Similarly, pharmacological inhibition of IP3 receptors enhanced LTP at CA1 synapses in guinea pig hippocampal slices (Taufiq et al., 2005). Our findings suggest that, whereas activation of muscarinic receptors enhances LTP in normal neurons, the enhanced ER Ca2+ release caused by mutant presenilin-1 may impair LTP. We found that the intracellular Ca2+ chelator BAPTA restored NMDA currents in hippocampal neurons in slices from PS1KI mice, again suggesting that the presenilin-1 mutation causes an elevation of the intracellular Ca2+ concentration above that which is optimal for LTP induction.
The first studies that revealed adverse effects of PS1 mutations on neuronal Ca2+ homeostasis did so in the context of neuronal vulnerability to excitotoxicity, and oxidative and metabolic stress in cell culture and in animal models of epilepsy and ischemic stroke (Guo et al., 1997, 1999; Mattson et al., 2000). It was shown that PS1 mutations cause excessive release of Ca2+ from endoplasmic reticulum stores when neurons are exposed to glutamate, ischemia or oxidative stress, an alteration that renders neurons more vulnerable to death induced by those insults. While PS1 mutant mice do not exhibit neuronal degeneration under normal conditions, hippocampal and cortical neurons are more prone to Ca2+-mediated degeneration when stressed. Enhanced elevations in synaptic Ca2+ responses to depolarization and oxidative stress have been documented in studies of PS1 mutant mice (Begley et al., 1999). When taken together with previous findings, the present data therefore suggest that an early and perhaps pivotal event in the pathogenic actions of PS1 mutations is a perturbation of synaptic Ca2+ regulation which first impairs synaptic function and, with advancing age, results in synaptic degeneration and neuronal death.
Acknowledgments
This work was supported by the Intramural Research Program of the National Institute on Aging, National Institutes of Health.
Footnotes
Disclosure statement: The authors certify that no conflict of interest exists.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barrow PA, Empson RM, Gladwell SJ, Anderson CM, Killick R, Yu X, Jefferys JG, Duff K. Functional phenotype in transgenic mice expressing mutant human presenilin-1. Neurobiol Dis. 2000;7:119–126. doi: 10.1006/nbdi.1999.0276. [DOI] [PubMed] [Google Scholar]
- Begley JG, Duan S, Chan W, Duff K, Mattson MP. Altered calcium homeostasis and mitochondrial dysfunction in cortical synaptic compartments of presenilin-1 mutant mice. J Neurochem. 1999;72:1030–1039. doi: 10.1046/j.1471-4159.1999.0721030.x. [DOI] [PubMed] [Google Scholar]
- Billings LM, Oddo S, Green KN, McGaugh JL, LaFerla FM. Intraneuronal Abeta causes the onset of early Alzheimer's disease-related cognitive deficits in transgenic mice. Neuron. 2005;45:675–688. doi: 10.1016/j.neuron.2005.01.040. [DOI] [PubMed] [Google Scholar]
- Blitzer RD, Gil O, Landau EM. Cholinergic stimulation enhances long-term potentiation in the CA1 region of rat hippocampus. Neurosci Lett. 1990;119:207–210. doi: 10.1016/0304-3940(90)90835-w. [DOI] [PubMed] [Google Scholar]
- Chan SL, Mayne M, Holden CP, Geiger JD, Mattson MP. Presenilin-1 mutations increase levels of ryanodine receptors and calcium release in PC12 cells and cortical neurons. J Biol Chem. 2000;275:18195–18200. doi: 10.1074/jbc.M000040200. [DOI] [PubMed] [Google Scholar]
- Chapman PF, White GL, Jones MW, Cooper-Blacketer D, Marshall VJ, Irizarry M, Younkin L, Good MA, Bliss TV, Hyman BT, Younkin SG, Hsiao KK. Impaired synaptic plasticity and learning in aged amyloid precursor protein transgenic mice. Nat Neurosci. 1999;2:271–276. doi: 10.1038/6374. [DOI] [PubMed] [Google Scholar]
- Coleman P, Federoff H, Kurlan R. A focus on the synapse for neuroprotection in Alzheimer disease and other dementias. Neurology. 2004;63:1155–1162. doi: 10.1212/01.wnl.0000140626.48118.0a. [DOI] [PubMed] [Google Scholar]
