Abstract
The impact of ageing in innate immunity is poorly understood. Studies in the mouse model have described altered innate immune functions in aged macrophages, although these were not generally linked to altered expression of receptors or regulatory molecules. Moreover, the influence of ageing in the expression of these molecules has not been systematically examined. We investigated age-dependent expression differences in selected Toll-like and other pattern-recognition receptors, receptors involved in inflammatory amplification, and in transmembrane and intracellular regulators of inflammatory signaling. Young and aged macrophages were examined under resting conditions or upon activation with Porphyromonas gingivalis, a major pathogen in periodontal disease, the prevalence and severity of which increase in old age. We detected a limited number of age-dependent alterations, involving both reduction and increase of immune activity. Interestingly, surface expression of receptors that amplify inflammation (C5a anaphylatoxin receptor and triggering receptor expressed on myeloid cells [TREM]-1) was elevated in aged macrophages. No significant age-dependent differences were observed regarding the phagocytosis and intracellular killing of P. gingivalis, consistent with lack of significant changes in phagocytic receptor expression and induction of antimicrobial molecules. Therefore, at least at the cellular level, certain aspects of innate immune function may not necessarily decline with age.
Keywords: Innate immunity, macrophages, pattern-recognition receptors, ageing
1. Introduction
Infection-driven chronic inflammatory diseases generally appear rather late in life, although it is not clear whether, or what kind of, age-related alterations in innate immune recognition are responsible. Despite compelling evidence that adaptive immunity declines with age (Miller, 1996), our current state of knowledge on age-related alterations in innate immunity is less clear. A plausible hypothesis linking innate immune alterations to immunosenescence is that innate recognition of microbial pathogens declines with age, consequently resulting in defective immunosurveillance. However, this notion is not completely supported by the literature, which has not yet reached a consensus conclusion (Gomez et al., 2008; Plowden et al., 2004; Solana et al., 2006).
In addition to predisposing to increased susceptibility to a number of infectious or autoimmune diseases (Gomez et al., 2008; Miller, 1996), advanced age is also associated with poor periodontal health and increased prevalence and severity of periodontitis (Minaya-Sanchez et al., 2007; Shizukuishi et al., 1998; Streckfus et al., 1999). This oral inflammatory disease afflicts about 30% of the adult population (Oliver et al., 1998). However, an estimated 10–15% develops severe periodontitis (Papapanou, 1996), which has a systemic impact on the patients who thereby run increased risk for atherosclerotic heart disease, diabetes, pulmonary disease, adverse pregnancy outcomes, and other systemic conditions (Pihlstrom et al., 2005). In terms of etiology, periodontitis is associated with a finite group of oral pathogens that includes Porphyromonas gingivalis (Hajishengallis, 2009; Socransky et al., 1998) and periodontal tissue damage, including bone and tooth loss, may result from inadequate or excessive host responses to bacterial challenge (Baker, 2000; Gaffen and Hajishengallis, 2008; Waldrop et al., 1987). The mouse model has been productively used for in vitro and in vivo investigation of host-bacterial interactions and elucidation of the host response in periodontitis (Graves et al., 2008). Macrophages play important roles in the innate host response in periodontitis, as well as in other chronic infections (Linton and Fazio, 2003; Teng, 2006).
As a professional phagocyte that mediates first-line defense, the macrophage is equipped with Toll-like and other pattern-recognition receptors which recognize and respond to conserved microbial structures (Beutler et al., 2003). Since Toll-like receptors (TLRs) generally respond to different types of microbial structures, this property endows the macrophage (and other innate immune cells) with a degree of specificity. For instance, TLR2 responds to bacterial lipoproteins, TLR3 to double-stranded viral RNA, TLR4 to lipopolysaccharide (LPS), and TLR5 to flagellin (Beutler et al., 2003). Although most TLRs (except for TLR3) signal through the key adaptor molecule MyD88, TLR3 and TLR4 can activate MyD88-independent signaling which is mediated by the TRIF adaptor (TIR-domain-containing adapter inducing IFN-β). Moreover, TLR2 and TLR4 also utilize a MyD88-like adaptor (Mal) (O'Neill, 2006). These differences and the compartmentalization of TLRs (TLR-1, −2, −4, −5, −6 expressed on the cell surface; TLR-3, −7, −8, −9 located in endocytic vesicles) may result in qualitatively different innate responses depending on the nature of the microbial challenge.
Studies in humans suggest that a number of monocyte/macrophage functions become compromised with advancing age; these include chemotaxis, phagocytic and scavenger receptor activity, production of reactive oxygen species, the inflammatory wound healing response, and induction of certain cytokine responses (reviewed by Gomez et al., 2008; Lloberas and Celada, 2002). However, discrepant results have often been reported attributable to differences in experimental conditions and the health status of the donor subjects (Gomez et al., 2008; Lloberas and Celada, 2002). Due to several limitations associated with the use of macrophages from “healthy elderly subjects”, most studies on macrophage ageing have been performed using cells from aged mice and rats (Gomez et al., 2008; Lloberas and Celada, 2002). Despite progress in understanding which macrophage activities may be affected as a function of age, the underlying mechanisms remain poorly characterized.
In that regard, age-related alterations in macrophage functions have not generally been linked to changes in expression of receptors responsible for mediating those activities. For example, although phagocytosis appears to decline in aged mouse macrophages (reviewed by Plowden et al., 2004), the impact of ageing on phagocytic receptors is largely unknown. In fact, the impact of ageing on innate immune receptor expression in macrophages has not been systematically examined, although several studies have examined a limited number of receptors. CD14, an important co-receptor for TLR2 and TLR4 (Beutler et al., 2003), was shown to be expressed at lower levels in macrophages from aged mice compared to their young counterparts (Vega et al., 2004). Another study found that aged mouse macrophages display reduced expression of TLR1–9 at the mRNA level, although at the protein level the results were confirmed only for TLR4 (Renshaw et al., 2002). Accordingly, LPS-induced cytokine responses were found to decline with age (Renshaw et al., 2002). This observation was confirmed by an independent study, although the age-dependent reduction in cytokine responses was not attributed to decreased TLR4 expression (remained intact) but rather to decreased expression of mitogen-activated protein kinases (Boehmer et al., 2004).
In this paper, we compared macrophages from young (8–10 week old) and old (≥ 18 months of age) mice for expression of selected innate immune receptors at the mRNA and protein levels, including examination of the inducibility of these receptors in response to P. gingivalis challenge. Since age-dependent changes in macrophage responses may also involve alterations in regulatory mechanisms, we additionally investigated expression and inducibility of negative regulators of innate immune signaling. The data from these assays were then considered for correlation with findings from functional assays, including antimicrobial and cytokine responses to P. gingivalis and phagocytosis and intracellular killing of this pathogen.
2. Materials and Methods
2.1. Bacteria
P. gingivalis ATCC 32277 was grown anaerobically at 37°C in modified GAM medium (contains 5 µg/ml hemin and 1 µg/ml menadione upon reconstitution) (Nissui Pharmaceutical).
2.2. Mice, cell isolation, and culture
BALB/cByJ mice (8–10 weeks of age [young] or ≥ 18 months of age [old]) were obtained from the National Institute of Ageing. All animal procedures were approved by the Institutional Animal Care and Use Committee, in compliance with established Federal and State policies. Thioglycollate-elicited macrophages were isolated from the peritoneal cavity of mice as previously described (Hajishengallis et al., 2005b). Specifically, mice were intraperitoneally injected with 1 ml of sterile 3% thioglycollate, and cells were harvested 5 days later by flushing the peritoneal cavity with 10 ml of ice-cold PBS three times. Isolated cells were then subjected to density gradient centrifugation (Histopaque 1.083) to remove dead cells and red blood cell contamination. The purity of macrophage preparations (> 90%) was confirmed by flow cytometry using phycoerythrin-labeled anti-F4/80 (clone BM8; eBioscience). The macrophages were rested overnight (at 37°C and 5% CO2 atmosphere) prior to use in experiments. The culture medium used was RPMI 1640 (InVitrogen) supplemented with 10% heat-inactivated fetal bovine serum, 2 mM L-glutamine, 10 mM HEPES, 100 U/ml penicillin G, 100 µg/ml streptomycin, and 0.05 mM 2-mercaptoethanol. Cell viability was monitored using the CellTiter-Blue™ assay kit (Promega). None of the experimental treatments affected cell viability compared to medium-only control treatments.
