Abstract
Viruses have adapted through millennia of evolution to effectively invade and lyse cells through diverse mechanisms. Strains of the attenuated measles virus Edmonston (MV-Edm) vaccine lineage can preferentially infect and destroy cancerous cells while sparing the surrounding tissues. This specificity is predominantly due to overexpression of the measles virus receptor CD46 in tumor cells. To facilitate in vivo monitoring of viral gene expression and replication, these oncolytic strains have been engineered to either express soluble marker peptides, such as the human carcinoembryonic antigen (CEA; MV-CEA virus), or genes that facilitate imaging and therapy, such as the human thyroidal sodium iodide symporter (NIS) gene (MV-NIS). Preclinical efficacy and safety data for engineered oncolytic MV-Edm derivatives that led to their clinical translation are discussed in this review, and an overview of the early experience in three ongoing clinical trials of patients with ovarian cancer, glioblastoma multiforme and multiple myeloma is provided. The information obtained from these ongoing trials will guide the future clinical application and further development of MV strains as anticancer agents.
Keywords: Brain tumor, clinical trial, measles virus, multiple myeloma, ovarian cancer, virotherapy
Introduction
Despite significant progress in recent years, most advanced malignancies remain incurable, and there is therefore an urgent need for the development of novel therapeutics. Among them, gene-based therapeutics have considerable promise. Even though gene therapy was initially conceived as a strategy for treating monogenic diseases, its scope was eventually broadened to include the in vivo expression of foreign gene products that can cause tumor cell lysis. This antitumoral effect has been achieved through a variety of different mechanisms, including the insertion of tumor suppressor genes, the stimulation of antitumor immunity and the introduction of genes encoding enzymes that can convert prodrugs into cytotoxic drugs. Cancer gene therapy is far less affected by the technical hurdles that complicate the somatic cell treatment of inherited diseases. As a result, the gene therapy field has shifted its focus toward the treatment of malignancies and, as of 2007, > 66% of all gene therapy clinical trials have investigated the treatment of cancer [1]. However, an important lesson learned from the first generation of clinical trials pertains to the realization of the limitations of non-replicating vectors, including their limited ability to transfer the gene of interest into the majority of tumor cells, which negatively impacts on their therapeutic efficacy [2]. Oncolytic viruses offer certain advantages over non-replicating vectors. Viruses have adapted through millennia of evolution to effectively invade, replicate in, and kill cells through diverse mechanisms. They excel at usurping the cellular biosynthetic pathways to produce viral progenies and spread to neighboring cells, thus augmenting their antitumor effect.
It is of note that a number of viral strains, including certain derivatives of the attenuated live measles virus Edmonston (MV-Edm) vaccine strain, demonstrate a propensity to preferentially infect, propagate in, and destroy cancerous tissues. On this basis, a large number of research groups have tested the ability of replicative viral agents to, directly or indirectly, induce targeted tumor cell death, and such agents have been translated into virotherapy clinical trials [2]. This review summarizes the rationale and preclinical research that led to the first clinical trials of engineered oncolytic measles virus (MV) strains in the treatment of cancer and describes the experimental approach used in these studies.
History of oncolytic measles virotherapy
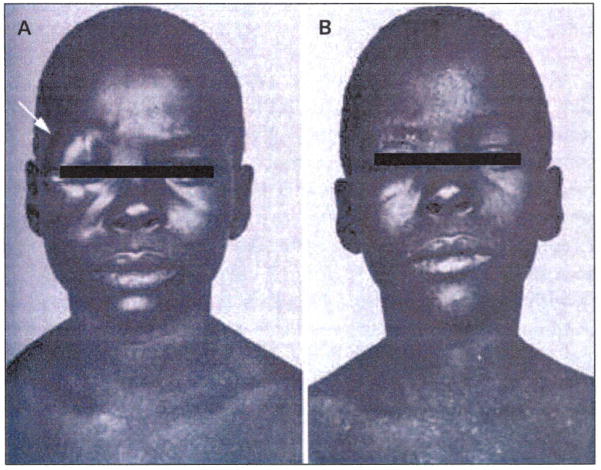
Over the past century, there has been a slow yet steady flow of clinical publications documenting remissions, usually in patients with hematological malignancies, following natural viral infections or vaccinations [2,3]. Historically, evidence of measles oncolytic activity was first provided by several case reports in the mid-twentieth century that documented the spontaneous regression of hematological cancers (ie, leukemias, Hodgkin’s disease and Burkitt’s lymphoma) after wild-type MV infection (Figure 1) [4–9].
Figure 1. Burkitt’s lymphoma regression following measles virus infection.
(A) An eight-year-old African boy with painless right orbital swelling due to Burkitt’s lymphoma. (B) The appearance of generalized measles exanthema coincided with complete tumor regression. The patient remained in complete remission for > 4 months after the measles infection.
(Reproduced with permission from Elsevier Ltd and Bluming AZ, Ziegler: Regression of Burkitt's lymphoma in association with measles infection. Lancet (1971) 2(7715):105-106 © 1971 Elsevier Ltd).
The initial enthusiasm for virotherapy as a therapeutic approach in cancer treatment, however, diminished in later years until its recent resurgence. Possible reasons for this include concerns regarding the potential of wild-type viruses to cause serious side effects [10], technical limitations in manufacturing viral lots of high purity for clinical use, as well as the overwhelming excitement and fervent support for the, at the time, newly emerging chemotherapy approaches that slowed research on alternative treatment strategies. Consequently, only during the last decade have clinicians begun to re-evaluate oncolytic virotherapy and exploit its largely untapped potential. The evolution of molecular biology techniques has facilitated this resurgence. As it pertains to MV, the expanding knowledge of MV biology, as well as the development of a reverse genetics system that allows rescue of recombinant MV strains and viral engineering, has significantly contributed to the recent therapeutic application of MV in cancer treatment [11,12].
Advantages of measles virus strains as cancer therapeutics
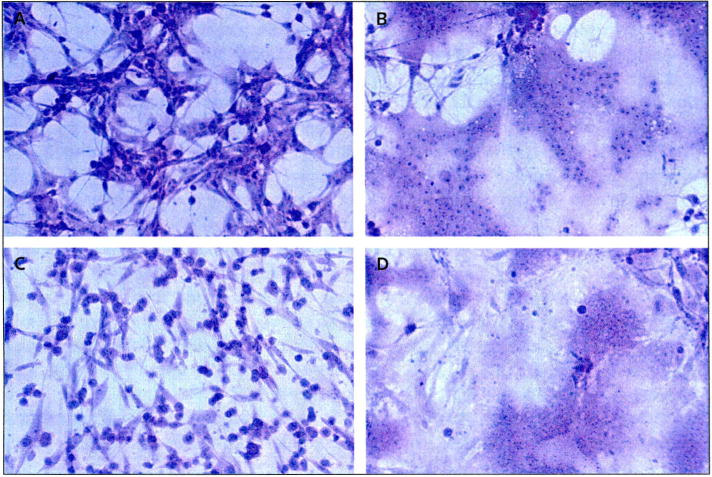
MV is a negative-strand RNA paramyxovirus of the genus Morbillivirus that is the causative agent of the common, acute, exanthemous, infectious measles disease. The MV genome contains six genes that encode eight proteins: the nucleocapsid (N), phospho- (P), matrix (M), fusion (F), hemagglutinin (H) and large (L) proteins, as well as two accessory proteins, C and V. The virus enters the target cells via pH-independent membrane fusion. Receptor binding and membrane fusion are mediated by the H and F proteins, respectively [13]. More specifically, MV enters the cells via the interaction of the surface H glycoprotein with the two known MV receptors: CD46, which is ubiquitously present on nucleated primate cells but is overexpressed in tumors [14,15], and the signaling lymphocyte-activation molecule (SLAM), which is primarily located on B- and T-lymphocytes [16]. CD46 is a membrane-associated complement regulatory protein that protects human cells against autologous complement lysis by acting as a cofactor in the proteolytic inactivation of C3b and C4b complement products. CD46 is frequently overexpressed in tumor cells, serving as a mechanism of tumor cell protection against complement-mediated lysis [17,18]. Receptor recognition by the H protein leads to conformational changes of the F protein that trigger fusion and viral entry. Infected cells, including tumor cells, express the viral F and H proteins on the cell surface. Recognition of the viral receptor in neighboring infected or uninfected cells similarly triggers cell to cell fusion. Therefore, the typical cytopathic effect of MV is the formation of giant mononuclear cell aggregates (syncytia) (Figure 2) [19].
