Abstract
Changes in cellular microtubule organization often accompany developmental progression. In the Caenorhabditis elegans embryo, the centrosome, which is attached to the nucleus via ZYG-12, organizes the microtubule network. In this study, we investigate ZYG-12 function and microtubule organization before embryo formation in the gonad. Surprisingly, ZYG-12 is dispensable for centrosome attachment in the germline. However, ZYG-12–mediated recruitment of dynein to the nuclear envelope is required to maintain microtubule organization, membrane architecture, and nuclear positioning within the syncytial gonad. We examined γ-tubulin localization and microtubule regrowth after depolymerization to identify sites of nucleation in germ cells. γ-Tubulin localizes to the plasma membrane in addition to the centrosome, and regrowth initiates at both sites. Because we do not observe organized microtubules around zyg-12(ct350) mutant nuclei with attached centrosomes, we propose that gonad architecture, including membrane and nuclear positioning, is determined by microtubule nucleation at the plasma membrane combined with tension on the microtubules by dynein anchored at the nucleus by ZYG-12.
Introduction
Tissue and organ architecture are key to biological function. Tissue architecture, in turn, is largely determined by constituent cell architecture, including global cell shape as well as subcellular positioning of the nucleus and other organelles. The cytoskeleton is responsible for generating and maintaining morphology and organelle organization in cells with a variety of shapes and functions (for review see Li and Gundersen, 2008). We do not yet understand how diverse cells use a common set of cytoskeletal elements and similar basic tools to manipulate these elements to generate distinct architectures. Cells manipulate these cytoskeletal elements using regulatory proteins, including nucleators, depolymerases, severing proteins, and other binding proteins. Additionally, motor proteins transport cargo along cytoskeletal tracks, and anchored motor proteins pull on the cytoskeleton itself (Nguyen-Ngoc et al., 2007) to achieve the desired organization.
Although all cytoskeletal components make significant contributions to the establishment and maintenance of architectural organization, dramatic changes in microtubule organization upon differentiation and the extensive diversity in microtubule organization among cell types speaks to their key role in cell architecture. The concept of a microtubule-organizing center (MTOC), a site of γ-tubulin localization that nucleates microtubule formation, is used to explain the origins of various microtubule arrangements. In dividing cells, the nucleus-attached centrosome nucleates a characteristic array with the plus ends of the microtubules extending radially toward the cell periphery. However, differentiated cells have various alternative organizations in which microtubules are nucleated at noncentrosomal sites via mechanisms that are less well understood. For example, during differentiation of myoblasts, the microtubule cytoskeleton is remodeled from a centrosome-nucleated radial structure to a series of parallel arrays along the long axis of the myotube that are likely nucleated at nuclei with plus ends extending to the poles (Tassin et al., 1985; Bugnard et al., 2005). Polarized epithelia provide a second dramatic example of an alternative microtubule cytoskeleton; epithelial cells contain linear arrays of microtubules oriented with minus ends at the apex and plus ends extending to the basal side (Bre et al., 1990; Meads and Schroer, 1995). In epidermal development, epidermal cells progress from a stage with a centrosomal array to a stage with a parallel array as microtubule-anchoring proteins are relocalized from the centrosome to the cell junctions near the apical membrane (Lechler and Fuchs, 2007; Meng et al., 2008). Thus, dramatic alterations in microtubule organization often accompany differentiation. To comprehend the events that underlie cell differentiation and development of tissue architecture, it is important to understand how diverse microtubule arrays are organized.
We use the Caenorhabditis elegans hermaphrodite gonad as a model to probe the molecular mechanisms that establish and maintain tissue architecture. The gonad consists of an orderly arrangement of nuclei within a large syncytium that is surrounded by somatic gonad sheath cells (Fig. 1 A). The global architecture of the gonad as well as the cell cycle state of the nuclei changes as nuclei progress from the distal gonad tips to the proximal region. A distal tip cell caps each gonad arm and acts to maintain a mass of mitotic nuclei. As new cell divisions push nuclei proximally and away from the distal tip cells, nuclei initiate a meiotic program in the transition zone. Each meiotic nucleus is maintained at the periphery of the gonad in a partially enclosed membrane compartment with a cytoplasmic bridge that connects to a central cytoplasmic pool, the rachis (Hirsh et al., 1976; Hall et al., 1999). Nuclei progress through the pachytene region into the loop of the gonad where changes in cell shape, including acquisition of extra cytoplasm, and full cellularization lead to production of large oocytes. A transverse view of the distal gonad arm reveals ∼12 germline nuclei abutting the gonad sheath and surrounding the rachis (Fig. 1 B). When viewed from the lateral side, germline nuclei are evenly spaced at the periphery throughout the distal gonad arm (Fig. 1 C). This regularly arrayed pattern of germline nuclei within the gonad seems important for proper oogenesis and reproduction. Nuclear position in C. elegans, including that of germline nuclei, is subject to active control by the cytoskeleton and by extracellular matrix proteins (Hedgecock and Thomson, 1982; Vogel and Hedgecock, 2001; Thompson et al., 2002; Lu et al., 2008). However, the mechanisms that maintain the evenly distributed peripheral pattern of germline nuclei are mysterious.
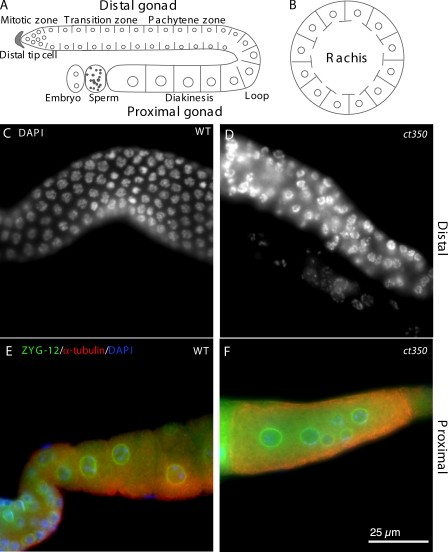
Figure 1.
zyg-12(ct350ts) disrupts the regular arrangement of germline nuclei. (A and B) Schematic lateral (A) and transverse (B) view of germline nuclear position in the C. elegans gonad. (C and D) DAPI-stained germline nuclei are regularly arrayed in the distal gonad arm of adult wild-type animals (C) but disarrayed in zyg-12(ct350ts) gonads (D). (E and F) Immunofluorescent images with ZYG-12 in green, α-tubulin in red, and nuclei (DAPI) in blue. Oocytes contain a single nucleus in the proximal gonad of wild-type animals (E) but have multiple nuclei in zyg-12(ct350ts) gonads (F). All animals were shifted to 25°C during L1.
Nuclear positioning in other C. elegans tissues and in other systems often depends on a conserved pair of protein families called Sad1/unc-84 (SUN) and Klarsicht/Anc-1/Syne-1 homology (KASH) domain proteins (for review see Starr, 2007). Current models posit that conserved SUN domain proteins bind chromatin or the nuclear lamina in the nucleus and span the inner nuclear membrane to bind to a conserved KASH domain protein partner in the inter membrane space. The KASH domain proteins span the outer nuclear membrane and interact with cytoskeletal elements in the cytoplasm. Thus, a SUN–KASH pair can facilitate interaction of the nucleus with a cytoskeletal system, including the microtubules (Starr and Han, 2002; Fischer et al., 2004; Yu et al., 2006; Zhang et al., 2007).
In the C. elegans embryo, centrosome–nucleus attachment is maintained via the SUN–KASH pair, SUN-1 and ZYG-12 (Malone et al., 2003). Two of three alternative ZYG-12 isoforms have a transmembrane-containing KASH domain and are localized to the nuclear envelope by SUN-1, but the third ZYG-12 isoform lacks the KASH domain and localizes to the centrosome (Malone et al., 2003). Interaction of these nuclear and centrosomal isoforms is proposed to mediate centrosome–nucleus attachment, and nuclear migration clearly depends on the connection between the nucleus and the centrosome MTOC. Dynein also plays a role in embryonic pronuclear migration; depletion of C. elegans dynein subunits DLI-1 or DHC-1 causes a penetrant pronuclear congression defect (Gonczy et al., 1999; Yoder and Han, 2001). These data are consistent with a model in which dynein provides the force for movement of the centrosome, whereas ZYG-12 provides centrosome–nucleus attachment, permitting nuclear movement. ZYG-12 also interacts directly with DLI-1, the light intermediate chain of dynein, and recruits DLI-1 to the nuclear envelope in the early embryo and the gonad (Malone et al., 2003), suggesting that ZYG-12 functions as a molecular anchor on the nuclear envelope for cytoplasmic dynein. However, the role of dynein at the nuclear envelope in embryos is not clear; a zyg-12 allele (or577) that abrogates ZYG-12 homodimerization but retains the dynein interaction has migration and centrosome detachment defects as severe as those of zyg-12(ct350), which disrupts the dynein interaction (Malone et al., 2003), suggesting that the key function for ZYG-12 in the embryo is centrosome–nucleus attachment.
