Abstract
Objective
This paper examines the hypothesis that the dermatan sulfate (DS) chain on decorin is a load carrying element in cartilage and that its damage or removal will alter the material properties.
Methods
To test this hypothesis, indentation and tensile testing of cartilage from bovine patella was performed before and after digestion with chondroitinase B (cB). Removal of significant amounts of DS by cB digestion was verified by Western blot analysis of proteoglycans extracted from whole and sectioned specimens. Specimens (control and treated) were subjected to a series of step-hold displacements. Elastic modulus during the step rise (rapid modulus) and at equilibrium (equilibrium modulus), and the relaxation function during each step were measured for test (cB and buffer) and control (buffer alone) conditions.
Results
cB had no effect on any of the viscoelastic mechanical properties measured, either in indentation or tension
Conclusion
Removing or damaging approximately 50% of the dermatan sulfate had no effect on the mechanical properties, strongly suggesting that dermatan sulfate either carries very low load or no load.
Introduction
The mechanical properties of cartilage derive from its complex composition and micro organization. Damage to this microstructure is thought to be involved in disease processes such as osteoarthritis, in which cartilage fibrillates and wears away1. It is thought that the fibrillation is a consequence of the weakening, but it is unknown which of the molecular elements are critical in this weakening process. Cartilage is composed primarily of water, Type II collagen and the large aggregating proteoglycan, aggrecan. However, it contains many other minor molecules, such as decorin, that have been speculated to play a critical role in the mechanical properties2,3,4.
Decorin is a member of the small leucine-rich proteoglycans (SLRP)5. Decorin in cartilage is composed of a protein core of approximately 45 kD and an attached glycosylaminoglycan (GAG) chain, dermatan sulfate (DS) of approximately 55 kD2. The core protein of decorin is found attached circumferentially to the collagen fibrils6 and the covalently attached DS chain is thought to project out from the collagen fibril into the interfibrilar space7,8.
There is evidence that decorin influences collagen fibril diameter during fibril formation9,10,11,12 and that it plays a role in the mechanical properties of healing tendons and ligaments. Scott13 proposed that decorin acts as a spacer and linker between Type I collagen fibrils in tendon. Others have proposed a more direct load carrying function for decorin through the DS GAG chain2,3,4. Nakamura14 showed that reduction of decorin concentration with antisense gene therapy increased the strength of healing rabbit tendons. Soslowsky and coworkers9,15 measured the mechanical properties of tendons/ligaments from decorin knockout mice. There was evidence that decorin influences tendon/ligament mechanical properties, but it is not clear if the effect was due directly to decorin as a load carrying element or to its effect on regulating fibril assembly in the developing animal. A recent study16 measured the elastic properties of human medial collateral ligament before and after digestion with chondroitinase B (cB) (an enzyme that removes the dermatan sulfate GAG chains from the decorin protein) and found no change in either tensile or shear modulus with cB treatment, arguing against a direct mechanical load carrying role by dermatan sulfate in ligament and tendon.
Decorin has also been found in cartilage attached to the collagen fibrils17,18,19,20,21 with the highest concentration in the surface layer, decreasing toward the deep zone1. Although it makes up a much smaller mass/mass (m/m) ratio of tissue than aggrecan in cartilage, it is present in a similar molar concentration1. Heinegard19 speculated that in cartilage, the extending DS chains aid adhesion between collagen fibrils, forming a sort of ‘glue’. Likewise, Scott and Stockwell22 applied methods of analysis to cartilage similar to those they applied to tendon and proposed that the same mechanism occurs in cartilage, i.e., that the DS chains in decorin provide a bridge and bond between Type II collagen fibrils by overlapping DS chains. DS also occurs at other sites in cartilage, such as in biglycan and other DSPGs (dermatan sulfate proteoglycans), although there has been no suggestion that they contribute a mechanical function at these sites. Like in tendons, decorin is proposed to contribute to the mechanical properties of the cartilage matrix by direct load transfer through the DS, but there is no direct data to support this hypothesis.
An approach to determine the function of a particular molecule on the mechanical properties of soft tissues is to test the tissue before and after digestion with selective enzymes23,24,25. To our knowledge, the effect of DS, specifically, on the mechanical properties of cartilage has not been studied by this method.
The goal of this study is to test the hypothesis that dermatan sulfate is a load carrying structural molecule in cartilage. To test this hypothesis, we chose to use cB enzymatic digestion for the disruption of the DS side chain to see if this caused a change in tensile and compressive mechanical properties. If dermatan sulfate does support load, and therefore plays a role in the mechanical properties, damage to this molecule could lead to degradation in mechanical properties, such as seen in osteoarthritis.
Methods
The experimental design was to indent or test in tension cartilage specimens after equilibration in phosphate buffered saline (PBS), apply the enzyme in buffer to the cB treated specimen and the buffer alone to the control specimen for the digestion period, and then retest each specimen after equilibration in PBS. Biochemical analysis was performed to ensure that DS was removed for altered. The details of this procedure were as follows.