2.3. Quantitative real-time PCR
Gene expression in resting or activated macrophages was quantified using quantitative real-time PCR. Briefly, RNA was extracted from cell lysates using the PerfectPure RNA cell kit (5 Prime, Fisher) and quantified by spectrometry at 260 and 280 nm. The RNA was reversetranscribed using the High-Capacity cDNA Archive kit (Applied Biosystems) and quantitative real-time PCR with cDNA was performed using the ABI 7500 Fast System, according to the manufacturer's protocol (Applied Biosystems). TaqMan probes, sense primers, and antisense primers for expression of genes shown in Figure 1, Figure 2, and Figure 4, or a house-keeping gene (GAPDH) were purchased from Applied Biosystems.
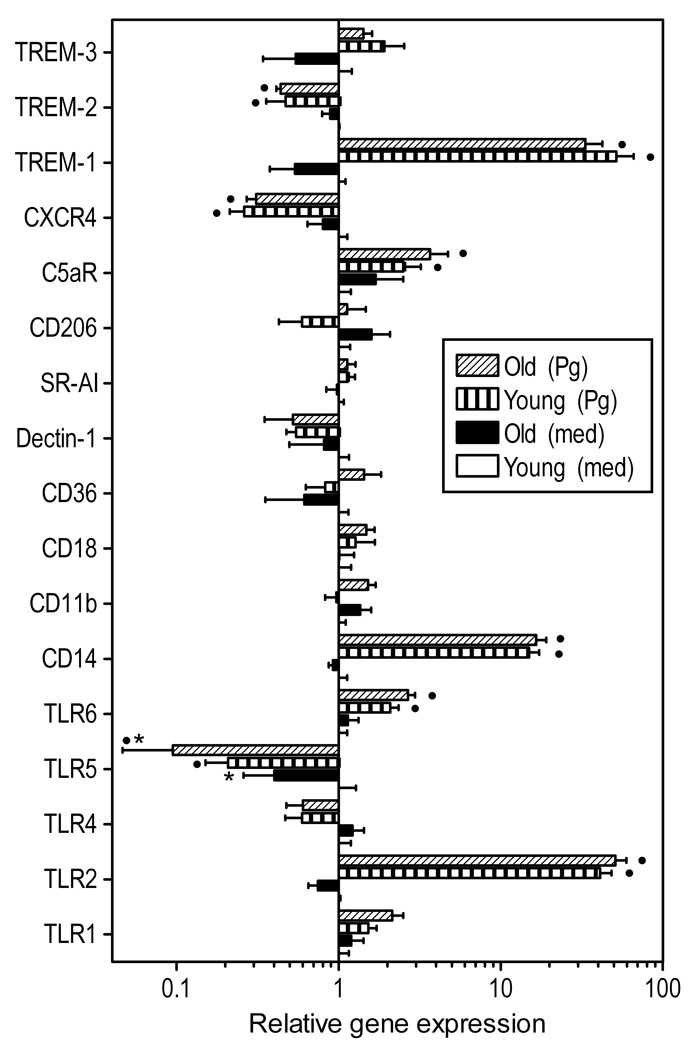
Fig. 1. Relative expression of innate immune receptors in young and aged macrophages.
Freshly explanted peritoneal macrophages from young or old mice were stimulated with P. gingivalis (Pg; MOI=10:1) or medium (med) control for 4h. Quantitative real-time PCR (qPCR) was used to determine mRNA expression levels for the indicated receptors (normalized against GAPDH mRNA levels). Results are shown as fold induction relative to unstimulated “young” macrophages. Each data point represents the mean (with standard deviation; SD) of 10 to 15 separate expression values, corresponding to qPCR analysis of total macrophage RNA from individual mice. A minimum of two-fold difference was a requirement for further testing of statistical significance. Asterisks indicate statistically significant (p < 0.05) differences between “old” and “young” macrophages, within the same activation status. Black circles show statistically significant (p < 0.05) differences compared to unstimulated macrophages, within the same age group.
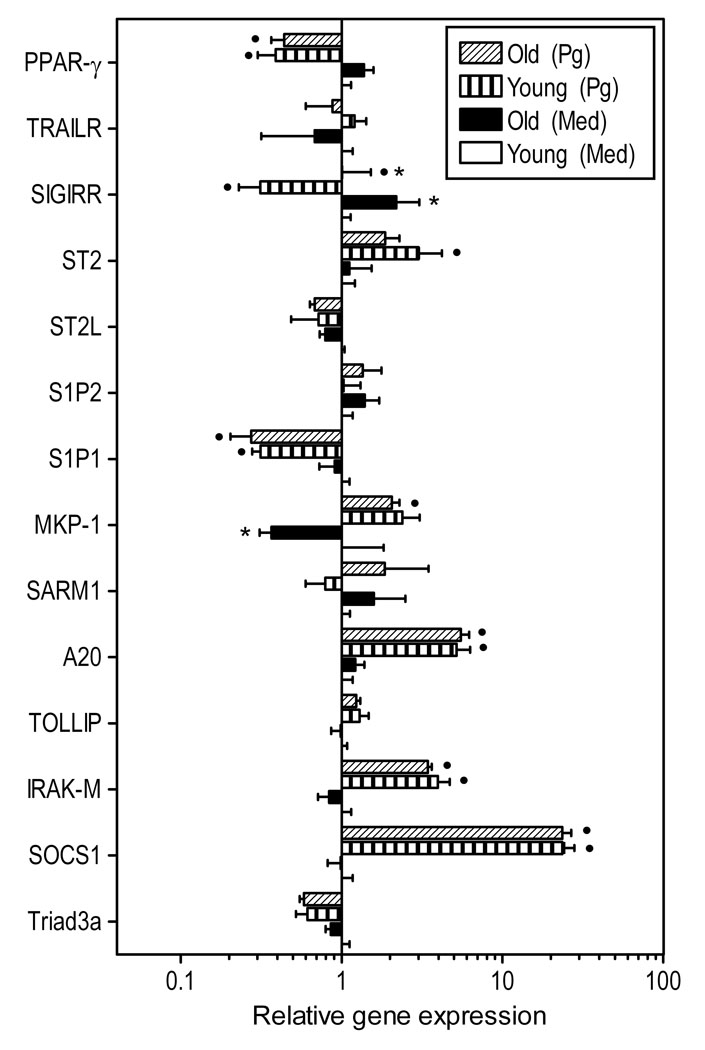
Fig. 2. Relative expression of negative regulators of TLR signaling in young and aged macrophages.
Freshly explanted peritoneal macrophages from young or old mice were stimulated with P. gingivalis (Pg; MOI = 10:1) or medium (med) control for 4h. Quantitative real-time PCR (qPCR) was used to determine mRNA expression levels for the indicated molecules (normalized against GAPDH mRNA levels). Results are shown as fold induction relative to unstimulated “young” macrophages. Each data point represents the mean (with SD) of 5 to 10 separate expression values corresponding to qPCR analysis of total macrophage RNA from individual mice. A minimum of two-fold difference was a requirement for further testing of statistical significance. Asterisks indicate statistically significant (p < 0.05) differences between “old” and “young” macrophages, within the same activation status. Black circles show statistically significant (p < 0.05) differences compared to unstimulated macrophages, within the same age group.
