Abstract
Newly synthesized apical and basolateral membrane proteins are sorted from one another in polarized epithelial cells. The trans-Golgi network participates in this sorting process, but some basolateral proteins travel from the Golgi to recycling endosomes (REs) before their surface delivery. Using a novel system for pulse–chase microscopy, we have visualized the postsynthetic route pursued by a newly synthesized cohort of Na,K-ATPase. We find that the basolateral delivery of newly synthesized Na,K-ATPase occurs via a pathway distinct from that pursued by the vesicular stomatitis virus G protein (VSV-G). Na,K-ATPase surface delivery occurs at a faster rate than that observed for VSV-G. The Na,K-ATPase does not pass through the RE compartment en route to the plasma membrane, and Na,K-ATPase trafficking is not regulated by the same small GTPases as other basolateral proteins. Finally, Na,K-ATPase and VSV-G travel in separate post-Golgi transport intermediates, demonstrating directly that multiple routes exist for transport from the Golgi to the basolateral membrane in polarized epithelial cells.
Introduction
Polarized epithelial cells establish separate and functionally discrete apical and basolateral plasma membrane (PM) domains (Mellman and Nelson, 2008). The maintenance of the distinct protein compositions of these domains requires that newly synthesized membrane proteins be sorted to their sites of ultimate functional residence. This sorting can be achieved through the delivery of newly synthesized membrane proteins to the appropriate domains of the PM or through indirect pathways involving the selective stabilization or redistribution of cell surface proteins.
The TGN has long been thought to serve as the major sorting nexus for newly synthesized membrane and secretory proteins (Rindler et al., 1985; Griffiths and Simons, 1986; Keller et al., 2001). Upon reaching the TGN, apical and basolateral cargoes can be separated into different post-Golgi transport intermediates (PGTIs) for delivery to their respective surfaces (Mellman, 1996; Keller et al., 2001; Rodriguez-Boulan et al., 2005). However, recent studies have indicated that some basolateral PM proteins leave the TGN and traffic through recycling endosomes (REs) before their arrival at the PM (Ang et al., 2004; Cancino et al., 2007; Cresawn et al., 2007). The formation of basolateral PGTIs is mediated through the direct or indirect interaction of their cargo proteins' basolateral sorting signals with adapter and coat proteins (Bonifacino and Dell'Angelica, 1999; Gravotta et al., 2007). AP-1B, the best characterized of the epithelial-specific adapter proteins, is required for efficient trafficking of several different proteins to the basolateral PM (Folsch et al., 1999; Gravotta et al., 2007). AP-1B is localized to REs in polarized MDCK cells and in stably transfected LLC-PK1 cells (Folsch et al., 2003; Cancino et al., 2007). Vesicular stomatitis virus G protein (VSV-G), which is sorted to the basolateral PM in an AP-1B–dependent manner, passes through REs after departing the TGN en route to the basolateral cell surface (Ang et al., 2004). Epithelial cadherin (E-cadherin) also uses REs for transport to the cell surface (Desclozeaux et al., 2008) and interacts with AP-1B via phosphatidylinositol phosphate kinase Iγ (Ling et al., 2007); however, E-cadherin targets to the lateral PM in cells lacking AP-1B, indicating that it can use an AP-1B–independent trafficking route (Miranda et al., 2001).
In this study, we have used a novel and powerful labeling technique to follow the cell surface delivery of the Na,K-ATPase (Na pump) to observe the trafficking of a protein that pursues AP-1B–independent basolateral delivery. In almost all epithelial cells, the Na pump is localized at the basolateral PM. This polarized distribution enables the Na pump, in conjunction with many other ion transporters and channels, to drive the fluxes of fluid and solutes across epithelial barriers (Muth et al., 1997). The minimal functional unit of the Na pump includes two subunits. The α subunit binds the substrates involved in the pump's enzymatic catalysis, undergoes conformational changes that drive vectorial ion transport, and harbors basolateral sorting information within its fourth transmembrane-spanning domain (Muth et al., 1998; Dunbar et al., 2000). The glycosylated β subunit is required for the exit of the pump complex from the ER (Geering et al., 1989; Gottardi et al., 1993). Basolateral localization of the pump is independent of expression of AP-1B, as the pump localizes to the basolateral surface in the µ1B-deficient cell line LLC-PK1 (Duffield et al., 2004) and in MDCK cells, in which µ1B expression has been suppressed via RNAi (Gravotta et al., 2007).
By taking advantage of the SNAP tag system to reveal the trafficking itinerary of the newly synthesized Na pump, we find that basolateral delivery of the Na,K-ATPase does not involve passage through REs. Furthermore, we find that although AP-1B–dependent and –independent cargoes are initially co-distributed within the TGN, they pursue different pathways involving distinct PGTIs and subsets of the cellular sorting machinery en route to their common destination in the basolateral domain of the epithelial PM. Our results demonstrate directly and conclusively that alternate pathways exist for delivery of cargo to the basolateral surface.
Results
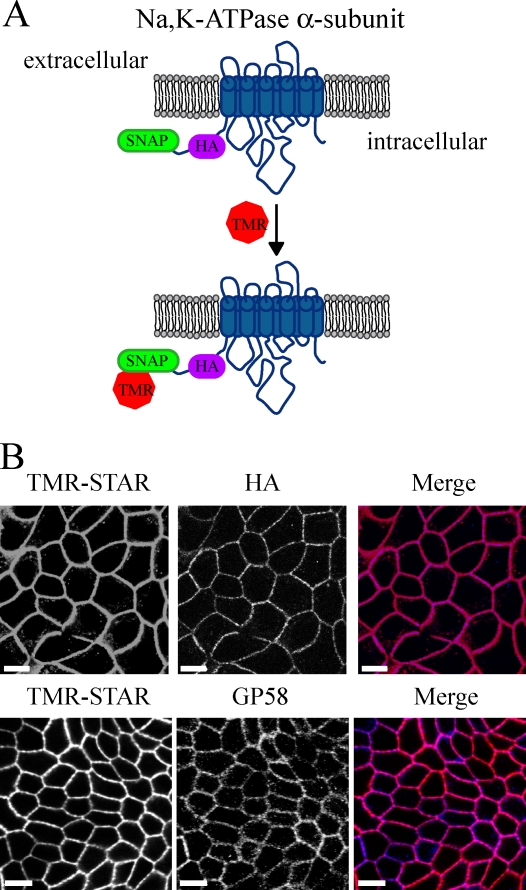
To study the intracellular trafficking of the Na pump, we have used a new method that permits the direct observation of temporally defined cohorts of proteins via the combination of fluorescence microscopy with pulse–chase labeling protocols. The 20-kD SNAP tag is a modified version of the DNA repair protein 06-alkylguanine-DNA alkyltransferase, which cleaves para-substituted benzylguanines (BGs) by covalently and irreversibly transferring the substituted benzyl group to its active thiol. Fluorescent BG derivatives allow for the labeling and detection of SNAP-tagged fusion proteins in either live or fixed cells (Keppler et al., 2004). We engineered a Na pump construct harboring a SNAP tag connected at the α subunit N terminus via a short linker region containing an HA tag (Fig. 1 A). MDCK cells stably transfected with the SNAP-tagged Na pump and labeled with tetramethylrhodamine-conjugated BG (TMR-STAR) yield a robust signal that colocalizes with gp58. gp58 detects the endogenous β subunit of the Na,K-ATPase (Füllekrug et al., 2006), indicating that PM localization is not affected by addition of the SNAP tag (Fig. 1 B). In addition, several lines of evidence indicate that the fusion of the SNAP tag with Na,K-ATPase does not perturb the trafficking behavior or enzymatic activity of the pump (Fig. S1).
Figure 1.
Detection of Na pump localization via the SNAP tag. (A) Diagram of the SNAP tag labeling reaction for the N-terminally tagged Na,K-ATPase α subunit. TMR, tetramethylrhodamine. (B) Stably transfected MDCK cells expressing both SNAP–Na,K-ATPase α subunit and Na,K-ATPase β subunit (SNAP cells) were fixed and stained with TMR-STAR (red) and processed for immunofluorescence with anti-HA or gp58 (blue). Bars, 10 µm.