Both SUN-1 and ZYG-12 are expressed in the germline, but neither the role of these proteins nor the role of the microtubules in germline architecture, nuclear positioning, or centrosome attachment has been investigated in the gonad. Therefore, we started our investigation of germline cytoarchitecture with ZYG-12. In this study, we reveal that, in the gonad, ZYG-12 is dispensable for centrosome attachment. However, ZYG-12 recruitment of dynein to the germ cell nuclear envelope is critical for nuclear positioning. Because both ZYG-12 function and nuclear positioning in the gonad are independent of the centrosome, we examined alternative sites of microtubule nucleation. Based on the localization of γ-tubulin and microtubule regrowth initiation at the cytoplasmic membrane after depolymerization, we propose that gonad architecture, including germline nuclear positioning, is determined by microtubule nucleation at the plasma membrane in combination with tension on these microtubules provided by dynein that is anchored to the nuclear membrane by ZYG-12.
Results
Mutation of zyg-12 results in nuclear position and oogenesis defects in the C. elegans gonad
In the embryo, nuclear positioning requires ZYG-12 at the nuclear membrane. ZYG-12 is also localized to the nuclear envelope of germline nuclei in the syncytial C. elegans gonad (Malone et al., 2003). To determine whether ZYG-12 has essential function in the gonad, we examined zyg-12 mutant gonads for defects. To avoid the early embryonic lethality associated with disruption of ZYG-12 function, we used a temperature-sensitive allele of zyg-12. We shifted postembryonic, first larval stage (L1) zyg-12(ct350 ts) animals to the restrictive temperature (25°C) and examined the gonad of the adults. Intriguingly, we observed disruption of the regular arrangement of nuclei within the syncytial gonad of all mutant animals. Although germline nuclei are arranged in a regular array at the periphery of the gonad in wild type (Fig. 1, A–C), germline nuclei appear randomly distributed within the gonad of the zyg-12(ct350) mutants (Fig. 1 D). Furthermore, large oocytes with multiple nuclei form in the proximal gonad arm of the mutants (Fig. 1 F) in contrast to the single nucleus in each wild-type oocyte (Fig. 1 E).
We used immunofluorescence and confocal microscopy to further characterize nuclear mispositioning. We extracted gonads from zyg-12(ct350) or wild-type animals at the restrictive temperature and performed immunofluorescence with antibodies against ZYG-12 to visualize nuclear envelopes and against α-tubulin to visualize cytoplasm. In wild type, germline nuclei are evenly arranged directly under the surface of gonad (Fig. 2 A), and no nuclei occupy the central rachis (Fig. 2 B). In contrast, in the zyg-12(ct350) mutant, the nuclei are disarrayed at the surface (Fig. 2 D), and large numbers of nuclei have collapsed into the central rachis (Fig. 2 E). We also observed abnormal clusters of small nuclei that do not appear to have undergone oogenesis in the proximal gonad (Fig. 2 F).
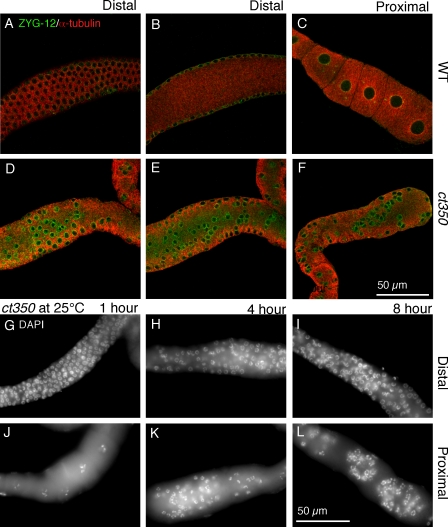
Figure 2.
zyg-12(ct350) germline nuclei relocalize rapidly to the central rachis in response to a temperature shift. (A–F) Confocal images of gonads from egg-laying adults incubated at 25°C for 6 h. Gonads are labeled with ZYG-12 (green) to mark the nuclear envelope and α-tubulin (red). Wild-type (WT) gonads maintain the regular position of the nuclei at the periphery (A) and have no nuclei in the rachis (B). (C) Furthermore, the proximal gonads of wild-type animals have evenly spaced oocytes with one nucleus each. (D and E) In contrast, the nuclei at the periphery of zyg-12(ct350) gonads are disorganized (D), and many nuclei occupy the central rachis (E). (F) The proximal gonad of zyg-12(ct350) animals has abnormal clusters of small nuclei that resemble the distal nuclei. (G–L) DAPI-stained proximal (G–I) and distal (J–L) gonads of egg-laying adult zyg-12(ct350) animals incubated at 25°C for short intervals, as indicated. The nuclei are rapidly displaced in response to exposure to the restrictive temperature.
Because zyg-12 is required for centrosome–nucleus attachment in the embryo, it is required for embryonic mitotic divisions (Malone et al., 2003). To rule out a mitotic defect in the rapidly proliferating germline as the underlying cause of the disruption in nuclear arrangement, we examined gonads at shorter intervals after the shift to the restrictive temperature. We transferred zyg-12(ct350) egg-laying hermaphrodites that had been maintained at 15°C to the restrictive temperature (25°C) for 1, 4, or 8 h and examined the postmitotic region of their gonads. Germline nuclei retained their position after 1 h at 25°C (Fig. 2 G). However, within 4 h, germline nuclei were displaced in the distal (Fig. 2 H) and the proximal (Fig. 2 K) gonad. 8 h after the temperature shift, the gonad was heavily disrupted (Fig. 2, I and L). The majority of the distal nuclei are unlikely to have undergone mitosis during the time course of the experiment. Moreover, the nuclei in the pachytene zone of the proximal gonad are postmitotic before the temperature shift and yet become mispositioned. Wild-type germline nuclei maintained their well-aligned position when cultured at the restrictive temperature starting in L1 (Fig. 1 C) or after 6 h at the restrictive temperature (Fig. 2, A–C). The rapid nuclear repositioning throughout the gonad after 4 h at the restrictive temperature suggests that the phenotype is independent of any mitotic defect.
Germ cell membranes are also disrupted in zyg-12(ct350) mutant gonads
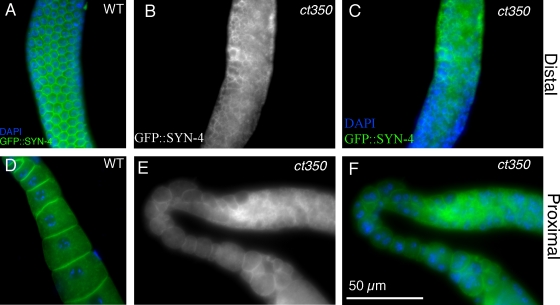
To further characterize the disrupted gonad phenotype caused by zyg-12(ct350), we examined cell membranes. We used a germ cell membrane–localized syntaxin-GFP fusion, GFP::SYN-4 (Jantsch-Plunger and Glotzer, 1999), to visualize the membranes that partially surround each germ cell nucleus in the syncytial gonad. In parallel with the disruption of nuclear position, the membranes are rearranged in the zyg-12(ct350) mutants. In wild type, the membranes that partially surround each germ cell nucleus are well organized in a honeycomb-like structure (Fig. 3 A). However, within 6 h at the restrictive temperature, zyg-12(ct350) membranes are disorganized (Fig. 3, B and C) and, in the proximal gonad, sometimes incorporate >1 nucleus (Fig. 3, E and F). We conclude that zyg-12 is involved in a mechanism that helps to maintain the well-aligned, evenly spaced peripheral position of the germline nuclei and the regularly arrayed surrounding membranes within the gonad syncytium.