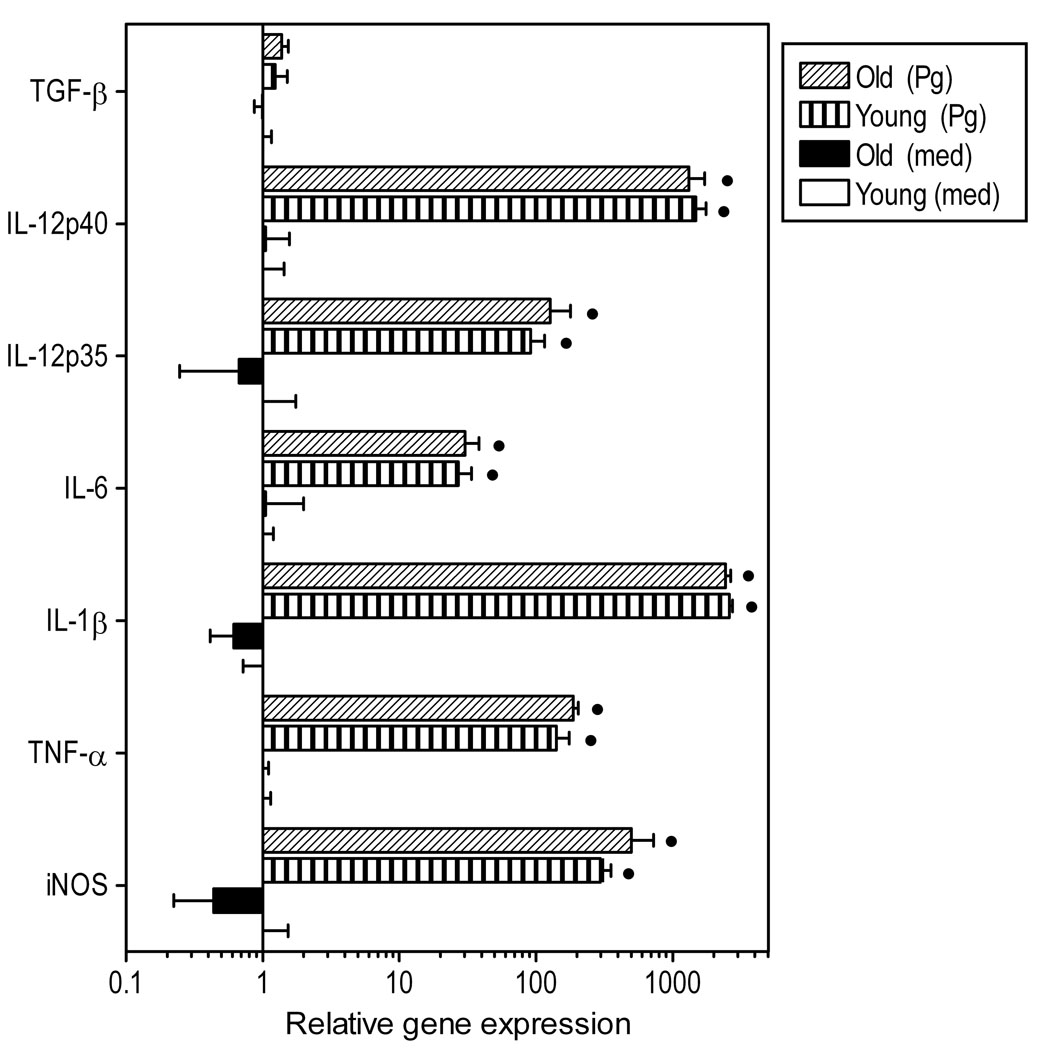
Fig. 4. Induction of innate immune response gene expression in P. gingivalis-stimulated macrophages from young or old mice.
Freshly explanted peritoneal macrophages from young or old mice were stimulated with P. gingivalis (Pg; MOI = 10:1) or medium (med) control for 4h. Quantitative real-time PCR (qPCR) was used to determine mRNA expression levels for the indicated molecules (normalized against GAPDH mRNA levels). Results are shown as fold induction relative to unstimulated “young” macrophages. Each data point represents the mean (with SD) of 5 to 10 separate expression values corresponding to qPCR analysis of total macrophage RNA from individual mice. Asterisks indicate statistically significant (p < 0.05) differences between “old” and “young” macrophages, within the same activation status. Black circles show statistically significant (p < 0.05) differences compared to unstimulated macrophages, within the same age group.
2.4 Flow cytometry and antibodies
Macrophages were incubated with fluorescently labeled specific monoclonal antibodies (mAbs) or Ig isotype controls (or with fluorescently labeled streptavidin in assays using biotinylated mAbs), in a total volume of 100 µl staining buffer (ice-cold Dulbecco's PBS containing 0.1% BSA and 0.01% azide). Subsequently, the cells were washed, fixed, and analyzed by flow cytometry (FACSCalibur, Becton-Dickinson) and the CellQuest software. The antibodies used were from the following sources. Mouse-specific mAbs to TLR1 (clone TR23), TLR2 (6C2), TLR4 (UT41), CD14 (Sa2–8), CD11b (Ml/70), CD18 (M18/2), CD36 (72-1), CXCR4 (2B11), and their isotype controls were obtained from e-Bioscience. MAbs to TLR5 (85B152.5) and Dectin-1 (2A11), and polyclonal antibody to SR-AI were from Abcam. MAbs to TLR6 (418601), TREM-1 (174031) and TREM-2 (237920), as well as polyclonal antibody to TREM-3 were from the R&D Systems. Anti-C5aR mAb (20/70) was from Cedarlane Laboratories and anti-CD206 mAb (MR5D3) was from Biolegend.
2.5. Cell activation assays
Induction of cytokine release in culture supernatants of activated mouse macrophages (plated at 2×105 cells/well) was measured using ELISA kits (eBioscience). Induction of nitric oxide (NO) production was assessed by measuring the amount of NO2− (stable metabolite of NO) in stimulated culture supernatants using a Griess reaction-based assay kit (R&D Systems).
2.6. Phagocytosis
A flow cytometric method was performed to assess the uptake of FITC-labeled P. gingivalis, as we previously described (Wang et al., 2007). Briefly, primary mouse macrophages were incubated at 37°C with FITC-labeled P. gingivalis at a MOI of 10:1 for 30 min. Phagocytosis was stopped by cooling the incubation tubes on ice. After cell washing to remove nonadherent bacteria, in some groups extracellular fluorescence (representing attached but not internalized bacteria) was quenched with 0.2% trypan blue. The cells were washed again, fixed with 1% paraforlmadehyde, and analyzed by flow cytometry (% positive cells for FITC-P. gingivalis and mean fluorescence intensity [MFI]) using the FACSCalibur and the CellQuest software (Becton-Dickinson). Association (i.e., representing both adherence and phagocytosis) or phagocytic indices were calculated using the formula (% positive cells x MFI)/100. Control experiments using cytochalasin D to block phagocytosis indicated that cytochalasin D-pretreated macrophages incubated with FITC-P. gingivalis and subsequently exposed to trypan blue did not show significant fluorescence, thus confirming that trypan blue effectively quenched extracellular fluorescence.
2.7. Antibiotic protection-based intracellular killing assay
The intracellular fate of phagocytosed P. gingivalis in macrophages was determined by an antibiotic protection-based survival assay, as we previously described (Wang et al., 2007). Briefly, following incubation of P. gingivalis with macrophages (at a multiplicity of infection [MOI] of 10:1) for various time points, extracellular bacteria were eliminated by washing and antibiotic treatment (gentamicin at 300 µg/ml and metronidazole at 200 µg/ml). Viable internalized bacteria were released by macrophage lysis and serial dilutions of the lysates were plated onto blood agar plates for anaerobic culture and CFU enumeration.
2.8. Statistical analysis
Data were evaluated by analysis of variance and the Tukey-Kramer Multiple Comparisons Test using the InStat program (GraphPad Software, San Diego, CA). Where appropriate (comparison of two groups only), two-tailed t tests were performed. P < 0.05 was taken as the level of significance. All experiments were performed at least twice for verification.
3. Results
3.1. Innate immune receptor gene expression in young and aged macrophages
Differences in innate receptor gene expression were examined in freshly explanted macrophages in a resting state or upon stimulation with P. gingivalis. Macrophages obtained from 8–10 week-old mice will be referred to as “young” and those from ≥ 18 month-old mice will be referred to as “old” or ‘aged”. We examined a selected subset of TLRs (those expressed on the cell surface; TLR-1, −2, −4, 5, and 6) and functionally associated receptors (CD14, CD1lb, CD18, CD36, and CXCR4) that are relevant to the innate immune response to P. gingivalis (Darveau et al., 2004; Hajishengallis et al., 2005a; Hajishengallis et al., 2006a; Hajishengallis et al., 2008b; Triantafilou et al., 2007). Also included in the analysis were important non-opsonindependent phagocytic receptors (the mannose receptor CD206, the β-glucan receptor Dectin-1, and the scavenger receptor SR-AI) (Linehan et al., 2000; Underhill, 2007), the complement receptor for the C5a anaphylatoxin (C5aR; CD88) (Guo and Ward, 2005), and the family of triggering receptors expressed on myeloid cells (TREM) including both activating (TREM-1 and −3) and inhibitory (TREM-2) receptors, which are thought to fine-tune the TLR-induced inflammatory response (Klesney-Tait et al., 2006). No significant differences were observed in basal gene expression between young and old macrophages, with the exception of TLR5 which was expressed at lower levels in old macrophages (Fig. 1). P. gingivalis induced significant upregulation (TLR2, TLR6, CD14, C5aR, TREM-1) or downregulation (TLR5, CXCR4, TREM-2) of about half of the receptors examined (Fig. 1). However, both young and old macrophages exhibited quantitatively comparable up- or down-regulation of receptor gene expression in response to P. gingivalis (Fig. 1). The only exception was TLR5, which was still expressed at significantly lower levels in P. gingivalis-stimulated old macrophages relative to their young counterparts (Fig. 1). These data suggest that, in general, age is not an important factor in determining the level of innate immune receptor gene expression in macrophages. Moreover, age does not seem to play an important role in how macrophages regulate their receptor expression in response to bacterial challenge.