Newly synthesized Na,K-ATPase is labeled by TMR-STAR and rapidly traffics to the PM
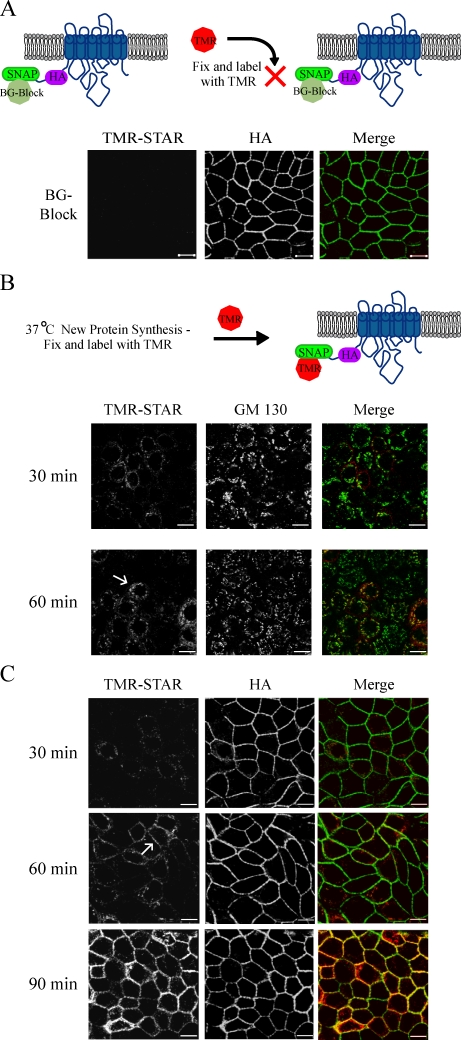
To follow the postsynthetic fate of the Na,K-ATPase, we used a block–chase strategy using our MDCK cell line expressing the SNAP-tagged Na,K-ATPase α subunit (Fig. 2). Preincubation of live cultures with the nonfluorescent BG-Block substrate was found to saturate the SNAP tags on all previously synthesized pumps and effectively blocked this pool of pump from labeling with TMR-STAR. BG-Block treatment did not achieve this effect by inducing pump degradation, as the distribution of the total cellular pool of the SNAP-tagged Na pump was unaltered when detected by HA staining (Fig. 2 A). In addition, we monitored the rate of protein turnover in metabolically labeled cultures after treatment with BG-Block and did not observe any effects relative to mock-treated samples (unpublished data).
Figure 2.
Newly synthesized Na pump trafficking imaged via the SNAP tag. (A) Live SNAP cells were preincubated with nonfluorescent BG (BG-Block) for 30 min, fixed, stained with TMR-STAR (red), and processed for immunofluorescence with the indicated antibodies (green). (B and C) SNAP cells pretreated with BG-Block were washed and allowed to synthesize new protein for the indicated times at 37°C before fixation and labeling as in A. Arrows highlight examples of colocalization with the Golgi marker GM130 or with the lateral membrane stained with anti-HA. TMR, tetramethylrhodamine. Bars, 10 µm.
We next investigated the recovery of TMR-STAR labeling in BG-blocked cultures to determine how soon after cessation of the block newly synthesized SNAP-tagged Na,K-ATPase could be detected. Within 30 min after the block, a perinuclear reticular staining pattern reminiscent of the ER was visible. The magnitude of signal increased over time, and within 1 h, we could detect significant colocalization of the TMR-STAR label with the Golgi marker protein GM130 (Fig. 2 B). To analyze the reappearance of the TMR-STAR–labeled pump at the membrane, we used the HA epitope as a marker for Na pump resident at the PM. When an area in a plane of focus below that of the Golgi was imaged, we could detect TMR-STAR signal colocalizing with the basolateral PM as early as 1 h after block, which increased dramatically by 90 min (Fig. 2 C).
Golgi-accumulated Na pump is rapidly trafficked to the lateral membrane in polarized MDCK cells
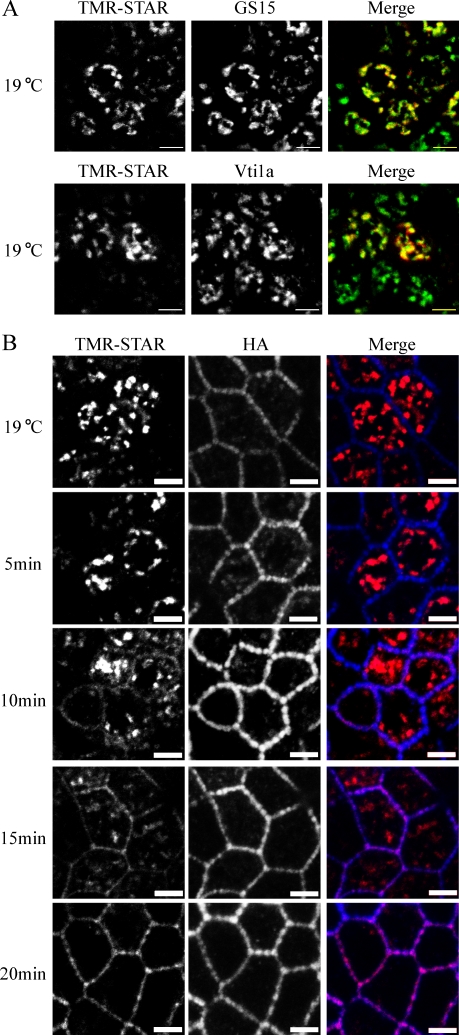
To accumulate a cohort of newly synthesized Na pump into a single organelle, we used a Golgi block step by incubating BG-blocked cells at 19°C for 2 h after a short post–BG-Block recovery period at 37°C. Using this protocol, we were able to synchronize the pump specifically in the Golgi complex, where it colocalized with the Golgi markers GS15 and Vti1a (Fig. 3 A), whereas no significant signal was detected in the ER or at the PM (Fig. 3 B). In this and subsequent experiments, all post-Golgi trafficking incubations were performed at 31°C to allow direct comparison with previous studies that used a temperature-sensitive mutant of the VSV-G protein (Ts 045; Ang et al., 2003, 2004; Schuck et al., 2007). Ts 045 trafficking is more efficient when assayed below 37°C because of misfolding of the mutant protein at higher temperatures (Ang et al., 2004). When samples were warmed to 31°C for 5 min, very little delivery to the PM was observed (Fig. 3 B). However, by 10 min, Na pump was readily detected at the lateral PM, and the majority of the Golgi signal was depleted by 20 min after release (Fig. 3 B). It is interesting to note that the HA staining corresponding to the pool of old Na pump was clustered into beadlike plaques strung along the length of the lateral PM, whereas the newly delivered pump exhibited a uniform distribution (10 min) and only later localized with HA into these clusters (20 min). It has been suggested that the Na pump is localized into this beaded arrangement in association with contacts between adjacent cells (Vagin et al., 2006). Our data indicate that the pump is first delivered randomly over the entire surface of the lateral PM and subsequently becomes incorporated into the preexisting aggregates at the sites of these contacts.
Figure 3.
TGN-accumulated Na pump is rapidly trafficked to the lateral membrane. (A) SNAP cells were subjected to BG-Block, incubated at 37°C for 30 min to begin synthesis of new Na pump, and placed at 19°C for 2 h to accumulate newly synthesized protein in the TGN. TMR-STAR is depicted in red, and Golgi markers are shown in green. (B) SNAP cells were treated as in A and either fixed immediately (19°C) or warmed to 31°C for the indicated times. Samples were labeled with TMR-STAR (red) and with anti-HA (blue). Bars, 5 µm.
To analyze quantitatively the trafficking of SNAP-tagged Na pump to the PM, z stacks were generated for each time point, and the percentage of newly synthesized Na pump colocalizing with the PM was determined (Fig. S2 A). In samples maintained at 19°C, ∼6 ± 3% of the TMR-STAR–labeled Na pump signal colocalized with the PM, indicating that there may be a small degree of leakage of Na pump out of the Golgi during incubation at 19°C. When the temperature was raised to 31°C, the TMR-STAR Na pump signal at the PM increased to 12 ± 8%, 27 ± 9%, 53 ± 11%, 84 ± 2%, 90 ± 4%, and 88 ± 3% at 5 min, 10 min, 15 min, 20 min, 40 min, and 60 min after Golgi block release, respectively.
A previous study of VSV-G trafficking in polarized MDCK cells demonstrated that upon release from Golgi block, only 40% of the Golgi-accumulated VSV-G signal reached the PM after 30 min (Hua et al., 2006). Because this suggests that the Na pump and VSV-G traffic to the PM at quite different rates, we compared VSV-G trafficking with that of the Na pump in our assay system (Fig. S2 A). For this experiment, we used VSV-G Ts 045, which is blocked from exiting the ER when host cells are incubated at 40°C. Next, cells were shifted from 40 to 19°C to allow the VSV-G protein to depart the ER and accumulate in the Golgi. Under Golgi block conditions, we detected slightly more signal for VSV-G at the lateral PM (7 ± 4%) than was observed for Na pump. When the temperature was shifted to 31°C, lateral PM accumulation increased to 11 ± 6%, 21 ± 4%, 28 ± 9%, 35 ± 4%, 52 ± 4%, 66 ± 12%, and 76 ± 9% for 5 min, 10 min, 15 min, 20 min, 30 min, 40 min, and 60 min, respectively, demonstrating that these two proteins traffic from the Golgi to the PM at quite different rates. It is possible that the relatively slow accumulation of VSV-G at the PM in comparison with the Na,K-ATPase is the result of comparatively rapid internalization of newly delivered VSV-G back into REs. To test this possibility, we monitored the uptake of newly delivered VSV-G from the PM by carrying out the temperature shift Golgi release protocol in the presence of an antibody directed against the extracellular domain of VSV-G (Fig. S2 B). Only a small fraction of the externally labeled VSV-G is endocytosed in samples released from the Golgi block for 20–40 min. Furthermore, much of the intracellular pool of VSV-G that is detected after release from the Golgi block does not colocalize with transferrin (Tfn) receptor (Tfn-R), indicating that it is not associated with endosomes and is instead most probably still resident in the TGN. Collectively, these findings suggest that rapid internalization does not account for the slow accumulation of VSV-G protein at the PM after release from the Golgi block.
