Abstract
We previously reported that vaccination with Freund’s adjuvant plus the recombinant N-terminus of the candidal adhesin, Als3p (rAls3p-N), protects mice from disseminated candidiasis. Here we report that the rAls3p-N vaccine is effective when combined with aluminum hydroxide adjuvant. Antibody titers of ≥1:6400 accurately predicted protection from infection. Nevertheless, neither B lymphocytes nor serum from immunized animals transferred protection to vaccine-naive animals. In contrast, CD3+, CD4+, or CD8+ T lymphocytes from immunized animals transferred protection, and the vaccine was efficacious in IL-4–deficient mice but not in IFN-γ–deficient mice. These data have significant implications for the development and interpretation of vaccine surrogate markers.
We have developed vaccines based on the Als family of adhesions from Candida albicans that protect mice against otherwise-lethal disseminated candidiasis [1– 4]. Previous work with the rAls3p-N vaccine used complete Freund’s adjuvant, which resulted in ~50% survival [1] during otherwise 100% fatal, candidal septic shock in mice [5]. Because complete Freund’s adjuvant is too toxic for use in humans, it was critical to achieve efficacy with an alternative adjuvant that is acceptable to regulatory agencies for testing in humans.
Aluminum derivatives are the only adjuvants used in vaccines that are approved for use in humans by the US Food and Drug Administration (FDA) [6]. One standard formulation of aluminum adjuvant is a gelatinous matrix of aluminum hydroxide (Alhydrogel), which minimizes lot-to-lot variability and has become a standard formulation used in FDA-approved vaccines [7, 8]. We therefore sought to identify the efficacy of rAls3p-N plus Alhydrogel. We also sought to define the rapidity of onset of protection, the role of humoral versus cell-mediated immunity in vaccine-induced protection, and the potential for antibody titers to serve as accurate surrogate markers of protection.
Methods
C. albicans SC5314 [5] was supplied by W. Fonzi (Georgetown University, Washington, DC). C. albicans 15563 was a clinical bloodstream isolate from a patient at Harbor–University of California, Los Angeles, Medical Center, which was also virulent in our murine model [3]. The organisms were serially passaged 3 times in yeast peptone dextrose broth (Difco) prior to infection. Female Balb/c retired breeder mice (>6 months old) were obtained from the National Cancer Institute (Bethesda, Maryland). For some experiments, congenic, IFN-γ deficient mice (129S-Ifngtm1Ts; Jackson Laboratories) or congenic, IL-4 deficient mice (Balb/c-Il4tm2Nnt/J) were used.
rAls3p-N (amino acids 17–432 of Als1p) and rAls5p (amino acids 20–664) were produced in Saccharomyces cerevisiae and purified by Ni+ nitrilotriacetic acid matrix affinity purification as described elsewhere [1, 9]. Mice were immunized by subcutaneous injection of 300µg of rAls3p-N or rAls5p in 0.1% Alhydrogel (Brenntag Biosector) in PBS. Control mice received adjuvant alone on the same schedule. Some mice were boosted at 21 days. Mice were infected 2 weeks after the boost or 3 weeks after the single dose. Vaccination doses were staggered so that single and dual-dose vaccinated mice were infected on the same day.
Vaccinated mice were infected via the tail vein with the appropriate inocula of C. albicans blastospores in PBS. All procedures involving mice were approved by the institutional animal use and care committee, in accordance with the National Institutes of Health guidelines for animal housing and care.
Serum antibody titers were determined by ELISA in 96-well plates coated with 5µg/mL rAls3p-N, as we have described elsewhere [2, 4]. IgG or IgG2a antibody titers were measured by use of peroxidase conjugated goat anti-mouse IgG or IgG2a secondary antibodies, respectively. The ELISA titer was taken as the reciprocal of the last serum dilution that gave a positive optical density reading (defined as an optical density value > 2 SDs above the mean for negative control samples).
Serum and splenocytes were harvested from vaccinated or control mice, as we have described elsewhere [10]. CD3+, CD4+, or CD8+ T lymphocytes, or B220+ B lymphocytes, were purified by use of the IMag system (BD Pharmingen). The purity (>95%) of the cells was confirmed by surface staining followed by flow cytometry.
Purified lymphocytes (107 per mouse for CD3+ or B220+ cells and 5 × 106 per mouse for CD4+ or CD8+ cells) were administered intravenously to congenic, unvaccinated recipient mice. Serum (0.25 mL) was administered intraperitoneally to other recipient mice. Mice were infected via the tail vein with C. albicans SC5314 either 24 h after splenocyte adoptive transfer or 3 h after serum administration. Serum doses were repeated weekly.