3.2. Gene expression analysis of negative regulators of TLR signaling
The magnitude of the innate immune and inflammatory response is not exclusively dependent upon the level of pattern-recognition receptor expression but is additionally determined by intracellular regulatory molecules (Liew et al., 2005; O'Neill, 2008). We thus examined intracellular negative regulators of TLR signaling in young and old macrophages for possible differential expression and inducibility. The molecules examined and a brief rationale for their selection as important regulators are as follows. The E3 ubiquitin-protein ligase known as Triad3a promotes the degradation of certain TLRs (Liew et al., 2005), whereas the suppressor of cytokine signaling-1 (SOCS-1) promotes the degradation of the TLR2- and TLR4-associated adaptor molecule Mal (O'Neill, 2008). The Toll-interacting protein (TOLLIP) and the interleukin-1 receptor-associated kinase M (IRAK-M) interfere with TLR signaling at the IRAK-1 level, whereas A20, a key ubiquitin-editing enzyme, interferes immediately downstream by de-ubiquitinating tumour-necrosis factor-receptor-associated factor (TRAF6) (Liew et al., 2005). The mitogen-activated protein kinase phosphatase-1 (MKP-1) suppresses TLR signaling by inhibiting the mitogen-activated protein kinase pathway (O'Neill, 2008). The adaptor known as sterile alpha and HEAT/Armadillo motif protein-1(SARM-1) is a specific inhibitor of TRIF-dependent TLR signaling.
With the exception of MKP-1, the basal expression of which was significantly lower in old macrophages, there were no significant differences in gene expression between unstimulated young and old macrophages (Fig. 2). P. gingivalis induced significant upregulation of SOCS-1, IRAK-M, A20, and MKP-1, although the elevated expression of these genes was similar between young and old macrophages (Fig. 2). In contrast, the expression of Triad3a, TOLLIP, and SARM-1 was not significantly affected by P. gingivalis stimulation (Fig. 2).
We have moreover investigated the following transmembrane regulators of TLR signaling. The sphingosine 1-phosphate receptors type 1 and 2 (S1P1 and S1P2), which attenuate TLR signaling through molecular cross-talk (Duenas et al., 2008; Hughes et al., 2008). ST2L is a type I transmembrane protein which sequesters MyD88 and Mal and thereby inhibits downstream TLR signaling; its soluble form (ST2) also inhibits TLR proinflammatory signaling via a different but poorly understood mechanism (Liew et al., 2005). The single immunoglobulin interleukin-1-related receptor (SIGIRR) interferes at the IRAK level of signal transduction (Li and Qin, 2005), whereas the tumour-necrosis factor-related (TRAILR) suppresses downstream events proximate to NF-κB activation (Liew et al., 2005). The peroxisome proliferative activated receptor-γ (PPAR-γ) is a nuclear receptor which inhibits TLR proinflammatory signaling, at least in part, by binding the relA subunit of NF-kB and mediating its nuclear export (Shibolet and Podolsky, 2007).
SIGIRR was the only gene that was expressed differentially by young and old macrophages under resting conditions; specifically, it was expressed at significantly higher levels in old macrophages (Fig. 2). Although SIGIRR was significantly downregulated by P. gingivalis, old macrophages still exhibited increased SIGIRR expression relative to their young counterparts (Fig. 2). Moreover, P. gingivalis induced significant downregulation of the expression of S1P1 and PPAR-γ in both young and old macrophages, whereas ST2 was significantly upregulated only in young macrophages (Fig. 2). The expression of S1P2, ST2L, and TRAILR was not significantly affected by the presence of P. gingivalis (Fig. 2).
In summary, P. gingivalis significantly affected the expression of about half of TLR negative regulators examined, although it tended to upregulate intracellular regulators (SOCS-1, IRAK-M, A20, and MKP-1) and downregulate transmembrane regulators (S1P1, SIGIRR, and PPAR-γ). However, significant age-dependent differential expression was observed only for SIGIRR (higher in old macrophages) and MKP-1 (lower in old macrophages, though only under resting conditions).
3.3. Flow cytometric analysis of innate receptor expression in young and aged macrophages
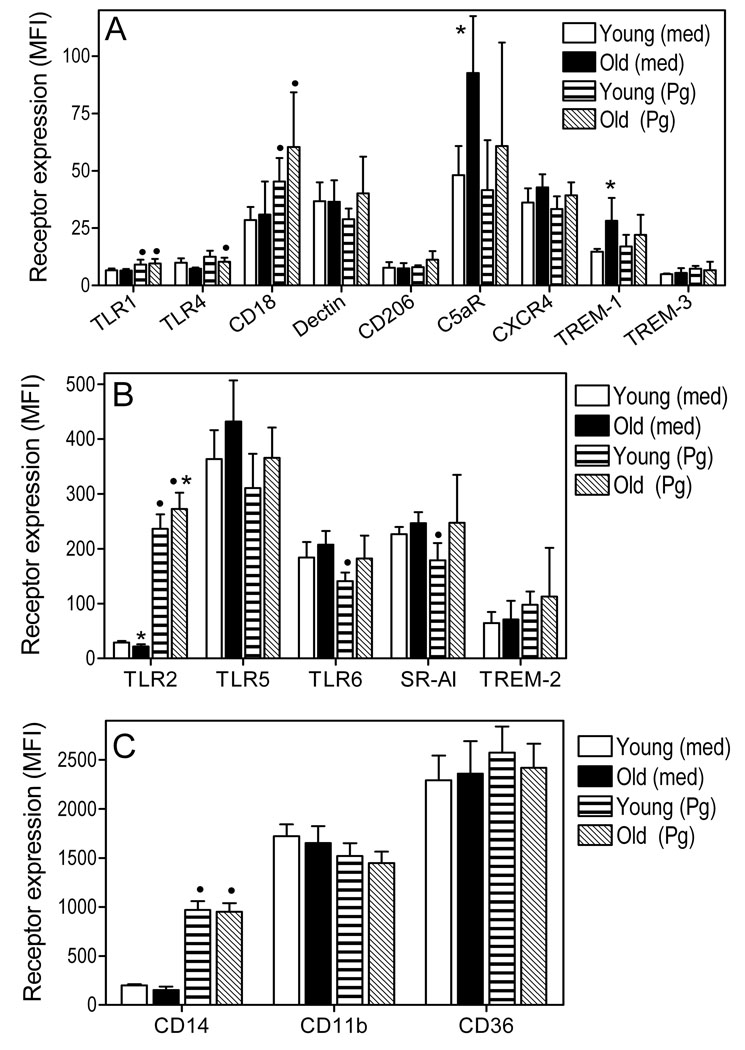
Possible differences in innate receptor expression at the protein level were investigated in freshly explanted macrophages from young and old mice. The macrophages were examined in a resting state or upon stimulation with P. gingivalis. As might be expected from the corresponding gene expression data (Fig. 1), we did not observe significant age-dependent differences in surface expression for most of the receptors investigated (Fig. 3). Statistically significant differences (p < 0.05) were observed for C5aR, TREM-1 (Fig. 3A) and TLR2 (Fig. 3B), but TLR2 involved minor differences which may not be biologically important. Resting old macrophages exhibited about two-fold higher surface expression of C5aR and TREM-1 compared to their young counterparts, although these differences were blunted upon activation with P. gingivalis (Fig. 3A). Upon stimulation, both young and old macrophages displayed significant (p < 0.05) upregulation of TLR2 (≥ 8-fold), CD14 (≥ 5-fold), CD18 (1.6- to 2-fold), and TLR1 (40 to 50 %) (Fig. 3). The findings for the highly upregulated receptors (TLR2 and CD14) were anticipated by the corresponding mRNA expression data (Fig. 1), although this was not the case for the modestly upregulated CD18 and TLR1 (in fact, CD18 and TLR1 mRNA expression was upregulated from a statistical point of view, but the two-fold cut-off difference criterion was not met; Fig. 1). Macrophage stimulation with P. gingivalis also resulted in reduced surface receptor expression; these observations were interestingly restricted to young macrophages and involved TLR6 and SR-AI (Fig. 3). In summary, although macrophages regulate innate immune receptor expression at the protein level in response to P. gingivalis, only C5aR and TREM-1 display age-dependent differential surface expression among 17 receptors investigated.
Fig. 3. Flow cytometric expression analysis of selected innate immune receptors in young and aged macrophages.
Freshly explanted peritoneal macrophages from young or old mice were stimulated with P. gingivalis (Pg; MOI = 10:1) or medium (med) control for 18h. The expression levels of the indicated receptors was determined by flow cytometry after cell staining with appropriate fluorescently labeled antibodies, and results are shown as mean fluorescent intensity (MFI). The data were assigned to three panels (A–C) according to receptor expression level (A, low; B, medium; and C, high) to facilitate visualization of differences. Each data point represents the mean (with SD) of 5 to 10 separate MFI values corresponding to flow cytometric analysis of macrophages from individual mice. Asterisks indicate statistically significant (p < 0.05) differences between “old” and “young” macrophages, within the same activation status. Black circles show statistically significant (p < 0.05) differences compared to unstimulated macrophages, within the same age group.