The Na pump traffics directly to and uniformly across the entire length of the lateral membrane
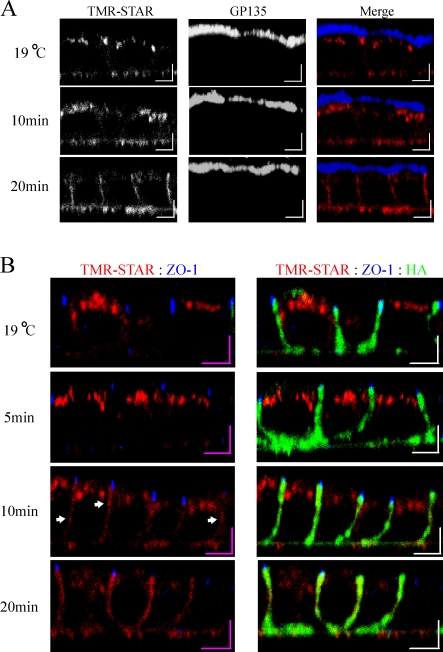
Previous studies analyzing the delivery of the Na,K-ATPase to the PM in the Heidelberg strain of MDCK cells demonstrated that newly synthesized pumps are delivered directly to the lateral PM without significant appearance at the apical surface (Caplan et al., 1986; Gottardi and Caplan, 1993; Mays et al., 1995). As biochemically based membrane delivery assays often involve complex procedures to label a single membrane surface, we investigated whether we could detect similar vectorial delivery directly using the SNAP system. As expected from the data depicted in Fig. 3 A, under the 19°C Golgi block, we detected a globular staining pattern concentrated beneath the apical PM (Fig. 4 A) that colocalizes with Golgi markers (not depicted). When samples were warmed for 5–20 min at 31°C, we were unable to detect colocalization of the Na pump with the apical marker gp135 at any time point, whereas a rapid accumulation of this Na pump population at the lateral PM was observed. Our data are consistent with those of previous biochemical studies (Caplan et al., 1986; Gottardi and Caplan, 1993; Mays et al., 1995), suggesting that the SNAP system can be applied successfully to follow the postsynthetic trafficking of membrane proteins to the PM.
Figure 4.
TGN-accumulated Na pump is trafficked directly to and randomly throughout the lateral membrane. SNAP cells were BG blocked, and newly synthesized Na,K-ATPase was accumulated in the TGN for 2 h at 19°C. After the Golgi block, samples were fixed immediately (19°C) or warmed to 31°C for the indicated times. (A) Samples labeled for TMR-STAR (red) and the apical membrane marker gp135 (blue). (B) Samples labeled with TMR-STAR (red), the junctional marker ZO-1 (blue), and for lateral membrane via anti-HA (green). Arrows denote areas of relatively high intensity TMR-STAR labeling along the lateral membrane. Bars, 5 µm.
Basolateral vesicle delivery to the lateral PM is often mediated through the specific tethering of these vesicles to the membrane via the exocyst complex (Grindstaff et al., 1998; Yeaman et al., 2001). This multicomponent protein complex is localized, in polarized epithelial cells, at the apical–lateral junction (Grindstaff et al., 1998) and in association with REs (Oztan et al., 2007). The localization of the exocyst at the site of tight junctions suggests that basolaterally targeted proteins must first be delivered to this region followed by redistribution across the entire surface of the lateral PM (Rodriguez-Boulan et al., 2005). However, conflicting evidence exists supporting both junctional and random delivery to the lateral PM. Live cell imaging experiments with GFP-tagged low density lipoprotein receptor (LDLR), an AP-1B–dependent basolateral cargo, showed that vesicles fuse with the lateral PM primarily in a region between 6–12 µm above the basal surface (Kreitzer et al., 2003). In addition, a GFP-tagged version of the VSV-G protein was found to accumulate in the region of the junction when cells were treated with tannic acid to block membrane fusion (Polishchuk et al., 2004). However, when VSV-G–YFP trafficking was monitored in live MDCK cells, specific delivery to the region of the junction was not observed (Hua et al., 2006).
To analyze the initial delivery of newly synthesized Na,K-ATPase to the PM, we monitored the sites of first appearance of the Na pump relative to the localization of the tight junctional marker ZO-1 (Fig. 4 B). As expected from the data depicted in Fig. 3, there was a lag period over which little pump was delivered to the PM after 5 min. Interestingly, at both 10 and 20 min after release from the 19°C Golgi block, we were unable to detect any specific accumulation at the region of the junction. When we monitored delivery of VSV-G, a protein known to use the exocyst in its trafficking to the PM (Yeaman et al., 2001; Moskalenko et al., 2002), random delivery was also observed (unpublished data). At early time points, we noted the appearance of concentrated zones of comparatively intense TMR-STAR–labeled Na pump signal that were distributed at random over the entire length of the lateral PM (Fig. 4 B, arrows). Because the Na pump is known to interact with the spectrin–ankyrin cytoskeleton (Woroniecki et al., 2003), it is possible that these local concentrations are attributable to the selective stabilization of the pump in subdomains of the PM rather than corresponding to sites of initial vesicle delivery. Of course, it remains possible that carrier vesicle fusion is occurring for both proteins at the site of the junction, and subsequent rapid diffusion in the plane of the PM accounts for their initial detection near the basal surface. However, the data presented in this study are not consistent with an obvious requirement for delivery of basolaterally directed cargo vesicles to spatially discrete fusion hot spots localized to specific subdomains of the lateral PM.
Unlike the VSV-G protein, the Na pump does not pass through REs en route to the PM
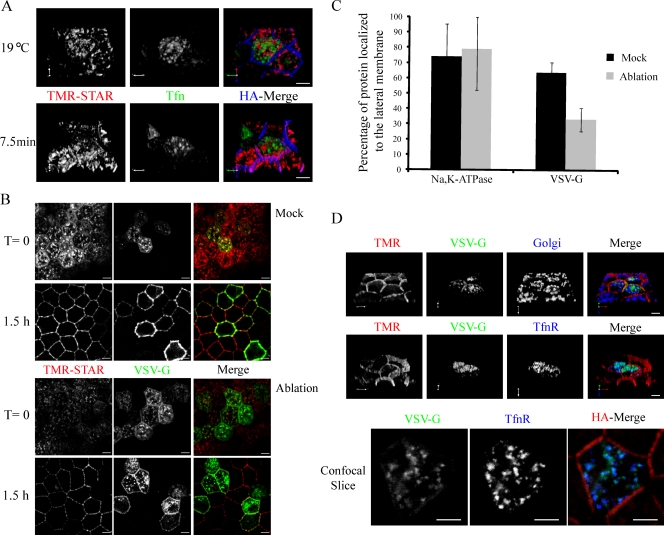
Previous studies have shown that REs are primary sorting stations for AP-1B–dependent cargoes en route to the PM (Ang et al., 2004; Cancino et al., 2007). However, little is known about the pathway taken by AP-1B–independent cargoes, as they traffic to the basolateral surface. To analyze whether the Na pump traverses REs before its arrival at the PM, we assessed whether newly synthesized Na pump colocalized with internalized fluorescently labeled Tfn, a marker for the RE, after release from the Golgi block. Under Golgi block conditions, the Alexa Fluor 488 Tfn signal corresponding to REs was detected adjacent to but not colocalized with elements of the Golgi complex (Fig. 5 A). Interestingly, when the temperature was raised to 31°C for periods ranging from 2 to 10 min, no colocalization between the Na,K-ATPase and Tfn was detected (Fig. 5 A and not depicted). Using a similar strategy, Ang et al. (2004) demonstrated marked colocalization of VSV-G with Tfn after a 10-min release from a 19°C Golgi block in nonpolarized MDCK cells. We confirmed that VSV-G protein behaves in this fashion when it is expressed in our SNAP tag–expressing MDCK cells. We also found that VSV-G colocalizes with Tfn in REs when cells have been treated with tannic acid, a membrane impermeant fixative which blocks vesicle delivery to the PM. Thus, the localization of VSV-G to REs after release from Golgi block must occur as the VSV-G protein is en route to the cell surface and is not caused by endocytosis after surface delivery (Fig. S3).
Figure 5.
The Na,K-ATPase does not pass through REs en route to the lateral membrane. (A) Cells were infected with adenovirus expressing the human Tfn-R, after which they were BG blocked, and newly synthesized Na pump was accumulated in the TGN (red) for 2 h at 19°C. Alexa Fluor 488–Tfn was added to the medium during the Golgi block to label REs (green). Golgi-blocked cells were fixed (19°C) or warmed for the indicated period and stained with anti-HA (blue). (B) Cells were infected to express the human Tfn-R and VSV-G–YFP Ts 045 (green) and incubated overnight at 40°C. Cells were BG blocked at 40°C and incubated for 40 min at 40°C with Tfn-HRP along with 1 µM TMR-STAR to live label newly synthesized pump (red). Next, cells were washed and incubated for a further 20 min at 40°C to remove excess TMR-STAR and to allow for the accumulation of Tfn-HRP into REs. Tfn-HRP–loaded REs were ablated on ice by exposing cells to DAB and H2O2 for 1 h, whereas control cells were treated with DAB alone. After the ablation reaction, cells were fixed or incubated in media plus CHX for 1.5 h at 31°C to allow trafficking of the proteins to the PM. (C) The percentage of protein that reached the lateral membrane 1.5 h after mock or ablation treatment was quantified using the enhanced colocalization tool (LSM Image Examiner software; Carl Zeiss, Inc.). 30 VSV-G–infected cells were analyzed, and data are represented as mean ± SD. (D) Samples were treated as in B and processed for immunofluorescence with a cocktail of Golgi antibodies (GM130, Vti1a, and GS15) or antibody against the Tfn-R shown in blue. The bottom panel depicts an individual confocal slice highlighting the colocalization of VSV-G with Tfn-R. TMR, tetramethylrhodamine. Bars, 5 µm.