Specimen preparation and experimental design
Indentation tests
Bovine patella were obtained from a local slaughterhouse. Articular cartilage specimens were cut from both medial and lateral facets to dimensions of 12 mm by 12 mm. Approximately 1 mm of subchondral bone was left attached to the cut specimens. The thickness of the cartilage for each specimen was determined with a calibrated reticle and stereoscope by measuring the thickness of cartilage at the specimen edge in multiple locations. Adjacent specimens were used for cB treated and control pair; 4 pairs were taken from 4 patellae. Specimens were placed in a 3 cm diameter plastic dish and fixed with dental cement, taking care not to get the cement on the cartilage surface. The tissue was then flooded with PBS and tested in indentation to obtain a 0 hour time point.
Following the time 0 hour indentation tests, the specimens were removed from the plastic dish and submersed in cB buffer (details in Supplementary Materials) with or without 0.1U/ml cB (Seikagaku 100337). Specimens were incubated at 37 °C for 24 hours under gentle agitation, after which the specimens were removed, solutions changed to PBS, equilibrated to room temperature and indented again for the 24 hour time point.
Tension tests
For tensile testing, cartilage from bovine patella was cut approximately 15 mm long by 3 mm wide and further sectioned in a cryomicrotome to approximately 0.3 mm thick. Slices were divided into surface (S), middle (M), or deep (D) zones. Specimens were equilibrated in PBS at room temperature, and a more accurate measurement of the thickness was determined using a custom device26. The specimens were then tested in tension to obtain a 0 hour time point. Following the 0 hour tensile tests, the specimens were separated into control (no cB) and cB treated specimens (cB at 1.0 U/ml; Sigma cat no. C-8058) in cB buffer defined above for approximately 38 hours at 37°C with gentle agitation. cB was used with higher concentrations in the tensile tests, compared to the indentation tests, in order to increase the amount of DS removed, based on that removed in the indentation test. In addition, enzyme from Seikagaku has a specific activity 6 times greater than the Sigma preparation. After the incubation, the specimens were equilibrated in PBS at room temperature, and the tensile test repeated to obtain a 38 hour time point.
Biochemical analysis
All biochemical analyses were performed after digestion and mechanical testing.
Proteoglycan Assay
In order to account for a possible difference in loss of aggrecan, in addition to DS, during incubation with cB, for both the indentation and tensile specimens, proteoglycan content was measured by the binding of dimethylmethylene blue (DMB) as previously described using purified proteoglycan monomer as the standard26. For indentation specimens, the proteoglycan content was determined directly from proteoglycans extracted in 4M guanidine chloride (GuCl) and data reduced to mg of proteoglycan per mg protein (details in Supplementary Material). This was done on both an entire specimen that had been cut from the bone and also a specimen that had been cut from the bone and then sectioned into 14 pieces (Figure 3). Similar procedures were used for the tensile specimens (see Supplementary Materials).
Figure 3.
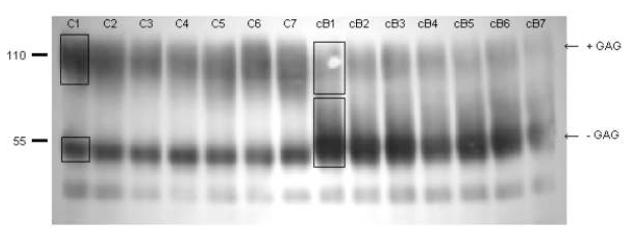
Ratio of (decorin + GAG)/((decorin + GAG) + (decorin − GAG)) for control tissue (regular font) and cB treated tissue (bold) in each section of the top half of an indentation specimen, as determined from densitometry of the Western blot in Figure 2. The number in the upper left hand corner of each section corresponds to the section number. Object not to scale.
Western blot analysis
Equal amounts of protein extracted in 4M GuCl from both control and cB specimens were separated on a Tris-HCL 10% T-acrylamide gel under reducing conditions. Resolved proteins were transferred to a nitrocellulose membrane (Millipore) and blotted for decorin using the anti-decorin antibody “6D6”27 diluted 1:10 in PBS 0.1% tween-20 followed by secondary labeling using Sigma’s rabbit anti-mouse HRP antibody diluted 1:5000 in 5 % non-fat dried milk in tris buffered saline and 0.4% tween-20. Immunoreactive bands were detected using ECL Western blotting detection reagents from Amersham Biosciences (RPN2109) following manufacturer’s instructions. Relative average amounts of decorin with GAG (+) and without GAG (-) were visually approximated in each lane and quantified by densitometry of the gels.