3.4. Induction of innate immune responses in macrophages from young and aged mice
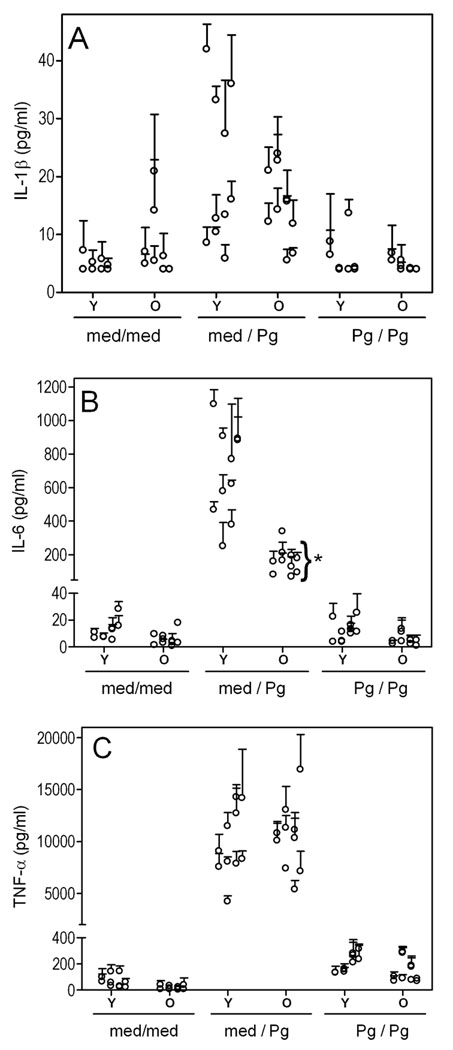
We next determined possible age-dependent differences in the inducibility of a number of immune response genes. Stimulation of young and old macrophages with P. gingivalis resulted in comparable upregulation of gene expression of the inducible nitric oxide synthase (iNOS) and of proinflammatory cytokines (TNF-α, IL-lβ, IL-6, IL-12p35, and IL-12p40), although mRNA expression of the immunosuppressive cytokine TGF-β was not significantly affected (Fig. 4). At the protein level, P. gingivalis induced low levels of IL-lβ (Fig. 5A) but high levels of IL-6 (Fig. 5B) and especially TNF-α (Fig. 5C). However, significant age-dependent differences were observed only for IL-6 (p < 0.05). Therefore, in general, there was no strong correlation between cytokine induction data at the mRNA and the protein levels. We additionally examined cytokine production following a secondary challenge with P. gingivalis to determine possible age-dependent differences in the induction of tolerance, i.e., a transient state of reduced ability to elicit proinflammatory responses to repeated stimulation. We found that young and old macrophages elicited similarly attenuated secondary cytokine responses, suggesting that they were tolerized to a comparable extent (Fig. 5).
Fig. 5. Induction of primary and secondary cytokine responses in P. gingivalis-stimulated young and aged macrophages.
Freshly explanted peritoneal macrophages from young or old mice were incubated overnight with medium only (med) or with P. gingivalis (Pg; MOI = 10:1). The following day, the cells were washed and restimulated or not, as indicated. After an additional overnight incubation, culture supernatants were collected and assayed for IL-lβ (A), IL-6 (B), or TNF-α (C). Data points are means with SD (n = 3); each point represents a triplicate group of macrophages harvested from individual mice (10 young or old mice were used for each condition; unstimulated [med/med], primary response [med/Pg], secondary response [Pg/Pg]). Asterisks indicate statistically significant (p < 0.05) differences between “old” and “young” macrophages, within the same activation status.
3.5. Comparison of young and aged macrophages in terms of bacterial uptake and killing
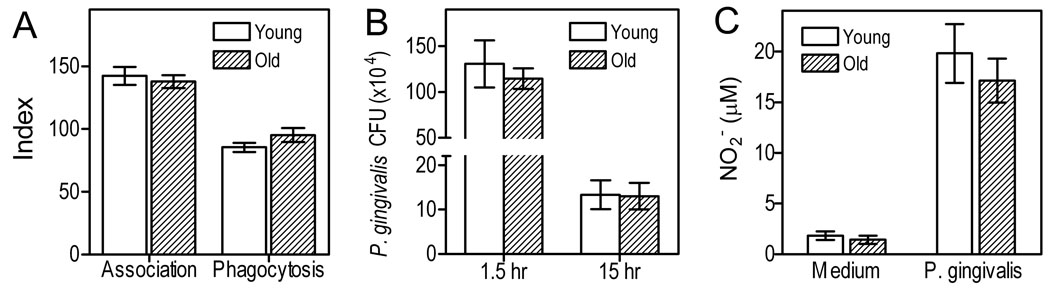
We next determined the comparative abilities of young and old macrophages for uptake and intracellular killing of P. gingivalis. We observed no differences with regard to the association (measuring both adherent and phagocytosed bacteria) of P. gingivalis with either age group, or in terms of the phagocytic capacity of young and aged macrophages (Fig. 6A). Since the intracellular bacterial load was similar between the two age groups, we could validly determine their respective intracellular killing efficiency by monitoring the intracellular survival of P. gingivalis. Enumeration of viable CFU at 1.5h and 15h post-challenge indicated comparable killing of the pathogen by either age group. Consistent with these results, we found that the production of nitric oxide, a key effector for P. gingivalis killing (Hajishengallis et al., 2008b), was not significantly different between young and old macrophages (Fig. 6C). The latter finding is also consistent with comparable induction of iNOS in young and aged macrophages (Fig. 4).
Fig. 6. Compative uptake and killing of P. gingivalis in young vs. aged macrophages.
(A) Mouse macrophages were incubated for 30 min with FITC-labeled P. gingivalis strains (MOI = 10:1). Association (i.e., representing both adherence and phagocytosis) or phagocytic indices were determined by flow cytometry, as outlined in Materials and Methods, using the following formula: % positive cells for FITC-P. gingivalis x MFI/100. (B–C) Mouse macrophages were infected with P. gingivalis (MOI = 10:1) and viable CFU of internalized bacteria were determined using an intracellular survival assay at 1.5h and 15h postinfection (B). After 15 h, macrophages were also assayed for production of NO2 (stable metabolite of NO) using a Griess reaction-based kit (C). Data are means (with SD) of triplicate determinations. Asterisks indicate statistically significant (p < 0.05) differences between “old” and “young” macrophages.
4. Discussion
In this comprehensive but targeted study on age-related changes of innate immune receptor expression and function, we detected only limited differences in macrophages from young and old mice. Aged macrophages exhibited about two-fold higher surface expression of C5aR and TREM-1. C5aR has been implicated in various local or systemic autoimmune and inflammatory conditions and, therefore, has been targeted for antagonistic therapeutic intervention (Ricklin and Lambris, 2007). TREM-1 is less well characterized but also functions to amplify inflammation (Klesney-Tait et al., 2006). It is possible that increased surface expression of C5aR and TREM-1 by macrophages (or other inflammatory cells) in advanced age may be a mechanism that contributes, at least in part, to the heightened inflammatory status associated with advanced age (Franceschi et al., 2000b). Moreover, the observed age-related differential mRNA expression of SIGIRR and MKP-1, which can regulate TLR signal transduction (O'Neill, 2008; Li and Qin, 2005), may impact on the way macrophages respond to microbial TLR ligands, although additional studies are warranted to confirm the significance of these results.
In addition to alterations in the expression of receptors or signaling molecules, signal transduction may be altered in old age as a consequence of changes in the composition of membrane lipid rafts (Fulop et al., 2004), which serve as cellular signaling platforms (Simons and Ikonen, 1997). For example, altered TLR4 signaling in aged human neutrophils was attributed to changes in the recruitability of this receptor to lipid rafts, which display age-related physicochemical changes (Fulop et al., 2004). Similarly, altered TREM-1 signaling in the same cell type was explained by defective recruitment of TREM-1 to lipid rafts (Fortin et al., 2007). Whether lipid raft-dependent changes also affect signaling in other innate immune cell types and occur across species has not been specifically addressed. However, given the similar structural organization and the global importance of lipid rafts in cell signaling (Hajishengallis et al., 2006a; Simons and Ikonen, 1997; Triantafilou et al., 2002), this possibility seems plausible. It is conceivable that age-associated alterations in lipid raft composition and function could affect intracellular signaling even when surface receptor expression does not change with age.