To further analyze whether delivery of the Na pump to the PM depends on the participation of functional REs, we used a modified version of an RE inactivation strategy (Ang et al., 2004). HRP is capable of reacting with DAB in the presence of H2O2 to form an insoluble precipitate, and using HRP that is conjugated to Tfn (Tfn-HRP), it has been shown that VSV-G is blocked from reaching the PM in both nonpolarized (Ang et al., 2004) and polarized MDCK cells (Cresawn et al., 2007) subjected to HRP-mediated RE ablation.
In this experiment, cells were infected with adenoviruses expressing Tfn-R to aid in Tfn uptake and VSV-G Ts 045 to verify whether the ablation manipulation was successful. Cells were treated as described in Materials and methods to accumulate both newly synthesized Na pump and VSV-G intracellularly and to accumulate Tfn-HRP in REs. Cells were subjected to RE ablation and either fixed immediately or allowed to recover for 1.5 h at 31°C in the presence of cycloheximide (CHX). Under these conditions, we detected a predominantly intracellular pool of both VSV-G and Na,K-ATPase at time 0 for both ablated and control cells (Fig. 5 B). In the absence of ablation, both VSV-G and the Na pump readily trafficked to the PM, with 74% of the Na pump and 64% of VSV-G resident at the membrane after incubation at 31°C (Fig. 5, B [mock] and C). Furthermore, in control experiments in which cells were either subjected to the DAB reaction without prior exposure to Tfn-HRP or in which DAB was omitted, both VSV-G and the Na,K-ATPase reached the membrane (unpublished data). In samples that were subjected to HRP- and DAB-mediated ablation, VSV-G was retained intracellularly after release from the Golgi block, with only 33% of the protein reaching the membrane (Fig. 5, B and C). In these ablated cells, the bulk of VSV-G had trafficked out of the TGN and was retained within the RE compartment, as revealed by costaining with an antibody directed against the Tfn-R (Fig. 5 D). In marked contrast to the behavior of the VSV-G protein, 79% of the Na pump reached the PM unimpeded in cells that had been subjected to the ablation protocol (Fig. 5, B [ablation] and C). Our results demonstrate that the ablation reaction does not globally affect the TGN, as the Na pump is able to depart the Golgi complex after ablation and to traffic to the PM. Furthermore, these experiments show that the newly synthesized Na,K-ATPase bypasses the RE en route from the Golgi complex to the PM.
Trafficking of the Na pump is not governed by the same set of small GTPase regulators that are involved in the trafficking of AP-1B–dependent membrane proteins
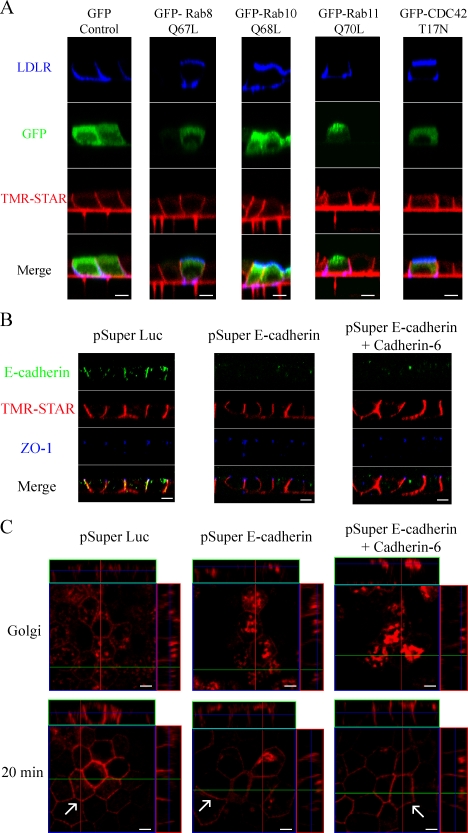
We next analyzed the effects of mutant forms of selected Rab and Rho family GTPases on Na pump trafficking. Small GTPases are important regulators of many membrane trafficking events (Grosshans et al., 2006). In polarized MDCK cells, expression of constitutively active forms of Rab8 and Rab10 or dominant-negative CDC42 has been shown to induce the missorting of AP-1B–dependent basolateral cargo proteins to the apical membrane (Kroschewski et al., 1999; Ang et al., 2003; Schuck et al., 2007). Rab11, which is resident in apical REs, appears to have no effect on AP-1B–dependent basolateral trafficking (Ang et al., 2003); however, it has been shown to disrupt sorting of E-cadherin, an AP-1B–independent cargo (Desclozeaux et al., 2008).
To test the effects of these proteins on Na pump trafficking, cells were microinjected with constructs encoding fusion proteins in which GFP is appended to constitutively active forms of Rab8 (Q67L), Rab10 (Q68L), Rab11 (Q70L), or dominant-negative CDC42 (T17N). As a positive control to demonstrate that these mutated proteins disrupt AP-1B–dependent trafficking, each Rab construct was simultaneously injected with a plasmid encoding LDLR. To ensure that the mutant Rab proteins were expressed at significant levels before analyzing pump trafficking, cells were allowed to synthesize the mutant Rab proteins for 45 min before the BG-Block reaction was initiated, and then labeling proceeded as described in Materials and methods. When cells were injected with a plasmid expressing GFP alone, both LDLR and the Na pump were detected at the lateral PM (Fig. 6 A). Expression of either Rab8 Q67L or CDC42 T17N routed LDLR to the apical surface (Fig. 6 A), whereas they had no visible effect on the trafficking of the Na pump, which is consistent with the apparent independence of Na,K-ATPase sorting from AP-1B–dependent mechanisms. Rab10 is known to participate in the sorting of at least two AP-1B–dependent cargoes; however, its effects on AP-1B–independent proteins have not been tested. Our assay demonstrated that expression of activated Rab10 led to apical accumulation of the LDLR, whereas Na pump trafficking was completely unaffected. In cells expressing levels of Rab11 Q70L equivalent to those obtained with Rab8 Q67L, there was no apparent effect on either LDLR or Na pump distribution, which is in agreement with previous observations (Fig. 6; Ang et al., 2003).
Figure 6.
Na pump trafficking is not regulated by the same small GTPases as AP-1B–dependent cargo and does not require E-cadherin. (A) SNAP cells were grown on clear polyester filters for 4 d to form a fully polarized monolayer and microinjected with plasmids expressing human LDLR and one of the following: GFP, GFP–Rab8 Q67L, GFP–Rab10 Q68L, GFP–Rab11 Q70L, or GFP–CDC42 T17N. Cells were then allowed to recover for 45 min before BG-Block addition. After BG blocking, cells were washed and allowed to synthesize new SNAP-tagged Na pump for 3 h, followed by incubation in the presence of CHX for 45 min. Samples were labeled with TMR-STAR (red) after being processed for surface immunofluorescence for LDLR (blue). (B). SNAP cells were transfected with shRNA expression plasmids directed against E-cadherin, Cadherin-6, or luciferase (Luc) as a negative control and cultured to allow monolayer polarization as previously described (Capaldo and Macara, 2007). 72 h after transfection, samples were stained with antibodies directed against E-cadherin (green) and ZO-1 (blue) and were labeled with TMR-STAR. (C) Cells were transfected and grown as in B and then subjected to the Golgi block protocol described in Fig. 3. Images depict samples fixed immediately after Golgi block or after incubation for 20 min at 31°C to allow Na pump exit from the TGN. Newly synthesized Na,K-ATPase traffics directly to the basolateral surface in cadherin-depleted cells. Arrows highlight lateral membrane delivery for each sample. Bars, 5 µm.
E-cadherin is not required for the polarized delivery of the Na pump
E-cadherin is a Ca-dependent cell adhesion molecule that links the lateral membranes of adjacent cells together through homotypic interactions (Takeichi, 1990). The introduction of E-cadherin into mouse fibroblasts induces the formation of cell–cell contacts and results in the localization of the Na,K-ATPase into these junctions (McNeill et al., 1990). Suppression of E-cadherin expression in MDCK cells by RNAi demonstrated that E-cadherin is required for the establishment of epithelial polarity, but it does not appear to be needed for its maintenance once polarity is established (Capaldo and Macara, 2007). In these experiments, even though E-cadherin depletion approached 100% in transfected cells, tight junctions remained intact, and both apical and basolateral markers (including the Na pump) were properly localized. However, in at least one strain of MDCK cells, Na pump polarity is achieved through selective stabilization of the pump in the basolateral PM after random delivery to both surfaces (Mays et al., 1995). Thus, the fact that the steady-state basolateral localization of the Na,K-ATPase is maintained in the E-cadherin knockdown MDCK cells does not provide any insight into the question of whether E-cadherin is required for the direct targeting of newly synthesized Na,K-ATPase to the basolateral surface.