Figure 3.
The membranes of zyg-12(ct350) gonads are rearranged at the restrictive temperature. (A–D) GFP::SYN-4 permits visualization of membranes in a wild-type distal (A) and proximal (D) gonad. This patterned array of germ cell membranes is disorganized in the distal (B and E) and proximal (C and F) arm of zyg-12(ct350) gonads.
ZYG-12–dynein and ZYG-12–SUN-1 interactions are important for normal gonad architecture
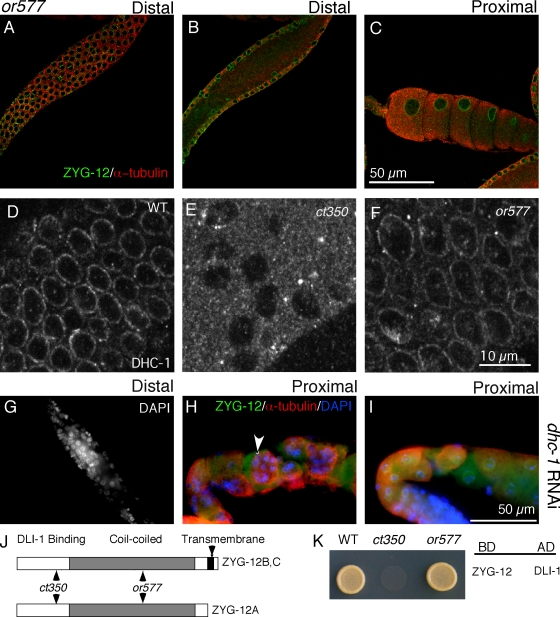
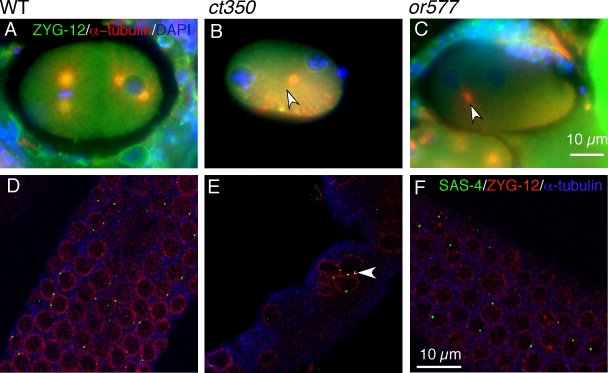
To understand how disruption of ZYG-12 could alter both nuclear position and membrane architecture, we tested alleles of zyg-12 that affect distinct protein–protein interactions (Fig. 4 J). ZYG-12 interacts with itself and with dynein light intermediate chain DLI-1 in yeast two-hybrid assays (Fig. 4 K; Malone et al., 2003). The zyg-12(ct350) allele mutation (Q44P) lies in the N-terminal region (Fig. 4 J) and renders both the ZYG-12–ZYG-12 and ZYG-12–DLI-1 interactions sensitive to high temperature in a yeast two-hybrid assay (Malone et al., 2003). In contrast, the mutation (Q367P) present in another zyg-12 ts allele, or577, lies in the coiled-coil domain of ZYG-12 (Fig. 4 J) and abrogates the ZYG-12–ZYG-12 interaction without affecting the ability of ZYG-12 to bind DLI-1 in a yeast two-hybrid assay (Fig. 4 K; Malone et al., 2003). Although zyg-12(or577ts) embryos exhibit detached centrosomes and pronuclear migration defects similar to those of zyg-12(ct350ts) embryos (see Fig. 6, B and C), we surprisingly found that zyg-12(or577ts) mutants do not display the gonad defect of zyg-12(ct350). The gonads of zyg-12(or577) hermaphrodites shifted to 25°C for 6 h are indistinguishable from wild-type animals (Fig. 4, A–C). zyg-12(or577) hermaphrodites shifted to the restrictive temperature during L1 also have evenly spaced germline nuclei and oocytes with single nuclei as adults (Fig. S1, A and B). Using an antibody to the heavy chain of cytoplasmic dynein, DHC-1, we confirmed that DHC-1 was recruited to the nuclear membrane of all germline nuclei in wild-type (Fig. 4 D) but not zyg-12(ct350) mutant gonads at 25°C (Fig. 4 E; Malone et al., 2003). zyg-12(or577) animals had normal DHC-1 localization at the nuclear membrane (Fig. 4 F).
Figure 4.
The zyg-12(or577) mutant, which does not affect the ZYG-12–dynein interaction, has normal germline nuclear positioning. (A–C) Confocal fluorescent images of zyg-12(or577) incubated at 25°C for 6 h. Gonads are labeled with ZYG-12 (green) to mark the nuclear envelope and α-tubulin (red). (A and B) The nuclei at the periphery of the gonad maintain their regular positioning (A), and no nuclei are in the central rachis (B). (C) Furthermore, the proximal gonad has evenly spaced oocytes with one nucleus each, which is similar to wild-type animals (Fig. 2 C). The or577 allele has no detectable effect on ZYG-12–DLI-1 interactions in this assay. (D–F) Immunofluorescent images obtained with DHC-1 antibody stained gonads. (D) We confirmed that DHC-1 is recruited to the nuclear envelope of all nuclei in the wild-type (WT) germline. (E) In contrast, robust DHC-1 signal was never observed in zyg-12(ct350) mutant germlines (Malone et al., 2003), and <10% of the zyg-12(ct350) nuclei display a weak DLI-1 signal. (F) DHC-1 is retained at the nuclear envelope of all nuclei in zyg-12(or577) animals at 25°C. (G–I) RNAi of dynein heavy chain (dhc-1) disrupts gonad nuclear arrangement. (G) DAPI-stained distal gonad shows disorganized nuclei. (H and I) Immunofluorescent images of proximal gonads stained with ZYG-12 (green) to mark nuclei, α-tubulin (red), and DAPI (blue) show multiple nuclei (arrowhead) in single oocytes. (J) ct350 and or577 affect different domains of ZYG-12 (Malone et al., 2003). (K) We confirmed the ZYG-12 interaction with dynein light intermediate chain DLI-1 using a yeast two-hybrid assay as well as the lack of interaction caused by the ct350 allele (Malone et al., 2003). BD, DNA-binding domain vector; AD, activation domain vector.
Figure 6.
There are different mechanisms controlling centrosome attachment in the embryo and the germline. (A–C) ZYG-12 is required for centrosomal attachment in the embryo. Immunofluorescent images of embryos incubated at the restrictive temperature for 6 h labeled with ZYG-12 (green) to mark the nuclear envelope and α-tubulin (red) to mark the centrosome. (A) The centrosomes are attached to the nuclei in a two-cell stage embryo. (B and C) Centrosomes (arrowheads) are detached from the nuclei in zyg-12(ct350) and zyg-12(or577) embryos. (D–F) ZYG-12 is dispensable for centrosome attachment in the gonad, and loss of nuclear anchoring is independent of centrosome attachment. Immunofluorescent images of gonads incubated at the restrictive temperature for 6 h labeled with antibodies to SAS-4 (green) to mark centrosomes, ZYG-12 (red) to mark nuclei, and α-tubulin (blue). Centrosomes remain closely apposed to the nuclear envelope of wild-type (WT) nuclei (D), zyg-12(ct350) nuclei that have been displaced (E), and zyg-12(or577) nuclei (F). Note that in wild-type and both mutants, the SAS-4 signal is visible at all nuclei but does not appear in images only because of the focal plane of the confocal image. Although nuclear position was disrupted, centrosomes still remain on the nuclear envelope of the zyg-12(ct350) mutants (E, arrowhead).