The age-dependent differences in C5aR and TREM-1 surface expression were not anticipated by the gene expression data. For example, although C5aR mRNA was expressed at higher levels in resting old macrophages than in young controls (in terms of simple statistical difference), the two-fold cut-off difference criterion was not met. On the other hand, although the gene expression data suggested that TLR5 may be downregulated in old age, this notion was not supported by flow cytometric analysis at the protein level. Even though there were instances where great differences in gene expression (e.g., upregulation of TLR2 and CD14 mRNA upon activation of both young and old macrophages) were fully confirmed at the protein level, gene expression data would generally be considered as suggestive. Indeed, possible posttranscriptional and/or post-translational regulation may have a great impact on protein expression levels.
Consistent with limited age-dependent expression differences in innate immune receptors or in regulatory molecules of TLR signaling pathways, we did not observe significant differences in the abilities of young and old macrophages to respond to P. gingivalis upon a primary or secondary challenge. A notable exception, however, was the production of IL-6 which was dramatically reduced in old macrophages. Reduced induction of IL-6 production has also been seen in aged human monocytes (Delpedro et al., 1998). Interestingly, production of IL-6 reverses the suppressive function of T regulatory cells and this is thought to be an effective mechanism whereby adjuvants stimulate immunity and host defense (Pasare and Medzhitov, 2003). Aged individuals display impaired immune responses after vaccination (van Duin et al., 2007) but whether this could be attributed to decreased IL-6 production has not been addressed as yet.
Reduced IL-6 production in aged mouse macrophages was also seen in response to purified TLR ligands, such as LPS, although different explanations were proposed (Boehmer et al., 2004; Boehmer et al., 2005; Renshaw et al., 2002). Renshaw et al attributed the observed reduced cytokine production to age-dependent decrease of TLR4 surface expression (Renshaw et al., 2002). On the other hand, Boehmer et al did not observe reduced TLR4 surface expression in old macrophages, and attributed their reduced responsiveness to reduced expression of mitogen-activated protein kinases (Boehmer et al., 2004; Boehmer et al., 2005). In a subsequent study by the same group, decreased TLR2-induced cytokine production was similarly linked to age-dependent reduced expression of mitogen-activated protein kinases, rather than changes in receptor surface expression (Boehmer et al., 2005). Interestingly, our study and those by Boehmer et al used BALB/c mice, whereas Renshaw et al used C57BL/6 mice. Whether the use of different mouse strains may result in distinct outcomes regarding the impact of ageing on TLR expression is uncertain, but it is a possibility that needs to be considered.
No age-dependent differences in the phagocytosis of P. gingivalis were found in our study, as also seen with Candida albicans, which was phagocytosed comparably by young and old mouse macrophages (Chen and Johnson, 1993). Although in general phagocytosis is believed to decline as a function of age (reviewed by Plowden et al., 2004), our findings are consistent with the fact that no age-dependent differences were seen in the surface expression of TLR2, TLR1, CD14, CD11b, CD18. Indeed, these receptors play a major role in the macrophage uptake of P. gingivalis (Hajishengallis et al., 2006b). Specifically, we previously showed that P. gingivalis activates the ligand-binding capacity of CR3 (CD11b/CD18) via CD14-TLR2/TLR1 inside-out signaling, leading to its internalization through a relatively safe pathway where the pathogen can persist in a viable state (Hajishengallis et al., 2007; Wang et al., 2007). Interestingly, these receptors (especially TLR2 and CD14) were upregulated in both age groups by P. gingivalis. It is conceivable that the macrophage phagocytic activity is not determined solely by host-related factors such as age, but it could also be regulated by the pathogens, especially those that proactively stimulate their phagocytosis for immune evasion.
Young and aged macrophages also exhibited comparable induction of iNOS and production of nitric oxide. This antimicrobial molecule is critical for the intracellular killing of P. gingivalis (Hajishengallis et al., 2008b), which is exquisitely resistant to killing by the oxidative burst (Hajishengallis et al., 2008a; Mydel et al., 2006). Not surprisingly, therefore, the intracellular killing of P. gingivalis did not differ between young and old macrophages. Our data are not in accordance with a study by Kissin et al who found that the ability of activated mouse macrophages to express iNOS and produce nitric oxide declines with age (Kissin et al., 1997). These investigators used purified Escherichia coli LPS as stimulus, whereas we used whole cells of P. gingivalis, which produces atypical LPS molecules that are biochemically and biologically distinct from enterobacterial LPS (Dixon and Darveau, 2005). It is possible that the nature of the stimuli used in these assays may influence the outcome, although this possibility has not been systematically addressed.
In contrast to our in vitro intracellular killing assays, in on-going in vivo studies we have found that old mice show impaired killing of P. gingivalis in comparison with their young counterparts (unpublished data). Superficially, this appears paradoxical and needs to be discussed further. It has been recently argued that the various tissues may have greater control over the initiation of immunity than previously thought (Matzinger, 2007). In this regard, the function of macrophages may be dictated, in large part, by the type and condition of the tissue they are located. In the context of ageing, therefore, it is likely that the aged local microenvironment may influence the function of macrophages in ways that cannot be replicated under in vitro culture conditions. For example, at least some of the innate immune dysfunction in old age may be due to inefficient communication between macrophages (or other innate immune cells) and the tissues, which appear to express lower levels of adhesion molecules and display reduced responsiveness to growth factors (Stout and Suttles, 2005).
These notions may account for discrepancies between in vitro and in vivo studies on the impact of ageing on innate immunity and the control of bacterial infection. In this regard, a recent study could not correlate the in vitro fungicidal activities of human neutrophils from young and old donors to the increased susceptibility to C. albicans infections in elderly individuals (Murciano et al., 2007). Moreover, the concept that the tissues are very important in regulating cellular function may also reconcile the observed limited in vitro phenotypic and functional differences between young and aged macrophages with the elevated chronic inflammatory status in old age (Franceschi et al., 2000b; Gomez et al., 2008). Alternatively, or in addition, the progressively increased proinflammatory status seen in advanced age may be secondary to inability to control infections. Specifically, uncontrolled overgrowth of microbes and persistent challenge may lead to elevated inflammatory responses, even if the inflammatory cells in aged individuals are not intrinsically more proinflammatory than their younger counterparts. The above discussed considerations raise concerns on the transferability of in vitro data to in vivo situations, although in vitro studies may have suggestive value for understanding the impact of ageing on macrophage function and innate immunity in general.
The heightened chronic inflammatory status associated with advanced age in humans has been aptly coined as “inflamm-aging” (Franceschi et al., 2000b). Although it is generally uncertain whether experimental mice undergo inflamm-aging like humans do, it is possible that mouse inflamm-aging may be manifested at least in certain tissues. In this regard, we have recently found that mice develop inflammatory periodontitis as a function of age, induced by their indigenous oral microbiota and characterized by dramatic bone loss and elevated expression of proinflammatory cytokines in the periodontal tissue (unpublished observations). It is thus possible that chronic inflammatory periodontal bone loss may be an example of inflamm-aging that is shared by both humans and mice.
To our knowledge, this is the first study that has comprehensively examined the impact of ageing on macrophage expression of innate immune receptors and signaling regulators, and on the macrophage ability to respond to and control a microbial challenge. Although in vitro studies may reveal intrinsic age-related alterations in macrophage function, as seen in our study with the elevated C5aR and TREM-1 surface expression in old macrophages, ageing may exert additional influence when the cells function in their native microenvironments. In summary, our data support the notion that, at least at the cellular level, certain aspects of innate immune function may not necessarily decline with age. In fact, any age-associated changes that could dysregulate innate immunity are likely to be dynamic, involving both loss and gain of immune activity (DeVeale et al., 2004; Franceschi et al., 2000a; Gomez et al., 2008; Plackett et al., 2004; Solana et al., 2006).