The nonparametric log rank test was used to determine differences in survival times. Antibody titers were compared by the Mann Whitney U test for unpaired comparisons or the Wilcoxon signed rank test for paired comparisons, as appropriate. Correlations were calculated with the Spearman rank test. P values <.05 were considered significant. To analyze test characteristics, standard Bayesian methods were used to calculate sensitivity, specificity, and accuracy [11].
Results
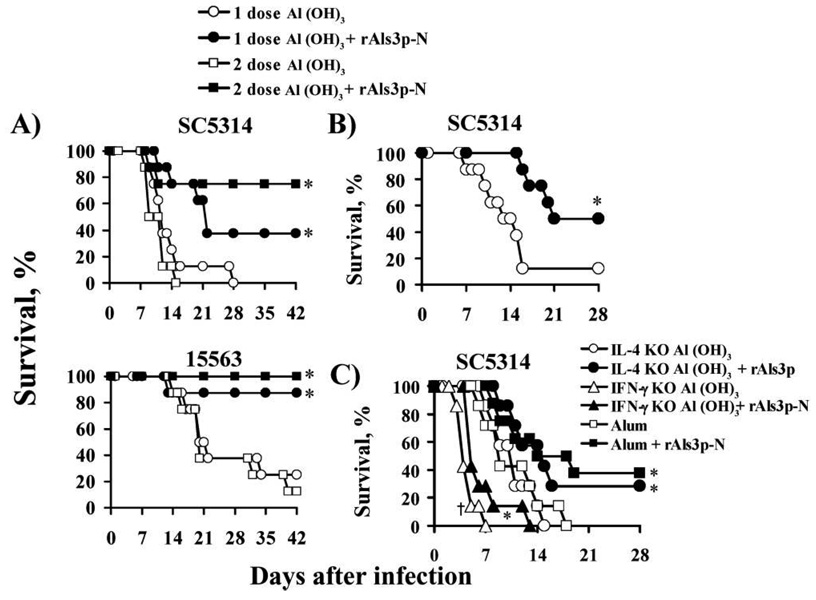
Balb/c mice were vaccinated subcutaneously with rAls3p-N (300 µg) plus aluminum hydroxide or with aluminum hydroxide alone. Control mice were also vaccinated with rAls3p-N without aluminum hydroxide, or with rAls5p plus aluminum hydroxide to determine specificity of Als vaccine protection. Vaccinated and control mice were infected via the tail vein with 1 of 2 clinical isolates of C. albicans, SC5314 or 15563. Both 1 and 2 doses of vaccine resulted in significant enhancement in survival rates, compared with aluminum hydroxide alone (figure 1A). Mice vaccinated with rAls3p-N without aluminum hydroxide or rAls5p with aluminum hydroxide adjuvant did not have significantly improved survival rates (data not shown).
Figure 1.
Survival of mice with disseminated candidiasis after vaccination with rAls3p-N plus aluminum hydroxide. A, Balb/c mice (8 per group) vaccinated with 1 or 2 doses of rAls3p-N plus aluminum hydroxide vs. aluminum hydroxide alone. Mice were infected via the tail vein with Candida albicans SC5314 (inoculum, 2.2 × 105 blastospores) or 15563 (inoculum, 5 × 105 blastospores) 3 weeks after the single dose or 2 weeks after theboost. *P < .05 vs. adjuvant control, by log rank test. B, Balb/c mice (8 per group) were vaccinated with rAls3p-N plus aluminum hydroxide or aluminum hydroxide alone. One week later, mice were infected via the tail vein with 1.8 × 105 C. albicans SC5314. *P = .01 vs. aluminum hydroxide alone, by log rank test. C, Seven Balb/c mice per group, except for the vaccinated wild-type group, which contained 8 mice. Vaccinated and control mice were infected with 2 × 105 blastospores of C. albicans SC5314. *P < .05 vs. control mice that received aluminum hydroxide; † P = .001 for IFN-γ knockout mice that received aluminum hydroxide vs. wild-type mice that received aluminum hydroxide.
To determine the rapidity of onset of protection, mice were infected via the tail vein with C. albicans only 1 week after the first dose of vaccine or aluminum hydroxide alone. Vaccinated mice had significantly improved survival rates, compared to wild-type controls (figure 1B).