Figure 2. Prominent syncytia formation of glioma cell lines following MV-CEA infection.
Cells were stained using crystal violet stain (× 200 magnification). The human malignant glioma cell lines U87 and U118 were infected with measles virus (MV) expressing human carcinoembryonic antigen (CEA; MV-CEA virus), resulting in the characteristic cytopathic effect, including extensive syncytia formation. (A) Uninfected U87 cells, (B) U87 cells 120 h after infection with MV-CEA (multiplicity of infection = 0.05), (C) uninfected U118 cells, (D) U118 cells 120 h after infection with MV-CEA (multiplicity of infection = 0.05).
In contrast to non-replicating or non-viral vector systems, MV-Edm is a replicating virus that offers the advantage of increased tumor dissemination, resulting in potentially enhanced therapeutic benefit. An important issue to address when replicating viral vectors are used as cancer therapeutics relates to their potential for virulence and toxicity. The MV vaccine strain was isolated in 1954 from the throat washings of an 11-year-old measles patient named David Edmonston and was attenuated by serial tissue culture passages [20,21]. Although the wild-type MV can result in potentially serious infectious disease, the MV strains have an excellent safety record, with millions of vaccine doses having been safely administered in > 40 years of use, while reversion of the vaccine strain to pathogenicity has never been reported [21,22].
Another safety consideration pertains to the possible spread of the oncolytic virus from treated patients to other individuals. The hypothetical scenario whereby genetically re-engineered viruses not normally found in nature would spread in the general population would be undesirable. This represents less of a concern for engineered MV-Edm derivatives because most individuals in industrialized countries are immune to the virus as a result of natural infection or vaccination. Of note, human to human MV vaccine transmission has never been confirmed [21]. In contrast to the wild-type MV, which enters cells more efficiently via the SLAM receptor, the attenuated MV-Edm enters cells more efficiently via the CD46 receptor and kills cells that overexpress this receptor, without significant cytopathic effect against non-transformed cells expressing low receptor levels [23]. Consistent with the above mechanism, MV-Edm derivatives are tumor-selective and cause minimal cytopathic effect in non-transformed cells, including normal ovarian surface epithelial cells, astrocytes, mesothelial cells, hepatocytes, peripheral blood lymphocytes and coronary artery smooth muscle cells [23–26].
The poor delivery efficiency of standard viral vectors has strengthened the requirement of potent bystander killing effects capable of destroying those tumor cells that the virus cannot reach. MV-Edm and its engineered derivatives, which include the human carcinoembryonic antigen (CEA; MV-CEA) gene and the human thyroidal sodium iodide symporter (NIS; MV-NIS virus) gene, produce a considerable local bystander effect mediated by massive cell-cell fusion [25,27–30]. For example, in the glioblastoma multiforme cell line U87, transfection of the measles fusogenic membrane glycoproteins F and H in a single U87 cell can kill 40 to 80 bystander (untransfected) cells [30]. Therefore, these derivatives can achieve significant neoplastic tissue destruction without infecting all tumor cells. It must also be noted that MV-Edm retains its considerable oncolytic activity when it is genetically modified [25,27,28,31,32] and can be effectively retargeted [11].
Measles virus derivatives: Efficacy and safety in preclinical models
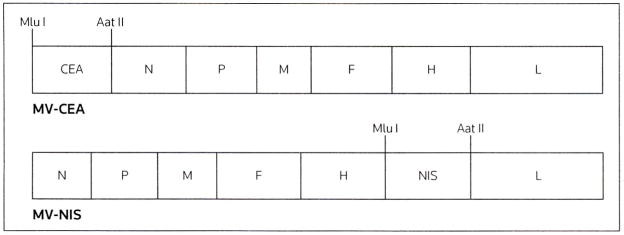
A major challenge in clinical cancer virotherapy efforts is the lack of convenient methods to monitor the in vivo spread and elimination of the virus, as well as to measure the profile of viral gene expression and kinetics over time. Knowledge of these parameters is important when deciding on the dose size intervals between repeat treatment cycles and could facilitate the tailoring of individualized treatment protocols. The MV-Edm vector has been effectively engineered to express soluble marker peptides, such as the CEA and β-human chorionic gonadotrophin [29,32]. Important characteristics of a clinically applicable marker peptide include lack of known biological activity, constant circulation half-life, limited immunogenicity, and the existence of a clinically validated assay that allows measurement. The replication-competent MV-CEA virus was engineered to express the soluble extracellular N-terminal domain of human CEA as an additional transcription unit before the viral N gene (Figure 3) [32]. Viral replication and gene expression following infection of the target cells results in CEA production. Measurement of this inert marker peptide in the serum could therefore provide important feedback on viral gene expression kinetics.
Figure 3. A schematic representation of MV-CEA and MV-NIS.
The measles virus (MV) strain producing the human carcinoembryonic antigen (CEA; MV-CEA) was constructed with insertion of the sequence corresponding to the soluble extracellular N-terminal domain of human CEA upstream of the viral nucleocapsid (N) gene. The MV strain containing the human thyroidal sodium iodide symporter gene (NIS; MV-NIS) shares a similar vector backbone with MV-CEA except that the NIS cDNA was inserted downstream of the viral hemagglutinin (H) gene. Aat II Aat II restriction site, F viral fusion gene, L viral polymerase gene, M viral matrix gene, Mlu I an Mlu I restriction site, P viral phosphoprotein gene.
(Reproduced with permission from the American Association for Cancer Research and Hasegawa K, Pham L, O'Connor MK, Federspiel MJ, Russell SJ, Peng K-W: Dual therapy of ovarian cancer using measles viruses expressing carcinoembryonic antigen and sodium iodide symporter. Clin Cancer (2006) 12(6):1868-1875 © 2006 American Association for Cancer Research).
While CEA expression can provide the opportunity of convenient real-time monitoring of viral gene expression, it cannot address questions pertaining to the localization and spread of the oncolytic virus. To address this issue, a recombinant oncolytic MV (MV-NIS) was designed to express the NIS gene. NIS is a membrane ion channel that can concentrate radioisotopes inside cells [33,34]. Isotope trapping can then be non-invasively detected by γ camera, PET or SPECT/CT by using radionuclide isotopes, such as 123[I], 124[I] or 99M[Tc], as tracers. Therefore, NIS can act as a reporter gene that enables the non-invasive tracking of viral localization, spread, gene expression and replication over time. An additional benefit is that NIS may also be used as a therapeutic transgene by allowing intracellular uptake of isotopes, such as 131[I], which can cause direct radiation damage to tumor cells, thereby enhancing the therapeutic efficacy of MV-NIS (radiovirotherapy). Preclinical data suggest the potential added benefits of a radiovirotherapy approach against radiosensitive tumors, such as multiple myeloma [27,34]. The MV-NIS shares a similar vector backbone with MV-CEA, except that the NIS cDNA was subcloned downstream of the H protein (Figure 3).