Acknowledgements
This study was supported by U.S. Public Health Service Grants DE015254, and DE018292 (to G.H.)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baker PJ. The role of immune responses in bone loss during periodontal disease. Microbes Infect. 2000;2:1181–1192. doi: 10.1016/s1286-4579(00)01272-7. [DOI] [PubMed] [Google Scholar]
- Beutler B, Hoebe K, Du X, Ulevitch RJ. How we detect microbes and respond to them: the Toll-like receptors and their transducers. J. Leukoc. Biol. 2003;74:479–485. doi: 10.1189/jlb.0203082. [DOI] [PubMed] [Google Scholar]
- Boehmer ED, Goral J, Faunce DE, Kovacs EJ. Age-dependent decrease in Tolllike receptor 4-mediated proinflammatory cytokine production and mitogen-activated protein kinase expression. J. Leukoc. Biol. 2004;75:342–349. doi: 10.1189/jlb.0803389. [DOI] [PubMed] [Google Scholar]
- Boehmer ED, Meehan MJ, Cutro BT, Kovacs EJ. Aging negatively skews macrophage TLR2- and TLR4-mediated pro-inflammatory responses without affecting the IL-2-stimulated pathway. Mech. Ageing Dev. 2005;126:1305–1313. doi: 10.1016/j.mad.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Chen Y, Johnson AG. In vivo activation of macrophages by prolactin from young and aging mice. Int. J. Immunopharmacol. 1993;15:39–45. doi: 10.1016/0192-0561(93)90029-x. [DOI] [PubMed] [Google Scholar]
- Darveau RP, Pham TT, Lemley K, Reife RA, Bainbridge BW, Coats SR, Howald WN, Way SS, Hajjar AM. Porphyromonas gingivalis lipopolysaccharide contains multiple lipid A species that functionally interact with both toll-like receptors 2 and 4. Infect. Immun. 2004;72:5041–5051. doi: 10.1128/IAI.72.9.5041-5051.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delpedro AD, Barjavel MJ, Mamdouh Z, Faure S, Bakouche O. Signal transduction in LPS-activated aged and young monocytes. J. Interferon Cytokine Res. 1998;18:429–437. doi: 10.1089/jir.1998.18.429. [DOI] [PubMed] [Google Scholar]
- DeVeale B, Brummel T, Seroude L. Immunity and aging: the enemy within? Aging Cell. 2004;3:195–208. doi: 10.1111/j.1474-9728.2004.00106.x. [DOI] [PubMed] [Google Scholar]
- Dixon DR, Darveau RP. Lipopolysaccharide heterogeneity: innate host responses to bacterial modification of lipid a structure. J. Dent. Res. 2005;84:584–595. doi: 10.1177/154405910508400702. [DOI] [PubMed] [Google Scholar]
- Duenas AI, Aceves M, Fernandez-Pisonero I, Gomez C, Orduna A, Crespo MS, Garcia-Rodriguez C. Selective attenuation of Toll-like receptor 2 signalling may explain the atheroprotective effect of sphingosine 1-phosphate. Cardiovasc. Res. 2008;79:537–544. doi: 10.1093/cvr/cvn087. [DOI] [PubMed] [Google Scholar]
- Fortin CF, Lesur O, Fulop T., Jr Effects of aging on triggering receptor expressed on myeloid cells (TREM)-1-induced PMN functions. FEBS Lett. 2007;581:1173–1178. doi: 10.1016/j.febslet.2007.02.029. [DOI] [PubMed] [Google Scholar]
- Franceschi C, Bonafe M, Valensin S. Human immunosenescence: the prevailing of innate immunity, the failing of clonotypic immunity, and the filling of immunological space. Vaccine. 2000a;18:1717–1720. doi: 10.1016/s0264-410x(99)00513-7. [DOI] [PubMed] [Google Scholar]
- Franceschi C, Bonafe M, Valensin S, Olivieri F, De Luca M, Ottaviani E, De Benedictis G. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann. N. Y. Acad. Sci. 2000b;908:244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- Fulop T, Larbi A, Douziech N, Fortin C, Guerard KP, Lesur O, Khalil A, Dupuis G. Signal transduction and functional changes in neutrophils with aging. Aging Cell. 2004;3:217–226. doi: 10.1111/j.1474-9728.2004.00110.x. [DOI] [PubMed] [Google Scholar]
- Gaffen SL, Hajishengallis G. A new inflammatory cytokine on the block: rethinking periodontal disease and the Th1/Th2 paradigm in the context of Th17 cells and IL-17. J. Dent. Res. 2008;87:817–828. doi: 10.1177/154405910808700908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez CR, Nomellini V, Faunce DE, Kovacs EJ. Innate immunity and aging. Exp. Gerontol. 2008;43:718–728. doi: 10.1016/j.exger.2008.05.0168.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves DT, Fine D, Teng Y-TA, Van Dyke TE, Hajishengallis G. The use of rodent models to investigate host-bacteria interactions related to periodontal diseases. J. Clin. Periodontol. 2008;35:89–105. doi: 10.1111/j.1600-051X.2007.01172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo RF, Ward PA. Role of C5a in inflammatory responses. Annu. Rev. Immunol. 2005;23:821–852. doi: 10.1146/annurev.immunol.23.021704.115835. [DOI] [PubMed] [Google Scholar]
- Hajishengallis G. Porphyromonas gingivalis-host interactions: Open war or intelligent guerilla tactics? Microbes Infect. 2009 doi: 10.1016/j.micinf.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajishengallis G, Ratti P, Harokopakis E. Peptide mapping of bacterial fimbrial epitopes interacting with pattern recognition receptors. J. Biol. Chem. 2005a;280:38902–38913. doi: 10.1074/jbc.M507326200. [DOI] [PubMed] [Google Scholar]
- Hajishengallis G, Shakhatreh M-AK, Wang M, Liang S. Complement receptor 3 blockade promotes IL-12-mediated clearance of Porphyromonas gingivalis and negates its virulence in vivo. J. Immunol. 2007;179:2359–2367. doi: 10.4049/jimmunol.179.4.2359. [DOI] [PubMed] [Google Scholar]
- Hajishengallis G, Tapping RI, Harokopakis E, Nishiyama S-I, Ratti P, Schifferle RE, Lyle EA, Triantafilou M, Triantafilou K, Yoshimura F. Differential interactions of fimbriae and lipopolysaccharide from Porphyromonas gingivalis with the Toll-like receptor 2-centred pattern recognition apparatus. Cell. Microbiol. 2006a;8:1557–1570. doi: 10.1111/j.1462-5822.2006.00730.x. [DOI] [PubMed] [Google Scholar]
- Hajishengallis G, Tapping RI, Martin MH, Nawar H, Lyle EA, Russell MW, Connell TD. Toll-like receptor 2 mediates cellular activation by the B subunits of type II heat-labile enterotoxins. Infect. Immun. 2005b;73:1343–1349. doi: 10.1128/IAI.73.3.1343-1349.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajishengallis G, Wang M, Bagby GJ, Nelson S. Importance of TLR2 in early innate immune response to acute pulmonary infection with Porphyromonas gingivalis in mice. J. Immunol. 2008a;181:4141–4149. doi: 10.4049/jimmunol.181.6.4141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajishengallis G, Wang M, Harokopakis E, Triantafilou M, Triantafilou K. Porphyromonas gingivalis fimbriae proactively modulate β2 integrin adhesive activity and promote binding to and internalization by macrophages. Infect. Immun. 2006b;74:5658–5666. doi: 10.1128/IAI.00784-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajishengallis G, Wang M, Liang S, Triantafilou M, Triantafilou K. Pathogen induction of CXCR4/TLR2 cross-talk impairs host defense function. Proc. Natl. Acad. Sci. U S A. 2008b;105:13532–13537. doi: 10.1073/pnas.0803852105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JE, Srinivasan S, Lynch KR, Proia RL, Ferdek P, Hedrick CC. Sphingosine-1-phosphate induces an antiinflammatory phenotype in macrophages. Circ. Res. 2008;102:950–958. doi: 10.1161/CIRCRESAHA.107.170779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissin E, Tomasi M, McCartney-Francis N, Gibbs CL, Smith PD. Age-related decline in murine macrophage production of nitric oxide. J. Infect. Dis. 1997;175:1004–1007. doi: 10.1086/513959. [DOI] [PubMed] [Google Scholar]
- Klesney-Tait J, Turnbull IR, Colonna M. The TREM receptor family and signal integration. Nat. Immunol. 2006;7:1266–1273. doi: 10.1038/ni1411. [DOI] [PubMed] [Google Scholar]
- Li X, Qin J. Modulation of Toll-interleukin 1 receptor mediated signaling. J. Mol. Med. 2005;83:258–266. doi: 10.1007/s00109-004-0622-4. [DOI] [PubMed] [Google Scholar]