Vaccination significantly increased IgG and IgG2a antibody titers at 3 weeks after the first dose (median [interquartile range {IQR}] for vaccinated vs. control mice: IgG titers, 12,800 [4884–23,150] vs. 400 [172–545], P < .001; IgG2a titers, 1,600 [898–2852] vs. 50 [46 –73], P < .001). The boost resulted in an additional increase in titers 2 weeks later (median [IQR] for vaccinated vs. control mice: IgG titers, 51,200 [27,513–117,550] vs. 565 [345–1296], P < .001 for vaccinated vs. control mice and vaccinated boost vs. vaccinated single dose mice; IgG2a titers, 18,102 [2691–29,652] vs. 100 [50 –189], P < .001 for vaccinated vs. control mice and P = .01 for booster titer vs. titer after the first dose).
The time to death of individually marked mice correlated with their total IgG (ρ =0.6; P = .0008, by Spearman rank test) and IgG2a antibody titers (ρ =0.5; P = .003). Similarly, median antibody titers in surviving mice were higher than in mice that died from infection (median IgG titers for survivors vs. nonsurvivors, 25,600 vs. 400; median IgG2a titers for survivors vs. nonsurvivors, 25,600 vs. 100; P < .001 for both comparisons). However, the difference between antibody titers in surviving versus nonsurviving mice was driven entirely by differences between vaccinated and unvaccinated mice. When only vaccinated mice were analyzed, neither IgG (ρ = 0.3; P = .2) nor IgG2a (ρ = 0.2; P = .3) antibody titers correlated with time to death, and antibody titers did not differ between surviving and nonsurviving mice (median IgG titers, 25,600 vs. 16,000; median IgG2a titers, 25,600 vs. 1,600; P = .2 for both comparisons).
Serum antibody titers from 64 individually marked, infected mice were evaluated to define the sensitivity, specificity, and overall accuracy (i.e., true-positives plus true-negatives divided by total number of data points) of various titer thresholds as predictors of protection from or susceptibility to infection. IgG or IgG2a titers ≥1:800 were highly sensitive, being present in 96% and 100% of surviving mice, respectively, but were also highly nonspecific (49% or 39% of mice that died had IgG or IgG2a titers ≥1:800), resulting in overall accuracies of 70% or 69%. In contrast, IgG and IgG2a titers of ≥1:102,400 had specificities of 100% (i.e., no mice with titers that high died from infection), but were highly insensitive (only 19% or 11% of surviving mice had IgG or IgG2a titers≥1:102,400, respectively), resulting in overall accuracies of 66% or 50%.
An IgG titer cutoff of ≥1:6400 had optimal sensitivities and specificities of 84% and 86%, respectively, resulting in an overall accuracy of 86%. Furthermore, even when only vaccinated mice were evaluated, an IgG titer threshold of≥1:6400 remained predictive of survival, with an overall accuracy of 81%.
To determine the relative importance of cell-mediated versus humoral immunity in vaccine-mediated protection, vaccine efficacy was determined in mice that were congenitally deficient in IL-4 or IFN-γ, compared with wild-type controls. Mice deficient in IFN-γ were significantly more susceptible to candidemia than wild-type mice (figure 1C); mice deficient in IL-4 demonstrated susceptibility similar to that of wild-type mice (figure 1C). Vaccination with rAls3p-N was as effective in IL-4–deficient mice as in wild-type controls. Vaccination marginally improved the survival rate of mice with IFN-γ deficiency, but vaccinated IFN-γ–deficient mice had a significantly worse survival rate than wild-type control mice.
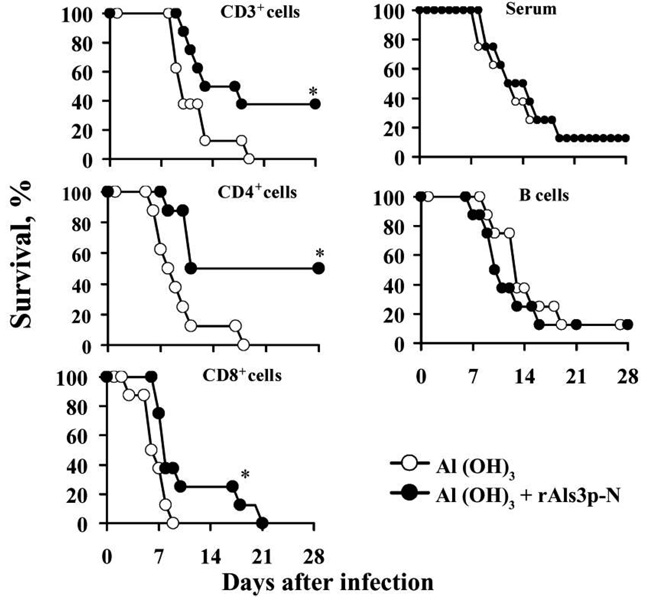
To confirm the relative roles of cell-mediated and humoral immunity, splenocyte subsets or pooled serum were adoptively transferred from vaccinated or control donor mice into congenic, unvaccinated recipient mice. Recipient mice were infected via the tail vein with C. albicans SC5314 (2 × 105 blastospores). CD3+, CD4+, or CD8+ T lymphocytes from vaccinated donors, but not from control donors, transferred protection to vaccinenaive recipient mice (figure 2). The survival rate of mice that received CD4+ splenocytes from vaccinated mice was greater than the survival rate of CD8+ splenocyte recipients (P = .03) (figure 2). In contrast, B220+ lymphocytes did not transfer protection, nor did immune serum (figure 2).