MV-CEA
The therapeutic potential of these MV-Edm derivatives against a variety of solid tumors and hematological malignancies, including ovarian cancer [32,35], glioblastoma multiforme [25], breast cancer [36], multiple myeloma [27,28], hepatocellular carcinoma [26] and prostate cancer [37], has been demonstrated. Ovarian cancer and glioblastoma multiforme were chosen as targets for two of the first clinical applications of the virus because of the tropism of recurrent disease in the majority of patients (peritoneal cavity and CNS, respectively), which justifies targeted delivery and decreases the impact of the immune system on viral efficacy. MV-CEA has demonstrated considerable preclinical efficacy against ovarian cancer and glioblastoma multiforme. The virus has been evaluated in vitro against a variety of glioma and ovarian cancer cell lines, both established (including the U87, U118 and U251 glioma cell lines, as well as the SKOv3ip.1 and OVCAR3 ovarian cancer cell lines) and primary cell cultures, with significant cytopathic effect being observed in all tumor cell lines tested (0 to 20% of cells were viable at day 7 post-infection at a multiplicity of infection of 0.5 to 1) [25,32]. This potent antitumor efficacy is predominantly associated with apoptotic cell death [25,36]. One step viral growth curves demonstrated significant growth of MV-CEA in tumor cells, and the virally encoded CEA levels in culture supernatants correlated well with viral replication/gene expression [25,32]. Furthermore, MV-CEA demonstrated potent in vivo therapeutic efficacy in murine subcutaneous, orthotopic, intracranial (gliomas) and intraperitoneal (ovarian cancer) xenograft models [25,32]. In the orthotopic glioma model, approximately 60% of the MV-CEA-treated mice remained tumor-free for at least 80 days after tumor engraftment while none of the untreated mice survived by this time point [25]. Furthermore, in the orthotopic ovarian cancer model, the MV-CEA-treated group demonstrated prolonged survival by 250% compared with the control group (mice treated with ultraviolet-inactivated MV-CEA) [32]. The MV-CEA-infected tumors released CEA into the murine circulation, resulting in increased serum concentrations of the marker (> 5 ng/ml) compared with uninfected controls and corresponding to the xenograft tumor burden [25,32,38].
In contrast to ovarian cancer and gliomas, most human malignancies present as systemic disease. In this context, pre-existing immunity against an oncolytic virus can decrease antitumor activity if the oncolytic agent is administered systemically. Multiple myeloma patients are significantly immunocompromised and their humoral immunity against viruses, including measles, is impaired. In preclinical research, MV-NIS has demonstrated potent oncolytic activity against myeloma cell lines, as well as primary myeloma cells [27]. Infected multiple myeloma cells take up radioiodide in vitro [27]. In vivo, the sites of gene expression can be mapped by radioiodide-based γ camera, SPECT or PET imaging. These images allow the quantification of relative radioiodide uptake at the predominant sites of viral gene expression. Increased radioiodide uptake reflects MV-NIS replication and NIS expression by tumor cells [27]. Furthermore, therapeutic administration of 131[I] following treatment with MV-NIS resulted in enhanced tumor regression compared with tumors treated with MV-NIS alone in multiple myeloma xenografts, including MV-resistant lines [27]. Xenografts of the multiple myeloma cell line MM1 did not respond to MV-NIS infection alone, but the combination of MV-NIS with 131[I] resulted in complete tumor regression in all mice tested [27].
Toxicology and biodistribution studies were conducted prior to clinical application of engineered MV strains in the treatment of cancer [39–43]. Important considerations when designing these studies include the choice of the appropriate animal model, designing a study that mimics the proposed clinical trial as it pertains to the route of administration and the proposed dosing scheme, and testing viral doses that are several times higher than the highest dose proposed for the clinical trials to ensure safety. The murine xenograft models used in the efficacy studies described earlier have significant limitations with regard to toxicity assessment. Rodents do not normally express CD46 and SLAM. Therefore, MV does not infect murine cells and such models cannot be used for toxicity assessment of measles-based therapeutics. The IFN type I receptor deficient (IFNARKO) CD46 Ge mouse strain, a transgenic model that is permissive to MV infection, has been developed and can be used for toxicity assessment of oncolytic MV-Edm strains [44–47]. These IFNα and IFNβ receptor knockout mice express human CD46 with tissue distribution and levels of expression that parallel human CD46 expression [47]. MV-Edm inoculation via the intranasal route causes acute lung inflammation and diffuse lung hemorrhage [44]. IFNARKO CD46 Ge mice have been accepted by the FDA as a model of measles toxicity and were used in toxicology studies prior to initiation of the three ongoing clinical trials of engineered MV strains.
Ovarian cancer typically spreads into, and remains confined in, the peritoneal cavity. Therefore, intraperitoneal MV-CEA administration should maximize viral infection of the tumor cells. Conceptually, there is a potential danger of the virus infecting non-transformed cells in the peritoneal cavity or spreading beyond the peritoneal cavity and throughout the body via the bloodstream. Toxicology studies were performed in measles-naive IFNARKO CD46 Ge mice. There was no evidence of viral replication outside the peritoneal cavity (ie, the brain, heart and skeletal muscle) and no signs of clinical toxicity following repeat administration, even at doses equivalent to 35-fold the maximum human dose in the proposed clinical protocol [39].
Prior to the phase I clinical trial of MV-CEA administered to the tumor and resection cavity in patients with recurrent glioblastoma multiforme, toxicology studies were performed in two animal models: IFNARKO CD46 Ge mice and Rhesus macaques. The transgenic IFNARKO CD46 Ge mice represent a very sensitive model used for the assessment of measles neurotoxicity [44–47], and CNS inoculation with MV-Edm strains can be lethal [45]. Although patients do not lack the IFNα and IFNβ receptor genes, the decision was made, in consultation with the FDA, to mimic the worst case scenario for the patient population by assessing MV-CEA neurotoxicity and biodistribution in measles-immune IFNARKO CD46 Ge mice. Following CNS administration of doses 35-fold higher than the maximum dose proposed for use in clinical trials, the general appearance, activity level and key hematological/biochemical parameters of MV-CEA treated animals remained normal and no signs of clinical toxicity or neurotoxicity were observed. Vital organs (ie, the brain, heart, lungs, liver, pancreas, kidneys, spleen, ovary, testes, peritoneum and skeletal muscles) underwent histological analysis that revealed no significant differences between mice receiving vehicle and MV-CEA-treated animals. No elevation of circulating CEA levels was observed and there was no evidence of MV-CEA replication outside the CNS [40].
Rhesus macaques represent the gold standard for the assessment of measles neurotoxicity. CNS inoculation of MV vaccine strains is the standard method by which MV vaccine lots are tested for neurotoxicity. Therefore, the safety of MV-CEA following CNS administration in Rhesus macaques was evaluated. Measles-immune macaques were used to accurately replicate the study population in the proposed clinical trials. Using previously established stereotactic coordinates, the virus was administered to the frontal lobe of the animals, which is a common site of glioblastoma multiforme appearance [48]. Repeat administration on days 1 and 5 was used to mimic the repeat dosing schedule proposed in the clinical trial. By direct brain conversion, the highest dose was 3-fold higher than the maximum dose that the normal brain would be exposed to in the clinical trial [41]. The animals were closely monitored for fever, rash and conjunctivitis, as well as clinical signs of neurological toxicity. All clinical, neurological and hematological/biochemical parameters remained normal. Laboratory examination of CSF demonstrated no evidence of encephalitis, and MRI imaging revealed no inflammation or other abnormalities [41].
MV-NIS
The protocol for phase I clinical trial of MV-NIS in patients with multiple myeloma includes intravenous administration of MV-NIS, with or without pretreatment with cyclophosphamide. In addition to its chemotherapeutic activity, cyclophosphamide is an immunosuppressive agent. Therefore, it can potentially prolong and enhance viral dissemination and replication in the tumor [49–51]. To evaluate the biodistribution and toxicity of intravenous MV-NIS administration, IFNARKO CD46 Ge mice were administered MV-NIS systemically with or without cyclophosphamide [26,42]. Mice that were pretreated with cyclophosphamide demonstrated alterations in the biodistribution of MV-NIS-infected cells and the kinetics of MV-NIS elimination [42]. Cyclophosphamide enhanced viral propagation initially after MV-NIS administration, but viral elimination was accelerated at later time points [42]. No clinical signs nor toxicity related to MV-NIS were observed. Cyclophosphamide treatment resulted in anticipated toxicity to the bone marrow, urinary bladder, lymphoid tissue and sex organs, which, however, was not increased by the combination of Cyclophosphamide with MV-NIS, except for a slightly lower total white blood cell count [42]. In addition to the transgenic mouse model, squirrel monkeys were chosen to test the toxicity and biodistribution of MV-NIS as a model that is susceptible to wild-type MV replication via interaction with the SLAM receptors. In contrast to Rhesus macaques, squirrel monkeys express a truncated CD46 variant on their erythrocytes that does not bind with MV [52]. Therefore, similarly to humans, MV-Edm strains do not agglutinate squirrel monkey erythrocytes, and this animal model can be used for studying viral distribution and safety after systemic MV-NIS administration. Treatment of squirrel monkeys with Cyclophosphamide resulted in non-significant toxicity (eg, modest bone marrow suppression indicated by the decreased reticulocyte counts in the cyclophosphamide-treated squirrel monkeys compared with animals that were not pretreated with Cyclophosphamide) that was not increased by its combination with MV-NIS. Cyclophosphamide suppression of anti-measles immunity resulted in prolongation of MV-NIS persistence before its clearance by the immune system. By day 29 following MV-NIS injection, viral RNA was detectable in buccal swabs of cyclophosphamide-treated squirrel monkeys, while animals not pretreated with cyclophosphamide were negative for viral RNA [42].