Mechanical testing
Indentation tests
Indentation tests were performed on an ELF 3100 test machine with 22 N load cell (Bose/EnduraTec, Inc., Minneapolis, MN) using a 2 mm diameter non-porous flat-ended cylindrical indenter tip. The plastic dishes with specimens in PBS were glued to a rotating holder on the test machine to allow indentation on a flat surface. The test protocol was displacement controlled, consisting of 3 step-holds of 0.02 mm for 240 sec and 4 step-holds of 0.04 mm for 300 sec. The step displacement was at 0.1 mm/sec. In the Step 7 hold, load relaxation rate at 300 sec was approximately - 0.2 mN/sec. Five indent tests were performed at different sites for each specimen.
For each indentation specimen pair (4 pairs), there were 4 test conditions: cB 0 hr, control 0 hr, cB 24 hr, and control 24 hr. Data for each indent (cB 0 hr N = 19; control 0 hr N = 19; cB 24 N = 18; control 24 hr N = 18) was reduced to a ‘rapid’ Young’s Modulus (E0) from the increasing displacement phase, a relaxation function (R(t)) from the hold phase for each step, and an equilibrium Young’s Modulus (Eeq) from the end of all of the hold phases. E0 and R(t) were determined at each step-hold; Eeq was determined from the 7 equilibrium points. E0 and Eeq were calculated using the Hayes solution for a flat ended indenter indenting an elastic layer28, with an assumed Poisson’s Ratio of 0.530 for rapid loading and 0.2531,32 for equilibrium using previously published methods29. The moduli for the 5 indents for a particular test condition were averaged. The average moduli data for each specimen were then reduced to E(24hr)/ E(0hr), reflecting the difference in modulus between testing at 0 hr and 24 hr due to both the effect of the cB and any change due to other factors, for example, loss of aggrecan. To assess whether cB caused a change in properties, this ratio was compared with the equivalent values for the control specimens, which would only be effected by the loss of factors other than DS.
The relaxation function for the indentation test was defined as the load minus the load at the last equilibrium, normalized by the maximum step load change during the rapid load for that step. This is not a true relaxation function, but it was a reasonable approximation and did not require committing to a specific time dependent constitutive equation. For each specimen at a particular test condition, the five normalized relaxation functions for each test condition were averaged at each step to give a single relaxation function at each step for that specimen. At each step, the normalized relaxation function of the specimen at 24 hr was divided by the value of the normalized relaxation function of the 0 hr test (same specimen) at the same time to give a function representing the change in relaxation function due to the time in solution versus time, for both the cB and control specimens. This function should always be 1 if there was no change in R(t) due to the digestion. These functions were then averaged over time for all specimens at that condition to give AvgR(t) for the cB and control specimens. The AvgR(t) for the control specimen was compared to the AvgR(t) for the cB specimen using a paired t-test at a time point near the end of the hold period since the function was monotonic and differences were greatest at the end.
Tension Tests
Tensile testing was performed on an MTS MicroBionix (MTS, Minnetonka, MN) test machine with 5 N load cell (MTS, Inc., Minneapolis, MN). Specimens were held in place by sandpaper surfaced grips. In preliminary work, ink marks were made on a series of specimens at the grip site and the test recorded on video to ensure no grip slippage. None was observed in any tests. The gage length for the test was approximately 11 mm (measured for each test) and grip displacement was used for strain calculation. The test protocol was displacement controlled, consisting of 3 step-holds of 0.35 mm for 180 sec, 0.35 mm for 240 sec, and 0.35 mm for 300 sec. The step displacement rate was at 1 mm/sec, for a step rise time of 0.35 sec. In the Step 3 hold, load relaxation rate at 300 sec was approximately − 0.06 mN/sec. Nine cB specimens were tested and nine control specimens were tested.
Data for each step was reduced to a ‘rapid’ Young’s Modulus (E0) from the increasing displacement phase, a relaxation function (R(t)) from the hold phase, and an equilibrium Young’s Modulus (Eeq) from the end of the hold phase (see Supplemental Material for justification of this method for data reduction). E0 was determined at each step-hold; Eeq was determined from the 3 equilibrium points, assuming one-dimensional stress. The relaxation data for before and after digest was treated in the same manner as the indentation data, but only the second step was used for comparison.
Several of the tensile specimens had stiffness of the same order of the error due to not achieving equilibrium. This will be discussed further in the Discussion. One specimen was extreme and data for this was not used. For seven others, the equilibrium data could not be used, but the rapid modulus data was used. This resulted in 3 control and 7 cB treated tensile specimens with equilibrium and relaxation data and 8 control and 9 cB treated specimens with rapid modulus data.
Statistical analysis
Mechanical data was analyzed by forming ratios of mechanical parameters for after/before treatment (cB in buffer or buffer alone) and comparing groups with an unpaired t-test, with p < 0.05 for significance. Statistical analysis of the western blots and proteoglycan content was performed using an unpaired t-test assuming unequal variances, except a paired (on location) t-test was used for western blot data for sectioned specimens.