Figure 2.
Adoptive transfer of T lymphocytes, but not B lymphocytes, transferred protection against disseminated candidiasis. Eight Balb/c mice per group. Mice received 107 CD3+ or B220+ lymphocytes, 5 × 106 CD4+ or CD8+ lymphocytes, or 250 µL of serum from rAls3p-N vaccinated congenic donor mice or from control congenic donor mice that received aluminum hydroxide alone. Mice were infected with 2 × 105 blastospores of C. albicans SC5314 24 h after the adoptive transfer of lymphocytes, or 3 h after intraperitoneal administration of serum. *P < .05 vs. control mice that received aluminum hydroxide; P = .03 for vaccinated mice that received CD4+ lymphocytes vs. vaccinated mice that received CD8+ lymphocytes.
Discussion
Efficacy of the rAls3p-N vaccine with an aluminum adjuvant is a critical advance in development of the vaccine because these are the only adjuvants used in clinical vaccines that are approved by both United States and European regulatory agencies. Furthermore, we found significant protection against otherwise-lethal disseminated candidiasis as early as 1 week after vaccination. Because the mean time to onset of disseminated candidiasis is 22 days of hospitalization [12], the presence of protection by 1 week after the first dose of vaccine emphasizes the practicality of vaccinating acutely at-risk patients prior to disease onset.
One should not extrapolate on a milligrams-per-kilogram basis from the doses of recombinant protein vaccines that were effective in mice to those that would be effective in humans. Rather, the doses used in mice tend to reflect those that are effective in humans. Both a widely used FDA guideline [13] and the World Health Organization Guidelines on Non-Clinical Evaluation of Vaccines [14] indicate that the highest dose planned for testing in humans is the dose that should be used in preclinical vaccine studies. For example, the doses of hepatitis B surface antigen, as wells as the tetanus toxoid, diphtheria, and pertussis components of the Tdap vaccine, that are approved for use in humans are generally similar to those tested in preclinical rodent studies. Hence, the use of a 300 µg dose in mice does not represent a barrier to testing the vaccine in humans.
The predominant cellular effector of vaccine efficacy was CD4+ T lymphocytes. Several groups have reported the critical role of CD4+ lymphocytes in regulating phagocytic effectors against invasive candidiasis [15]. In contrast, B220+ lymphocytes did not transfer protection, nor did passive immunization with serum from vaccinated mice. These data are concordant with clinical data indicating that B cell– deficient patients are not predisposed to developing disseminated candidiasis. Finally, full vaccine efficacy required IFN-γ but not IL-4, further indicating that efficacy depended on type 1 rather than type 2 immunity.
The establishment of an accurate and facile surrogate marker is a critical step in development of a vaccine. Unfortunately, cell-mediated immune surrogate marker assays are not available clinically. We identified an antibody titer threshold that was highly accurate at predicting which mice would live or die from infection even though our vaccine did not work by inducing humoral immunity. Theses data have 2 important implications. First, the correlation of a predefined antibody titer threshold with protective immunity cannot be used to predict which form of immunity is responsible for vaccine-mediated protection. Second, even if a vaccine’s mechanism of protection is the induction of cell-mediated immunity, antibody titer thresholds may still serve as useful surrogate markers of vaccine-mediated protection during subsequent investigations.
Collectively, these data support the continued development of the rAls3p-N vaccine against disseminated candidiasis. They also highlight the potential dissociation between the immune mechanism of protection and the accuracy of immune surrogate markers of protection.
Acknowledgments
Financial support: Public Health Service grants (R01 AI19990 and AI063382 to J.E.E.; R01 AI072052 and K08 AI060641 to B.J.S.); Bristol Myers Squibb (unrestricted Freedom to Discover Grant for Infectious Disease to J.E.E.); Burroughs Wellcome (New Investigator Award in Molecular Pathogenic Mycology to A.S.I.); Los Angeles Biomedical Research Institute (Liu Young Investigator Award to B.J.S.); American Heart Association (Beginning Grant-in-Aid 0665154Y to B.J.S.).