The studies described above in measles-susceptible transgenic mice and non-human primates support the safety and feasibility of intraperitoneal, intratumoral and resection cavity administration of MV-CEA into primary ovarian or CNS tumors, respectively, as well as systemic administration of MV-NIS with or without concomitant administration of cyclophosphamide in multiple myeloma patients.
Clinical trials of measles virotherapy
The substantial oncolytic potential of both MV-NIS and MV-CEA, in conjunction with the preclinical efficacy, toxicity and biodistribution data discussed above, has prompted three ongoing phase I clinical trials: (i) a trial of intraperitoneal administration of MV-CEA in patients with recurrent ovarian cancer [53–55], (ii) a trial of intratumoral and resection cavity administration of MV-CEA in patients with recurrent glioblastoma multiforme [56], and (iii) a trial of intravenous administration of MV-NIS with or without cyclophosphamide in patients with multiple myeloma [57]. A primary objective in phase I clinical trials of cancer treatments is to determine the MTD of the new treatment. A common strategy to design such trials is by using the standard ‘cohorts of three’ design, wherein patients are treated in groups of three with doses successively escalating between consecutive groups if acceptable toxicity is observed. This design was applied to all three ongoing measles virotherapy clinical trials. No intrapatient dose escalation was permitted.
The phase I clinical trial of MV-CEA in patients with advanced ovarian cancer is the most advanced clinical trial of an engineered MV strain [53]. Doses ranging from 103 to 109 TCID50 (50% tissue culture infective dose) were administered intraperitoneally in measles-immune patients with platinum-resistant ovarian cancer and disease limited to the peritoneal cavity. Eligible patients should not have experienced CEA elevation at any time during their disease course and, therefore, CEA elevation during treatment can only be attributed to viral replication. Patients (n = 21) were treated at seven dose levels (103 to 109 TCID50). No dose-limiting toxicity has been observed. The most common side effects included grade 1 to 2 fatigue, grade 1 fever, grade 1 to 2 anorexia, grade 1 to 2 abdominal pain, and grade 1 to 2 nausea. No treatment-induced immunosuppression as assessed by delayed-type hypersensitivity, and CD4, CD8, immunoglobulin and complement levels, no development of anti-CEA antibodies, and no shedding of MV in urine or mouth gargle specimens has been observed. CEA elevation was detected in the peritoneal fluid of one patient (108 TCID50-dose group) and in the serum of three patients (109 TICD50-dose group). Viremia was detected in four asymptomatic patients by quantitative RT-PCR. CD46 receptor overexpression was observed in seven of eight patient tumor samples tested, as assessed by immunohistochemistry. The Response Evaluation Criteria in Solid Tumors (RECIST) were used for assessment of antitumor response [54]. The best objective response was stable disease in 14 patients, with a median duration of 88 days and a range of 55 to 277 days. Clinical outcome was dose dependent, with all patients (n = 9) treated in the higher dose levels (107 to 109 TCID50) accomplishing stable disease compared with only 5 of 12 patients treated in the lower dose levels (103 to 106 TCID50). All patients with viremia or CEA elevation achieved disease stability. Significant decreases in cancer antigen-125 levels by 43.5, 77.7, 71.8, 31.6 and 33.9% were reported in five patients [55].
A phase I clinical trial evaluating MV-CEA in patients with recurrent glioblastoma multiforme has been initiated and is currently recruiting patients [56]. Patients with recurrent glioblastoma multiforme and pre-existing immunity to measles who are candidates for gross total or subtotal tumor resection are eligible for enrollment. The starting dose of MV-CEA is to be 105 TCID50 per patient and will be escalated to a maximum dose level of 2 × 107 TCID50 per patient. Patients will be assigned to one of two groups. Group A will receive direct MV-CEA injections in the resection cavity. After dose escalation up to a dose of 107 TCID50 is completed in Group A, patient assignment to Group B will be initiated. Virus will be administered into the recurrent tumors of patients in group B. At the time of maximum viral replication 5 days later, tumors will be resected, and a second dose will be administered into the resection cavity. Resected tumors will be examined for viral distribution by in situ hybridization and immunohistochemistry relative to distance from the injection site. To date, three patients have received 105 TCID50 doses and three patients have received 106 TCID50 doses in the resection cavity, with no dose-limiting toxicity observed. Correlative analysis will be performed to assess viral distribution in PBMC shedding, and to identify any associations between tumor CD46 receptor levels, CEA levels and viral replication in the tumor [E Galanis, unpublished data].
A phase I clinical trial evaluating MV-NIS as an intravenous treatment for patients with recurrent or refractory multiple myeloma has also been initiated. MV-NIS doses ranging from 106 to 109 TCID50 per patient will be administered [57]. The trial protocol includes two steps. In the first, patients will be treated with one systemic MV-NIS injection (dose ranging from 106 to 109 TCID50). The second step will begin once the MTD of MV-NIS is reached in step 1. During step 2, new cohorts of patients (n = 3) will be pretreated with cyclophosphamide (10 mg/kg) 2 days prior to MV-NIS injection. The MV-NIS dose will range from the MTD divided by 100 (MTD/100) to 81-fold the MTD/100. Hematological and biochemical parameters, and anti-measles immunity will be determined pre- and post-therapy. MV-NIS levels will be measured in blood, urine and gargle samples. Serial imaging of MV-NIS biodistribution following 123[I] administration will also be performed. Myeloma cells from bone-marrow aspirates/biopsies will be examined for CD46 expression and MV infectivity. Three patients have been treated at a dose of 106 TCID50, three patients at 107 TCID50, and two at 108 TCID50, without any dose-limiting toxicities observed despite low or absent levels of MV neutralizing antibodies. Notwithstanding the low viral dose, one patient had 123[I] uptake as detected by SPECT/CT 8 days after treatment with MV-NIS, which was localized to an area of a multiple myeloma tumor in the pelvis [A Dispenzieri, unpublished data].
These trials represent the first clinical studies of oncolytic engineered MV strains in the treatment of cancer. Prior efforts in this field include one open-label, non-randomized, dose-escalation, phase I clinical trial of the unmodified vaccine strain MV-Edm Zagreb (MV-EZ) in patients (n = 5) with cutaneous T-cell lymphomas (CTCLs) of stage IIb or higher [58]. One or two treatment cycles were administered to each patient. All patients had detectable anti-measles antibodies in their serum. Each treatment cycle included two intratumoral MV-EZ injections on days 4 and 17, which were preceded by subcutaneous IFNα injections administered 72 and 24 h before each of the two MV-EZ injections. The maximum dose was 103 TCID50. In one patient, the treated CTCL lesion regressed completely after the first treatment cycle and another lesion was therefore injected in the second treatment cycle. Partial regression was observed in four of the treated lesions, while one of the treated lesions demonstrated no response. Even though CTCL is an immunosuppressive disease, the anti-measles antibody titers were significantly increased in all five patients following MV-EZ treatment. The treatment was well tolerated at all dose levels, with minimal toxicity after intratumoral administration [58]. Because of the study design, the impact of IFNα on the observed antitumor effect could not be determined.