Results
Model system
There was no detectable difference in proteoglycan content between the indented control and cB treated specimens (control: 1.7 ± 0.01, cB: 1.77 ± 0.05 mg pg/mg protein, p = 0.14). However western blot analysis of the extracted proteoglycans from the full cartilage thickness of an indentation specimen (Figure 1) shows that cB removed much of the GAG from decorin, although the digestion was not complete. For the whole specimen, the ratio of GAG(+)/(GAG(+) + GAG(-)) after digestion for the cB specimen was 0.52, compared to values for controls of 0.75. For a sectioned cB indentation specimen there was a small, but insignificant change in decorin in the lower half (control 0.65 ± 0.05, cB 0.59 ± 0.22, p = 0.12), but each section in the top half had less GAG(+) in the cB treated samples (Figures 2 and 3). The ratio of GAG(+)/(GAG(+) + GAG(-)) was control 0.71 ± 0.04, cB 0.28 ± 0.02, p = 5 × 10-7. From this data, approximately one-half of the DS was removed from the top half of the specimen by the cB treatment.
Figure 1.
Western blot of guanidine extracted proteoglycans from full thickness layer of indentation specimens blotted with 6D6 anti-decorin antibody. Decorin with the DS chain resolves at approximately 110 kD, whereas decorin without the DS chain resolves at approximately at 55 kD. The (+) refers to digestion with cB; the (-) is the control incubated in buffer only. DS digestion is evident by the smear below the 110 kD and greater staining at the 55 kD band (DS digestion). The blot was quantified with densitometry (see Figure 2 for areas measured), as reported in the text.
Figure 2.
Western blot for decorin in 7 sections from top layer of control and cB treated cartilage from the indentation tests. Numbers after C and cB refer to sections shown in Figure 3. Arrows indicate decorin with (+GAG) and without (-GAG) dermatan sulfate attached. Boxes indicate the regions evaluated by densitometry.
Tensile test specimens showed similar results by Western blot to the indentation specimens. There was clear evidence of removal of DS by the cB (Figure 4). (GAG(+)/(GAG(+) + GAG(-)) was control 0.76 ± 0.04, cB 0.38 ± 0.06, p = 9 × 10-10. There was no difference in total proteoglycan by DMB (control 11.3 ± 4.7, cB 11.4 ± 4.2 μg pg/mg ww tissue, p = 0.95).
Figure 4.
Western blot of guanidine extracted proteoglycans from representative tension specimens from the deep, middle, and surface regions blotted with 6D6 anti-decorin antibody. (-) represents control specimens incubated in buffer only; (+) represents incubation in cB and buffer.
Mechanical testing
For the indentation tests, there was no detectable difference between controls and cB treated specimens in either E0 or Eeq (Table 1). Since the Eeq for controls and cB treated at 0 hr were the same (0.49 ± 0.37 MPa for controls and 0.56 ± 0.19 MPa for cB treated at 0 hr, p>0.1), Eeq for all control tests at 24 hr (Eeq = 0.45 ± 0.34, N = 18) was compared with Eeq for all cB treated tests at 24 hr (Eeq = 0.47 ± 0.15, N = 18). There was no difference (p>0.8). Both controls and cB treated specimens showed reduced Eeq and Erapid due to the incubation (Table 1). Figure 5 shows the average ratio of the relaxations at 24 hr and 0 hr, for the cB treated specimens and controls for a typical step, Step 5. There was no statistical difference between the R(t) before and after digestion with cB for any of the steps. Both controls and cB digested specimens showed changes in the relaxation curve, but this was due to the incubation over the 24 hours, not the cB, since it occurred for the controls as well.
Table 1.
Equilibrium and rapid modulii ratios for indentation tests (E at 24 hr after digest or control buffer/ E at 0 hrbefore digest or buffer) for both control and cB specimens.
| Eeq (24 hr)/ Eeq (0 hr) |
E1rapid (24 hr)/ E1rapid (0 hr) |
E2rapid (24 hr)/ E2rapid (0 hr) |
E3rapid (24 hr)/ E3rapid (0 hr) |
E4rapid (24 hr)/ E4rapid (0 hr) |
E5rapid (24 hr)/ E5rapid (0 hr) |
E6rapid (24 hr)/ E6rapid (0 hr) |
E7rapid (24 hr)/ E7rapid (0 hr) |
|
|---|---|---|---|---|---|---|---|---|
| CON | 0.85 ± 0.06 (N = 4)* | 1.00 ± 0.25+ | 0.99 ± 0.24 | 0.96 ± 0.27 | 0.94 ± 0.20 | 0.96 ± 0.17 | 0.92 ± 0.19 | 0.92 ± 0.16 |
| cB | 0.86 ± 0.14 (N = 4)* | 1.72 ± 1.62** | 1.05 ± 0.31 | 0.92 ± 0.21 | 0.89 ± 0.19 | 0.91 ± 0.18 | 0.94 ± 0.16 | 0.95 ± 0.14 |
Figure 5.