The lack of a gonad phenotype in zyg-12(or577), which binds to and recruits dynein to the nuclear envelope, compared with the severe nuclear positioning and membrane morphology defects of zyg-12(ct350), which fails to bind dynein or recruit it to the nuclear envelope, suggests that a ZYG-12 interaction with dynein is important for maintenance of gonad architecture. To further investigate the role of dynein, we used RNAi to deplete DHC-1. dhc-1(RNAi) causes a larval arrest phenotype (unpublished data). However, adult animals that escape larval arrest have a gonad phenotype similar to that of zyg-12(ct350) animals. In the distal gonad of dhc-1(RNAi) hermaphrodites, germline nuclei were displaced from the periphery of the gonad (Fig. 4 G) as previously noted (Wolke et al., 2007). Phenotypes in the proximal gonad in dhc-1(RNAi) were somewhat variable. However, there were many oocytes with multiple nuclei (Fig. 4 H), and the arrangement of the oocytes during their formation is abnormal (Fig. 4 I).
In the zygote and the gonad, the localization of ZYG-12 to the outer nuclear membrane is dependent on the inner nuclear envelope protein SUN-1 (Malone et al., 2003; Penkner et al., 2007). As expected, depletion of sun-1 via RNAi also causes disorganization of the nuclei in the distal gonad and clumping of multiple nuclei in the proximal gonad (Fig. S2). We conclude that in addition to ZYG-12, both known binding partners of ZYG-12, SUN-1 and DLI-1, are required for maintenance of gonad architecture.
The cytoplasmic microtubule network is disrupted in the syncytial gonad of zyg-12(ct350)
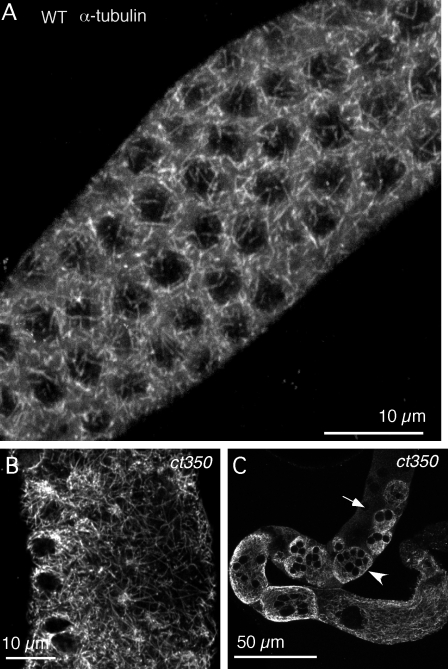
Given the drastic rearrangement of gonad morphology in the dynein-binding–defective zyg-12 mutant or upon RNAi of dhc-1, we hypothesized that microtubule organization might be perturbed. To visualize microtubule structure with high resolution, we stained glutaraldehyde-fixed gonads with an anti–α-tubulin antibody and acquired an immunofluorescent confocal z series at 0.1-µm intervals along the z axis. In a single frame, the α-tubulin appears in a punctate pattern throughout the cytoplasm (Fig. S3 A and see Fig. 7 G). A 3D projection of the z series reveals a meshwork arrangement of microtubule filaments criss crossed throughout the cytoplasm (Fig. 5 A and Fig. S4 A′). The zyg-12(ct350) mutant displays the same microtubule arrangement as wild type when maintained at 15°C (Fig. S3 B and Fig. S4 B′). To examine microtubule reorganization upon disruption of zyg-12 function, we shifted ct350 adults to 25°C and fixed the gonad at different time points. 1 h after the temperature shift, the microtubules show no obvious differences compared with wild type (Fig. S3 C and Fig. S4 C′), and nuclei maintained their peripheral positions (Fig. S4 C). 2 h after the temperature shift, microtubule structure is altered; longer filaments can be seen in a single frame (Fig. S3 D) compared with the short filaments seen in wild type (see Fig. 7 A). Coincident with this microtubule rearrangement, the well-aligned nuclear position is partially disrupted (Fig. S4 D). 3 h after the temperature shift, dramatic rearrangement of microtubules is accompanied by disruption of nuclear position (Fig. S3 E; and Fig. S4, E and E′). By 8 h, gonad architecture is completely rearranged (Fig. 5, B and C; and Fig. S3 F). There are longer microtubule filaments that are no longer associated with nuclei (Fig. 5 B and Fig. S3 F). Aberrant microtubule arrays encapsulate large clusters of nuclei, whereas other nuclei are excluded from these structures (Fig. 5 C; and S4, F and F′).
Figure 7.
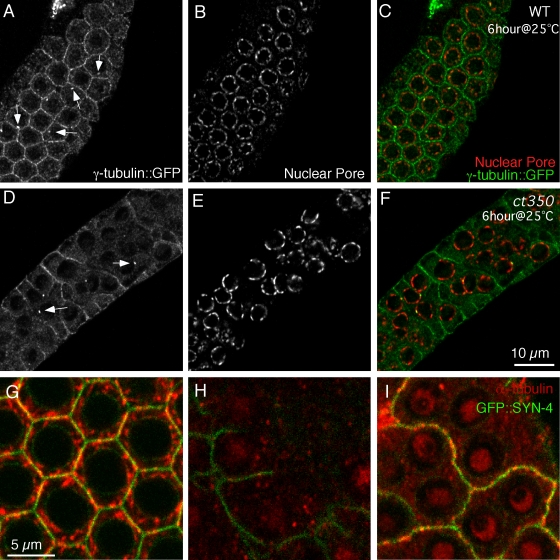
Plasma membrane as the nucleation site of microtubules in the distal gonad. (A–E) γ-Tubulin localizes to germ cell membrane in both wild-type (WT) and zyg-12(ct350) gonads. (A) In the distal gonad of wild-type animals, γ-tubulin localizes to germ cell membranes and centrosomes (white arrows). (B) The nuclear pore complex marks the nuclei. (D–F) In zyg-12(ct350), neither the membrane nor the centrosomal localization (D, white arrows) of γ-tubulin::GFP is altered. (F) However, nuclear position has been disrupted. (G–I) Microtubules regrow from the plasma membrane (marked by GFP::SYN-4) after the removal of depolymerizing drug from cultured gonads. (G) Filamentous microtubules occupy the cytoplasm and partially colocalize with the plasma membrane in a cultured gonad not subjected to nocodazole. (H) 1 h of nocodazole treatment effectively depolymerizes microtubules in the gonad. (I) 30 min after the removal of nocodazole, microtubules regrow at the plasma membrane.
Figure 5.
Microtubule architecture accounts for nuclear positioning in both wild-type and ct350 mutants. (A) Distal gonad of wild-type (WT) animal. 3D projection of a z series of confocal immunofluorescent images stained with α-tubulin reveals a meshwork-like structure of microtubules surrounding the nuclei. Microtubules in the gonad of zyg-12(ct350) mutants are severely disorganized after 8 h at the restrictive temperature. (B) In a view of the distal gonad region, longer microtubule filaments crisscross through a cytoplasm devoid of nuclei. (C) In a view of the whole gonad arm, large microtubule networks encapsulate multiple nuclei. The arrowhead indicates multiple nuclei surrounded by the same microtubule network, and the arrow indicates nuclei not surrounded by microtubules.
Maintenance of microtubule organization within the gonad is centrosome independent
The best-characterized MTOC is the centrosome, and ZYG-12 is required for the juxtaposition of the centrosome and the nucleus in the embryo. Thus, one simple hypothesis to explain disruption of microtubules in zyg-12(ct350) gonads is that mispositioning of the centrosomal MTOC underlies the microtubule disorganization. Therefore, we determined whether zyg-12(ct350) at the nonpermissive temperature resulted in loss of the centrosome from its perinuclear position in the gonad. We examined attachment of the centrosome to the nucleus at the restrictive temperature in wild-type and zyg-12(or577) gonads, in which germline nuclei are always properly positioned, and in zyg-12(ct350) gonads, in which the germline nuclei are mispositioned. We confirmed that both zyg-12(ct350) and zyg-12(or577) result in detached centrosomes in embryos (Fig. 6, A–C), but, strikingly, the centrosome remains attached to the germline nuclei regardless of zyg-12 genotype or nuclear positioning phenotype (Fig. 6, D–F). In wild-type and zyg-12(or577) gonads, centrosomes are always juxtaposed to the regularly arrayed nuclei in the peripheral plane (Fig. 6, D and F). Similarly, the centrosomes remain attached to all displaced nuclei in zyg-12(ct350) gonads, as most strikingly illustrated by the maintenance of attachment to nuclei that have collapsed into the central rachis (Fig. 6 E). We conclude that the mechanism for centrosome attachment differs between the embryo and the germline and does not require ZYG-12 in the gonad. Furthermore, nuclear and microtubule rearrangement upon shift to the restrictive temperature is not associated with loss of centrosomal–nuclear attachment. Misplaced mutant nuclei maintain their centrosome attachments (Fig. 6 E), and misplaced nuclei with attached centrosomes are sometimes completely outside any microtubule network (Fig. 5 C). Thus, the centrosome is not sufficient to organize the global microtubule architecture in the postmitotic gonad, and the mechanism for maintaining nuclear positioning and microtubule organization within the gonad is likely centrosome independent.
