Abstract
Fibrates are lipid lowering drugs and found as ligands for peroxisome proliferator-activated receptors (PPARs). A clinical study has shown that one type of fibrate gemfibrozil reduces stroke incidence in men. However, it remains unknown whether gemfibrozil improves outcome after stroke. We hypothesized that prophylactic administration of gemfibrozil improves outcome after ischemic stroke. In this study, we measured the impact of gemfibrozil in two permanent middle cerebral artery occlusion (MCAO) models in young adult male mice on normal diet. First, we tested gemfibrozil in a filamentous MCAO model. Pretreatment with gemfibrozil (30 mg/kg) for 7 days moderately but significantly reduced infarct size at 24 h after MCAO. A higher dose (120 mg/kg) did not attenuate infarct size. Rather, it tended to increase brain swelling. Second, we tested in a distal MCAO model. Gemfibrozil (30 mg/kg) for 7 days before and after stroke significantly attenuated cortical lesion size at 7 days after MCAO. Cortical blood flow measured by laser speckle imaging was improved by gemfibrozil in the ischemic hemisphere. In non-stroke animals gemfibrozil also altered gene expression levels of PPARs in both the aorta and brain in organ specific manners; however, endothelial nitric oxide synthase (eNOS) was not significantly affected. These findings suggested the possibility that the observed infarct reductions and cortical blood flow improvements in ischemic brains were not through eNOS-mediated mechanisms. Further investigations may be meritorious to examine whether prophylactic usage of gemfibrozil against stroke is beneficial.
Keywords: brain, focal ischemia, fibrate, PPAR, MCAO, mouse, aorta, eNOS
1. Introduction
Peroxisome proliferator-activated receptors (PPARs) are ligand-activated transcriptional factors belonging to the so-called nuclear receptor family (Chinetti et al., 2000). Several classes of PPARs have been identified, namely PPARα, PPARβ, and PPARγ. PPARγ consists of two isoforms, PPARγ1 and PPARγ2. All of them are involved in regulation of lipid or glucose metabolism. PPAR agonists have been demonstrated to induce pleiotropic effects in different organs, such as vessels, heart, and kidney (Chinetti et al., 2000). For example, beyond the metabolic effects, the activation of PPARα and PPARγ has been shown to exert multiple cellular functions, such as anti-inflammatory and anti-oxidative effects (Staels et al., 1998; Escher and Wahli, 2000; Von Knethen and Brune, 2001). These pleiotropic effects may be attributable to the mode of tissue protection by PPAR agonists against ischemia in several different organs (Yue et al., 2001; Sivarajah et al., 2002; Wayman et al., 2002; Cuzzocrea et al., 2004).
PPAR agonists have been shown to protect the nervous system under several disease conditions. For example, experimental studies have shown that fibrates attenuate brain tissue injury associated with inflammation (for review, Bordet et al., 2006; Heneka and Landreth, 2007). Lovett-Racke et al. (2004) demonstrated the effect of fenofibrate and gemfibrozil on clinical signs of experimental autoimmune encephalomyelitis in mice. Regarding stroke, two independent studies demonstrated that fenofibrate attenuated brain infarct formation in mice subjected to focal cerebral ischemia (Deplanque et al., 2003; Inoue et al., 2003). Inoue et al. (2003) also showed that another PPARα agonist Wy-14643 protected the brain against permanent focal cerebral ischemia in mice. Moreover, PPARγ agonists and PPARβ agonists have been shown to protect the brain against focal ischemia (Shimazu et al., 2005; Zhao et al., 2005; Iwashita et al., 2007). Thus, PPARs seem to be potential therapeutic molecular targets to predispose the brain to be more resistant against ischemic stroke.
Gemfibrozil (Fig. 1), one of PPAR agonists, has been prescribed in hyperlipidemia patients to lower the level of triglycerides. Gemfibrozil has a higher affinity to PPARα compared to PPARγ and PPARβ. This drug has been shown to decrease the incidence of stroke in men who previously had coronary heart disease (the Veterans Affairs HDL Intervention Trial (VA-HIT)) (Bloomfield Rubins et al., 2001). However, its effects on stroke outcome have not been studied. Since other PPARα agonists attenuated infarct size after permanent and transient focal cerebral ischemia in mice (Deplanque et al., 2003; Inoue et al., 2003), we hypothesized that gemfibrozil protects brain against ischemic stroke. Therefore, we investigated the effects of gemfibrozil on brain infarct formation using two mouse models of ischemic stroke, permanent middle cerebral artery occlusion (MCAO). We tested 7 days pretreatment with gemfibrozil at 30 or 120 mg/kg bw. The two doses were selected based on the following three facts: (1) the VA-HIT study (Bloomfield Rubins et al., 2001) administered gemfibrozil at 1200 mg daily; (2) the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study (Keech et al., 2005) administered fenofibrate at 200 mg daily; and (3) in our previous study, fenofibrate (30 mg/kg bw daily) was effective to significantly attenuate infarct size in young adult mice after permanent MCAO (Inoue et al., 2003).
Fig. 1.
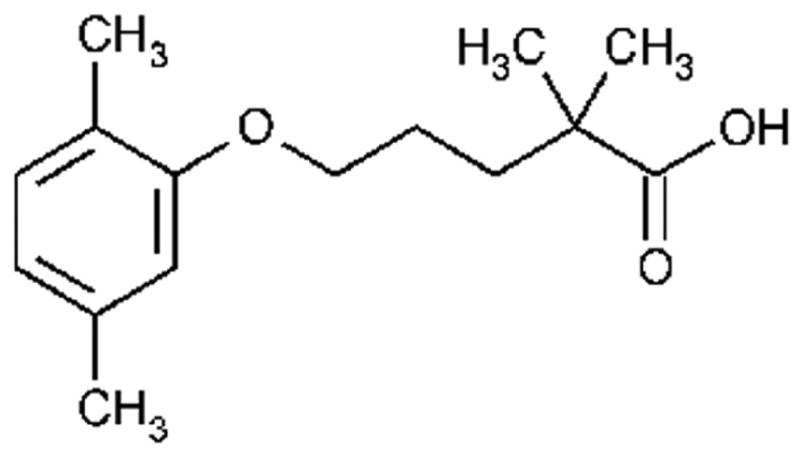
Chemical structure of gemfibrozil.
2. Results
2.1. Effects on stroke outcome after filamentous MCAO
Body temperature measured before and after filamentous MCAO did not show significant difference among the vehicle and gemfibrozil groups (Table 1). The difference of regional cerebral blood flow (rCBF) at the ischemic core area after MCAO was not statistically significant among the three groups (Table 1).
Table 1.