Effect of cB on indentation relaxation function, R(t), for step 5 as a typical step, for controls (grey line) and cB specimens (dark line). Relaxation function at 24 hr is divided by relaxation function at 0 hr to give the curves for control and cB. Error bars represent the standard deviation near the end of the test for the specimens in that group. Left error bar is for the controls; right error bar is for the cB digested specimens. There was no statistically significant difference between control and cB for any of the steps.
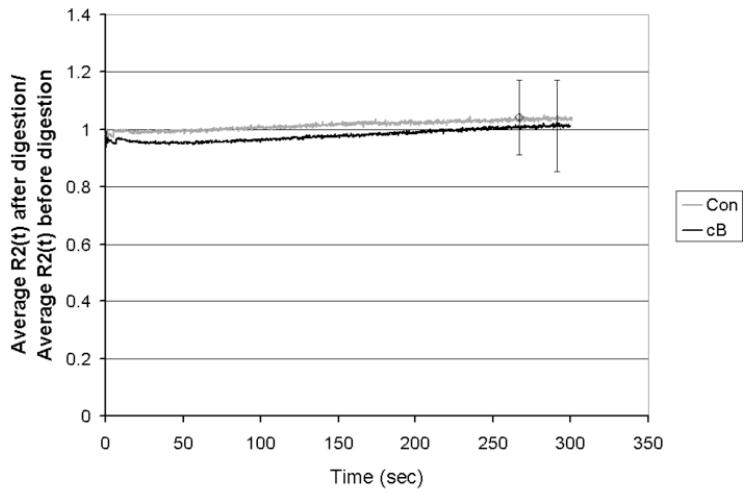
For the tensile tests, there was no change in equilibrium or rapid modulus between before and after digestion with cB and the moduli for the cB did not differ from the moduli for the controls (Table 2). There was also no statistically significant difference in the relaxation function for the second step between before and after digestion with cB (Figure 6).
Table 2.
Equilibrium and rapid modulii ratios for tension tests (after digest or control buffer/ before digest or buffer) for both control and cB specimens.
| Eeq (38 hr)/ Eeq (0 hr) |
E1rapid (38 hr)/ E1rapid (0 hr) |
E2rapid (38 hr)/ E2rapid (0 hr) |
E3rapid (38 hr)/ E3rapid (0 hr) |
|
|---|---|---|---|---|
| CON | 1.01 ± 0.10 (N = 3) | 1.09 ± 0.18 (N = 8) | 1.19 ± 0.22 (N = 8) | 1.20 ± 0.21 (N = 8) |
| cB | 1.02 ± 0.22 (N = 7) | 0.98 ± 0.16 (N = 9) | 1.02 ± 0.18 (N = 9) | 1.03 ± 0.17 (N = 9) |
cB was not different from 1 for any of the four modulii. There was no difference in the modulii between before and after cB digest. CON was not different from cB for any of the four modulii.
Figure 6.
Average relaxation function for the tension tests for the second step, R2(t), after digestion divided by R2(t) before digestion, for control specimens (CONS/S; N = 3) and specimens treated with cB (cBS,M/S,M; N = 7). There was no statistically significant difference from one in either average curve due to the incubation and CON is not different from cB at any time point.
Discussion
The goal of this study was to assess the effect of dermatan sulfate digestion on the mechanical properties of articular cartilage. Digestion of dermatan sulfate by cB had no detectable effect on E0, Eeq, or the relaxation function for either indentation or tension tests. These results refute the hypothesis that DS is a load carrying element in cartilage. A power analysis of our indentation equilibrium data was done by comparing the ratios of equilibrium modulus before and after treatment, for control and cB specimens. This indicates that we would have been able to detect a change of Eeq of 23 % due to loss of DS at a power of 80% and p<0.0533. If there was a change in the material properties of the tissue due to digestion of the dermatan sulfate chain, it was small.
Our results are consistent with the results of Lujan16 in which they found no effect of cB digestion on the quasi-static material properties of human medial collateral ligament, supporting the notion that decorin and the DS chain do not carry load and function the same in cartilage as they do in ligament. In subsequent work by this group21, they confirmed the work of Scott13 in which they demonstrated by TEM the DS chains surrounding collagen fibrils in ligament. However, their digestion studies suggest that the DS chains do not carry load, as we too have concluded. The DS chains could still perform some mechanical function, such as spacing collagen fibrils in low force conditions, but the attachment forces involved must be very low and would not resist functional external forces on the structures. Although we did not measure strength of the cartilage, it would be surprising if there were a significant effect on strength and no effect on prefailure properties, both elastic and viscous. Assuming the DS chains do not affect mechanical properties, there remains the question of what is their function. It is quite likely that they influence fibril formation as others have suggested12,18, but it is still confounding as to why they are so dense in mature ligament21 and cartilage22 if their only function is in tissue formation.