γ-Tubulin localization suggests plasma membrane nucleation of microtubules in the distal gonad
As the centrosome is not the key to the loss of microtubule organization in zyg-12(ct350) mutants, we hypothesized that another major MTOC is present in the gonad. To identify potential sites of microtubule nucleation, we visualized γ-tubulin, the core component of the canonical microtubule nucleation pathway. We first visualized γ-tubulin using live animals expressing γ-tubulin::GFP (Dammermann et al., 2004). Although the γ-tubulin::GFP signal is weak, it demonstrates that the bulk of the γ-tubulin in the distal region of the gonad syncytium localizes to the germ cell membranes and to the centrosome (Fig. S5 A). In the proximal gonad, we see γ-tubulin on the nuclear and plasma membranes of oocytes with some signal emanating from structures in the cytoplasm as well (Fig. S5 B).
As a more sensitive method to confirm the expression pattern, we used immunofluorescence with a polyclonal GFP antibody in combination with a nuclear pore complex antibody to mark the nuclear membrane. As observed in live animals, γ-tubulin::GFP localizes to both the germ cell membranes and to the nucleus-associated centrosome in the distal gonad (Fig. 7, A–C). In the zyg-12(ct350) mutant at the restrictive temperature, γ-tubulin::GFP still localizes to the cell membranes and the centrosomes (Fig. 7, D–F). Based on the localization of γ-tubulin and the phenotype of zyg-12(ct350), we hypothesized that the plasma membrane may be a major site of microtubule nucleation in the distal gonad.
To investigate this hypothesis, we used nocodazole to depolymerize microtubules and then removed the drug to observe regrowth in gonads in which the plasma and nuclear membranes were marked with GFP. Filamentous microtubules are found in the space between the nuclear envelope and plasma membrane, with some overlap at each membrane, before treatment with the drug (Fig. 7 G). Microtubules were effectively depolymerized after 1 h of nocodazole treatment (Fig. 7 H). Consistent with the idea that the microtubule network is critical for maintaining germline nuclear position and germ cell membrane organization, both are disrupted upon microtubule depolymerization (Fig. 7 H). Within 30 min of nocodazole removal, staining for microtubules reveals numerous puncta that are associated with the plasma membrane (Fig. 7 I; and Fig. S5, C–E). No microtubules are observed along the circumference of the nuclear membrane at these early time points (Fig. 7 I; and Fig. S5, C and E) with the exception of those at the centrosome (Fig. S5, G and H). However, microtubules do localize along the circumference of the nuclear membrane by 1 h after nocodazole removal (Fig. S5 F). This temporal pattern of microtubule regrowth suggests that the plasma membrane is a primary microtubule nucleation site. Microtubule nucleation at the centrosome is also observed in disorganized gonads of the zyg-12(ct350) mutants (Fig. S5 I), again suggesting that the centrosome has the capacity to nucleate some microtubules but is insufficient to organize the global germline microtubule array. We propose that the majority of microtubules in the gonad are nucleated at the plasma membrane and that dynein localized to the nuclear envelope by ZYG-12 subsequently captures these microtubules to organize the microtubule array and to position nuclei at the gonad periphery.
Ultrastructural analysis of gonad subcellular architecture
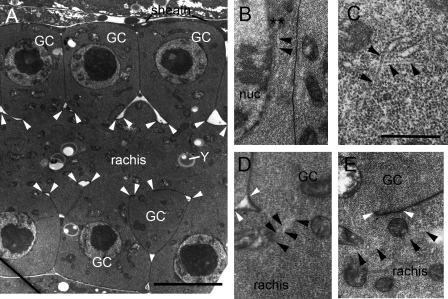
We also used ultrastructural analysis to examine gonad architecture and microtubule organization to gain a deeper understanding of how microtubules relate to other organelles and major features of the gonad. Electron micrographs of the proximal gonad reveal several interesting features. First, there is a striking uniformity in cytoplasmic contents in the partially cellularized germ cells and the rachis, consisting primarily of an abundance of free ribosomes, some mitochondria, and rather little ER or yolk (Fig. 8). Second, organelles such as mitochondria cross the cytoplasmic bridge from germ cell to rachis, illustrating the need for holding the larger nucleus in place and restricting its proximity to the rachis bridge (Fig. 8). Third, nuclei are maintained in close proximity to the outer membrane of the germ cell (Fig. 8 A). Fourth, microtubule organization in the germ cells is nonuniform. There are relatively few microtubules, and they are not found in parallel arrays. They criss cross (Fig. 8 C) and approach both the plasma membrane and the nucleus, specifically contacting the nucleus at areas of electron density (Fig. 8 B) in proximity to the nuclear pores (not depicted). Microtubules also contact (unpublished data) the rachis along its structural support feature, “the rachis coat” (Vogel and Hedgecock, 2001; Hall and Altun, 2008). The coat is an electron-dense structure (Fig. 8, A, D, and E) that covers the outer surface of the rachis, with slight thickening near each germ cell opening. The coat is comprised principally of hemicentin, which is required to prevent early germ cell membranes from encapsulating multiple nuclei (Vogel and Hedgecock, 2001). Mitochondria (and other potential cargoes) are often observed to lie very near to microtubules by transmission EM (TEM; Fig. 8 E). Finally, bundles of microtubules cross the cytoplasmic bridge between the germ cell and the rachis (Fig. 8 D).
Figure 8.
TEM reveals microtubule organization in the germline. (A) Low power view of the distal arm of the gonad seen in lengthwise view. Rows of germ cells (GC) lie on either side of the rachis, connected by cytoplasmic bridges to the rachis. A somatic sheath cell partially covers the germline at this level. The cytoplasm of the germ cells contains a large central nucleus and scattered mitochondria. The rachis is also filled with mitochondria and contains yolk granules (Y) and some ER. Some organelles span the bridges between the germ cell and rachis. The outside surface of the rachis membrane has an electron-dense rachis coat between closely spaced arrowheads. (B–E) Higher power views of germ cells oriented so that the germ cell lies to the top of each panel with the rachis beneath it, running left to right as in A. Note that free ribosomes fill most of the cytoplasm in all distal germline cells and in the rachis. (B) A microtubule appears to attach to an electron-dense projection (asterisks) on the cytoplasmic face of a germ cell nucleus (nuc). These projections appear to extend from the nuclear pore. (C) Two microtubules pass one another at almost orthogonal angles near the top of a germ cell. (D) A bundle of microtubules passes across the cytoplasmic bridge between the rachis and a germ cell, running close to several mitochondria. (E) Microtubules can be seen running lengthwise along the long axis of the rachis close to several mitochondria. Black arrowheads indicate microtubules, and white arrowheads mark rachis openings for each germ cell. Bars: (A) 5 µm; (C) 0.5 µm.
Discussion
Distinct molecular interactions by ZYG-12 are required for gonad and embryonic functions
Recruitment of ZYG-12 to the nuclear envelope is critical to embryonic development (Malone et al., 2003). In this study, we show that ZYG-12 at the nuclear envelope is crucial for maintenance of the global architecture of the gonad as well. Interestingly, the key binding partners for ZYG-12 in the embryo appear to be distinct from those in the gonad.