Future perspectives and challenges
Further development of engineered oncolytic MV strains as antitumor agents will depend on the results of the ongoing phase I clinical trials. Information on safety, efficacy, biodistribution and merit of the CEA and NIS monitoring strategies will guide the development strategy. However, some potential challenges are worth discussing. The majority of cancer patients who are candidates for measles-based therapeutics are immune to the virus as a result of natural infection or vaccination. Immunity could have a negative impact on the therapeutic efficacy of the virus, an issue that needs to be addressed, especially if systemic administration of the virus is considered. Although malignancy-related immunosuppression, such as in multiple myeloma patients, can hamper antiviral immune response in some patients, the development of strategies which circumvent or modulate the immune response against MV has the potential to increase the applicability of measles-based therapeutics. The combination of measles virotherapy with cyclophosphamide could represent such a strategy. Experiments performed with oncolytic herpes virus strains have demonstrated that cyclophosphamide can decrease the innate immune response, prolong viral gene expression/proliferation in tumors and enhance oncolytic viral effects [49,50,59]. Cyclophosphamide can similarly increase the antitumor effect of reoviruses [60]. Preclinical biodistribution data of MV-NIS in squirrel monkeys suggest that the addition of cyclophosphamide can reduce the primary immune response to MV and prolong viral gene expression [42]. The impact of this approach on the efficacy of measles virotherapy remains to be determined and data derived from the phase I multiple myeloma clinical trial will be pivotal in this regard.
In addition to cyclophosphamide, other novel strategies are currently being developed to circumvent anti-measles immunity and thus preserve or enhance the oncolytic potency of MV following in situ delivery, as well as facilitate systemic delivery in future applications of this technology. One such promising concept is the use of cell carriers, such as monocytoid cell lines, which would ideally traffic to tumors, protect MV from the immune system and efficiently deliver the virus to tumor cells [61–63]. MV may also propagate inside such delivery vehicles and then be released to infect adjacent tumor cells.
Furthermore, viruses used as oncolytic agents have unique mechanisms of action that can be synergistic with conventional therapies. Oncolytic viruses, including the MV-Edm derivatives, represent a novel therapeutic class of agents that demonstrates no cross-resistance with existing approaches and can therefore be combined with conventional treatment modalities. Examples include the combination of MV-Edm derivatives with external beam radiation therapy that has synergistic activity in the treatment of glioblastoma multiforme [64], and radioisotopes such as 131[I], which demonstrated synergistic activity in the treatment of multiple myeloma in preclinical studies of MV-NIS [27]. Another approach to investigate is the combination of MV-Edm derivatives with small molecules, which could augment the MV fusogenicity and antitumor effect. For example, this effect has been demonstrated when MV was combined with Hsp inhibitors; Hsps increase tumor cell susceptibility to fusion, possibly via cytoskeletal modulation [65].
An advantage of the MV vector is that its attachment and fusion functions are mediated by two different proteins (H and F, respectively). The H protein determines the viral binding specificity [66]. This is particularly convenient because retargeting strategies can focus on H protein modification while the fusogenic capacity of the virus remains unaffected. Several studies have demonstrated that MV-Edm retains its potent oncolytic activity when it is genetically modified [25,27,28,31,32]. Moreover, MV-Edm can be effectively retargeted with the introduction of H protein mutations that ablate viral recognition and entry via the natural receptors CD46 and SLAM, combined with display on the H protein of a ligand that can recognize tumor-specific targets [11,67,68]. Retargeted strains have been designed that display single-chain antibodies against receptors such as the EGF receptor (EGFR) or the EGFR variant type III (EGFRvIII) [69,70], or that display cytokines such as IL-13 [71], which recognize the tumor-specific IL-13Rα2 receptor. Despite the increase in specificity, there is no loss of antitumor potency against cells or xenografts expressing the target receptors [69–71]. Although dose-limiting toxicity has not been observed in the ongoing phase I trials, viral retargeting can allow any toxicity concerns associated with higher viral doses to be addressed, as well as to overcome tumor variability in viral receptor expression.
Conclusions
MV-Edm derivatives have demonstrated potent and tumor-specific oncolytic activity against a variety of preclinical tumor models. The antitumor potential of measles derivatives is further supported by the results of the first completed clinical trial using intratumoral administration of an unmodified MV-EZ strain in patients with CTCL. Genetic manipulation of MV-Edm strains to express human CEA or NIS allows non-invasive real-time monitoring of virus propagation (MV-CEA and MV-NIS strains) and biodistribution (MV-NIS strain), and their antitumor activity can potentially be additionally increased using radioisotopes (MV-NIS). These may be the first steps on the pathway toward ‘redemption’ for MV, a virus that currently causes significant child mortality in unvaccinated populations, but may nevertheless prove to be a promising anticancer agent. To this end, three phase I clinical trials have been initiated to evaluate the efficacy and safety of engineered oncolytic MV strains against refractory ovarian cancer, recurrent glioblastoma multiforme, and recurrent or refractory multiple myeloma. Safety and efficacy data obtained from these ongoing trials will guide future clinical testing and development of engineered MV derivatives as cancer therapeutics.
Acknowledgments
The authors would like to acknowledge Dr Stephen J Russell, the Mayo Clinic Vector Production Laboratory, the Molecular Medicine Toxicology Core, the Animal Core of the Mayo Clinic Brain SPORE, and the Mayo Clinic Cancer Center Women’s Cancer Program, Neurooncology Program and Myeloma Group for their support of the clinical measles virotherapy program. This research is supported by grants CA108961 (EG), CA123831 (EG), CA103276 (EG), CA125614 (AD), and the Siebens and Goodwin Foundations.
References
•• of outstanding interest
• of special interest
- 1.Edelstein ML, Abedi MR, Wixon J. Gene therapy clinical trials worldwide to 2007 – An update. J Gene Med. 2007;9(10):833–842. doi: 10.1002/jgm.1100. [DOI] [PubMed] [Google Scholar]
- 2.Liu TC, Galanis E, Kirn D. Clinical trial results with oncolytic virotherapy: A century of promise, a decade of progress. Nat din Pract Oncol. 2007;4(2):101–117. doi: 10.1038/ncponc0736. [DOI] [PubMed] [Google Scholar]
- 3.Kelly E, Russell SJ. History of oncolytic viruses: Genesis to genetic engineering. Mol Ther. 2007;15(4):651–659. doi: 10.1038/sj.mt.6300108. [DOI] [PubMed] [Google Scholar]
- 4.Bluming AZ, Ziegler JL. Regression of Burkitt’s lymphoma in association with measles infection. Lancet. 1971;2(7715):105–106. doi: 10.1016/s0140-6736(71)92086-1. [DOI] [PubMed] [Google Scholar]