The Western blot analysis supported the effectiveness of the cB and confirmed that a significant portion of the DS was removed or damaged from the indentation specimen, although the alteration was neither spatially uniform, nor complete. Evidence from the data supports that a significant portion of the DS was digested in the top layer. This is the region (top approx. 0.8 mm) where the indentation would be expected to be the most effected34,35, since the indentation depth was less than 0.22 mm. The Western blot analysis of the tension tests also showed that approximately one-half of the DS was removed by the cB. For both indentation and tension specimens, it would be expected that this degree of damage to DS would change the mechanical properties if DS were a load carrying structure. Although we performed densitometry analysis in an attempt to quantify DS removed, based on our prior use of this method we do not believe it is a very accurate measure of the quantity of DS in the tissue.
It is known that proteoglycans can be lost from cartilage during long term incubation, and this loss could be reflected in change in mechanical properties24,25. To account for this possibility, we measured total proteoglycans in all specimens after mechanical testing. We did not assess proteoglycans before testing, since that would destroy the specimens. There was equal reduction in Eeq and Erapid for both test groups due to the digestion and the total proteoglycans of both groups after testing was the same. Since there was neither a difference in proteoglycan content nor mechanical properties betweencontrol and cB groups, but DS content did differ between the groups, the conclusion must be that the difference in DS did not cause a change in mechanical properties even though there was a change in proteoglycans.
We have focused on the dermatan sulfate attached to decorin because it has been speculated to have a direct mechanical role19,22. However, dermatan sulfate is known to attach to molecules such as biglycan and other DSPGs (dermatan sulfate proteoglycans)18 so our enzyme digestions do not uniquely attack decorin. Since there was no effect on the mechanical properties, however, this is not an issue because the conclusion remains that dermatan sulfate has no direct effect on mechanical properties, wherever it is in the matrix.
There were limitations to our study. We chose a relatively short time for the hold phase of the steps because we were concerned with specimen degradation during the test, and we also wanted to minimize the time of testing since many tests were performed. Although we did not see a problem with the indentation tests in this regard, there was a problem with the tensile tests. We found that in the tension tests the deep and mid level specimens had stiffness of the same order as the error due to not achieving equilibrium. We estimated this error by simulating the tensile experiment with a linear viscoelastic model using parameters we had fit for cartilage in a previous study35. Based on this information we estimated that the error in deduced equilibrium modulus was approximately 0.2 MPa. If the equilibrium modulus we were trying to measure were of this size we could get a substantial error. Therefore, we chose not to use any equilibrium or relaxation data if the calculated equilibrium modulus was less than 0.5 MPa. This mainly affected the deep and some mid range specimens. It was fortunate that most of the decorin is in the surface region1; our selection criterion was such that we could use all surface specimens.
Another limitation in the study was the considerable scatter in our data, especially the indentation tests. We noticed this in pilot tests and hence decided to perform 5 indents per specimen. Unfortunately, a consequence of this scatter was the relatively low power of our study; however we felt we could detect a difference of 23% in Eeq with a power of 80% in the indentation tests. This raises the possibility that the effect we were trying to detect was smaller than our resolution. Our test methods were typical for cartilage testing so we believe that others would have similar limitations. Improving resolution will be a challenge; and until these limitations are overcome, this study provides direct evidence that DS does not carry load in cartilage.