In the embryo, a homodimeric interaction between ZYG-12 proteins localized separately to the nuclear envelope and the centrosome is presumed to mediate the necessary attachment between the nucleus and the centrosome (Malone et al., 2003). In the gonad, ZYG-12 has no role in nucleus–centrosome attachment. ZYG-12 is not localized to the centrosome in the gonad (Penkner et al., 2007; unpublished data), and nucleus–centrosome attachment is maintained in the gonad in both zyg-12(ts) alleles examined. Furthermore, ZYG-12–ZYG-12 interactions are dispensable in the gonad, as indicated by the normal gonad phenotype of the ZYG-12 homodimerization-defective mutant zyg-12(or577). This is further illustrated by comparing the gross phenotype of zyg-12(ct350), which is sterile, indicating a germline defect, and zyg-12(or577), which is embryonic lethal but not sterile, suggesting normal gonad and germline function. Thus, we have revealed a ZYG-12–independent mechanism for centrosome attachment in the postmitotic gonad. The protein emerin appears to function in nucleus–centrosome attachment in human fibroblasts (Salpingidou et al., 2007). It will be interesting to determine whether C. elegans emerin shares this function in the gonad or whether other as yet unidentified proteins attach germline nuclei and perhaps nuclei in other tissues to centrosomes.
In the germline and embryos, ZYG-12 appears to function as a molecular anchor for recruiting dynein to the outer nuclear membrane. Although the function of the dynein at the nuclear envelope in embryos may play a minor role in centrosome attachment (Malone et al., 2003), we have shown that recruitment of dynein to the nuclear membrane by ZYG-12 is critical for maintenance of gonad architecture; loss of ZYG-12–DLI-1 interactions likely underlies the zyg-12(ct350) mutant phenotype. Although we cannot rule out the possibility that an interaction between ZYG-12 and another unknown protein is simultaneously disrupted by the ct350 allele, our data are consistent with a central role for dynein. Disruption of dynein expression mimics the zyg-12(ct350) phenotype, and the lack of dynein recruitment to the nuclear envelope is correlated with the zyg-12(ct350) phenotype. ZYG-12 has also been implicated in meiotic chromosome pairing (Penkner et al., 2007), and it remains to be determined which molecular interactions of ZYG-12 are key to this function.
Model for architecture of the distal gonad
We propose that cellular architecture in the distal gonad is dictated by microtubules nucleated at the plasma membrane by γ-tubulin and captured at the nuclear envelope by dynein, whose motor activity, directed toward microtubule minus ends, creates tension between the nucleus and plasma membrane (Fig. 9). This tension could explain the major structural features of the distal gonad, including the peripheral position of the nuclei in the gonad, with a central rachis devoid of nuclei, and the architecture of the plasma membrane that appears to be pulled around each nucleus (Hall et al., 1999). The predicted resulting haphazard arrangement of microtubules in the cytoplasm is supported by the ultrastructural analysis.
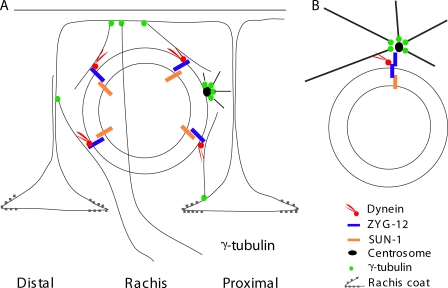
Figure 9.
A model for MTOC-independent nuclear anchoring in the germline. (A) A model for germline nuclear anchoring in the gonad. ZYG-12 recruits dynein to the outer nuclear envelope. Dynein promotes nuclear anchoring via direct interactions with the microtubule filaments nucleated from membrane-localized γ-tubulin. The centrosome is not involved in nuclear positioning of germline nuclei. (B) The centrosome is the key to nuclear positioning in the embryo. ZYG-12 homodimerization is essential to maintain the nucleus–centrosome interaction in the embryo, permitting control of nuclear positioning.
If the only considerations in the distal gonad were positioning nuclei near the edge and bringing plasma membrane extensions between nuclei, other arrangements of microtubules might work equally well. For example, microtubules could be nucleated at or near the nucleus and be pulled on by interactions with the cell cortex. This general arrangement is used frequently (Siegrist and Doe, 2007). However, the distal gonad is not simply a storage shed for future oocyte nuclei; it is hypothesized to function as a nurse compartment that provides material to the oocyte (Wolke et al., 2007). Oocytes, which are transcriptionally quiescent, are packed with material for early embryogenesis via actin-based cytoplasmic streaming from the rachis (Wolke et al., 2007). The transcriptionally active germline nuclei distal to the developing oocytes are thought to synthesize the rachis cytoplasm (Starck, 1977; Gibert et al., 1984; Wolke et al., 2007). The high free ribosome density observed in electron micrographs is consistent with a role for these cells in synthesizing material for loading into the oocyte during cytoplasmic streaming. Thus, most trafficking from the peripheral nuclei should be directed into the central rachis of the gonad, and this transport is likely to be microtubule based. Microtubule nucleation at the plasma membrane and tension along the side of microtubules by dynein at the nuclear envelope allows the tips of microtubules to extend past the nuclei into the rachis (Fig. 8 D and Fig. 9), whereas other microtubule arrangements that could account for peripheral localization of nuclei as mentioned previously, would not. Interestingly, microtubules appear to make closest contact with the nucleus at sites containing electron-dense projections that lie in proximity to the nuclear pore (Fig. 8 D and not depicted). This may provide a link for trafficking from the nucleus to the rachis. Microtubule extension from the peripheral germ cells into the rachis has been described previously (Wolke et al., 2007), but the organizing factors were not known. Interactions between microtubules and the rachis coat are also likely to contribute to the integrity of the membrane in the area of the bridge to the rachis. The rachis coat appears to reinforce the tubular shape of the rachis and probably the cytoplasmic bridges between the germ cells and the rachis, as the coat covers the rachis surface with slight thickening at each opening but never extends over the surface of the germ cells (Hall et al., 1999). Interactions between this coat and local microtubules could maintain each rachis opening between a germ cell and the syncytium as the maturing germ cell migrates proximally along the rachis.
Several other biologically important features of the gonad are likely to be facilitated by anchoring nuclei at its outside edge. Unanchored nuclei could float through cytoplasmic bridges to the rachis, as observed for mitochondria (Fig. 8). This might block the bridge or disrupt cytoplasmic streaming in the rachis. It could also result in multiple nuclei streaming into the oocyte as seen in zyg-12(ct350) (Fig. 1 F and Fig. 2 F). Other mutants are known to cause similar defects in the distal gonad, such as nuclei in the central rachis (Thompson et al., 2002), chains of membrane-covered germline cells with extended cytoplasmic bridges connecting them to a malformed rachis (Lu et al., 2008), or germline cells containing multiple nuclei per membranous compartment but connected normally to the rachis (Vogel and Hedgecock, 2001). The gonads of these mutants appear superficially similar to that of zyg-12(ct350). However, molecular analyses suggest that these phenotypes are caused by different mechanisms such as a mitotic defect or malformed membranes.
Aspects of the phenotype observed after loss of dynein from the nuclear envelope in zyg-12(ct350) are also consistent with a plasma membrane–nucleus tension model for architecture of the distal gonad. After loss of dynein from the nuclear envelope in the zyg-12(ct350) mutant, the distal gonad is rearranged such that the microtubule network encapsulates groups of nuclei while excluding others. The excluded nuclei with no surrounding microtubules clearly illustrate the lack of microtubule-organizing activity by the nuclei and their attached centrosomes. The nuclei that are encapsulated by the microtubule network no longer pull membrane around themselves and are not pulled to the periphery because tension between nuclei and the membrane is lost.
Although the proposed model for microtubule arrangement seems to make sense for the biology of the C. elegans gonad, it appears to conflict with what is known about microtubule arrays in other cell types. Typically, microtubule minus ends are thought to be near the center of the cell and plus ends at the periphery (Siegrist and Doe, 2007). However, the canonical rearrangement is reversed in oocyte development in other animals. For example, in the middle of Drosophila melanogaster oocyte development, microtubules are rearranged so that minus ends emanate from the cortex (Steinhauer and Kalderon, 2006), although in this case, the principles that underlie the arrangement of microtubules have not been elucidated (Steinhauer and Kalderon, 2006). Further afield, minus ends are also known to be far from the cell center in mammalian (Baas et al., 1988) and Drosophila (Stone et al., 2008) neurons. In all of these examples, the positioning of the nucleation sites is not well understood. This system will provide an opportunity to identify, using a simple genetic organism, how these sites are regulated and to test whether this regulation is the key to setting up different types of architecture in different regions of a single organ.