- 5.Gross S. Measles and leukaemia. Lancet. 1971;l(7695):397–398. doi: 10.1016/s0140-6736(71)92232-x. [DOI] [PubMed] [Google Scholar]
- 6.Mota HC. Infantile Hodgkin’s disease: Remission after measles. Br Med J. 1973;2(5863):421. doi: 10.1136/bmj.2.5863.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pasquinucci G. Possible effect of measles on leukaemia. Lancet. 1971;1(7690):136. doi: 10.1016/s0140-6736(71)90869-5. [DOI] [PubMed] [Google Scholar]
- 8.Zygiert Z. Hodgkin’s disease: Remissions after measles. Lancet. 1971;1(7699):593. doi: 10.1016/s0140-6736(71)91186-x. [DOI] [PubMed] [Google Scholar]
- 9.Hernandez SA. Observacion de un caso de enfermedad de Hodgkin, con regresion de los sintomas e infartos ganglionares, post-sarampion. Arch Cubanos Cancer. 1949;8:26–31. [Google Scholar]
- 10.Southam CM. Present status of oncolytic virus studies. Trans NY Acad Sci. 1960;22:657–673. doi: 10.1111/j.2164-0947.1960.tb00739.x. [DOI] [PubMed] [Google Scholar]
- 11••.Nakamura T, Peng KW, Harvey M, Greiner S, Lorimer IA, James CD, Russell SJ. Rescue and propagation of fully retargeted oncolytic measles viruses. Nat Biotechnol. 2005;23(2):209–214. doi: 10.1038/nbt1060. This paper describes the development of the pseudoreceptor STAR system that allows rescue and propagation of fully retargeted MV-Edm strains. [DOI] [PubMed] [Google Scholar]
- 12••.Radecke F, Spielhofer P, Schneider H, Kaelin K, Huber M, Dötsch C, Christiansen G, Billeter MA. Rescue of measles viruses from cloned DNA. EMBO J. 1995;14(23):5773–5784. doi: 10.1002/j.1460-2075.1995.tb00266.x. This paper first established a system for rescuing measles viruses from cloned DNA without relying on any helper virus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yanagi Y, Takeda M, Ohno S. Measles virus: Cellular receptors, tropism and pathogenesis. J Gen Virol. 2006;87(10):2767–2779. doi: 10.1099/vir.0.82221-0. [DOI] [PubMed] [Google Scholar]
- 14.Naniche D, Varior-Krishnan G, Cervoni F, Wild TF, Rossi B, Rabourdin- Combe C, Gerlier D. Human membrane cofactor protein (CD46) acts as a cellular receptor for measles virus. J Virol. 1993;67(10):6025–6032. doi: 10.1128/jvi.67.10.6025-6032.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dorig RE, Marcil A, Chopra A, Richardson CD. The human CD46 molecule is a receptor for measles virus (Edmonston strain) Cell. 1993;75(2):295–305. doi: 10.1016/0092-8674(93)80071-l. [DOI] [PubMed] [Google Scholar]
- 16.Tatsuo H, Ono N, Tanaka K, Yanagi Y. SLAM (CDw150) is a cellular receptor for measles virus. Nature. 2000;406(6798):893–897. doi: 10.1038/35022579. [DOI] [PubMed] [Google Scholar]
- 17.Jurianz K, Ziegler S, Garcia-Schuler H, Kraus S, Bohana-Kashtan O, Fishelson Z, Kirschfink M. Complement resistance of tumor cells: Basal and induced mechanisms. Mol Immunol. 1999;36(13–14):929–939. doi: 10.1016/s0161-5890(99)00115-7. [DOI] [PubMed] [Google Scholar]
- 18.Fishelson Z, Donin N, Zell S, Schultz S, Kirschfink M. Obstacles to cancer immunotherapy: Expression of membrane complement regulatory proteins (mCRPs) in tumors. Mol Immunol. 2003;40(2–4):109–123. doi: 10.1016/s0161-5890(03)00112-3. [DOI] [PubMed] [Google Scholar]
- 19.Wild TF, Malvoisin E, Buckland R. Measles virus: Both the haemagglutinin and fusion glycoproteins are required for fusion. J Gen Virol. 1991;72(2):439–442. doi: 10.1099/0022-1317-72-2-439. [DOI] [PubMed] [Google Scholar]
- 20.Live attenuated measles vaccine. EPI Newsl. 1980;2(1):6. No authors listed. [PubMed] [Google Scholar]
- 21.Hilleman MR. Current overview of the pathogenesis and prophylaxis of measles with focus on practical implications. Vaccine. 2001;20(5–6):651–665. doi: 10.1016/s0264-410x(01)00384-x. [DOI] [PubMed] [Google Scholar]
- 22.Cutts FT, Markowitz LE. Successes and failures in measles control. J Infect Dis. 1994;170(Suppl 1):S32–S41. doi: 10.1093/infdis/170.supplement_1.s32. [DOI] [PubMed] [Google Scholar]
- 23•.Anderson BD, Nakamura T, Russell SJ, Peng KW. High CD46 receptor density determines preferential killing of tumor cells by oncolytic measles virus. Cancer Res. 2004;64(14):4919–4926. doi: 10.1158/0008-5472.CAN-04-0884. This study suggested that variations in CD46 density determine the tumor-specificity of MV-Edm strains. [DOI] [PubMed] [Google Scholar]
- 24.Peng KW, Donovan KA, Schneider U, Cattaneo R, Lust JA, Russell SJ. Oncolytic measles viruses displaying a single-chain antibody against CD38, a myeloma cell marker. Blood. 2003;101(7):2557–2562. doi: 10.1182/blood-2002-07-2195. [DOI] [PubMed] [Google Scholar]
- 25••.Phuong LK, Allen C, Peng KW, Giannini C, Greiner S, TenEyck CJ, Mishra PK, Macura SI, Russell SJ, Galanis EC. Use of a vaccine strain of measles virus genetically engineered to produce carcinoembryonic antigen as a novel therapeutic agent against glioblastoma multiforme. Cancer Res. 2003;63(10):2462–2469. This paper describes the preclinical evaluation of MV-CEA efficacy against human glioblastoma multiforme xenografts. MV-CEA demonstrated potent therapeutic efficacy against gliomas in both subcutaneous and intracranial orthotopic mouse models. [PubMed] [Google Scholar]
- 26•.Blechacz B, Splinter PL, Greiner S, Myers R, Peng KW, Federspiel MJ, Russell SJ, LaRusso NF. Engineered measles virus as a novel oncolytic viral therapy system for hepatocellular carcinoma. Hepatology. 2006;44(6):1465–1477. doi: 10.1002/hep.21437. This study investigated the antitumor effect of MV-CEA and MV-NIS against human hepatocellular carcinoma xenografts. Both viruses demonstrated significant therapeutic effect against hepatocellular carcinoma in vitro and in vivo. [DOI] [PubMed] [Google Scholar]
- 27••.Dingli D, Peng KW, Harvey ME, Greipp PR, O’Connor MK, Cattaneo R, Morris JC, Russell SJ. Image-guided radiovirotherapy for multiple myeloma using a recombinant measles virus expressing the thyroidal sodium iodide symporter. Blood. 2004;103(5):1641–1646. doi: 10.1182/blood-2003-07-2233. This paper describes the design of an MV-NIS strain and the assessment of the efficacy of MV-NIS against multiple myeloma xenografts. Non-invasive tracking of virus localization was achieved by 123[I] imaging, and combination treatment with 131[I] resulted in significant regression of MV-resistant tumors. [DOI] [PubMed] [Google Scholar]
- 28•.Peng KW, Ahmann GJ, Pham L, Greipp PR, Cattaneo R, Russell SJ. Systemic therapy of myeloma xenografts by an attenuated measles virus. Blood. 2001;98(7):2002–2007. doi: 10.1182/blood.v98.7.2002. This study investigated the antitumor effect of MV-Edm against human myeloma xenografts. The virus induced significant tumor regression. [DOI] [PubMed] [Google Scholar]
- 29••.Peng KW, Facteau S, Wegman T, O’Kane D, Russell SJ. Non-invasive in vivo monitoring of trackable viruses expressing soluble marker peptides. Nat Med. 2002;8(5):527–531. doi: 10.1038/nm0502-527. This paper describes the generation and characterization of MV-CEA. Monitoring of viral gene expression kinetics was achievable by measuring CEA levels in blood serum. [DOI] [PubMed] [Google Scholar]
- 30.Galanis E, Bateman A, Johnson K, Diaz RM, James CD, Vile R, Russell SJ. Use of viral fusogenic membrane glycoproteins as novel therapeutic transgenes in gliomas. Hum Gene Ther. 2001;12(7):811–821. doi: 10.1089/104303401750148766. [DOI] [PubMed] [Google Scholar]
- 31•.Grote D, Russell SJ, Cornu TI, Cattaneo R, Vile R, Poland GA, Fielding AK. Live attenuated measles virus induces regression of human lymphoma xenografts in immunodeficient mice. Blood. 2001;97(12):3746–3754. doi: 10.1182/blood.v97.12.3746. This study evaluated the antitumor effect of MV-Edm against human lymphoma xenografts. MV-Edm infection resulted in significant regression of large established human lymphoma xenografts. [DOI] [PubMed] [Google Scholar]