Footnotes
Presented in part: 47th Interscience Convention on Antimicrobial Agents and Chemotherapy (ICAAC), Chicago, IL, September 2007 (abstract B-1447).
Potential conflicts of interest: B.J.S, A.S.I, Y.F, and J.E.E. own equity in NovaDigm Therapeutics, Inc., which is developing vaccine technologies. NovaDigm Therapeutics, Inc. provided no financial support for these studies.
References
- 1.Spellberg BJ, Ibrahim AS, Avanesian V, et al. Efficacy of the anti-Candida rAls3p-N or rAls1p-N vaccines against disseminated and mucosal candidiasis. J Infect Dis. 2006;194:256–260. doi: 10.1086/504691. [DOI] [PubMed] [Google Scholar]
- 2.Spellberg BJ, Ibrahim AS, Avenissian V, et al. The anti-Candida albicans vaccine composed of the recombinant N terminus of Als1p reduces fungal burden and improves survival in both immunocompetent and immunocompromised mice. Infect Immun. 2005;73:6191–6193. doi: 10.1128/IAI.73.9.6191-6193.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ibrahim AS, Spellberg BJ, Avanesian V, Fu Y, Edwards JEJ. The anti-Candida rAls1p-N vaccine is broadly active against disseminated candidiasis. Infect Immun. 2006;74:3039–3041. doi: 10.1128/IAI.74.5.3039-3041.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ibrahim AS, Spellberg BJ, Avenissian V, Fu Y, Filler SG, Edwards JE., Jr Vaccination with recombinant N-terminal domain of Als1p improves survival during murine disseminated candidiasis by enhancing cell-mediated, not humoral, immunity. Infect Immun. 2005;73:999–1005. doi: 10.1128/IAI.73.2.999-1005.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spellberg B, Ibrahim AS, Edwards JE, Jr, Filler SG. Mice with disseminated candidiasis die of progressive sepsis. J Infect Dis. 2005;192:336–343. doi: 10.1086/430952. [DOI] [PubMed] [Google Scholar]
- 6.Baylor NW, Egan W, Richman P. Aluminum salts in vaccines—US perspective. Vaccine. 2002;20 Suppl 3:S18–S23. doi: 10.1016/s0264-410x(02)00166-4. [DOI] [PubMed] [Google Scholar]
- 7.Gupta RK, Relyveld EH, Lindblad EB, Bizzini B, Ben-Efraim S, Gupta CK. Adjuvants—a balance between toxicity and adjuvanticity. Vaccine. 1993;11:293–306. doi: 10.1016/0264-410x(93)90190-9. [DOI] [PubMed] [Google Scholar]
- 8.Lindblad EB. Aluminium compounds for use in vaccines. Immunol Cell Biol. 2004;82:497–505. doi: 10.1111/j.0818-9641.2004.01286.x. [DOI] [PubMed] [Google Scholar]
- 9.Rauceo JM, De Armond R, Otoo H, et al. Threonine-rich repeats increase fibronectin binding in the Candida albicans adhesin Als5p. Eukaryot Cell. 2006;5:1664–1673. doi: 10.1128/EC.00120-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spellberg BJ, Johnston D, Phan QT, et al. Parenchymal organ, and not splenic, immunity correlates with host survival during disseminated candidiasis. Infect Immun. 2003;71:5756–5764. doi: 10.1128/IAI.71.10.5756-5764.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jaeschke R, Guyatt GH, Sackett DL. Users’ guides to the medical literature. III. How to use an article about a diagnostic test. B. What are the results and will they help me in caring for my patients? The Evidence-Based Medicine Working Group. JAMA. 1994;271:703–707. doi: 10.1001/jama.271.9.703. [DOI] [PubMed] [Google Scholar]
- 12.Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis. 2004;39:309–317. doi: 10.1086/421946. [DOI] [PubMed] [Google Scholar]
- 13.Gruber MF. US Food and Drug Administration. 2003. Non-clinical safety assessment of vaccines. Washington, DC: Center for Biological Evaluation and Research. [Google Scholar]
- 14.World Health Organization. Annex 1: guidelines on nonclinical evaluation of vaccines. [Accessed 6 February 2008];Adopted by the 54th Meeting of the World Health Organization Expert Committee on Biological Standardization. 2003 Available at: http://www.who.int/int/biologicals/publications/nonclinical_evaluation_vaccines_nov_2003.pdf.
- 15.Romani L. Innate and adaptive immunity in Candida albicans infections and saprophytism. J Leukoc Biol. 2000;68:175–179. [PubMed] [Google Scholar]