- Collingridge GL. The induction of N-methyl-D-aspartate receptor-dependent long-term potentiation. Philos Trans R Soc Lond B Biol Sci. 2003;358:635–641. doi: 10.1098/rstb.2002.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dineley KT, Weeber EJ, Atkins C, Adams JP, Anderson AE, Sweatt JD. Leitmotifs in the biochemistry of LTP induction: amplification, integration and coordination. J Neurochem. 2001;77:961–971. doi: 10.1046/j.1471-4159.2001.00321.x. [DOI] [PubMed] [Google Scholar]
- Furukawa K, Fu W, Li Y, Witke W, Kwiatkowski DJ, Mattson MP. The actin-severing protein gelsolin modulates calcium channel and NMDA receptor activities and vulnerability to excitotoxicity in hippocampal neurons. J Neurosci. 1997;17:8178–8186. doi: 10.1523/JNEUROSCI.17-21-08178.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futatsugi A, Kato K, Ogura H, Li ST, Nagata E, Kuwajima G, Tanaka K, Itohara S, Mikoshiba K. Facilitation of NMDAR-independent LTP and spatial learning in mutant mice lacking ryanodine receptor type 3. Neuron. 1999;24:701–713. doi: 10.1016/s0896-6273(00)81123-x. [DOI] [PubMed] [Google Scholar]
- Guo Q, Sopher BL, Furukawa K, Pham DG, Robinson N, Martin GM, Mattson MP. Alzheimer's presenilin mutation sensitizes neural cells to apoptosis induced by trophic factor withdrawal and amyloid beta-peptide: involvement of calcium and oxyradicals. J Neurosci. 1997;17:4212–4222. doi: 10.1523/JNEUROSCI.17-11-04212.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Q, Fu W, Sopher BL, Miller MW, Ware CB, Martin GM, Mattson MP. Increased vulnerability of hippocampal neurons to excitotoxic necrosis in presenilin-1 mutant knock-in mice. Nat Med. 1999;5:101–106. doi: 10.1038/4789. [DOI] [PubMed] [Google Scholar]
- Halagappa VK, Guo Z, Pearson M, Matsuoka Y, Cutler RG, Laferla FM, Mattson MP. Intermittent fasting and caloric restriction ameliorate age-related behavioral deficits in the triple-transgenic mouse model of Alzheimer's disease. Neurobiol Dis. 2007;26:212–220. doi: 10.1016/j.nbd.2006.12.019. [DOI] [PubMed] [Google Scholar]
- Hardy J. Amyloid, the presenilins and Alzheimer's disease. Trends Neurosci. 1997;20:154–159. doi: 10.1016/s0166-2236(96)01030-2. [DOI] [PubMed] [Google Scholar]
- Herms J, Schneider I, Dewachter I, Caluwaerts N, Kretzschmar H, Van Leuven F. Capacitive calcium entry is directly attenuated by mutant presenilin-1, independent of the expression of the amyloid precursor protein. J Biol Chem. 2003;278:2484–2489. doi: 10.1074/jbc.M206769200. [DOI] [PubMed] [Google Scholar]
- Hernandez D, Sugaya K, Qu T, McGowan E, Duff K, McKinney M. Survival and plasticity of basal forebrain cholinergic systems in mice transgenic for presenilin-1 and amyloid precursor protein mutant genes. Neuroreport. 2001;12:1377–1384. doi: 10.1097/00001756-200105250-00018. [DOI] [PubMed] [Google Scholar]
- Jouvenceau A, Potier B, Battini R, Ferrari S, Dutar P, Billard JM. Glutamatergic synaptic responses and long-term potentiation are impaired in the CA1 hippocampal area of calbindin D(28k)-deficient mice. Synapse. 1999;33:172–180. doi: 10.1002/(SICI)1098-2396(19990901)33:3<172::AID-SYN2>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Klyubin I, Walsh DM, Lemere CA, Cullen WK, Shankar GM, Betts V, Spooner ET, Jiang L, Anwyl R, Selkoe DJ, Rowan MJ. Amyloid beta protein immunotherapy neutralizes Abeta oligomers that disrupt synaptic plasticity in vivo. Nat Med. 2005;11:556–561. doi: 10.1038/nm1234. [DOI] [PubMed] [Google Scholar]
- LaFerla FM. Calcium dyshomeostasis and intracellular signalling in Alzheimer's disease. Nat Rev Neurosci. 2002;3:862–872. doi: 10.1038/nrn960. [DOI] [PubMed] [Google Scholar]