- 32••.Peng KW, TenEyck CJ, Galanis E, Kalli KR, Hartmann LC, Russell SJ. Intraperitoneal therapy of ovarian cancer using an engineered measles virus. Cancer Res. 2002;62(16):4656–4662. This paper describes the preclinical evaluation of MV-CEA efficacy against human ovarian cancer xenografts. MV-CEA demonstrated significant in vivo therapeutic benefits and viral gene expression could be monitored by serum CEA measurement. [PubMed] [Google Scholar]
- 33.Dingli D, Bergert ER, Bajzer Z, O’Connor MK, Russell SJ, Morris JC. Dynamic iodide trapping by tumor cells expressing the thyroidal sodium iodide symporter. Biochem Biophys Res Commun. 2004;325(1):157–166. doi: 10.1016/j.bbrc.2004.09.219. [DOI] [PubMed] [Google Scholar]
- 34.Dingli D, Russell SJ, Morris JC., 3rd In vivo imaging and tumor therapy with the sodium iodide symporter. J Cell Biochem. 2003;90(6):1079–1086. doi: 10.1002/jcb.10714. [DOI] [PubMed] [Google Scholar]
- 35.Hasegawa K, Pham L, O’Connor MK, Federspiel MJ, Russell SJ, Peng KW. Dual therapy of ovarian cancer using measles viruses expressing carcinoembryonic antigen and sodium iodide symporter. Clin Cancer Res. 2006;12(6):1868–1875. doi: 10.1158/1078-0432.CCR-05-1803. [DOI] [PubMed] [Google Scholar]
- 36••.McDonald CJ, Erlichman C, Ingle JN, Rosales GA, Allen C, Greiner SM, Harvey ME, Zollman PJ, Russell SJ, Galanis E. A measles virus vaccine strain derivative as a novel oncolytic agent against breast cancer. Breast Cancer Res Treat. 2006;99(2):177–184. doi: 10.1007/s10549-006-9200-5. This study determined the antitumor effect of MV-CEA against human breast cancer xenografts. Potent antitumor activity of MV-CEA against breast cancer cell lines and xenografts was demonstrated. [DOI] [PubMed] [Google Scholar]
- 37•.Msaouel P, Iankov ID, Allen C, Morris JC, von Messling V, Cattaneo R, Koutsilieris M, Russell SJ, Galanis E. Engineered measles virus as a novel oncolytic therapy against prostate cancer. Prostate. 2009;69(1):82–91. doi: 10.1002/pros.20857. This study determined the antitumor effect of MV-CEA against human prostate cancer xenografts. The virus demonstrated considerable oncolytic potency both in vitro and in vivo. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peng KW, Hadac EM, Anderson BD, Myers R, Harvey M, Greiner SM, Soeffker D, Federspiel MJ, Russell SJ. Pharmacokinetics of oncolytic measles virotherapy: Eventual equilibrium between virus and tumor in an ovarian cancer xenograft model. Cancer Gene Ther. 2006;13(8):732–738. doi: 10.1038/sj.cgt.7700948. [DOI] [PubMed] [Google Scholar]
- 39.Peng KW, Frenzke M, Myers R, Soeffker D, Harvey M, Greiner S, Galanis E, Cattaneo R, Federspiel MJ, Russell SJ. Biodistribution of oncolytic measles virus after intraperitoneal administration into Ifnar-CD46Ge transgenic mice. Hum Gene Ther. 2003;14(16):1565–1577. doi: 10.1089/104303403322495070. [DOI] [PubMed] [Google Scholar]
- 40.Allen C, Paraskevakou G, Liu C, Iankov ID, Msaouel P, Zollman P, Myers R, Peng KW, Russell SJ, Galanis E. Oncolytic measles virus strains in the treatment of gliomas. Expert Opin Biol Ther. 2008;8(2):213–220. doi: 10.1517/14712598.8.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Myers R, Harvey M, Kaufmann TJ, Greiner SM, Krempski JW, Raffel C, Shelton SE, Soeffker D, Zollman P, Federspiel MJ, Blanco M, Galanis E. Toxicology study of repeat intracerebral administration of a measles virus derivative producing carcinoembryonic antigen in rhesus macaques in support of a phase I/II clinical trial for patients with recurrent gliomas. Hum Gene Ther. 2008;19(7):690–698. doi: 10.1089/hum.2008.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42••.Myers RM, Greiner SM, Harvey ME, Griesmann G, Kuffel MJ, Buhrow SA, Reid JM, Federspiel M, Ames MM, Dingli D, Schweikart K, et al. Preclinical pharmacology and toxicology of intravenous MV-NIS, an oncolytic measles virus administered with or without cyclophosphamide. Clin Pharmacol Ther. 2007;82(6):700–710. doi: 10.1038/sj.clpt.6100409. This paper describes a preclinical toxicology study of MV-NIS in support of a phase I clinical trial of multiple myeloma. Cyclophosphamide suppression of anti-measles immunity resulted in prolongation of MV-NIS persistence before clearance by the immune system. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Myers RM, Harvey ME, Greiner SM, Soeffker DC, Krempski JW, Zollman PJ, Shelton SE, Raffel C, Goerss SJ, Kaufmann JT, Federspiel MJ, et al. Safety of repeat intracerebral administration of MV-CEA in Rhesus macaques in support of a phase I/II clinical trial for patients with recurrent gliomas. Mol Ther. 2006;13(S1) doi: 10.1089/hum.2008.035. Abs 297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mrkic B, Odermatt B, Klein MA, Billeter MA, Pavlovic J, Cattaneo R. Lymphatic dissemination and comparative pathology of recombinant measles viruses in genetically modified mice. J Virol. 2000;74(3):1364–1372. doi: 10.1128/jvi.74.3.1364-1372.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45•.Mrkic B, Pavlovic J, Rulicke T, Volpe P, Buchholz CJ, Hourcade D, Atkinson JP, Aguzzi A, Cattaneo R. Measles virus spread and pathogenesis in genetically modified mice. J Virol. 1998;72(9):7420–7427. doi: 10.1128/jvi.72.9.7420-7427.1998. This paper describes the design and characterization of the IFNARKO CD46 Ge mouse. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roscic-Mrkic B, Schwendener RA, Odermatt B, Zuniga A, Pavlovic J, Billeter MA, Cattaneo R. Roles of macrophages in measles virus infection of genetically modified mice. J Virol. 2001;75(7):3343–3351. doi: 10.1128/JVI.75.7.3343-3351.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kemper C, Leung M, Stephensen CB, Pinkert CA, Liszewski MK, Cattaneo R, Atkinson JP. Membrane cofactor protein (MCP; CD46) expression in transgenic mice. Clin Exp Immunol. 2001;124(2):180–189. doi: 10.1046/j.1365-2249.2001.01458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Driesse MJ, Vincent AJ, Sillevis Smitt PA, Kros JM, Hoogerbrugge PM, Avezaat CJ, Valerio D, Bout A. Intracerebral injection of adenovirus harboring the HSVtk gene combined with ganciclovir administration: Toxicity study in nonhuman primates. Gene Ther. 1998;5(8):1122–1129. doi: 10.1038/sj.gt.3300695. [DOI] [PubMed] [Google Scholar]
- 49.Fulci G, Breymann L, Gianni D, Kurozomi K, Rhee SS, Yu J, Kaur B, Louis DN, Weissleder R, Caligiuri MA, Chiocca EA. Cyclophosphamide enhances glioma virotherapy by inhibiting innate immune responses. Proc Natl Acad Sci USA. 2006;103(34):12873–12878. doi: 10.1073/pnas.0605496103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kambara H, Saeki Y, Chiocca EA. Cyclophosphamide allows for in vivo dose reduction of a potent oncolytic virus. Cancer Res. 2005;65(24):11255–11258. doi: 10.1158/0008-5472.CAN-05-2278. [DOI] [PubMed] [Google Scholar]