To determine whether newly synthesized Na pumps are vectorially targeted to the lateral membrane in E-cadherin–depleted cells, we transfected MDCK cells expressing the SNAP-tagged Na pump with a plasmid expressing a short hairpin RNA (shRNA) directed against either E-cadherin or against the luciferase gene, which served as a negative control. Transfected cells were then plated onto trans-well filters at a high enough density to immediately form a monolayer and allow cell–cell contacts to form before E-cadherin knockdown (Capaldo and Macara, 2007). Using this method, E-cadherin was significantly depleted in transfected cells, whereas steady-state Na pump distribution and tight junctions were unaffected (Fig. 6 B). In addition, we cotransfected cells with shRNAs directed against both E-cadherin and Cadherin-6, as codepletion of these two proteins is required for the loss of β-catenin localization at cell–cell contacts in MDCK cells (Capaldo and Macara, 2007). As expected, no effect was observed for ZO-1 staining in double knockdown cells relative to control cells (Fig. 6 B). The Na pump also remained localized to the lateral membrane, indicating that cadherin-mediated localization of β-catenin is not required to maintain the Na pump's distribution. To analyze whether cadherins are necessary for direct trafficking of the Na pump to the basolateral membrane, we accumulated newly synthesized Na pump into the Golgi in both control and knockdown cells, as described in the legend for Fig. 3, and followed its trafficking after release from the Golgi block (Fig. 6 C). Similar to the results of Fig. 3, the Na pump was rapidly trafficked from the Golgi to the lateral membrane in cells transfected with the luciferase RNAi construct. Similar results were observed in the cadherin-depleted cells, indicating that neither E-cadherin nor Cadherin-6 is required for the vectorial delivery of the Na pump once polarity has been established.
The Na pump and VSV-G are segregated into distinct PGTIs en route to the PM
It has been shown that some apical and basolateral cargoes segregate from one another in the Golgi complex and are delivered to the PM in separate PGTIs (Wandinger-Ness et al., 1990; Keller et al., 2001). However, the presence of separate PGTIs for different basolateral cargoes in the same cell has not been demonstrated. Our data suggest that VSV-G, and possibly all AP-1B–dependent cargo proteins, uses a pathway that is distinct from that pursued by the Na pump during its biosynthetic trafficking to the PM. This behavior suggests that during at least one presumably late step in their transport to the PM, the VSV-G protein and the Na,K-ATPase must occupy separate transport vesicles.
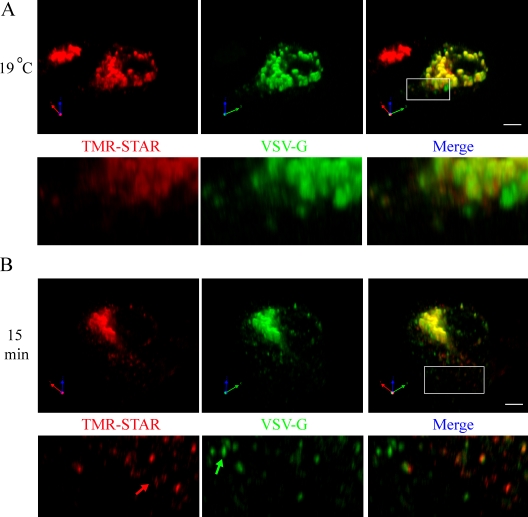
To test this possibility, we used a modified version of our Golgi block protocol on nonpolarized MDCK cells, as described in Materials and methods. Using this strategy, the vast majority of VSV-G and TMR-STAR–labeled Na pump colocalized in the TGN during the Golgi block (Fig. 7 A). Interestingly, even under Golgi block conditions, areas of local concentrations within the Golgi were observed for both Na pump and VSV-G, indicating that their initial distributions are partially segregated (Fig. 7 A, inset). Upon release from the block for 15 min, the VSV-G and Na pump signals diverged and appeared as populations of small puncta presumably corresponding to separate PGTIs (Fig. 7 B, arrows). Direct observation of 150 Na pump–containing carriers found that only 30 ± 6% of carriers costained for VSV-G. In some cases, individual puncta that were labeled exclusively for either the Na pump or the VSV-G protein appeared to reside in close proximity to one another. These observations support the surprising conclusion that at least some proteins destined for delivery to the same domain of the PM are segregated into distinct populations of vesicular carriers that pursue divergent courses before arriving at their common destination.
Figure 7.
Na pump and VSV-G exit the TGN in separate PGTIs en route to the lateral membrane. SNAP cells were transfected with a plasmid encoding Ts 045 VSV-G–YFP and incubated for 24 h at 40°C to accumulate newly synthesized VSV-G in the ER. Next, samples were BG blocked at 40°C, washed, and incubated for a short 10-min recovery period to permit synthesis of new SNAP-tagged Na pump protein. (A and B) Samples were subjected to 19°C Golgi block and fixed immediately (19°C; A) or warmed to 31°C for 15 min (B). Samples were labeled with TMR-STAR (red) and imaged for YFP (green). The boxed areas are shown at higher magnification in the panels below. The green arrow highlights a PGTI containing only VSV-G, whereas the red arrow highlights a carrier containing only the Na pump. Bars, 5 µm.
Discussion
In this study, we validated the SNAP tag as a tool for studying the trafficking of newly synthesized membrane proteins and used it to follow the AP-1B–independent trafficking of the Na,K-ATPase. With the SNAP system, we were able to select a cell line expressing near endogenous levels of the tagged protein and then analyze the delivery of a temporally defined cohort of Na pump. The incorporation of a Golgi block step allowed us to synchronize this newly synthesized pool of protein and to follow its subsequent trafficking to the lateral PM. These techniques should be applicable to study the trafficking of virtually any membrane protein of interest.
Our kinetic data on the trafficking of the Na pump from the Golgi to the PM indicate that the pump is delivered to the membrane much faster than VSV-G, with 80% of the Golgi-accumulated Na pump reaching the membrane within 20 min compared with only 30% for VSV-G. Another study has previously reported that biosynthetic delivery of basolateral membrane proteins can occur at different rates (Le Bivic et al., 1990). However, both of the basolateral proteins examined in this study arrived at the surface within 20 min after acquisition of endoglycosidase H resistance, indicating that the discrepancy in delivery times observed by these investigators is caused by a step in their trafficking that precedes their release from the Golgi. Our data indicate that for VSV-G and the Na pump, there is a significant difference in their post-Golgi trafficking rates. This difference may be attributable to the requirement that VSV-G engage with AP-1B and pass through REs before arriving at the membrane. Our data indicate that cell surface delivery of the Na,K-ATPase is independent of these steps.
Not all basolateral traffic uses REs during exocytosis, and AP-1B–dependent and –independent proteins occupy separate PGTIs before this step
The discovery that newly synthesized VSV-G must traffic through REs before its arrival at the cell surface has raised the question of whether all basolaterally directed PM proteins pass by default from the TGN to REs or whether trafficking through this pathway is limited to a subset of basolaterally directed cargo. The latter model receives support from a study in which knockdown of AP-1B in MDCK cells led to perturbations in the biosynthetic delivery of VSV-G, whereas the Tfn-R was delivered normally to the basolateral PM and then subsequently trafficked apically during recycling (Gravotta et al., 2007). In another study, microinjection of function-blocking antibodies directed against AP-1B was found to result in accumulation of newly synthesized VSV-G in the AP-1B–positive RE compartment, whereas newly synthesized LDLR trafficked first to the basolateral cell surface and was trapped in REs only upon endocytosis (Cancino et al., 2007). Together, these results suggest that AP-1B and obligate passage through REs are essential components of the initial exocytic trafficking of some but not all basolateral proteins. However, it is worth noting that a subset of proteins whose sorting is not AP-1B dependent also transit through REs en route to the basolateral surface (Desclozeaux et al., 2008). Using the endosome ablation technique, we found that the Na pump does not require REs for its delivery to the basolateral PM (Fig. 5). This result demonstrates that the Na pump traffics from the Golgi to the PM in a manner that functionally bypasses a step that constitutes a major intermediate in the trafficking itineraries of many other basolateral proteins. The SNAP tag technique allowed us to visualize this alternate pathway unambiguously, without any need to inactivate AP-1B or its cognate sorting signals to reveal its presence. Thus, our data provide very clear and direct proof that such a second pathway to the basolateral surface must coexist in parallel with the AP-1B pathway.
The fact that the Na pump does not require REs for membrane delivery demonstrates that VSV-G, the Na pump, and, more generally, basolateral proteins bearing different types of sorting motifs are segregated into distinct populations of PGTIs before their delivery to the basolateral cell surface. This hypothesis receives support from the observation that inactivation of the basolateral v-SNARE cellubrevin by expression of tetanus neurotoxin results in the mis-sorting of a specific subset of basolateral cargoes (Fields et al., 2007). In addition, it has recently been shown that the depletion of clathrin by RNAi results in the apical mislocalization of many basolateral proteins, including VSV-G and E-cadherin; however, the localization of the Na,K-ATPase was not affected (Deborde et al., 2008). The ability of the Na pump to accumulate at the basolateral membrane in the absence of clathrin, as well as the identification of a v-SNARE regulating the traffic of select basolateral cargoes, suggests that these cargoes may be delivered in separate vesicles.