Regional cerebral blood flow (rCBF) and body temperature (BT)
| Gemfibrozil (mg/kg) | 0 (n=9) | 30 (n=8) | 120 (n=5) | |
|---|---|---|---|---|
| % rCBF | Before | 100 | 100 | 100 |
| After | 12.1±2.7 | 13.6±3.3 | 15.8±3.0 | |
| BT (°C) | Before | 36.9±0.2 | 37.0±0.2 | 37.0±0.2 |
| After | 37.0±0.2 | 37.0±0.2 | 37.0±0.2 | |
At 24 h after MCAO, the infarct tissue was visible as the area of pallor which was sharply demarcated from the adjacent tissue (Fig 2A). Gemfibrozil had a dose dependent effect in affecting the infarct volume (p < 0.05 by ANOVA). When the dosage of gemfibrozil was 30 mg/kg bw, the infarct volume was significantly reduced by 20% compared with vehicle (Fig 2B). The dose at 120 mg/kg did not reduce the infarct volume compared with vehicle.
Fig. 2.
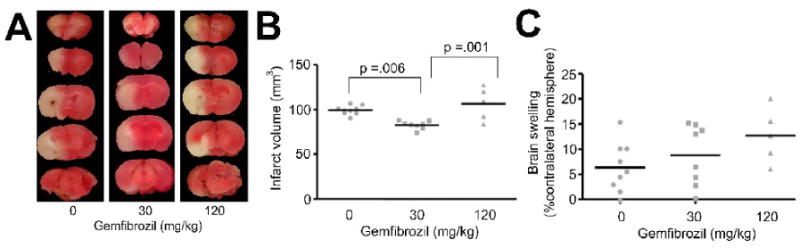
Effects of gemfibrozil on infarct formation after filamentous MCAO. A, Representative case from each treatment group showing five serial coronal brain slices stained with 2% 2,3,5-triphenyltetrazolium chloride (TTC) in saline. Animals were treated with vehicle, 30 or 120 mg/kg of gemfibrozil for 7 days and subjected to permanent MCAO. Brains were examined by TTC staining at 24 h after MCAO. Graphs showing infarct volume (B) and brain swelling (C) after 24 h permanent MCAO in mice. Gemfibrozil (30 mg/kg) significantly decreased infarct volume compared with vehicle and 120 mg/kg. Statistical analysis was made by one-way ANOVA followed by Scheffe. Bars, mean value.
Brain swelling in the vehicle group at 24 h after MCAO was 6.3±4.9% of the contralateral hemispheric volume. Gemfibrozil showed a tendency to aggravate brain swelling but the tendency was not statistically significant (Fig 2C). As the tendency of brain swelling suggested that gemfibrozil may worsen long-term outcome, we tested gemfibrozil in another model by distal MCAO combined with ipsilateral permanent carotid artery ligation, which allowed longer-term outcome measurements.
2.2. Effects on stroke outcome after distal MCAO
Based on the results from filamentous MCAO, we tested only 30 mg/kg in this distal MCAO model. In addition to the pre-stroke treatment, we continued treating until day 7 after MCAO. All animals survived for 7 days after distal MCAO in both gemfibrozil and vehicle groups. Body weight changes across MCAO did not significantly differ between the groups (Fig 3). On day 7, the infarct tissue was found mainly in the parietal cortex with minor involvements in the temporal and occipital cortices (Fig 4A, B). Many animals showed cortical atrophy. Gemfibrozil significantly reduced infarct size by 42% compared with vehicle (p < 0.001 by Mann-Whiteny) (Fig 4C).
Fig. 3.
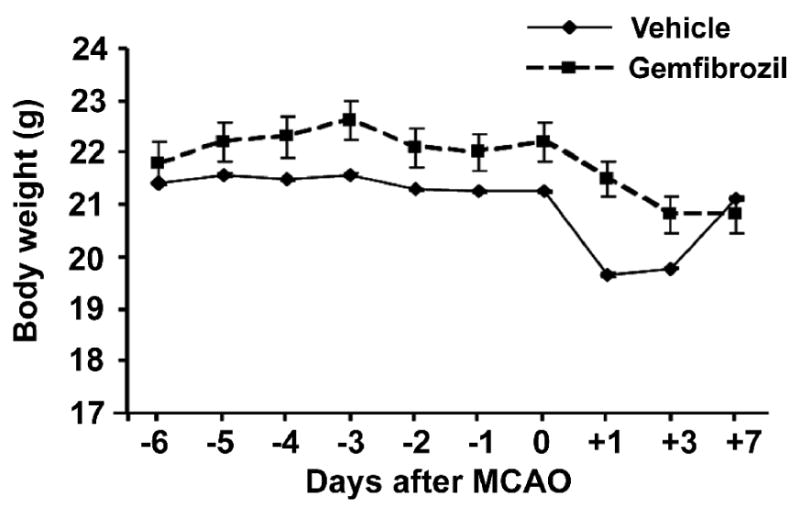
Effects of gemfibrozil on body weight. Vehicle or gemfibrozil (30 mg/kg) was administered once per day. Drug administration was started 6 days prior MCAO and continued until 7 days post MCAO. There was no significant difference between the groups. Error bars, 95% confidence interval.
Fig.4.
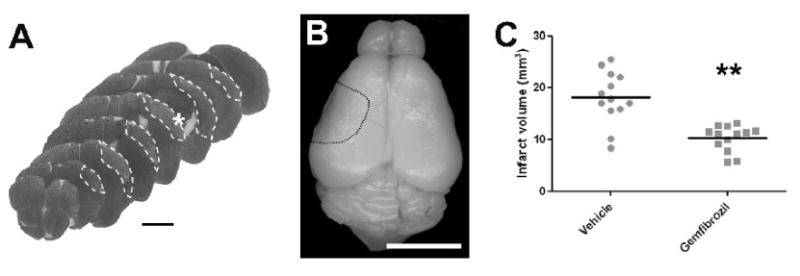
Effects of gemfibrozil (30 mg/kg) on brain tissue injury at 7 days after distal MCAO. A, Cresyl violet stained coronal brain sections from a representative animal in vehicle group. White dotted line demarcates infarct area. White asterisk indicates the point where laser Doppler probe was placed in the filamentous MCAO model. Scale bar, 2 mm. B, Macroscopic photograph showing dorsal aspect of the brain from the same animal as shown in (A). Dotted line demarcates damaged cortical tissue. Scale bar, 5 mm. C, Graph showing infarct volume. Gemfibrozil significantly decreased infarct volume compared with vehicle. **, p < 0.01 by Mann-Whiteny. Bars, mean value.