- Liew FY, Xu D, Brint EK, O'Neill LAJ. Negative regulation of Toll-like receptor-mediated immune responses. Nat. Rev. Immunol. 2005;5:446–458. doi: 10.1038/nri1630. [DOI] [PubMed] [Google Scholar]
- Linehan SA, Martinez-Pomares L, Gordon S. Mannose receptor and scavenger receptor: two macrophage pattern recognition receptors with diverse functions in tissue homeostasis and host defense. Adv. Exp. Med. Biol. 2000;479:1–14. doi: 10.1007/0-306-46831-X_1. [DOI] [PubMed] [Google Scholar]
- Linton MF, Fazio S. Macrophages inflammation, and atherosclerosis. Int. J. Obes. Relat. Metab. Disord. 2003;27 Suppl 3:S35–S40. doi: 10.1038/sj.ijo.0802498. [DOI] [PubMed] [Google Scholar]
- Lloberas J, Celada A. Effect of aging on macrophage function. Exp. Gerontol. 2002;37:1325–1331. doi: 10.1016/s0531-5565(02)00125-0. [DOI] [PubMed] [Google Scholar]
- Matzinger P. Friendly and dangerous signals: is the tissue in control? Nat. Immunol. 2007;8:11–13. doi: 10.1038/ni0107-11. [DOI] [PubMed] [Google Scholar]
- Miller RA. The aging immune system: primer and prospectus. Science. 1996;273:70–74. doi: 10.1126/science.273.5271.70. [DOI] [PubMed] [Google Scholar]
- Minaya-Sanchez M, Medina-Solis CE, Maupome G, Vallejos-Sanchez AA, Casanova-Rosado JF, Marquez-Corona MD. Prevalence of and risk indicators for chronic periodontitis in males from Campeche, Mexico. Rev. Salud. Publica. 2007;9:388–398. doi: 10.1590/s0124-00642007000300007. [DOI] [PubMed] [Google Scholar]
- Murciano C, Villamon E, Yanez A, Murciano J, Mir A, O'Connor JE, Gozalbo D, Gil ML. In vitro response to Candida albicans in cultures of whole human blood from young and aged donors. FEMS Immunol. Med. Microbiol. 2007;51:327–335. doi: 10.1111/j.1574-695X.2007.00309.x. [DOI] [PubMed] [Google Scholar]
- Mydel P, Takahashi Y, Yumoto H, Sztukowska M, Kubica M, Gibson FC, 3rd, Kurtz DM, Travis J, Collins LV, Nguyen KA, Genco CA, Potempa J. Roles of the host oxidative immune response and bacterial antioxidant rubrerythrin during Porphyromonas gingivalis infection. PLoS Pathog. 2006;2:e76. doi: 10.1371/journal.ppat.0020076. (712–725). [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill LA. How Toll-like receptors signal: what we know and what we don't know. Curr. Opin. Immunol. 2006;18:3–9. doi: 10.1016/j.coi.2005.11.012. [DOI] [PubMed] [Google Scholar]
- O'Neill LA. When signaling pathways collide: positive and negative regulation of tolllike receptor signal transduction. Immunity. 2008;29:12–20. doi: 10.1016/j.immuni.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Oliver RC, Brown LJ, Loe H. Periodontal diseases in the United States population. J. Periodontol. 1998;69:269–278. doi: 10.1902/jop.1998.69.2.269. [DOI] [PubMed] [Google Scholar]
- Papapanou PN. Periodontal diseases: epidemiology. Ann Periodontol. 1996;1:1–36. doi: 10.1902/annals.1996.1.1.1. [DOI] [PubMed] [Google Scholar]
- Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science. 2003;299:1033–1036. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]
- Pihlstrom BL, Michalowicz BS, Johnson NW. Periodontal diseases. Lancet. 2005;366:1809–1820. doi: 10.1016/S0140-6736(05)67728-8. [DOI] [PubMed] [Google Scholar]
- Plackett TP, Boehmer ED, Faunce DE, Kovacs EJ. Aging and innate immune cells. J. Leukoc. Biol. 2004;76:291–299. doi: 10.1189/jlb.1103592. [DOI] [PubMed] [Google Scholar]
- Plowden J, Renshaw-Hoelscher M, Engleman C, Katz J, Sambhara S. Innate immunity in aging: impact on macrophage function. Aging Cell. 2004;3:161–167. doi: 10.1111/j.1474-9728.2004.00102.x. [DOI] [PubMed] [Google Scholar]
- Renshaw M, Rockwell J, Engleman C, Gewirtz A, Katz J, Sambhara S. Cutting edge: impaired Toll-like receptor expression and function in aging. J. Immunol. 2002;169:4697–4701. doi: 10.4049/jimmunol.169.9.4697. [DOI] [PubMed] [Google Scholar]
- Ricklin D, Lambris JD. Complement-targeted therapeutics. Nat. Biotechnol. 2007;25:1265–1275. doi: 10.1038/nbt1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibolet O, Podolsky DK. TLRs in the Gut. IV. Negative regulation of Toll-like receptors and intestinal homeostasis: addition by subtraction. Am. J. Physiol. Gastrointest. Liver Physiol. 2007;292:G1469–G1473. doi: 10.1152/ajpgi.00531.2006. [DOI] [PubMed] [Google Scholar]
- Shizukuishi S, Hayashi N, Tamagawa H, Hanioka T, Maruyama S, Takeshita T, Morimoto K. Lifestyle and periodontal health status of Japanese factory workers. Ann. Periodontol. 1998;3:303–311. doi: 10.1902/annals.1998.3.1.303. [DOI] [PubMed] [Google Scholar]
- Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent RL., Jr Microbial complexes in subgingival plaque. J. Clin. Periodontol. 1998;25:134–144. doi: 10.1111/j.1600-051x.1998.tb02419.x. [DOI] [PubMed] [Google Scholar]
- Solana R, Pawelec G, Tarazona R. Aging and innate immunity. Immunity. 2006;24:491–494. doi: 10.1016/j.immuni.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Stout RD, Suttles J. Immunosenescence and macrophage functional plasticity: dysregulation of macrophage function by age-associated microenvironmental changes. Immunol. Rev. 2005;205:60–71. doi: 10.1111/j.0105-2896.2005.00260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streckfus CF, Parsell DE, Streckfus JE, Pennington W, Johnson RB. Relationship between oral alveolar bone loss and aging among African-American and Caucasian individuals. Gerontology. 1999;45:110–114. doi: 10.1159/000022072. [DOI] [PubMed] [Google Scholar]
- Teng YT. Protective and destructive immunity in the periodontium: Part 1-Innate and humoral immunity and the periodontium. J. Dent. Res. 2006;85:198–208. doi: 10.1177/154405910608500301. [DOI] [PubMed] [Google Scholar]
- Triantafilou M, Gamper FGJ, Lepper PM, Mouratis MA, Schumann C, Harokopakis E, Schifferle RE, Hajishengallis G, Triantafilou K. Lipopolysaccharides from atherosclerosis-associated bacteria antagonize TLR4, induce formation of TLR2/1/CD36 complexes in lipid rafts and trigger TLR2-induced inflammatory responses in human vascular endothelial cells. Cell. Microbiol. 2007;9:2030–2039. doi: 10.1111/j.1462-5822.2007.00935.x. [DOI] [PubMed] [Google Scholar]
- Triantafilou M, Miyake K, Golenbock DT, Triantafilou K. Mediators of innate immune recognition of bacteria concentrate in lipid rafts and facilitate lipopolysaccharideinduced cell activation. J. Cell Sci. 2002;115:2603–2611. doi: 10.1242/jcs.115.12.2603. [DOI] [PubMed] [Google Scholar]
- Underhill DM. Collaboration between the innate immune receptors dectin-1, TLRs, and Nods. Immunol. Rev. 2007;219:75–87. doi: 10.1111/j.1600-065X.2007.00548.x. [DOI] [PubMed] [Google Scholar]
- van Duin D, Mohanty S, Thomas V, Ginter S, Montgomery RR, Fikrig E, Allore HG, Medzhitov R, Shaw AC. Age-associated defect in human TLR-1/2 function. J. Immunol. 2007;178:970–975. doi: 10.4049/jimmunol.178.2.970. [DOI] [PubMed] [Google Scholar]
- Vega VL, De Cabo R, De Maio A. Age and caloric restriction diets are confounding factors that modify the response to lipopolysaccharide by peritoneal macrophages in C57BL/6 mice. Shock. 2004;22:248–253. doi: 10.1097/01.shk.0000133590.09659.a1. [DOI] [PubMed] [Google Scholar]
- Waldrop TC, Anderson DC, Hallmon WW, Schmalstieg FC, Jacobs RL. Periodontal manifestations of the heritable Mac-1, LFA-1, deficiency syndrome. Clinical, histopathologic and molecular characteristics. J. Periodontol. 1987;58:400–416. doi: 10.1902/jop.1987.58.6.400. [DOI] [PubMed] [Google Scholar]
- Wang M, Shakhatreh M-AK, James D, Liang S, Nishiyama S-i, Yoshimura F, Demuth DR, Hajishengallis G. Fimbrial proteins of Porphyromonas gingivalis mediate in vivo virulence and exploit TLR2 and complement receptor 3 to persist in macrophages. J. Immunol. 2007;179:2349–2358. doi: 10.4049/jimmunol.179.4.2349. [DOI] [PubMed] [Google Scholar]