In Fig. 7, we observed distinct PGTIs harboring either VSV-G or the Na pump shortly after MDCK cells were released from a 19°C Golgi block, which constitutes perhaps the most graphic, straightforward, and definitive evidence that two separate and parallel pathways to the basolateral surface exist side by side in normal cells. It remains to be determined whether these proteins are sorted into distinct vesicles at the time of or immediately after their departure from the Golgi complex. During the Golgi block, we observed areas within the TGN and in vesicle-like structures localized to the Golgi periphery that contained high concentrations of either VSV-G or the Na pump. This suggests that the sorting of these proteins may begin during their residence in the TGN or soon after their initial budding and release. However, 30% of the PGTIs produced after release from the Golgi block exhibited both Na,K-ATPase and VSV-G protein fluorescence signals, suggesting that some segregation events may be completed subsequent to departure from the TGN. This possibility is consistent with previous experiments performed in intestinal epithelial cells, in which apically directed proteins leave the TGN in common vesicles only to segregate into separate carriers before delivery to the cell surface (Jacob and Naim, 2001).
Insight into the regulation of AP-1B–independent protein trafficking will require characterization of the proteins that interact with the AP-1B–independent cargo protein at various steps in its postsynthetic trafficking pathway. It is possible that a nontraditional adapter may function in the trafficking of the Na pump to the basolateral surface. For example, ankyrin-G, which binds to various membrane proteins and links them to the cytoskeleton through interaction with β2-spectrin, regulates the delivery of E-cadherin from the TGN to the PM. Ankyrin-G most likely exerts this effect by linking E-cadherin–containing vesicles to microtubule-based motors (Kizhatil et al., 2007). Ankyrins have been well documented to bind to the Na pump (Jordan et al., 1995), and a recent study demonstrated that although depletion of ankyrin-G has no effect on Na pump steady-state localization, depletion of ankyrin-R results in a retention of the Na pump within the ER (Stabach et al., 2008). However, it is unlikely that if ankyrin plays a role in Na pump sorting, it is similar to its to its function in E-cadherin trafficking because depletion of microtubules with colchicine has no effect on Na pump trafficking (Boll et al., 1991), whereas disruption of microtubules leads to intracellular accumulation of E-cadherin. In fact, interaction with ankyrin appears to be required for the newly synthesized Na pump to exit from the ER and is thus involved at a pre-Golgi stage in the biosynthetic trafficking of the Na,K-ATPase (Stabach et al., 2008).
The utility in maintaining several overlapping but separate routes to either domain of the epithelial cell surface may arise from the requirement that the cellular sorting apparatus must be able to serve the individual needs of different epithelial cell types whose physiological functions require alternative distributions of particular classes of membrane proteins. For example, in retinal pigment epithelial cells and the epithelial cells of the choroid plexus, the Na pump is localized to the apical membrane rather than at the basolateral surface (Gundersen et al., 1991). However, other PM proteins retain their traditional distributions in these cell types (Bok et al., 1992). The existence of several distinct sorting pathways to the basolateral membrane may endow the cell with the plasticity required to direct a subset of normally basolateral polypeptides to the apical surface without perturbing the polarized distributions of other proteins. Interestingly, a previous study that used quantitative electrophysiological methods to count the number of newly synthesized acetyl choline receptors transported in a single PGTI found that, at least in this particular case, the number of these receptors per vesicle approached their theoretical packing density in the membrane (Andreose et al., 1996). This observation suggests the intriguing possibility that each individual PGTI may be dedicated to carrying only one species of closely packed cargo proteins.
The basolateral sorting of the Na pump appears to depend on the information contained within a novel sorting determinant. This signal involves the α subunit's fourth transmembrane domain and its flanking sequences in the formation of a conformationally defined motif (Dunbar et al., 2000). Future identification of the proteins that recognize the Na pump's sorting signal and interpret its message will no doubt shed light on the nature of the pathway that the Na pump pursues from the Golgi complex to the basolateral cell surface. These experiments will provide additional insights into the mechanisms through which Na pump delivery is differentiated from the pathway pursued by AP-1B–dependent cargoes.
Materials and methods
Antibodies
Antibodies used in this study are as follows: an mAb directed against the HA epitope was purchased from Covance; mAbs to gp135 (Gravotta et al., 2007) and gp58 (Ang et al., 2003) as well as against the human Tfn-R (Folsch et al., 1999) were previously described; antibodies targeted against Golgi proteins were purchased in the Golgi Sampler kit (BD); and antibody directed against E-cadherin (BD) and C7, an mAb to LDLR (American Type Culture Collection), was also used. Alexa Fluor–conjugated secondary antibodies were purchased from Invitrogen.
Constructs and cell lines
The SNAP tag sequence was amplified by PCR from pSS26m (New England Biolabs, Inc.) incorporating unique restriction sites and inserted at the N terminus of a HA-tagged version of the rat Na,K-ATPase α1 subunit, generating SNAP-HA–Na,K-ATPase (Fig. 1 A). MDCK cells were cultured in α-MEM supplemented with 10% FBS; however, MDCK cells stably expressing both SNAP-HA–Na,K-ATPase α subunit and the rat β1 subunit (SNAP cells) were selected in 500 µg/ml G418 and 500 µg/ml Zeocin, which select for transfection with the α subunit and β subunit, respectively. In addition, during the initial selection process, 10 µM ouabain was added to the selection medium to select for clones displaying active rat Na pump at the cell surface. Immunofluorescence experiments were performed on polarized cells grown for 4–6 d on trans-well polycarbonate filters (Hua et al., 2006) or on clear polyester filters for microinjection experiments.
Plasmids used throughout this study include GFP–Rab8 Q67L, GFP–Rab10 Q68L, GFP–Rab11 Q70L, GFP–CDC42 T17N, and the human LDLR (Ang et al., 2003, 2004; Schuck et al., 2007). A plasmid encoding VSV-G–YFP Ts 045 was a gift from D. Toomre (Yale University, New Haven, CT; Keller et al., 2001). Adenoviruses expressing VSV-G–YFP Ts 045 and the human Tfn-R were previously described (Folsch et al., 1999; Ang et al., 2004). shRNA expression plasmids directed against E-cadherin, Cadherin-6, and luciferase were gifts from I. Macara (University of Virginia School of Medicine, Charlottesville, VA).
SNAP tag labeling and immunofluorescence
To initiate pulse–chase experiments, SNAP tag activity was blocked by adding 1.4 µM BG-Block (New England Biolabs, Inc.) to complete medium and incubating cells at 37°C for 30 min. After the block, cells were washed three times and either immediately fixed, as described in the next paragraph, or incubated in complete medium for the indicated time before fixation. For samples undergoing a 19°C Golgi block protocol, after 30 min of post–BG-Block recovery at 37°C, cells were washed twice with CO2-independent medium (Invitrogen) and incubated for 2 h by suspending their growth chambers in a 19°C water bath. During the last 1 h of this incubation, 150 µg/ml CHX was added to prevent additional new SNAP-tagged protein synthesis.
For fixation, cells were washed twice with cold PBS (Sigma-Aldrich) with 1 mM MgCl2 and 100 µM CaCl2 (PBS2+) followed by fixation for 1 h at 4°C in 4% PFA. After fixation, cells were washed again in PBS2+. For both fluorescent SNAP tag labeling and immunofluorescence, fixed cells were permeabilized and blocked in PBS2+ containing 10% goat serum, 0.5% BSA, and 0.2% saponin for 20 min at RT. Next, cells were incubated for 1 h in primary antibody in blocking solution containing 0.25 µM TMR-STAR (New England Biolabs, Inc.). Cells were washed three times for 10 min in PBS2+ with 2% BSA and 0.2% saponin and incubated with the appropriate Alexa Fluor secondary antibody for 30 min as described for primary antibodies, followed by an additional three washes. Cells were rinsed once in PBS2+ and mounted in Vectashield (Vector Laboratories). For surface immunofluorescence, cells were fixed, blocked for 15 min in PBS2+ containing 10% goat serum, and labeled with primary antibody for 1 h. After washing in PBS2+, cells were fixed again to stabilize the primary antibody, and immunofluorescence was continued.
In experiments in which fluorescent Tfn was used, cells were infected with an adenovirus encoding the human Tfn-R and allowed to synthesize this protein for 18–24 h. The next day, cells were blocked with BG-Block, and newly synthesized protein was accumulated at the Golgi as described in the beginning of this section. Throughout the Golgi block incubation, Alexa Fluor 488–conjugated Tfn was present in the media, which allowed it to bind to Tfn-R and to accumulate in the RE compartment (Ang et al., 2004).
For analysis of PGTIs, we used subconfluent SNAP cells to maximize the lateral size of the cells to be examined and thus the spatial resolution of the technique. In addition, the VSV-G–YFP protein was expressed by transfection rather than by adenovirus infection to lower its expression to a level comparable with that of the SNAP-tagged Na pump. Transfected cells were incubated for 24 h at 37°C, followed by 24 h at 40°C. Cells were then BG blocked as before but only allowed to recover for 10 min before incubation at 19°C. This shortened recovery period ensured that no protein had exited the TGN before the imposition of the Golgi block.