We measured cortical surface blood flow by laser speckle imaging through intact skull. In the vehicle treated animals, at 10 min after MCAO, CBF within the hemispheric cortical area between the linea temporalis and midline was reduced to 60% of the basal value (Fig 5A, B). When measurement was limited to the MCA territory, the CBF was reduced to 40% of the basal (Fig 5C). At 1 day after MCAO, these numbers were recovered to 70% and 60% of the basal, respectively (Fig 5). Gemfibrozil improved both hemispheric CBF and MCA territory CBF (p < 0.05 for both hemispheric and MCA territory by repeated measurement) (Fig 5). There was a good correlation between infarct size determined at 7 days after MCAO and CBF in the MCA territory measured at 3 h after MCAO (Fig. 6).
Fig 5.
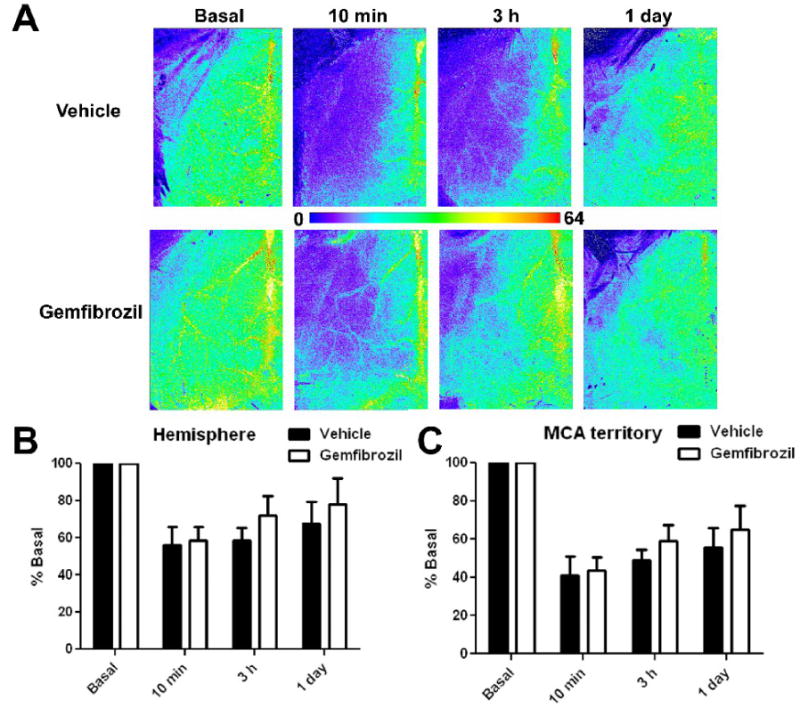
Effects of gemfibrozil (30 mg/kg) on CBF in the ischemic hemisphere after distal MCAO. A, Color coded laser speckle image taken from representative animals in vehicle and gemfibrozil groups. Ischemic hemisphere is shown. Note that the yellow vertical signal on the right edge reflects blood flow in the superior sagittal sinus. B, Graph showing %CBF in the ischemic hemisphere. C, Graph showing %CBF in the ischemic MCA territory. Error bars, SD. Vehicle and gemfibrozil groups are significantly different in both hemisphere and MCA territory(p < 0.05 by repeated measurements).
Fig. 6.
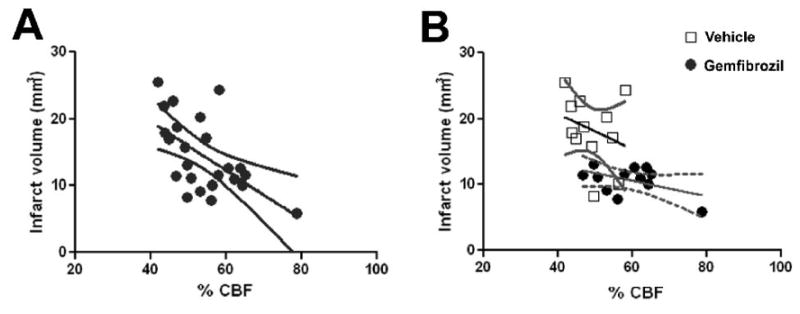
Graph showing a correlation between infarct volume at 7 days after distal MCAO and %CBF in the MCA territory measured by laser speckle imaging 3 h after distal MCAO. A, All animals from both vehicle and gemfibrozil (30 mg/kg) groups were plotted and analyzed. γ = -0.365. B, Vehicle-treated (square) and gemfibrozil-treated (circle) animals were separately plotted and analyzed. γ = −0.27 for vehicle. γ = −0.116 for gemfibrozil.
Functional outcomes were measured by neurological deficit (a modified Bederson score) (Bederson, et al., 1986), pole test, and accelerated rota-rod test. Only neurological score showed deficits after distal MCAO (Table 2); however, pole test and rota-rod test failed to detect deterioration by stroke (Fig 7). Gemfibrozil did not affect any functional outcomes up to 7 days after MCAO (Fig 7).
Table 2.
Effects of gemfibozil on neurological deficit after distal MCAO
| Gemfibrozil (n = 12) | Vehicle (n = 12) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Score | 0 | 1 | 2 | 3 | 4 | 0 | 1 | 2 | 3 | 4 |
| Time after MCAO | ||||||||||
| 3 h | 6 | 6 | 0 | 0 | 0 | 7 | 4 | 1 | 0 | 0 |
| 1 day | 10 | 2 | 0 | 0 | 0 | 8 | 4 | 0 | 0 | 0 |
| 2 day | 9 | 3 | 0 | 0 | 0 | 10 | 2 | 0 | 0 | 0 |
| 3 day | 9 | 3 | 0 | 0 | 0 | 9 | 3 | 0 | 0 | 0 |
| 7 day | 8 | 4 | 0 | 0 | 0 | 6 | 6 | 0 | 0 | 0 |
Neurological deficit was measured by a modified Bederson score; 0, normal; 1, decreased forepaw extension; 2, circling; 3, loss of postural reflex; 4, death.
Fig. 7.
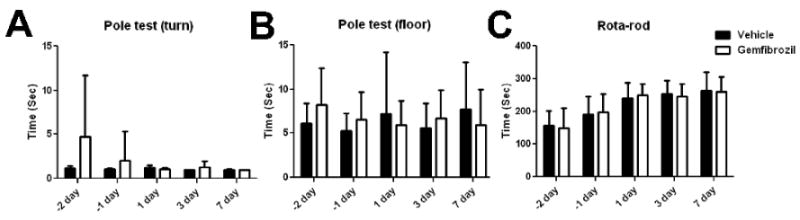
Effects of gemfibrozil (30 mg/kg) on behavior outcomes after distal MCAO. A, Time for turn in pole test. B, Time for reaching floor in pole test. C, Latency time in accelerating rota-rod test. There was no significant difference between the groups. Error bars, SD.