Supplementary Material
Acknowledgements
This work was supported by NIH grants AR050731 and AR049900, and the Catharine Mills Davis Chair in Biomechanical Engineering. We thank Dr. Ted Oegema, Jr. for helpful discussions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Poole AR, Rosenberg LC, Reiner A, Ionescu M, Bogoch E, Roughley PJ. Contents and distributions of the proteoglycans decorin and biglycan in normal and osteoarthritic human articular cartilage. J Orthop Res. 1996;14(5):681–689. doi: 10.1002/jor.1100140502. [DOI] [PubMed] [Google Scholar]
- 2.Redaelli A, Vesentini S, Soncini M, Vena P, Mantero S, Montevecchi FM. Possible role of decorin glycosaminoglycans in fibril to fibril force transfer in relative mature tendons — a computational study from molecular to microstructural level. J Biomech. 2003;36:1555–1569. doi: 10.1016/s0021-9290(03)00133-7. [DOI] [PubMed] [Google Scholar]
- 3.Liu X, Yeh M-L, Lewis JL, Luo Z-P. Direct measurement of the rupture force of single pair of decorin interactions. Biochemical and Biophysical Research Communications. 2005;338:1342–1345. doi: 10.1016/j.bbrc.2005.10.096. [DOI] [PubMed] [Google Scholar]
- 4.Vesentini S, Redaelli A, Montevecchi FM. Estimation of the binding force of the collagen molecule-decorin core protein complex in collagen fibril. J Biomech. 2005;38:433–443. doi: 10.1016/j.jbiomech.2004.04.032. [DOI] [PubMed] [Google Scholar]
- 5.Roughley PJ. Articular cartilage and changes in arthritis. Noncollagenous proteins and proteoglycans in the extracullular matrix of cartilage. Review. Arth Res. 2001;3(6):342–347. doi: 10.1186/ar326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vynios DH, Papageorgakopoulou N, Sazakli H, Tsiganos CP. The interactions of cartilage proteoglycans with collagens are determined by their structures. Biochimie. 2001;83(9):899–906. doi: 10.1016/s0300-9084(01)01332-3. [DOI] [PubMed] [Google Scholar]
- 7.Weber IT, Harrison RW, Iozzo RV. Model structure of decorin and implications for collagen fibrillogenesis. J Biol Chem. 1996;271(50):31767–31770. doi: 10.1074/jbc.271.50.31767. [DOI] [PubMed] [Google Scholar]
- 8.Raspanti M, Alessandrini A, Ottani V, Ruggeri A. Direct visualization of collagen-bound proteoglycans by tapping-mode atomic force microscopy. J Struct Biol. 1997;119:118–122. doi: 10.1006/jsbi.1997.3865. [DOI] [PubMed] [Google Scholar]
- 9.Robinson PS, Lin TW, Reynolds PR, Derwin KA, Iozzo RV, Soslowsky LJ. Strain rate sensitive mechanical properties of tendon fascicles from mice with genetically engineered alterations in collagen and decorin. J Biomech Eng. 2004;126:252–257. doi: 10.1115/1.1695570. [DOI] [PubMed] [Google Scholar]
- 10.Danielson KG, Baribault H, Holmes DF, Graham H, Kadler KE, Iozzo RV. Targeted disruption of decorin leads to abnormal collagen fibril morphology and skin fragility. J Cell Biol. 1997;136:729–743. doi: 10.1083/jcb.136.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pins GD, Christiansen DL, Patel R, Silver FH. Self-assembly of collagen fibers, influence of fibrillar alignment and decorin on mechanical properties. Biophys J. 1997;73:2164–2172. doi: 10.1016/S0006-3495(97)78247-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vogel KG, Trotter JA. The effects of proteoglycans on the morphology of collagen fibrils formed in vitro. Collagen Rel Res. 1987;7:105–114. doi: 10.1016/s0174-173x(87)80002-x. [DOI] [PubMed] [Google Scholar]
- 13.Scott JE. Proteoglycan-fibrillar collagen interactions. Biochem J. 1988;252:313–323. doi: 10.1042/bj2520313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakamura N, Hart DA, Boorman RS, Kaneda Y, Shrive NG, Marchuk LL, Shino K, Ochi T, Frank CB. Decorin antisense gene therapy improves functional healing of early rabbit ligament scar with enhanced collagen fibrillogenesis in vivo. J Orthop Res. 2000;18:517–523. doi: 10.1002/jor.1100180402. [DOI] [PubMed] [Google Scholar]
- 15.Elliott DM, Robinson PS, Gimbel JA, Sarver JJ, Abboud JA, Iozzo RV, Soslowsky LJ. Effect of altered matrix proteins on quasilinear viscoelastic properties in transgenic mouse tail tendons. Ann Biomed Eng. 2003;31:599–605. doi: 10.1114/1.1567282. [DOI] [PubMed] [Google Scholar]
- 16.Lujan TJ, Underwood CJ, Henninger HB, Thompson BM, Weiss JA. Effect of dermatan sulfate glycosaminoglycans on the quasi-static material properties of the human medial collateral ligament. J Orthop Res. 2007 doi: 10.1002/jor.20351. Online pub DOI 10.1002/jor.20351. [DOI] [PubMed] [Google Scholar]