Confocal microscopy was performed on a laser-scanning microscope (LSM 510; Carl Zeiss, Inc.) using a 63× water immersion lens (n = 1.5 at 25°C). Images were processed using LSM Image Viewer (Carl Zeiss, Inc.) and Photoshop version 6.0 (Adobe). Images are the product of eightfold line averaging, and contrast and brightness settings were chosen so that all pixels were in the linear range. 3D image reconstructions were performed with Volocity software 4.3 (PerkinElmer).
Microinjection of polarized MDCK cells
The cDNAs for GFP or GFP-tagged Rab/Rho GTPases were combined with the cDNA of wild-type LDLR (0.2 mg/ml each) and were injected into the nuclei of ∼200–400 cells using an Eppendorf Transjector microinjection system mounted on an inverted microscope (Axiovert; Carl Zeiss, Inc.). After injection, the filters were incubated at 37°C for 45 min, and pulse–chase analysis was performed as described in the previous section.
HRP-mediated RE ablation and live cell labeling
3 d after confluence, MDCK cells expressing the SNAP-tagged Na pump were infected with adenoviruses encoding Tfn-R and VSV-G–YFP Ts 045 and incubated overnight at 40°C. The next day, cells were blocked at 40°C by the addition of BG-Block in serum-free α-MEM containing 1% BSA to deplete cells of Tfn. Immediately after the block, cells were washed and incubated for 40 min at 40°C with 0.01 mg/ml Tfn-HRP (Accurate Chemical and Scientific Corp.) and 1 µM TMR-STAR (New England Biolabs, Inc.). Next, cells were washed twice and incubated for a further 20 min at 40°C to remove excess TMR-STAR and to allow for the accumulation of Tfn-HRP into REs. Finally, cells were transferred to ice, and RE inactivation was performed as previously described (Ang et al., 2004). In brief, cells were washed with ice-cold PBS2+ and resuspended in PBS2+ containing 0.1 mg/ml DAB (Sigma-Aldrich). H2O2 was added to a final concentration 0.025% to inactivate REs, and samples were incubated on ice for 60 min in the dark. The reaction was stopped by washing cells twice in PBS2+ containing 1% BSA. Next, samples were fixed immediately or incubated in warm media containing CHX before fixation and labeling.
Western blotting
Wild-type and SNAP cell lysates were prepared by incubation of cells in buffer containing 1% Triton X-100, and samples were analyzed by SDS-PAGE followed by Western blotting (Kimura et al., 2007). Total Na,K-ATPase α1 expression was detected with an antibody directed against residues 338–724 of chicken Na,K-ATPase (α5; Tamkun and Fambrough, 1986).
Online supplemental material
Fig. S1 shows transient and stable transfections of the SNAP-tagged Na,K-ATPase, demonstrating the specificity of SNAP labeling, the requirement of the Na pump β subunit for membrane delivery, and the level of expression of the Na pump in our MDCK stable cell line. Fig. S2 depicts our quantification of data measuring the delivery of the Na pump and VSV-G from the Golgi to the lateral membrane and the lack of internalization of newly delivered VSV-G from the PM. Fig. S3 shows that VSV-G enters REs even when delivery to the PM is inhibited by tannic acid treatment. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.200901021/DC1.
Acknowledgments
We would like to thank Dr. Ian Macara for providing us with the shRNA plasmids for cadherin knockdown and Dr. Pietro De Camilli for his critical reading of the manuscript. We also thank Emily Stoops for experimental assistance and the members of the Caplan laboratory for invaluable comments and discussions.
This work was supported by grants from the National Institutes of Health (DK072612 and DK17433). G.A. Farr was supported by The American Cancer Society New England Division Shoreline Circle of Hope postdoctoral fellowship (PF-06-243-01-CSM).
Footnotes
Abbreviations used in this paper: BG, benzylguanine; CHX, cycloheximide; E-cadherin, epithelial cadherin; LDLR, low density lipoprotein receptor; PGTI, post-Golgi transport intermediate; PM, plasma membrane; RE, recycling endosome; shRNA, short hairpin RNA; Tfn, transferrin; Tfn-R, Tfn receptor; VSV-G, vesicular stomatitis virus G protein.
References
- Andreose J.S., Fumagalli G., Sigworth F.J., Caplan M.J. 1996. Real-time detection of the surface delivery of newly synthesized membrane proteins.Proc. Natl. Acad. Sci. USA. 93:7661–7666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ang A.L., Folsch H., Koivisto U.M., Pypaert M., Mellman I. 2003. The Rab8 GTPase selectively regulates AP-1B–dependent basolateral transport in polarized Madin-Darby canine kidney cells.J. Cell Biol. 163:339–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ang A.L., Taguchi T., Francis S., Folsch H., Murrells L.J., Pypaert M., Warren G., Mellman I. 2004. Recycling endosomes can serve as intermediates during transport from the Golgi to the plasma membrane of MDCK cells.J. Cell Biol. 167:531–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bok D., O'Day W., Rodriguez-Boulan E. 1992. Polarized budding of vesicular stomatitis and influenza virus from cultured human and bovine retinal pigment epithelium.Exp. Eye Res. 55:853–860 [DOI] [PubMed] [Google Scholar]
- Boll W., Partin J., Katz A., Caplan M., Jamieson J. 1991. Distinct pathways for basolateral targeting of membrane and secretory proteins in polarized epithelial cells.Proc. Natl. Acad. Sci. USA. 88:8592–8596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifacino J.S., Dell'Angelica E.C. 1999. Molecular bases for the recognition of tyrosine-based sorting signals.J. Cell Biol. 145:923–926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancino J., Torrealba C., Soza A., Yuseff M.I., Gravotta D., Henklein P., Rodriguez-Boulan E., Gonzalez A. 2007. Antibody to AP1B adaptor blocks biosynthetic and recycling routes of basolateral proteins at recycling endosomes.Mol. Biol. Cell. 18:4872–4884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capaldo C.T., Macara I.G. 2007. Depletion of E-cadherin disrupts establishment but not maintenance of cell junctions in Madin-Darby canine kidney epithelial cells.Mol. Biol. Cell. 18:189–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan M.J., Anderson H.C., Palade G.E., Jamieson J.D. 1986. Intracellular sorting and polarized cell surface delivery of (Na+,K+)ATPase, an endogenous component of MDCK cell basolateral plasma membranes.Cell. 46:623–631 [DOI] [PubMed] [Google Scholar]
- Cresawn K.O., Potter B.A., Oztan A., Guerriero C.J., Ihrke G., Goldenring J.R., Apodaca G., Weisz O.A. 2007. Differential involvement of endocytic compartments in the biosynthetic traffic of apical proteins.EMBO J. 26:3737–3748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deborde S., Perret E., Gravotta D., Deora A., Salvarezza S., Schreiner R., Rodriguez-Boulan E. 2008. Clathrin is a key regulator of basolateral polarity.Nature. 452:719–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desclozeaux M., Venturato J., Wylie F., Kay J., Joseph S., Le H., Stow J. 2008. Active Rab11 and functional recycling endosome are required for E-cadherin trafficking and lumen formation during epithelial morphogenesis.Am. J. Physiol. Cell Physiol. 295:C545–C556 [DOI] [PubMed] [Google Scholar]
- Duffield A., Folsch H., Mellman I., Caplan M.J. 2004. Sorting of H,K-ATPase beta-subunit in MDCK and LLC-PK cells is independent of mu 1B adaptin expression.Traffic. 5:449–461 [DOI] [PubMed] [Google Scholar]
- Dunbar L.A., Aronson P., Caplan M.J. 2000. A transmembrane segment determines the steady-state localization of an ion-transporting adenosine triphosphatase.J. Cell Biol. 148:769–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields I.C., Shteyn E., Pypaert M., Proux-Gillardeaux V., Kang R.S., Galli T., Folsch H. 2007. v-SNARE cellubrevin is required for basolateral sorting of AP-1B–dependent cargo in polarized epithelial cells.J. Cell Biol. 177:477–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folsch H., Ohno H., Bonifacino J.S., Mellman I. 1999. A novel clathrin adaptor complex mediates basolateral targeting in polarized epithelial cells.Cell. 99:189–198 [DOI] [PubMed] [Google Scholar]
- Folsch H., Pypaert M., Maday S., Pelletier L., Mellman I. 2003. The AP-1A and AP-1B clathrin adaptor complexes define biochemically and functionally distinct membrane domains.J. Cell Biol. 163:351–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Füllekrug J., Shevchenko A., Shevchenko A., Simons K. 2006. Identification of glycosylated marker proteins of epithelial polarity in MDCK cells by homology driven proteomics.BMC Biochem. 7:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geering K., Theulaz I., Verrey F., Hauptle M.T., Rossier B.C. 1989. A role for the beta-subunit in the expression of functional Na+-K+-ATPase in Xenopus oocytes.Am. J. Physiol. 257:C851–C858 [DOI] [PubMed] [Google Scholar]