2.3. Effects on molecular expression in non-stroke animals
To seek insights into the molecular mechanisms of infarct reduction and CBF improvement by gemfibrozil, we measured the impact of gemfibrozil on gene expression levels of PPARs and endothelial nitric oxide synthase (eNOS) in non-stroke mice. For this purpose, animals on normal diet were treated with vehicle or gemfibrozil (30 or 120 mg/kg) for 7 days. At 1 h after the last drug administration, aorta and brain tissues were collected and analyzed.
In the aorta, mRNA levels of all PPARα, PPARβ, and PPARγ were increased by gemfibrozil (Fig 8A). In contrast, isolated brain microvessels showed decreases in PPARβ, but neither PPARα nor PPARγ was affected by gemfibrozil (Fig. 8B). The brain fraction containing neuron and glia showed reductions in all PPARα, PPARβ, and PPARγ (Fig 8C). By Western blot analysis, we confirmed protein expression of PPARα in the brain, both microvessels and neuro-glia fractions (Fig 8D).
Fig. 8.
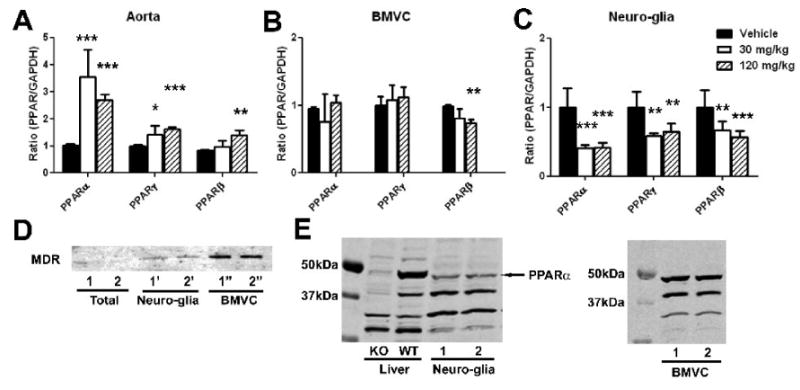
Effects of gemfibrozil on mRNA levels of PPARs in aorta (A), brain microvessels (BMVC) fraction (B), and neuro-glia fraction (C) in non-stroke mice. Mice on normal diet were treated with gemfibrozil for 7 days. mRNA levels were determined by quantitative PCR normalized by GAPDH mRNA levels in each sample. For each condition, the mean value in vehicle group was considered as 1 and relative ratio was calculated and expressed for gemfibrozil groups. *, p < 0.05; **, p < 0.01; ***, p < 0.001 by ANOVA followed by Scheffe (n = 6 or 7 in each group). D, Western blot analysis showing MDR immunoreactivity in total brain fraction, brain microvessels (BMVC), and residual fraction (Neuro-glia) containing neurons and glia. Two animals were used. E, Western blot analysis showing PPARα immunoreactivity in mouse brain. Liver tissues from PPARα knockout (KO) and wild type (WT) mouse were used to characterize the antibodies. The ∼50kDa band was weak in KO liver, suggesting that the band reflects PPARα. This band was detected in both neuro-glia fraction and brain microvessel (BMVC) fraction.
We next measured the effects on eNOS levels since another type of lipid lowering drug, statins, has been shown to improve CBF by upregulating eNOS (Endres, et al., 1998). Gemfibrozil did not significantly alter eNOS levels in both aorta and brain microvessels (Fig 9).
Fig. 9.
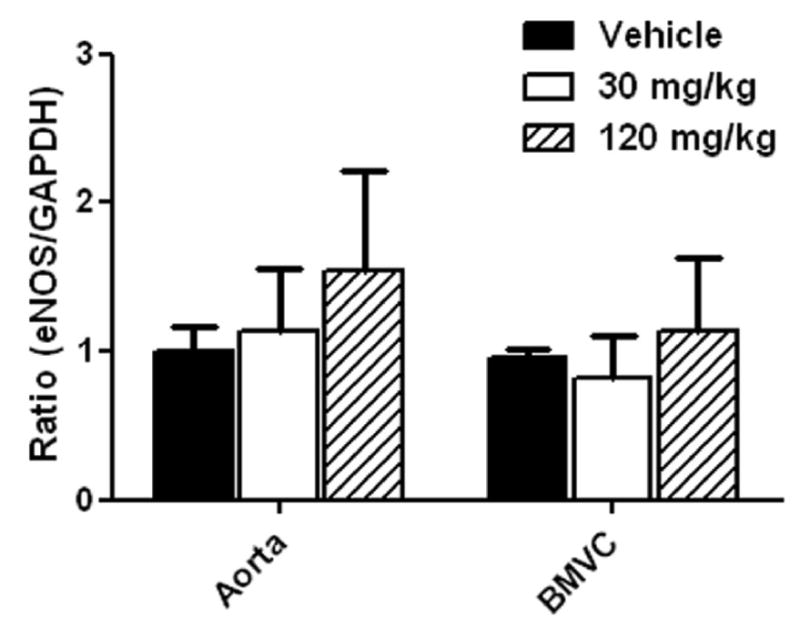
Effects of gemfibrozil on eNOS mRNA levels in aorta and brain microvessel (BMVC) fraction in non-stroke mice. Mice were treated with gemfibrozil for 7 days. eNOS mRNA levels were determined by quantitative PCR normalized by GAPDH mRNA levels in each sample. For each condition, the mean value in vehicle group was considered as 1 and relative ratio was calculated and expressed for gemfibrozil groups. There was no significant effect of gemfibrozil (n = 6 or 7 in each group).
2.4. Effects on cholesterol and triglyceride levels
The current treatment protocols with gemfibrozil in young male C57BL6 mice on normal diet did not affect total cholesterol and triglyceride levels (Table 3), suggesting that the infarct size reduction by gemfibrozil was not due to altered lipid levels.
Table 3.
Effects of gemfibozil on total cholesterol and triglycerides in non-ischemic mice
| Gemfibrozil (mg/kg) | 0 (n = 7) | 30 (n = 7) | 120 (n = 7) |
|---|---|---|---|
| Total cholesterol (mg/dL) | 67.5 ± 11.0 | 70.4 ± 16.5 | 62.3 ± 9.5 |
| Triglyceride (mg/dL) | 91.9 ± 29.4 | 77.9 ± 14.8 | 72.5 ± 25.6 |
Values are means ± SD.