- 17.Trowbridge JM, Gallo RL. Mini Review: Dermatan sulfate: new functions from an old glycosaminoglycan. Glycobiology. 2002;12:117R–125R. doi: 10.1093/glycob/cwf066. [DOI] [PubMed] [Google Scholar]
- 18.Roughly PJ. The structure and function of cartilage proteoglycans. European Cells and Materials. 2006;12:92–101. doi: 10.22203/ecm.v012a11. [DOI] [PubMed] [Google Scholar]
- 19.Heinegård D, Lorenzo P, Sommarin Y. Articular cartilage matrix proteins. In: Kuettner KE, Goldberg VM, editors. Osteoarthritic Disorders, American Academy of Orthopaedic Surgeons. 1995. pp. 229–237. Ch 16. [Google Scholar]
- 20.Visser NA, de Koning MH, Lammi MJ, Hakkinen T, Tammi M, van Kampen GP. Increase of decorin content in articular cartilage following running. Conn Tissue Res. 1998;37(34):295–302. doi: 10.3109/03008209809002446. [DOI] [PubMed] [Google Scholar]
- 21.Henninger HB, Maas SA, Underwood CJ, Whitaker RT, Weiss JA. Spatial distribution and orientation of dermatan sulfate in human medial collateral ligament. J Struct Biol. 2007;158:33–45. doi: 10.1016/j.jsb.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scott JE, Stockwell RA. Cartilage elasticity resides in shape module decoran and aggrecan sumps of damping fluid: implications in osteoarthrosis. J Physiol. 2006;574:643–650. doi: 10.1113/jphysiol.2006.108100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kempson BE, Tuke MA, Dingle JT, Barrett AJ, Horsfield PH. The effects of proteolytic enzymes on the mechanical properties of adult human articular cartilage. Biochi Biophy Acta. 1976;428:741–760. doi: 10.1016/0304-4165(76)90205-1. [DOI] [PubMed] [Google Scholar]
- 24.Korhonen RK, Laasanen MS, Töyräs J, Lappalainen R, Helminen HJ, Jurvelin JS. Fibril reinforced poroelastic model predicts specifically mechanical behavior of normal, proteoglycan depleted and collagen degraded articular cartilage. J Biomech. 2003;36:1373–1379. doi: 10.1016/s0021-9290(03)00069-1. [DOI] [PubMed] [Google Scholar]
- 25.Schmidt MB, Mow VC, Chun LE, Eyre DR. Effects of proteoglycan extraction on the tensile behavior of articular cartilage. J Orthop Res. 1990;8:353–363. doi: 10.1002/jor.1100080307. [DOI] [PubMed] [Google Scholar]
- 26.Fedewa MM, Oegema TR, Jr, Schwartz MH, MacLeod A, Lewis JL. Chondrocytes in culture produce a mechanically functional tissue. J Orthop Res. 1998;16:227–236. doi: 10.1002/jor.1100160210. [DOI] [PubMed] [Google Scholar]
- 27.Scott PG, Dodd CM, Pringle GA. Mapping the locations of the epitopes of five monoclonal antibodies to the core protein of dermatan sulfate proteoglycan II (decorin) J Biol Chem. 1993;268:11558–11564. [PubMed] [Google Scholar]
- 28.Hayes WC, Keer LM, Hermann G, Mockros LF. A mathematical analysis for indentation tests of articular cartilage. J Biomech. 1972;5:541–551. doi: 10.1016/0021-9290(72)90010-3. [DOI] [PubMed] [Google Scholar]
- 29.Simha NS, Jin H, Hall ML, Chiravarambath S, Lewis JL. Effect of indenter size on elastic modulus of cartilage measured by indentation. J Biomech Engr. 2006;129:767–775. doi: 10.1115/1.2768110. [DOI] [PubMed] [Google Scholar]
- 30.Armstrong CG, Lai WM, Mow VC. An analysis of the unconfined compression of articular cartilage. J Biomech Eng. 1984;106:165–173. doi: 10.1115/1.3138475. [DOI] [PubMed] [Google Scholar]
- 31.Athanasiou KA, Agarwal A, Dzida FJ. Comparative study of the intrinsic mechanical properties of the human acetabular and femoral head cartilage. J Ortho Res. 1994;12:340–349. doi: 10.1002/jor.1100120306. [DOI] [PubMed] [Google Scholar]
- 32.Korhonen RK, Laasanen MS, Töyräs J, Lappalainen R, Helminen HJ, Jurvelin JS. Comparison of the equilibrium response of articular cartilage in unconfined compression, confined compression and indentation. J Biomech. 2002;35:903–909. doi: 10.1016/s0021-9290(02)00052-0. [DOI] [PubMed] [Google Scholar]
- 33.Wilson DB. PowerAnalysis_V1_2.xls. 2002 http://mason.gmu.edu/∼dwilsonb/pa.html.
- 34.Korhonen RK, Wong W, Arokoski J, Lindgren R, Helminen HJ, Hunziker EB, Jurvelin JS. Importance of the superficial tissue lalyer for the indentation stiffness of articular cartilage. Med Engr Physics. 2002;24:99–108. doi: 10.1016/s1350-4533(01)00123-0. [DOI] [PubMed] [Google Scholar]
- 35.Chiravarambath S, Simha NK, Namani R, Lewis JL. Poroviscoelstic cartilage properties in the mouse from indentation. J Biomech Engr. 2008 doi: 10.1115/1.3005199. in press. [DOI] [PubMed] [Google Scholar]
- 36.Huang C-Y, Mow VC, Ateshian GA. The role of flow-independent viscoelasticity in the biphasic tensile and compressive responses of articular cartilage. J Biomech Engr. 2001;123:410–417. doi: 10.1115/1.1392316. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.