- Legendre P, Rosenmund C, Westbrook GL. Inactivation of NMDA channels in cultured hippocampal neurons by intracellular calcium. J Neurosci. 1993;13:674–684. doi: 10.1523/JNEUROSCI.13-02-00674.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leissring MA, Akbari Y, Fanger CM, Cahalan MD, Mattson MP, LaFerla FM. Capacitative calcium entry deficits and elevated luminal calcium content in mutant presenilin-1 knockin mice. J Cell Biol. 2000;149:793–798. doi: 10.1083/jcb.149.4.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Parks SB, Kushner JD, Nauman D, Burgess D, Ludwigsen S. Mutations of presenilin genes in dilated cardiomyopathy and heart failure. Am J Hum Genet. 2006;79:1030–1039. doi: 10.1086/509900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Cullen WK, Anwy R, Rowan MJ. Muscarinic acetylcholine receptor-dependent induction of persistent synaptic enhancement in rat hippocampus in vivo. Neuroscience. 2007;144:754–761. doi: 10.1016/j.neuroscience.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Lleo A, Greenberg SM, Growdon JH. Current pharmacotherapy for Alzheimer's disease. Annu Rev Med. 2006;57:513–533. doi: 10.1146/annurev.med.57.121304.131442. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Zhu H, Yu J, Kindy MS. Presenilin-1 mutation increases neuronal vulnerability to focal ischemia in vivo and to hypoxia and glucose deprivation in cell culture: involvement of perturbed calcium homeostasis. J Neurosci. 2000;20:1358–1364. doi: 10.1523/JNEUROSCI.20-04-01358.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP. Pathways towards and away from Alzheimer's disease. Nature. 2004;430:631–639. doi: 10.1038/nature02621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutchen E, Scheiderer CL, Dobrunz LE, McMahon LL. Coexistence of muscarinic long-term depression with electrically induced long-term potentiation and depression at CA3-CA1 synapses. J Neurophysiol. 2006;96:3114–3121. doi: 10.1152/jn.00144.2006. [DOI] [PubMed] [Google Scholar]
- Muller D, Joly M, Lynch G. Contributions of quisqualate and NMDA receptors to the induction and expression of LTP. Science. 1988;242:1694–1697. doi: 10.1126/science.2904701. [DOI] [PubMed] [Google Scholar]
- Nagase T, Ito KI, Kato K, Kaneko K, Kohda K, Matsumoto M. Long-term potentiation and long-term depression in hippocam pal CA1 neurons of mice lacking the IP(3) type 1 receptor. Neuroscience. 2003;117:821–830. doi: 10.1016/s0306-4522(02)00803-5. [DOI] [PubMed] [Google Scholar]
- Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R. Triple-transgenic model of Alzheimer's disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron. 2003;39:409–421. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- Oddo S, LaFerla FM. The role of nicotinic acetylcholine receptors in Alzheimer's disease. J Physiol (Paris) 2006;9:172–179. doi: 10.1016/j.jphysparis.2005.12.080. [DOI] [PubMed] [Google Scholar]
- Pack-Chung E, Meyers MB, Pettingell WP, Moir RD, Brownawelln AM, Cheng I. Presenilin 2 interacts with sorcin, a modulator of the ryanodine receptor. J Biol Chem. 2000;275:14440–14445. doi: 10.1074/jbc.m909882199. [DOI] [PubMed] [Google Scholar]
- Parent A, Linden DJ, Sisodia SS, Borchelt DR. Synaptic transmission and hippocampal long-term potentiation in transgenic mice expressing FAD-linked presenilin 1. Neurobiol Dis. 1999;1:56–62. doi: 10.1006/nbdi.1998.0207. [DOI] [PubMed] [Google Scholar]
- Price CJ, Rintoul GL, Baimbridge KG, Raymond LA. Inhibition of calcium-dependent NMDA receptor current rundown by calbindin-D28k. J Neurochem. 1999;72:634–642. doi: 10.1046/j.1471-4159.1999.0720634.x. [DOI] [PubMed] [Google Scholar]
- Rademakers R, Cruts M, Van Broeckhoven C. Genetics of early-onset Alzheimer dementia. Scientific World Journal. 2003;3:497–519. doi: 10.1100/tsw.2003.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond CR, Redman SJ. Different calcium sources are narrowly tuned to the induction of different forms of LTP. J Neurophysiol. 2002;88:249–255. doi: 10.1152/jn.2002.88.1.249. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer's disease is a synaptic failure. Science. 2002;298:789–791. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- Shen J, Kelleher RJ. The presenilin hypothesis of Alzheimer's disease: evidence for a loss-of-function pathogenic mechanism. Proc Natl Acad Sci USA. 2007;104:403–409. doi: 10.1073/pnas.0608332104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinoe T, Matsui M, Taketo MM, Manabe T. Modulation of synaptic plasticity by physiological activation of M1 muscarinic a cetylcholine receptors in the mouse hippocampus. J Neurosci. 2005;25:11194–11200. doi: 10.1523/JNEUROSCI.2338-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith IF, Hitt B, Green KN, Oddo S, LaFerla FM. Enhanced caffeine-induced Ca2+ release in the 3×Tg-AD mouse model of Alzheimer's disease. J Neurochem. 2005;9:1711–1718. doi: 10.1111/j.1471-4159.2005.03332.x. [DOI] [PubMed] [Google Scholar]
- Stutzmann GE, Smith I, Caccamo A, Oddo S, Laferla FM, Parker I. Enhanced ryanodine receptor recruitment contributes to Ca2+ disruptions in young, adult, and aged Alzheimer's disease mice. J Neurosci. 2006;26:5180–5189. doi: 10.1523/JNEUROSCI.0739-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taufiq AM, Fujii S, Yamazaki Y, Sasaki H, Kaneko K, Li J, Kato H, Mikoshiba K. Involvement of IP3 receptors in LTP and LTD induction in guinea pig hippocampal CA1 neurons. Learn Mem. 2005;12:594–600. doi: 10.1101/lm.17405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonkikh A, Janus C, El-Beheiry H, Pennefather PS, Samoilova M, McDonald P. Calcium chelation improves spatial learning and synaptic plasticity in aged rats. Exp Neurol. 2006;197:291–300. doi: 10.1016/j.expneurol.2005.06.014. [DOI] [PubMed] [Google Scholar]
- Tu H, Nelson O, Bezprozvanny A, Wang Z, Lee SF, Hao YH, Serneels L, Strooper B, Yu G, Bezprozvanny I. Presenilins form ER Ca2+ leak channels, a function disrupted by familial Alzheimer's disease-linked mutations. Cell. 2006;126:981–993. doi: 10.1016/j.cell.2006.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh DM, Klyubin I, Fadeeva JV, Cullen WK, Anwyl R, Wolfe MS, Rowan MJ, Selkoe DJ. Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2001;416:535–539. doi: 10.1038/416535a. [DOI] [PubMed] [Google Scholar]
- Wang Y, Wu J, Rowan MJ, Anwyl R. Role of protein kinase C in the induction of homosynaptic long-term depression by brief low frequency stimulation in the dentate gyrus of the rat hippocampus in vitro. J Physiol (Lond) 1998;513:467–475. doi: 10.1111/j.1469-7793.1998.467bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang KT, Dineley JD, Sweatt JD, Zheng H. Presenilin 1 familial Alzheimer's disease mutation leads to defective associative learning and impaired adult neurogenesis. Neuroscience. 2004a;126:305–312. doi: 10.1016/j.neuroscience.2004.03.048. [DOI] [PubMed] [Google Scholar]
- Wang Y, Chan SL, Miele L, Yao PJ, Mackes J, Ingram DK, Mattson MP, Furukawa K. Involvement of Notch signaling in hippocampal synaptic plasticity. Proc Natl Acad Sci USA. 2004b;101:9458–9462. doi: 10.1073/pnas.0308126101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Cook DG. Presenilin-1 deficiency impairs glutamate-evoked intracellular calcium responses in neurons. Neuroscience. 2004;124:501–505. doi: 10.1016/j.neuroscience.2003.11.029. [DOI] [PubMed] [Google Scholar]
- Yoo AS, et al. Presenilin-mediated modulation of capacitative calcium entry. Neuron. 2000;27:561–572. doi: 10.1016/s0896-6273(00)00066-0. [DOI] [PubMed] [Google Scholar]
- Yu QS, Pei XF, Holloway HW, Greig NH, Brossi A. Total syntheses and anticholinesterase activities of (3aS)-N(8)-norphysostigmine, (3aS)-N(8)-norphenserine, their antipodal isomers, and other N(8)-substituted analogues. J Med Chem. 1997;40:2895–2901. doi: 10.1021/jm970210v. [DOI] [PubMed] [Google Scholar]
- Zekanowski C, Golan MP, Krzysko KA, Lipczynska-Lojkowska W, Filipek S, Kowalska A. Two novel presenilin 1 gene mutations connected with frontotemporal dementia-like clinical phenotype: genetic and bioinformatic assessment. Exp Neurol. 2006;200:82–88. doi: 10.1016/j.expneurol.2006.01.022. [DOI] [PubMed] [Google Scholar]