3. Discussion
We demonstrated that gemfibrozil (30 mg/kg, 7 days pretreatment) improved stroke outcome (infarct volume reduction) in two mouse permanent MCAO models. In the distal MCAO model, the infarct size reduction by gemfibrozil was sustained at least for 7 days after stroke. Since gemfibrozil has been prescribed for patients as a drug to normalize the high level of triglycerides, its pharmacokinetics and safety-related adverse effects have been well characterized. Together with the previous clinical evidence that gemfibrozil reduced stroke incidence in patients (Bloomfield Rubins et al., 2001), the current findings suggest a merit of further studies to investigate the prophylactic effects of gemfibrozil on stroke outcome in different species.
One important caveat is that the current study failed to show behavioral improvement by gemfibrozil. Although statistical significance was not detected, there was a tendency of aggravation in brain swelling formation in the filamentous MCAO model. The ischemic condition in this model is too severe to follow long-term functional outcome. Therefore, we conducted the distal MCAO model and measured functional outcomes by conventional neurological score, pole test, and accelerating rota-rod test. Gerlai et al. (2000) reported significantly deteriorated performance in rota-rod test at 3 weeks after a similar ischemic model with distal MCAO in young C57BL6 mice. The study demonstrated infarct formation limited to the cortex, which was similar to our current observations. However, we did not detect impairments by ischemia alone in the rota-rod test. Royl et al. (2009) demonstrated prolonged time in pole test after 45 min filamentous MCAO in young C57BL6 mice. This model involved the striatum, which was not induced by the distal MCAO model that we used. The pole test is thought to detect striatal dysfunction (Matsuura et al., 1997). This difference in striatum involvement may explain why we did not see prolonged time by ischemia alone. In our current study, only neurological score showed very minor deficits after ischemia, which was not affected by gemfibrozil. Appropriate selections of species, ischemia model, and functional measurement will be important to future studies to detect the effects of gemfibrozil on behavioral outcome after stroke.
The current study demonstrated improved residual cortical cerebral blood flow by gemfibrozil in the ischemic hemisphere after distal MCAO (Fig. 5). Thus, the effects of gemfibrozil on the cerebral blood flow likely contribute to the brain protection in part. Moreover, additional brain protective mechanisms that are distinct from the improved cerebral blood flow were suggested. When animals showed comparable cerebral blood flow in the ischemic cortex, the gemfibrozil treated group demonstrated smaller infarct size (Fig. 6). Future studies are needed to determine the additional mechanisms.
The actual action site(s) of gemfibrozil for the in vivo brain protective efficacy still remains unknown. We here demonstrated PPARα expression in the mouse brain at the mRNA and protein levels. PPARα expression was detected in both isolated brain microvessels and the other neuro-glia fractions. Thus, gemfibrozil could possibly act directly on PPARα in the brain so that gemfibrozil exerted the protective effects against ischemia. Additionally or alternatively, the brain protection might be resulted from the action of gemfibrozil on peripheral organs that could release humoral factors, which subsequently protected the brain. Our study demonstrated significantly increased gene expressions by gemfibrozil in all of PPARα, PPARβ, and PPARγ in the aorta. The increase of PPARα by gemfibrozil (30 mg/kg) was more prominent (3.5 fold) compared with that of PPARβ and PPARγ. The findings agree with the notion that gemfibrozil is potentially bound to all PPARα, PPARβ, and PPARγ, but with a higher affinity to PPARα. In addition, the findings suggest that the gene expression of PPARs may be regulated by a positive feedback loop, at least in the aorta.
In contrast, gene expression of PPARβ was reduced by gemfibrozil in the brain microvessels. However, PPARα and PPARγ were not affected. Furthermore, all three PPARs were reduced by gemfibrozil in the neuro-glia fraction. Thus, PPARs gene expression appears to be regulated by cell type specific manners. Since enhanced infarct formation after permanent MCAO has been shown in PPARβ gene knockout mice (Arsenijevic et al., 2006), the observed reductions in PPARβ level in the brain might be attributed to the observed U-shaped dose-response of infarct size after filamentous MCAO.
Statins, inhibitors of hydroxymethylglutaryl coenzyme A reductase, and fibrates are frequently compared since they share several properties (Bloomfield, 2006). Both are lipid lowering drugs. In addition to their main actions on body energy homeostasis, both have pleiotropic actions, including anti-inflammatory and anti-oxidative actions. Both have been shown effective to prevent major cardiovascular events. The current study demonstrated a similarity and a difference between gemfibrozil and statins. We measured total cholesterol levels and triglyceride levels after 7 days treatment with gemfibrozil. Neither total cholesterol nor triglyceride was significantly decreased by the treatment protocol that was sufficient for infarct size reduction. Therefore, the brain protection by gemfibrozil was not attributed to its lipid lowering effects. Similarly, statins have been shown to protect the brain against ischemia through mechanisms independent of their lipid lowering effects (Endres et al., 1998). The difference is their effects on eNOS level. Statins have been shown to upregulate eNOS in aorta. eNOS null mice failed to show the infarct reduction by statins; therefore, CBF improvement mediated through eNOS-dependent mechanisms is believed to contribute to the brain protection against ischemia (Endres et al., 1998). In our study, gemfibrozil did not show a significant increase in eNOS mRNA levels in both of the aorta and the brain microvessels; therefore, the mechanisms of the improved CBF by gemfibrozil may be different from those by statins. Further studies are needed to determine the mechanisms.
4. Experimental procedures
4.1. Animals
Male SV129EV and C57BL6 mice (6–8 weeks old) were purchased from Taconic (Germantown, NY) and Charles River (Wilmington, MA), respectively, and housed with a 12 hour daily light/dark cycle. Food and water were provided ad libitum. All methods and procedures performed on mice conformed to the humane treatment of animals as prescribed by the Institute for Laboratory Animal Research (Guide for the Care and Use of Laboratory Animals) and were reviewed and approved by the Institutional Animal Care and Use Committee of the Morehouse School of Medicine.
4.2. Gemfibrozil treatment
Gemfibrozil was purchased from Sigma Aldrich (St. Louise, MO). Animals were randomly assigned to vehicle (5 ml/kg of 0.5% carboxymethyl cellulose) or gemfibrozil (30 and 120 mg/kg bw). Vehicle or gemfibrozil was orally given through a feeding needle once per day for 7 consecutive days. At 1 h after the last administration, the animals underwent surgery. After distal MCAO, animals were treated until post-stroke day 7.