Materials and methods
Maintenance and culture of C. elegans
Wild-type N2 animals were maintained at 20 or 15°C. The temperature-sensitive mutants zyg-12(ct350) and zyg-12(or577) (provided by J. White, University of Wisconsin, Madison, WI) were maintained at 15°C before temperature shifts. ct350 animals expressing GFP::syn-4 or γ-tubulin::GFP were obtained by crossing lin-31(n301) ct350; him-5(e1490) with strains syn-4(ok372); xuIs88 (gfp::syn-4) (provided by J. Nance, New York University, New York, NY) and unc-119(ed3); ddIs6 [tbg-1::GFP + unc-119(+)], respectively, then cloning green F2 animals with a multivulva phenotype. The F3 generation was shifted to the restrictive temperature to confirm the sterility phenotype of ct350. Temperature shifts were conducted on standard culture plates (Brenner, 1974). For sun-1 and dhc-1 RNAi, we transferred L1 stage animals to RNAi feeding plates with bacteria (strain HT115) expressing double-stranded RNA and assayed animals as adults (Malone et al., 2003). sun-1 and dhc-1 RNAi was performed by feeding animals bacteria that express double-stranded RNA as previously described (Malone et al., 2003).
Immunofluorescence and microscopy
We cut animals to release the gonad on polylysine-coated slides and fixed gonads using methanol at −20°C. Primary ZYG-12 (raised in rat; Malone et al., 2003), SUN-1 (rabbit), SAS-4 (rabbit), DHC-1 (rabbit; provided by S. Strome, University of California, Santa Cruz, Santa Cruz, CA; Schmidt et al., 2005), GFP (rabbit), and α-tubulin (T9026; Sigma-Aldrich) antibodies were diluted 1:200 in PBSBT buffer (PBS containing 0.1% Tween 20 and 3% BSA) and incubated with sample overnight at 4°C. 1:400 dilution of mAb 414 (Covance) was used to stain nuclear pore complex (Rolls et al., 1999). Corresponding Alexa Fluor 488– and 568–conjugated secondary antibodies (Invitrogen) or Cy5 AffiniPure goat anti–mouse IgG (Jackson ImmunoResearch Laboratories) were diluted in PBSBT buffer to a final concentration of 20 µg/ml and incubated with samples at room temperature for 1 h followed by incubation with 10 µg/ml DAPI (Sigma-Alrich) for 5 min. Because the zyg-12 alleles are temperature sensitive, we also fixed the gonads of mutants using paraformaldehyde at 25°C and performed the immunolabeling at 25°C. These conditions had no effect on staining patterns or observed phenotypes. To visualize microtubules structure with high resolution, we extracted gonads and fixed with 4% paraformaldehyde and 0.1% glutaraldehyde in PBST buffer containing 2% Triton-X 100 at room temperature for 15 min. After three washes with PBST buffer, the sample was quenched with 150 mM glycine in PBST for 10 min as described previously (Yu et al., 2008). When examining the position of germline nuclei, we took images at the pachytene region of distal gonad (just before nuclei enter the loop region) and oocytes in proximal gonad (postloop region). Images were acquired using a confocal microscope (LSM 510; Carl Zeiss, Inc.) with a Plan NeoFluar 63× 1.4 NA oil lens (Carl Zeiss, Inc.) operated by LSM 510 software (Carl Zeiss, Inc.) or using a microscope (Eclipse 90i; Nikon) equipped with a Plan Apochromat total internal reflection fluorescence 60× 1.45 NA oil lens (Nikon) and a camera (CoolSnap HQ; Photometrics) operated by SimplePCI software (Compix, Inc). We captured frames every 0.1 µm to create the z-axis series and used LSM 510 software to produce 3D projection images. Image levels were adjusted using Photoshop (CS; Adobe) without altering the γ value and were assembled by Canvas 9 (Deneba).
Yeast two-hybrid assay
The yeast two-hybrid assay was performed with ZYG-12 as bait using the Matchmaker 3 system (Clontech Laboratories, Inc.) as described previously (Malone et al., 2003). Full-length dli-1 was PCR amplified from cDNA yk102f4 using primers 5′-GACCATATGCCACCAACTGCGCAAC-3′ and 5′-GTCCTGCAGTTATGCATCACTGTCCCG-3′ cloned into NdeI and PstI sites of vector pGAKT7 and shifted to the pGADT7 vector. Plasmids were introduced into the yeast AH109 strain, and selection was performed on synthetic defined/−Leu/−Trp plates. Cotransformants were inoculated into liquid YPD medium and grown to OD600 0.8. 1 ml culture was centrifuged and washed once with 1 ml of water and resuspended in 1 ml of water. 10 µl of a 1:100 dilution in water was inoculated on a synthetic defined/−Leu/−Trp/−His/−Ade plate and incubated at 25°C.
Microtubule depolymerization and regrowth
We cultured gonads from strain syn-4(ok372); xuIs88 (gfp::syn-4) for drug treatments as described previously (Wolke et al., 2007). We placed animals in embryonic culture medium (ECM; 84% Leibovitz L-15 medium [Invitrogen], 9.3% FBS [Hyclone], 4.7% sucrose, 0.01% levamisole, and 2 mM EGTA) on a coverslip and cut at the pharynx. We transferred extruded gonads to a 1.5-ml centrifuge tube containing ECM and 10 µg/ml nocodazole. After 1 h, gonads were washed twice with 1 ml ECM then resuspended in ECM and incubated for 0, 20, 30, or 60 min. All gonads were fixed and stained with α-tubulin antibody as described in Immunofluorescence and microscopy.
Ultrastructural analysis
Intact young adult nematodes were fixed and embedded for electron microscopy by conventional methods (Hall, 1995). A two-step fixation was used: animals were first fixed in buffered aldehydes followed by postfixation with osmium tetroxide and en bloc staining with uranyl acetate. Serial thin sections were examined with an electron microscope (CM10; Phillips). The distal gonad was observed in lengthwise sections to better display the relationship between the developing germline, the rachis, and the overlying somatic gonad. Additional TEM images of the gonad in animal N533 can be viewed at http://www.wormimage.org.
Online supplemental material
Fig. S1 shows that the zyg-12(or577) has a normal gonad at 25°C. Fig. S2 demonstrates that sun-1 RNAi disrupts germline nuclear position. Fig. S3 presents confocal images of microtubule architecture in the germline of wild type and zyg-12(ct350) grown at 25°C. Fig. S4 shows 3D projection images of germline microtubules. Fig. S5 shows live and GFP antibody–stained images of the germline of animals expressing γ-tubulin::GFP and images showing regrowth of microtubules at time points after nocodazole removal. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.200902101/DC1.
Acknowledgments
We thank Floyd Mattie, Michelle Stone, Seung Kyu Lee, and Suting Zheng for technical help and John White and Jeremy Nance for worm strains. DHC-1 antisera were kindly provided by Susan Strome. Special thanks to I.L. Minn for valuable discussions and to all other members of Hanna-Rose and Malone laboratories. Some nematode strains were provided by the Caenorhabditis Genetics Center, which is funded by the National Institutes of Health National Center for Research Resources.
Electron microscopy was supported by the National Institutes of Health (grant RR 12596 to D.H. Hall).
Footnotes
Abbreviations used in this paper: ECM, embryonic culture medium; KASH, Klarsicht/Anc-1/Syne-1 homology; MTOC, microtubule-organizing center; SUN, Sad1/unc-84; TEM, transmission EM.