- 51.Lamfers ML, Fulci G, Gianni D, Tang Y, Kurozumi K, Kaur B, Moeniralm S, Saeki Y, Carette JE, Weissleder R, Vandertop WP, et al. Cyclophosphamide increases transgene expression mediated by an oncolytic adenovirus in glioma-bearing mice monitored by bioluminescence imaging. Mol Ther. 2006;14(6):779–788. doi: 10.1016/j.ymthe.2006.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hsu EC, Dong RE, Sarangi F, Marcil A, Iorio C, Richardson CD. Artificial mutations and natural variations in the CD46 molecules from human and monkey cells define regions important for measles virus binding. J Virol. 1997;71(8):6144–6154. doi: 10.1128/jvi.71.8.6144-6154.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53••. NCT00408590. Oncolytic virus therapy in treating patients with progressive, recurrent, or refractory ovarian epithelial cancer or primary peritoneal cancer. NIH, Bethesda, MD; USA: 2008. October 27. http://www.clinicaltrials.gov/ct2/show/NCT00408590. The design of the phase I clinical trial of MV-CEA in patients with recurrent ovarian cancer is described. [Google Scholar]
- 54.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92(3):205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 55••.Galanis E, Hartmann LC, Cliby W, Peethambaram PP, Long HJ, Kaur JS, Haluska P, Jr, Sloan JA, Peng K, Russell SJ. Phase I trial of intraperitoneal (IP) administration of a measles virus (MV) derivative expressing the human carcinoembryonic antigen (CEA) in recurrent ovarian cancer (OvCa) J Clin Oncol. 2008;26(15S) Abs 5538. This abstract reports results of a phase I clinical trial using MV-CEA against recurrent ovarian cancer. MV-CEA demonstrated evidence of dose-dependent biological activity and was well-tolerated in doses up to 109 TCID50. [Google Scholar]
- 56••. NCT00390299. Viral therapy in treating patients with recurrent glioblastoma multiforme. NIH, Bethesda, MD; USA: 2008. http://www.clinicaltrials.gov/ct2/show/NCT00390299. The study rationale, design, and accrual criteria of the phase I clinical trial of MV-CEA in patients with recurrent glioblastoma multiforme are described. [Google Scholar]
- 57••. NCT00450814. Vaccine therapy with or without Cyclophosphamide in treating patients with recurrent or refractory multiple myeloma. NIH, Bethesda, MD; USA: 2008. http://www.clinicaltrials.gov/ct2/show/NCT00450814. The study rationale, design, and accrual criteria of the phase I clinical trial of MV-NIS in patients with recurrent or refractory multiple myeloma are described. [Google Scholar]
- 58••.Heinzerling L, Künzi V, Oberholzer PA, Kündig T, Naim H, Dummer R. Oncolytic measles virus in cutaneous T-cell lymphomas mounts antitumor immune responses in vivo and targets interferon-resistant tumor cells. Blood. 2005;106(7):2287–2294. doi: 10.1182/blood-2004-11-4558. This paper reports results of a phase I, open-label, non-randomized, dose-escalation clinical trial of the unmodified MV vaccine strain MV-EZ in patients with CTCLs. Early signs of biological activity were observed, including complete tumor regression of one CTCL lesion. The treatment was well tolerated at all dose levels, with minimal toxicity after intratumoral administration. [DOI] [PubMed] [Google Scholar]
- 59.Ikeda K, Ichikawa T, Wakimoto H, Silver JS, Deisboeck TS, Finkelstein D, Harsh GR, 4th, Louis DN, Bartus RT, Hochberg FH, Chiocca EA. Oncolytic virus therapy of multiple tumors in the brain requires suppression of innate and elicited antiviral responses. Nat Med. 1999;5(8):881–887. doi: 10.1038/11320. [DOI] [PubMed] [Google Scholar]
- 60.Qiao J, Wang H, Kottke T, White C, Twigger K, Diaz RM, Thompson J, Selby P, de Bono J, Melcher A, Pandha H, et al. Cyclophosphamide facilitates antitumor efficacy against subcutaneous tumors following intravenous delivery of reovirus. Clin Cancer Res. 2008;14(1):259–269. doi: 10.1158/1078-0432.CCR-07-1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Iankov ID, Blechacz B, Liu C, Schmeckpeper JD, Tarara JE, Federspiel MJ, Caplice N, Russell SJ. Infected cell carriers: A new strategy for systemic delivery of oncolytic measles viruses in cancer virotherapy. Mol Ther. 2007;15(1):114–122. doi: 10.1038/sj.mt.6300020. [DOI] [PubMed] [Google Scholar]
- 62.Ong HT, Hasegawa K, Dietz AB, Russell SJ, Peng KW. Evaluation of T cells as carriers for systemic measles virotherapy in the presence of antiviral antibodies. Gene Ther. 2007;14(4):324–333. doi: 10.1038/sj.gt.3302880. [DOI] [PubMed] [Google Scholar]
- 63.Munguia A, Ota T, Miest T, Russell SJ. Cell carriers to deliver oncolytic viruses to sites of myeloma tumor growth. Gene Ther. 2008;15(10):797–806. doi: 10.1038/gt.2008.45. [DOI] [PubMed] [Google Scholar]
- 64.Liu C, Sarkaria JN, Petell CA, Paraskevakou G, Zollman PJ, Schroeder M, Carlson B, Decker PA, Wu W, James CD, Russell SJ, et al. Combination of measles virus virotherapy and radiation therapy has synergistic activity in the treatment of glioblastoma multiforme. Clin Cancer Res. 2007;13(23):7155–7165. doi: 10.1158/1078-0432.CCR-07-1306. [DOI] [PubMed] [Google Scholar]
- 65.Liu C, Erlichman C, McDonald CJ, Ingle JN, Zollman P, Iankov I, Russell SJ, Galanis E. Heat shock protein inhibitors increase the efficacy of measles virotherapy. Gene Ther. 2008;15(14):1024–1034. doi: 10.1038/gt.2008.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nussbaum O, Broder CC, Moss B, Stern LB, Rozenblatt S, Berger EA. Functional and structural interactions between measles virus hemagglutinin and CD46. J Virol. 1995;69(6):3341–3349. doi: 10.1128/jvi.69.6.3341-3349.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67•.Nakamura T, Peng KW, Vongpunsawad S, Harvey M, Mizuguchi H, Hayakawa T, Cattaneo R, Russell SJ. Antibody-targeted cell fusion. Nat Biotechnol. 2004;22(3):331–336. doi: 10.1038/nbt942. This study demonstrated that cells can fuse after MV-Edm retargeting and ablation of entry through CD46 and SLAM. [DOI] [PubMed] [Google Scholar]
- 68.Vongpunsawad S, Oezgun N, Braun W, Cattaneo R. Selectively receptor-blind measles viruses: Identification of residues necessary for SLAM- or CD46-induced fusion and their localization on a new hemagglutinin structural model. J Virol. 2004;78(1):302–313. doi: 10.1128/JVI.78.1.302-313.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69•.Allen C, Vongpunsawad S, Nakamura T, James CD, Schroeder M, Cattaneo R, Giannini C, Krempski J, Peng KW, Goble JM, Uhm JH, et al. Retargeted oncolytic measles strains entering via the EGFRvIII receptor maintain significant antitumor activity against gliomas with increased tumor specificity. Cancer Res. 2006;66(24):11840–11850. doi: 10.1158/0008-5472.CAN-06-1200. This paper describes the engineering of a retargeted MV-Edm strain designed to display a single-chain antibody against EGFRvIII. The EGFRvIII-retargeted virus demonstrated comparable therapeutic efficacy to the unmodified MV-CFP strain against EGFRvIII-expressing glioma lines. [DOI] [PubMed] [Google Scholar]
- 70•.Paraskevakou G, Allen C, Nakamura T, Zollman P, James CD, Peng KW, Schroeder M, Russell SJ, Galanis E. Epidermal growth factor receptor (EGFR)-retargeted measles virus strains effectively target EGFR- or EGFRvIII expressing gliomas. Mol Ther. 2007;15(4):677–686. doi: 10.1038/sj.mt.6300105. This paper describes the design of a retargeted MV-Edm strain engineered to display a single-chain antibody against EGFR. The ECFR-retargeted virus demonstrated comparable therapeutic efficacy to the unmodified virus in glioma cells overexpressing EGFR or EGFRvIII. [DOI] [PubMed] [Google Scholar]
- 71•.Allen C, Paraskevakou G, Iankov I, Schroeder M, Puri RK, Russell SJ, Galanis E. IL13 displaying retargeted oncolytic measles virus (MV) strains have significant activity and improved therapeutic index against gliomas. Mol Ther. 2007;15(Suppl 1s) Abs 51. This paper describes the engineering of a retargeted MV-Edm strain designed to display a natural ligand (IL-13). The retargeted virus demonstrated comparable therapeutic efficacy and improved specificity compared with the unmodified MV strain MV-GFP. [Google Scholar]