4.3. Permanent middle cerebral artery occlusion (MCAO)
Filamentous MCAO was induced as described previously (Namura et al., 2001). Animals were anesthetized with 1.5% isofluorane in 68.5% N2O and 30% O2. Rectal temperatures were maintained at 37°C throughout the surgical procedures with a thermostat-controlled heating pad (Neuroscience, Tokyo, Japan). Changes in rCBF before and after MCAO were measured using laser-Doppler flowmetry (FLO-C1, Omegawave, Tokyo, Japan). The tip of flexible probe was affixed with glue perpendicularly to the ipsilateral skull over the area between the secondary somatosensory cortex and the rostral part of the auditory cortex (Franklin and Paxinos, 1996). For 1 h after discontinuing anesthesia, animals were kept in a recovery chamber where ambient temperature was set at 32°C. MCAO was induced on the left side with an 8-0 nylon monofilament coated with silicone resin and hardener mixture (Heraeus). The common carotid artery (CCA) and external carotid artery (ECA) were ligated with 5-0 silk suture. Then, the filament was introduced into the left internal carotid artery, advanced until the rCBF dropped below 20% of the basal.
To induce distal MCAO, the left CCA was ligated with 5-0 silk suture under isoflurane anesthesia. A midline scalp incision was made to expose the skull. The left temporal muscle was separated from the skull and a cranial window (2 mm diameter) was made on the temporal bone by drilling at the junction with zygomatic arch. After the dura was incised, the middle cerebral artery was coagulated by electrical bipolar forceps and transected with microscissors at the point below the inferior cerebral vein.
4.4 Laser speckle imaging
After distal MCAO, cerebral cortical surface blood flow changes were monitored by using a laser speckle blood flow imaging system (Omegazone, Omegawave, Tokyo, Japan). The skull surface was diffusely illuminated by semiconductor laser (780 nm) and scattered light was filtered using a hybrid filter and detected by a CCD camera positioned above the head. The hybrid filter was used to detect only scattered light that had perpendicular polarization to the incident laser light so that stable and specific measurements were achieved. The current experimental setting allowed CBF measurements on the dorsal surface of the cerebrum, such as the motor cortex and parietal cortex including the primary somatosensory cortex. However, the lateral and ventral surface of the cerebrum could not be measured, including the piriform cortex and temporal cortex and the temporal half of the secondary somatosensory cortex (Franklin and Paxinos, 1996). Raw speckle images were recorded by using a video capture card and software (Ultra Edit 2). The raw speckle images were used to compute speckle contrast, which corresponds to the number and velocity of moving red blood cells, i.e. CBF. Signal processing was done by the algorithm developed by Forrester et al. (2002). Color-code blood flow image was obtained with the high resolution mode (638 pixels × 480 pixels; 1 image/second). One blood flow image was generated by averaging numbers obtained from 20 consecutive raw speckle images. The CBF in a region of interest was obtained by using the pallet software installed in the Omegazone imaging system. CBF was measured at 10 minutes, 3 hours, and 24 hours after dMCAO. During the CBF measurement, animals were maintained under 1.5% isoflurane anesthesia and the rectal temperature was maintained at 37°C.
4.5 Infarct size measurement
After filamentous MCAO, infarct volume was measured by staining with 2% 2,3,5-triphenyltetrazolium chloride (Sigma Aldrich) at 24 h after MCAO. The images of stained brain section were analyzed by an investigator who was unaware of the treatments that the animals received, using a commercial image-analysis software program (MCID, Cambridge, UK). The total volume of each hemisphere and infarct volume were calculated by integration of the five measured sections. Brain swelling was determined with the following formula: Swelling (% contralateral hemispheric volume) = [(ipsilateral hemispheric volume) – (contralateral hemispheric volume)]/(contralateral hemispheric volume) × 100.
After distal MCAO, infarct volume was determined at 7 days after MCAO. Animals were perfused and fixed with 4% paraformaldehyde and the collected brains were treated with PBS containing 20% sucrose. The brains were cut into 20-μm-thick coronal sections with a cryostat. Sections from eight levels with 1 mm intervals were stained with cresyl violet and infarct area was measured by the MCID imaging system.
4.6 Behavior outcome
Functional outcomes were measured by neurological deficit, pole test, and accelerated rota-rod test at post MCAO day 1, day 3, and day 7. Neurological deficit was measured by a modified Bederson score; 0, normal; 1, decreased forepaw extension; 2, circling; 3, loss of postural reflex; 4, death.
The pole test was performed according to Matsuura et al. (1997). Each animal was trained for 2 days before MCAO. Animals were positioned head upward on the top of the pole. Two parameters were counted: the time until the animal turned head downwards (t turn) and the total time to descend and reach the floor. Animals were tested on 5 trials per day and the average time was used as the pole test score.
Rota-rod test (Jones and Roberts, 1968) was performed using a commercially available apparatus (Ugo Basile, Italy). Animals were trained for two before MCAO. Animals were first habituated to a stationary rod (3 cm diameter) two days prior MCAO. After habituation, the rod was started at 7 revolutions per minutes (rpm) and accelerated linearly to 42 rpm over 270 sec. The walking time until animals fall from the rod was counted automatically. Two consecutive passive rotations were considered as a fall. Each animal was tested 5 times per day. Inter-trial interval was 2 min. The average time of 5 trials in each animal was used as the rota-rod score.
4.7 Brain microvessels isolation
Mouse brain capillaries were isolated as previously reported (Ospina et al., 2002) with minor modifications. Collected fresh brains were homogenized in ice-cold PBS (pH 7.4) with a loosely fitting Dounce homogenizer, and centrifuged at 2,000 g for 5 minutes at 4°C. The supernatant was removed and stored on ice. The pellet was re-suspended in PBS and centrifuged at 2,000 g for 5 minutes at 4°C. The supernatant was combined with the first supernatant and centrifuged at 3,000 g for 10 minutes at 4°C. The resulting pellet containing the neuro-glia fraction was stored at -80°C. The pellet that was obtained after the second 2,000 g centrifugation was re-suspended in PBS, carefully layered over a 15% dextran density gradient (molecular weight 35,000 to 40,000 kDa, Sigma Aldrich), and centrifuged in a swinging-bucket rotor at 3,500 g for 35 minutes at 4°C. The supernatant was discarded, and the pellet was re-suspended in PBS, layered over dextran and centrifuged at 3,500 g for additional 35 minutes. The resulting pellet was thoroughly washed with ice-cold PBS over a 70 μm nylon mesh and the collected cerebral vessels were stored at −80°C.
4.8 Protein and mRNA analysis
The neuro-glia and microvessel fractions were further homogenized with lysis buffer using a tightly fitting homogenizer. The homogenates were centrifuged at 13000g for 20 min, and the supernatants were used for Western blot analysis. Anti-PPARα antibodies were purchased from Cayman (Ann Arbor, MI) and used at 1:1000. Monoclonal anti-MDR antibody was purchased from Covance (Princeton, NJ) and used at 1:500. To control sample amount loaded, the blots were stripped and the membranes were reproved with monoclonal anti-β action antibody (Sigma, 1:500).