References
- Baas P.W., Deitch J.S., Black M.M., Banker G.A. 1988. Polarity orientation of microtubules in hippocampal neurons: uniformity in the axon and nonuniformity in the dendrite.Proc. Natl. Acad. Sci. USA. 85:8335–8339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bre M.H., Pepperkok R., Hill A.M., Levilliers N., Ansorge W., Stelzer E.H., Karsenti E. 1990. Regulation of microtubule dynamics and nucleation during polarization in MDCK II cells.J. Cell Biol. 111:3013–3021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner S. 1974. The genetics of Caenorhabditis elegans.Genetics. 77:71–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugnard E., Zaal K.J., Ralston E. 2005. Reorganization of microtubule nucleation during muscle differentiation.Cell Motil. Cytoskeleton. 60:1–13 [DOI] [PubMed] [Google Scholar]
- Dammermann A., Muller-Reichert T., Pelletier L., Habermann B., Desai A., Oegema K. 2004. Centriole assembly requires both centriolar and pericentriolar material proteins.Dev. Cell. 7:815–829 [DOI] [PubMed] [Google Scholar]
- Fischer J.A., Acosta S., Kenny A., Cater C., Robinson C., Hook J. 2004. Drosophila klarsicht has distinct subcellular localization domains for nuclear envelope and microtubule localization in the eye.Genetics. 168:1385–1393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibert M.A., Starck J., Beguet B. 1984. Role of the gonad cytoplasmic core during oogenesis of the nematode Caenorhabditis elegans.Biol. Cell. 50:77–85 [DOI] [PubMed] [Google Scholar]
- Gonczy P., Pichler S., Kirkham M., Hyman A.A. 1999. Cytoplasmic dynein is required for distinct aspects of MTOC positioning, including centrosome separation, in the one cell stage Caenorhabditis elegans embryo.J. Cell Biol. 147:135–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall D.H. 1995. Electron microscopy and three-dimensional image reconstruction.Methods Cell Biol. 48:395–436 [DOI] [PubMed] [Google Scholar]
- Hall D.H., Altun Z.F. 2008. C. elegans Atlas. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY: 348 pp [Google Scholar]
- Hall D.H., Winfrey V.P., Blaeuer G., Hoffman L.H., Furuta T., Rose K.L., Hobert O., Greenstein D. 1999. Ultrastructural features of the adult hermaphrodite gonad of Caenorhabditis elegans: relations between the germ line and soma.Dev. Biol. 212:101–123 [DOI] [PubMed] [Google Scholar]
- Hedgecock E.M., Thomson J.N. 1982. A gene required for nuclear and mitochondrial attachment in the nematode Caenorhabditis elegans.Cell. 30:321–330 [DOI] [PubMed] [Google Scholar]
- Hirsh D., Oppenheim D., Klass M. 1976. Development of the reproductive system of Caenorhabditis elegans.Dev. Biol. 49:200–219 [DOI] [PubMed] [Google Scholar]
- Jantsch-Plunger V., Glotzer M. 1999. Depletion of syntaxins in the early Caenorhabditis elegans embryo reveals a role for membrane fusion events in cytokinesis.Curr. Biol. 9:738–745 [DOI] [PubMed] [Google Scholar]
- Lechler T., Fuchs E. 2007. Desmoplakin: an unexpected regulator of microtubule organization in the epidermis.J. Cell Biol. 176:147–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R., Gundersen G.G. 2008. Beyond polymer polarity: how the cytoskeleton builds a polarized cell.Nat. Rev. Mol. Cell Biol. 9:860–873 [DOI] [PubMed] [Google Scholar]
- Lu J., Dentler W.L., Lundquist E.A. 2008. FLI-1 Flightless-1 and LET-60 Ras control germ line morphogenesis in C. elegans.BMC Dev. Biol. 8:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone C.J., Misner L., Le Bot N., Tsai M.C., Campbell J.M., Ahringer J., White J.G. 2003. The C. elegans hook protein, ZYG-12, mediates the essential attachment between the centrosome and nucleus.Cell. 115:825–836 [DOI] [PubMed] [Google Scholar]
- Meads T., Schroer T.A. 1995. Polarity and nucleation of microtubules in polarized epithelial cells.Cell Motil. Cytoskeleton. 32:273–288 [DOI] [PubMed] [Google Scholar]
- Meng W., Mushika Y., Ichii T., Takeichi M. 2008. Anchorage of microtubule minus ends to adherens junctions regulates epithelial cell-cell contacts.Cell. 135:948–959 [DOI] [PubMed] [Google Scholar]
- Nguyen-Ngoc T., Afshar K., Gonczy P. 2007. Coupling of cortical dynein and G alpha proteins mediates spindle positioning in Caenorhabditis elegans.Nat. Cell Biol. 9:1294–1302 [DOI] [PubMed] [Google Scholar]
- Penkner A., Tang L., Novatchkova M., Ladurner M., Fridkin A., Gruenbaum Y., Schweizer D., Loidl J., Jantsch V. 2007. The nuclear envelope protein Matefin/SUN-1 is required for homologous pairing in C. elegans meiosis.Dev. Cell. 12:873–885 [DOI] [PubMed] [Google Scholar]
- Rolls M.M., Stein P.A., Taylor S.S., Ha E., McKeon F., Rapoport T.A. 1999. A visual screen of a GFP-fusion library identifies a new type of nuclear envelope membrane protein.J. Cell Biol. 146:29–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salpingidou G., Smertenko A., Hausmanowa-Petrucewicz I., Hussey P.J., Hutchison C.J. 2007. A novel role for the nuclear membrane protein emerin in association of the centrosome to the outer nuclear membrane.J. Cell Biol. 178:897–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt D.J., Rose D.J., Saxton W.M., Strome S. 2005. Functional analysis of cytoplasmic dynein heavy chain in Caenorhabditis elegans with fast-acting temperature-sensitive mutations.Mol. Biol. Cell. 16:1200–1212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegrist S.E., Doe C.Q. 2007. Microtubule-induced cortical cell polarity.Genes Dev. 21:483–496 [DOI] [PubMed] [Google Scholar]
- Starck J. 1977. Radioautographic study of RNA synthesis in Caenorhabditis elegans (Bergerac Variety) oogenesis.Biol. Cell. 30:181–182 [Google Scholar]
- Starr D.A. 2007. Communication between the cytoskeleton and the nuclear envelope to position the nucleus.Mol. Biosyst. 3:583–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr D.A., Han M. 2002. Role of ANC-1 in tethering nuclei to the actin cytoskeleton.Science. 298:406–409 [DOI] [PubMed] [Google Scholar]
- Steinhauer J., Kalderon D. 2006. Microtubule polarity and axis formation in the Drosophila oocyte.Dev. Dyn. 235:1455–1468 [DOI] [PubMed] [Google Scholar]
- Stone M.C., Roegiers F., Rolls M.M. 2008. Microtubules have opposite orientation in axons and dendrites of Drosophila neurons.Mol. Biol. Cell. 19:4122–4129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tassin A.M., Maro B., Bornens M. 1985. Fate of microtubule-organizing centers during myogenesis in vitro.J. Cell Biol. 100:35–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson H.M., Skop A.R., Euteneuer U., Meyer B.J., McNiven M.A. 2002. The large GTPase dynamin associates with the spindle midzone and is required for cytokinesis.Curr. Biol. 12:2111–2117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel B.E., Hedgecock E.M. 2001. Hemicentin, a conserved extracellular member of the immunoglobulin superfamily, organizes epithelial and other cell attachments into oriented line-shaped junctions.Development. 128:883–894 [DOI] [PubMed] [Google Scholar]
- Wolke U., Jezuit E.A., Priess J.R. 2007. Actin-dependent cytoplasmic streaming in C. elegans oogenesis.Development. 134:2227–2236 [DOI] [PubMed] [Google Scholar]
- Yoder J.H., Han M. 2001. Cytoplasmic dynein light intermediate chain is required for discrete aspects of mitosis in Caenorhabditis elegans.Mol. Biol. Cell. 12:2921–2933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J., Starr D.A., Wu X., Parkhurst S.M., Zhuang Y., Xu T., Xu R., Han M. 2006. The KASH domain protein MSP-300 plays an essential role in nuclear anchoring during Drosophila oogenesis.Dev. Biol. 289:336–345 [DOI] [PubMed] [Google Scholar]
- Yu W., Qiang L., Solowska J.M., Karabay A., Korulu S., Baas P.W. 2008. The microtubule-severing proteins spastin and katanin participate differently in the formation of axonal branches.Mol. Biol. Cell. 19:1485–1498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Xu R., Zhu B., Yang X., Ding X., Duan S., Xu T., Zhuang Y., Han M. 2007. Syne-1 and Syne-2 play crucial roles in myonuclear anchorage and motor neuron innervation.Development. 134:901–908 [DOI] [PubMed] [Google Scholar]