- Gottardi C.J., Caplan M.J. 1993. Delivery of Na+,K(+)-ATPase in polarized epithelial cells.Science. 260:552–554 [DOI] [PubMed] [Google Scholar]
- Gottardi C.J., Pietrini G., Roush D.L., Caplan M.J. 1993. Sorting of ion transport proteins in polarized cells.J. Cell Sci. Suppl. 17:13–20 [DOI] [PubMed] [Google Scholar]
- Gravotta D., Deora A., Perret E., Oyanadel C., Soza A., Schreiner R., Gonzalez A., Rodriguez-Boulan E. 2007. AP1B sorts basolateral proteins in recycling and biosynthetic routes of MDCK cells.Proc. Natl. Acad. Sci. USA. 104:1564–1569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths G., Simons K. 1986. The trans Golgi network: sorting at the exit site of the Golgi complex.Science. 234:438–443 [DOI] [PubMed] [Google Scholar]
- Grindstaff K.K., Yeaman C., Anandasabapathy N., Hsu S.C., Rodriguez-Boulan E., Scheller R.H., Nelson W.J. 1998. Sec6/8 complex is recruited to cell-cell contacts and specifies transport vesicle delivery to the basal-lateral membrane in epithelial cells.Cell. 93:731–740 [DOI] [PubMed] [Google Scholar]
- Grosshans B.L., Ortiz D., Novick P. 2006. Rabs and their effectors: achieving specificity in membrane traffic.Proc. Natl. Acad. Sci. USA. 103:11821–11827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundersen D., Orlowski J., Rodriguez-Boulan E. 1991. Apical polarity of Na,K-ATPase in retinal pigment epithelium is linked to a reversal of the ankyrin-fodrin submembrane cytoskeleton.J. Cell Biol. 112:863–872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua W., Sheff D., Toomre D., Mellman I. 2006. Vectorial insertion of apical and basolateral membrane proteins in polarized epithelial cells revealed by quantitative 3D live cell imaging.J. Cell Biol. 172:1035–1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob R., Naim H. 2001. Apical membrane proteins are transported in distinct vesicular carriers.Curr. Biol. 11:1444–1450 [DOI] [PubMed] [Google Scholar]
- Jordan C., Püschel B., Koob R., Drenckhahn D. 1995. Identification of a binding motif for ankyrin on the alpha-subunit of Na+,K(+)-ATPase.J. Biol. Chem. 270:29971–29975 [DOI] [PubMed] [Google Scholar]
- Keller P., Toomre D., Diaz E., White J., Simons K. 2001. Multicolour imaging of post-Golgi sorting and trafficking in live cells.Nat. Cell Biol. 3:140–149 [DOI] [PubMed] [Google Scholar]
- Keppler A., Pick H., Arrivoli C., Vogel H., Johnsson K. 2004. Labeling of fusion proteins with synthetic fluorophores in live cells.Proc. Natl. Acad. Sci. USA. 101:9955–9959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura T., Allen P.B., Nairn A.C., Caplan M.J. 2007. Arrestins and spinophilin competitively regulate Na+,K+-ATPase trafficking through association with a large cytoplasmic loop of the Na+,K+-ATPase.Mol. Biol. Cell. 18:4508–4518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kizhatil K., Davis J., Davis L., Hoffman J., Hogan B., Bennett V. 2007. Ankyrin-G is a molecular partner of E-cadherin in epithelial cells and early embryos.J. Biol. Chem. 282:26552–26561 [DOI] [PubMed] [Google Scholar]
- Kreitzer G., Schmoranzer J., Low S.H., Li X., Gan Y., Weimbs T., Simon S.M., Rodriguez-Boulan E. 2003. Three-dimensional analysis of post-Golgi carrier exocytosis in epithelial cells.Nat. Cell Biol. 5:126–136 [DOI] [PubMed] [Google Scholar]
- Kroschewski R., Hall A., Mellman I. 1999. Cdc42 controls secretory and endocytic transport to the basolateral plasma membrane of MDCK cells.Nat. Cell Biol. 1:8–13 [DOI] [PubMed] [Google Scholar]
- Le Bivic A., Quaroni A., Nichols B., Rodriguez-Boulan E. 1990. Biogenetic pathways of plasma membrane proteins in Caco-2, a human intestinal epithelial cell line.J. Cell Biol. 111:1351–1361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling K., Bairstow S., Carbonara C., Turbin D., Huntsman D., Anderson R. 2007. Type Iγ phosphatidylinositol phosphate kinase modulates adherens junction and E-cadherin trafficking via a direct interaction with µ1B adaptin.J. Cell Biol. 176:343–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mays R.W., Siemers K.A., Fritz B.A., Lowe A.W., van Meer G., Nelson W.J. 1995. Hierarchy of mechanisms involved in generating Na/K-ATPase polarity in MDCK epithelial cells.J. Cell Biol. 130:1105–1115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeill H., Ozawa M., Kemler R., Nelson W. 1990. Novel function of the cell adhesion molecule uvomorulin as an inducer of cell surface polarity.Cell. 62:309–316 [DOI] [PubMed] [Google Scholar]
- Mellman I. 1996. Membranes and sorting.Curr. Opin. Cell Biol. 8:497–498 [DOI] [PubMed] [Google Scholar]
- Mellman I., Nelson W. 2008. Coordinated protein sorting, targeting and distribution in polarized cells.Nat. Rev. Mol. Cell Biol. 9:833–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda K.C., Khromykh T., Christy P., Le T.L., Gottardi C.J., Yap A.S., Stow J.L., Teasdale R.D. 2001. A dileucine motif targets E-cadherin to the basolateral cell surface in Madin-Darby canine kidney and LLC-PK1 epithelial cells.J. Biol. Chem. 276:22565–22572 [DOI] [PubMed] [Google Scholar]
- Moskalenko S., Henry D.O., Rosse C., Mirey G., Camonis J.H., White M.A. 2002. The exocyst is a Ral effector complex.Nat. Cell Biol. 4:66–72 [DOI] [PubMed] [Google Scholar]
- Muth T.R., Dunbar L.A., Cortois-Coutry N., Roush D.L., Caplan M.J. 1997. Sorting and trafficking of ion transport proteins in polarized epithelial cells.Curr. Opin. Nephrol. Hypertens. 6:455–459 [DOI] [PubMed] [Google Scholar]
- Muth T.R., Gottardi C.J., Roush D.L., Caplan M.J. 1998. A basolateral sorting signal is encoded in the alpha-subunit of Na-K-ATPase.Am. J. Physiol. 274:C688–C696 [DOI] [PubMed] [Google Scholar]
- Oztan A., Silvis M., Weisz O.A., Bradbury N.A., Hsu S.C., Goldenring J.R., Yeaman C., Apodaca G. 2007. Exocyst requirement for endocytic traffic directed toward the apical and basolateral poles of polarized MDCK cells.Mol. Biol. Cell. 18:3978–3992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polishchuk R., Di Pentima A., Lippincott-Schwartz J. 2004. Delivery of raft-associated, GPI-anchored proteins to the apical surface of polarized MDCK cells by a transcytotic pathway.Nat. Cell Biol. 6:297–307 [DOI] [PubMed] [Google Scholar]
- Rindler M.J., Ivanov I.E., Plesken H., Sabatini D.D. 1985. Polarized delivery of viral glycoproteins to the apical and basolateral plasma membranes of Madin-Darby canine kidney cells infected with temperature-sensitive viruses.J. Cell Biol. 100:136–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Boulan E., Kreitzer G., Musch A. 2005. Organization of vesicular trafficking in epithelia.Nat. Rev. Mol. Cell Biol. 6:233–247 [DOI] [PubMed] [Google Scholar]
- Schuck S., Gerl M.J., Ang A., Manninen A., Keller P., Mellman I., Simons K. 2007. Rab10 is involved in basolateral transport in polarized Madin-Darby canine kidney cells.Traffic. 8:47–60 [DOI] [PubMed] [Google Scholar]
- Stabach P.R., Devarajan P., Stankewich M.C., Bannykh S., Morrow J.S. 2008. Ankyrin facilitates intracellular trafficking of {alpha}1-Na+-K+-ATPase in polarized cells.Am. J. Physiol. Cell Physiol. 295:C1202–C1214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeichi M. 1990. Cadherins: a molecular family important in selective cell-cell adhesion.Annu. Rev. Biochem. 59:237–252 [DOI] [PubMed] [Google Scholar]
- Tamkun M.M., Fambrough D.M. 1986. The (Na+ + K+)-ATPase of chick sensory neurons. Studies on biosynthesis and intracellular transport.J. Biol. Chem. 261:1009–1019 [PubMed] [Google Scholar]
- Vagin O., Tokhtaeva E., Sachs G. 2006. The role of the beta1 subunit of the Na,K-ATPase and its glycosylation in cell-cell adhesion.J. Biol. Chem. 281:39573–39587 [DOI] [PubMed] [Google Scholar]
- Wandinger-Ness A., Bennett M.K., Antony C., Simons K. 1990. Distinct transport vesicles mediate the delivery of plasma membrane proteins to the apical and basolateral domains of MDCK cells.J. Cell Biol. 111:987–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woroniecki R., Ferdinand J.R., Morrow J.S., Devarajan P. 2003. Dissociation of spectrin-ankyrin complex as a basis for loss of Na-K-ATPase polarity after ischemia.Am. J. Physiol. Renal Physiol. 284:F358–F364 [DOI] [PubMed] [Google Scholar]
- Yeaman C., Grindstaff K.K., Wright J.R., Nelson W.J. 2001. Sec6/8 complexes on trans-Golgi network and plasma membrane regulate late stages of exocytosis in mammalian cells.J. Cell Biol. 155:593–604 [DOI] [PMC free article] [PubMed] [Google Scholar]