RNA was isolated with Trizol (Invitrogen) according to the manufacturer's instructions. The PCR amplifications were done in 50 μL reaction volume containing 2 μL cDNA, 25 μL of iQ SYBR Green Supermix (Bio-RAD), primer, and nuclease-free water. Primer sequences for the studied genes were as the followings: eNOS, forward, 5′-gaccctcaccgctacaacat-3′ and reverse, 5′-ctggccttctgctcattttc-3′; PPARα, forward, 5′-gcagctcgtacaggtcatca-3′ and reverse, 5′- ctcttcatccccaagcgtag-3′; PPARγ, forward, 5′-ttttcaagggtgccagtttc and reverse, 5′-aatccttggccctctgagat; PPARβ, forward, 5′-tggagctcgatgacagtgac reverse, 5′- gtactggctgtcagggtggt; GAPDH, forward, 5′-aactttggcattgtggaagg-3′ and reverse, 5′- acacattgggggtaggaaca-3′. The PCR was carried out in a iCycler iQ at 50°C for 2 min, 95°C for 10 min, and 35 cycles of 95°C for 10 s, 55°C for 10 s, and 72°C for 10 s. The fold changes in gene expression of eNOS and PPARs were calculated using the comparative Ct (cross-threshold) method. Briefly, the Ct of the housekeeping gene GAPDH was subtracted from the Ct of each target molecule to get ΔCt. The ΔCt value of control group sample was then subtracted from the ΔCt of the treatments to get the ΔΔCt value. Fold differences compared to control group sample are obtained by calculating 2-ΔΔCt for each treatment group.
4.9 Total cholesterol and triglyceride measurement
Total cholesterol and triglyceride levels were respectively measured by using the Wako Cholesterol E assay kit and Wako L-Type TG M assay kit (Wako Diagnostics, Richmond, VA) according to the manufacturer's instructions.
4.9 Statistical analysis
All values are expressed as means ± SD except for neurological deficit score, body weight. SPSS 15.0 software (SPSS, Chicago, IL, USA) was used for statistical analyses. Differences among groups in infarct volume and brain edema after filamentous MCAO, and mRNA levels were analyzed with one-way ANOVA followed by Scheffe post-hoc test. Differences in body weight, LSI data, Pole test and rota-rod test among groups were analyzed by repeated measurement. The criterion for statistical significance was set at p<0.05.
Acknowledgments
Supported by NS034194 and NS048532 from NIH/NINDS. The study was conducted in a facility constructed with support from Research Facilities Improvement Grant 1 C06 RR-07571 from the National Center for Research Resources, NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arsenijevic D, de Bilbao F, Plamondon J, Paradis E, Vallet P, Richard D, Langhans W, Giannakopoulos P. Increased infarct size and lack of hyperphagic response after focal cerebral ischemia in peroxisome proliferator-activated receptor beta-deficient mice. J Cereb Blood Flow Metab. 2006;26(3):433–445. doi: 10.1038/sj.jcbfm.9600200. [DOI] [PubMed] [Google Scholar]
- Bederson JB, Pitts LH, Tsuji M, Nishimura MC, Davis RL, Bartkowski H. Rat middle cerebral artery occlusion: evaluation of the model and development of a neurologic examination. Stroke. 1986;17(3):472–476. doi: 10.1161/01.str.17.3.472. [DOI] [PubMed] [Google Scholar]
- Bloomfield HE. The role of fibrates in a statin world. Arch Intern Med. 2006;166(7):715–716. doi: 10.1001/archinte.166.7.715. [DOI] [PubMed] [Google Scholar]
- Bloomfield Rubins H, Davenport J, Babikian V, Brass LM, Collins D, Wexler L, Wagner S, Papademetriou V, Rutan G, Robins SJ, VA-HIT Study Group Reduction in stroke with gemfibrozil in men with coronary heart disease and low HDL cholesterol: The Veterans Affairs HDL Intervention Trial (VA-HIT) Circulation. 2001;103(23):2828–2833. doi: 10.1161/01.cir.103.23.2828. [DOI] [PubMed] [Google Scholar]
- Bordet R, Ouk T, Petrault O, Gele P, Gautier S, Laprais M, Deplanque D, Duriez P, Staels B, Fruchart JC, Bastide M. PPAR: a new pharmacological target for neuroprotection in stroke and neurodegenerative diseases. Biochem Soc Trans. 2006;34(Pt6):1341–1346. doi: 10.1042/BST0341341. [DOI] [PubMed] [Google Scholar]
- Chinetti G, Fruchart JC, Staels B. Peroxisome proliferator-activated receptors (PPARs): nuclear receptors at the crossroads between lipid metabolism and inflammation. Inflamm Res. 2000;49(10):497–505. doi: 10.1007/s000110050622. [DOI] [PubMed] [Google Scholar]
- Cuzzocrea S, Di Paola R, Mazzon E, Genovese T, Muià C, Caputi AP. WY 14643, a potent exogenous PPAR-alpha ligand, reduces intestinal injury associated with splanchnic artery occlusion shock. Shock. 2004;22(4):340–346. doi: 10.1097/01.shk.0000136704.26372.2d. [DOI] [PubMed] [Google Scholar]
- Deplanque D, Gele P, Petrault O, Six I, Furman C, Bouly M, Nion S, Dupuis B, Leys D, Fruchart JC, Cecchelli R, Staels B, Duriez P, Bordet R. Peroxisome proliferator-activated receptor-alpha activation as a mechanism of preventive neuroprotection induced by chronic fenofibrate treatment. J Neurosci. 2003;23(15):6264–6271. doi: 10.1523/JNEUROSCI.23-15-06264.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endres M, Laufs U, Huang Z, Nakamura T, Huang P, Moskowitz MA, Liao JK. Stroke protection by 3-hydroxy-3-methylglutaryl (HMG)-CoA reductase inhibitors mediated by endothelial nitric oxide synthase. Proc Natl Acad Sci U S A. 1998;95(15):8880–8885. doi: 10.1073/pnas.95.15.8880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escher P, Wahli W. Peroxiome proliferator-activated receptors: insight into multiple cellular functions. Mutat Res. 2000;448(2):121–138. doi: 10.1016/s0027-5107(99)00231-6. [DOI] [PubMed] [Google Scholar]
- Forrester KR, Stewart C, Tulip J, Leonard C, Bray RC. Comparison of laser speckle and laser Doppler perfusion imaging: measurement in human skin and rabbit articular tissue. Med Biol Eng Comput. 2002;40(6):687–697. doi: 10.1007/BF02345307. [DOI] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G. The Mouse Brain in Stereotaxic Coordtinates. Academic Press; London: 1996. [Google Scholar]
- Gerlai R, Thibodeaux H, Palmer JT, van Lookeren Campagne M, Van Bruggen N. Transient focal cerebral ischemia induces sensorimotor deficits in mice. Behav Brain Res. 2000;108(1):63–71. doi: 10.1016/s0166-4328(99)00130-8. [DOI] [PubMed] [Google Scholar]
- Heneka MT, Landreth GE. PPARs in the brain. Biochim Biophts Acta. 2007;1771(8):1031–1045. doi: 10.1016/j.bbalip.2007.04.016. [DOI] [PubMed] [Google Scholar]
- Inoue H, Jiang XF, Katayama T, Osada S, Umesono K, Namura S. Brain protection by resveratrol and fenofibrate against stroke requires peroxisome proliferator-activated receptor alpha in mice. Neurosci Lett. 2003;352(3):203–206. doi: 10.1016/j.neulet.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Iwashita A, Muramatsu Y, Yamazaki T, Muramoto M, Kita Y, Yamazaki S, Mihara K, Moriguchi A, Matsuoka N. Neuroprotective efficacy of the peroxisome proliferator-activated receptor delta-selective agonists in vitro and in vivo. J Pharmacol Exp Ther. 2007;320:1087–1096. doi: 10.1124/jpet.106.115758. [DOI] [PubMed] [Google Scholar]
- Jones BJ, Roberts DJ. A rotarod suitable for quantitative measurements of motor incoordination in naive mice. Naunyn Schmiedebergs Arch Exp Pathol Pharmakol. 1968;259(2):211. doi: 10.1007/BF00537801. [DOI] [PubMed] [Google Scholar]
- Keech A, Simes RJ, Barter P, Best J, Scott R, Taskinen MR, Forder P, Pillai A, Davis T, Glasziou P, Drury P, Kesäniemi YA, Sullivan D, Hunt D, Colman P, d'Emden M, Whiting M, Ehnholm C, Laakso M, FIELD study investigators Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet. 2005;366(9500):1849–1861. doi: 10.1016/S0140-6736(05)67667-2. [DOI] [PubMed] [Google Scholar]
- Lovett-Racke AE, Hussain RZ, Northrop S, Choy J, Rocchini A, Matthes L, Chavis JA, Diab A, Drew PD, Racke MK. Peroxisome proliferator-activated receptor alpha agonists as therapy for autoimmune disease. J Immunol. 2004;172(9):5790–5798. doi: 10.4049/jimmunol.172.9.5790. [DOI] [PubMed] [Google Scholar]
- Matsuura K, Kabuto H, Makino H, Ogawa N. Pole test is a useful method for evaluating the mouse movement disorder caused by striatal dopamine depletion. J Neurosci Methods. 1997;73(1):45–48. doi: 10.1016/s0165-0270(96)02211-x. [DOI] [PubMed] [Google Scholar]
- Namura S, Iihara K, Takami S, Nagata I, Kikuchi H, Matsushita K, Moskowitz MA, Bonventre JV, Alessandrini A. Intravenous administration of MEK inhibitor U0126 affords brain protection against forebrain ischemia and focal cerebral ischemia. Proc Natl Acad Sci U S A. 2001;98(20):11569–11574. doi: 10.1073/pnas.181213498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ospina JA, Krause DN, Duckles SP. 17beta-estradiol increases rat cerebrovascular prostacyclin synthesis by elevating cyclooxygenase-1 and prostacyclin synthase. Stroke. 2002;33(2):600–605. doi: 10.1161/hs0202.102732. [DOI] [PubMed] [Google Scholar]
- Royl G, Balkaya M, Lehmann S, Lehnardt S, Stohlmann K, Lindauer U, Endres M, Dirnagl U, Meisel A. Effects of the PDE5-inhibitor vardenafil in a mouse stroke model. Brain Res. 2009;1265:148–157. doi: 10.1016/j.brainres.2009.01.061. [DOI] [PubMed] [Google Scholar]
- Shimazu T, Inoue I, Araki N, Asano Y, Sawada M, Furuya D, Nagoya H, Greenberg JH. A peroxisome proliferator-activated receptor-gamma agonist reduces infarct size in transient but not in permanent ischemia. Stroke. 2005;36(2):353–359. doi: 10.1161/01.STR.0000152271.21943.a2. [DOI] [PubMed] [Google Scholar]
- Sivarajah A, Chatterjee PK, Hattori Y, Brown PA, Stewart KN, Todorovic Z, Mota-Filipe H, Thiemermann C. Agonists of peroxisome-proliferator activated receptor-alpha (clofibrate and WY14643) reduce renal ischemia/reperfusion injury in the rat. Med Sci Monit. 2002;8(12):BR532–539. [PubMed] [Google Scholar]
- Staels B, Koenig W, Habib A, Merval R, Lebret M, Torra IP, Delerive P, Fadel A, Chinetti G, Fruchart JC, Najib J, Maclouf J, Tedgui A. Activation of human aortic smooth-muscle cells is inhibited by PPARalpha but not by PPARgamma activators. Nature. 1998;393(6687):790–793. doi: 10.1038/31701. [DOI] [PubMed] [Google Scholar]
- Von Knethen AA, Brune B. Delayed activation of PPARgamma by LPS and IFN-gamma attenuates the oxidative burst in macrophages. FASEB J. 2001;15(2):535–544. doi: 10.1096/fj.00-0187com. [DOI] [PubMed] [Google Scholar]
- Wayman NS, Hattori Y, McDonald MC, Mota-Filipe H, Cuzzocrea S, Pisano B, Chatterjee PK, Thiemermann C. Ligands of the peroxisome proliferator-activated receptors (PPAR-gamma and PPAR-alpha) reduce myocardial infarct size. FASEB J. 2002;16(9):1027–1040. doi: 10.1096/fj.01-0793com. [DOI] [PubMed] [Google Scholar]
- Yue TL, Chen J, Bao W, Narayanan PK, Bril A, Jiang W, Lysko PG, Gu JL, Boyce R, Zimmerman DM, Hart TK, Buckingham RE, Ohlstein EH. In vivo myocardial protection from ischemia/reperfusion injury by the peroxisome proliferator-activated receptor-g agonist rosiglitazone. Circulation. 2001;104(21):2588–2594. doi: 10.1161/hc4601.099403. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Patzer A, Gohlke P, Herdegen T, Culman J. The intracerebral application of the PPARgamma-ligand pioglitazone confersneuroprotection against focal ischaemia in the rat brain. Eur J Neurosci. 2005;22(1):278–282. doi: 10.1111/j.1460-9568.2005.04200.x. [DOI] [PubMed] [Google Scholar]


